Chapter 4 The Cosmeceutical Marketplace
EFFICACY
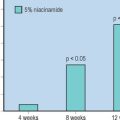
Efficacy is evaluated in numerous ways. There are two primary categories of measurement for ‘antiaging’ moisturizers: ‘health’ benefits (how good a moisturizer it is) and ‘beauty’ benefits (its effect on appearance of fine lines and wrinkles, skin tone and texture, etc.). For ‘health’ benefits, transepidermal water loss (TEWL) can be measured objectively, as can capacitance, an indirect measure with a correlation to moisturizer efficacy. Expert grading is carried out to evaluate visual dryness and redness.
 Is the product from a reputable company? As a general rule, large companies have much more stringent self-regulation on claims than smaller companies.
Is the product from a reputable company? As a general rule, large companies have much more stringent self-regulation on claims than smaller companies.
 Is proof offered via some professional interchange? If the manufacturer will not invest in a clinical study, rule out the product. It is possible, but unlikely, that a manufacturer with a great product just chooses not to prove it.
Is proof offered via some professional interchange? If the manufacturer will not invest in a clinical study, rule out the product. It is possible, but unlikely, that a manufacturer with a great product just chooses not to prove it.
 What is the quality of the proof? The manufacturer’s dilemma is that opportunities to profit from sales of new technology occur in the here and now, and publishing in some journals happens ‘in the future’, if at all. If the manufacturer has made reasonable attempts to do quality studies and has exposed them to peer review in some manner, they should be viewed favorably (posters at medical meetings, or engaging reputable dermatologists in the design and implementation of a clinical study would qualify). The term ‘data on file’ means a study was done, usually by the manufacturer, but not published, and so there is no citation to offer. If the reputation of the manufacturer is good, then this represents quality research.
What is the quality of the proof? The manufacturer’s dilemma is that opportunities to profit from sales of new technology occur in the here and now, and publishing in some journals happens ‘in the future’, if at all. If the manufacturer has made reasonable attempts to do quality studies and has exposed them to peer review in some manner, they should be viewed favorably (posters at medical meetings, or engaging reputable dermatologists in the design and implementation of a clinical study would qualify). The term ‘data on file’ means a study was done, usually by the manufacturer, but not published, and so there is no citation to offer. If the reputation of the manufacturer is good, then this represents quality research.
COMPLIANCE
Compliance in the category of cosmeceuticals is most often achieved when the final product is aesthetically pleasing and can be considered overall as a cosmetically elegant product (Fig. 4.1). Such attributes as having superior packaging, a nice feel and spread during application, absorbing readily into the skin, and improving the appearance of the skin immediately in a cosmetic or superficial way are all important when it comes to product usage compliance.
MARKETING TRENDS
The demand for cosmeceuticals has exploded since the introduction of hydroxy acids for exfoliation and wrinkle defense in the 1980s. The use of Botox, which began in the late 1990s, also coincides with increased demand from consumers for nonsurgical procedures to address the visible signs of aging. The US is the largest market for cosmeceuticals with sales of $4.2 billion in 2006 (Datamonitor, April 2007). Sales for 2007 exceeded $5 billion, with skin care products remaining dominant at approximately 80% of total sales. Forecasters estimate that demand will grow at an annual rate of between 8 and 11% through 2010.
EDUCATIONAL MARKETING
• Celebrity and media endorsements
It should also be noted that endorsements and testimonials from ‘everyday women and men’ are also used frequently by marketers. While the celebrity positions the product in a glamorous light, the ‘girl next door’ testimonial can reinforce the product’s accessibility and real life results.
MARKETING DISTRIBUTION CHANNELS
• Office dispensing
In a study published in the Archives of Dermatology, ‘Physicians’ and patients’ perspectives on office-based dispensing: the central role of the physician–patient relationship’ by Ogbogu et al (2001), the authors concluded:
• Infomercials
Direct-to-consumer (DTC) advertising has had a major impact on propelling market growth. It is estimated that more than $2 billion was spent on cosmetic advertising in 2006. One type of advertising, designed to ‘educate’ the consumer and provide testimonials, whether from customers, celebrities or physicians, is the paid television infomercial.
SUMMARY
 our aging population’s desire to live longer and ‘stop the clock’
our aging population’s desire to live longer and ‘stop the clock’
 the proliferation of and ease of access to trusted product lines
the proliferation of and ease of access to trusted product lines
 an increasing amount of scientific research invested in product development, efficacy, and safety testing
an increasing amount of scientific research invested in product development, efficacy, and safety testing
 the convenience and price of cosmeceuticals, as compared to invasive procedures such as plastic surgery or Botox
the convenience and price of cosmeceuticals, as compared to invasive procedures such as plastic surgery or Botox
 increased media attention and advertising that keep products top-of-mind for consumers
increased media attention and advertising that keep products top-of-mind for consumers
 the use of alternate distribution channels to reach a wider consumer audience.
the use of alternate distribution channels to reach a wider consumer audience.