Chapter 9 The Chiari Malformations and Syringohydromyelia
• Chiari malformations are pathological herniations of the hindbrain through the foramen magnum and into the cervical spinal canal. Chiari malformations are being recognized with increasing frequency because of the increased availability of magnetic resonance imaging (MRI).
• Chiari I malformation (CIM) represents downward herniation of the cerebellar tonsils into the cervical canal at least 3 to 5 mm on sagittal MRI. The impaction of the tonsils in the foramen magnum causes an anatomical and physiological block of cerebrospinal fluid (CSF), which normally flows from the posterior fossa into the cervical subarachnoid space. The symptoms of CIM commonly include headache or neck pain worsened by activity or Valsalva maneuver, or signs of brainstem compression. Syringomyelia may be associated with CIM, and those patients often present with signs of spinal cord dysfunction.
• The most widely accepted treatment of CIM is a posterior fossa decompression with removal of the posterior arch of C1 and dural augmentation graft. The goal of surgery is to enlarge the posterior fossa and permit normal flow of CSF from the posterior fossa to the cervical subarachnoid space. In the majority of patients, the symptoms of headache resolve and there is a concomitant collapse of the associated syrinx. Only rarely does the surgeon have to treat directly the associated syringomyelia.
• Chiari II malformation (CIIM), which is a downward migration of the cerebellar vermis and brainstem through the foramen magnum, is most commonly seen in patients with myelomeningocele. It is associated with hydrocephalus in 90% of the patients. Syringomyelia is also very common. In addition, on MRI, patients often have low-lying tentorium, tectal beaking, cervicomedullary kinks, agenesis of the corpus callosum, and other brain abnormalities. A small, but significant, number of patients will present with acute brainstem, cerebellar, or progressive spinal cord dysfunction. The initial step in this treatment should be the surgical establishment of a functioning shunt. If the symptoms progress or do not resolve in this setting, then a formal decompressive procedure may be required.
• Syringomyelia, or cavitation of the spinal cord, has many causes in addition to hindbrain herniation. Signs and symptoms of a syrinx include pain and temperature loss, reflex changes, or motor weakness. Treatment of the syrinx, when not the result of hindbrain herniation, often includes shunting of the syrinx into the peritoneum or pleura. The goal is to drain the syrinx and to reestablish a patent subarachnoid space.
In the early 1890s, the pathologist Hans Chiari1 described four congenital malformations that would later become known as the Chiari malformations. The four traditional varieties of Chiari malformations represent varying degrees of involvement of rhombencephalic derivatives. Three of these (types I to III) have progressively more severe herniation of these structures outside the posterior fossa as a common feature. These three also have in common a pathogenesis that involves a loss of free movement of cerebral spinal fluid (CSF) out of the normal outlet channels of the fourth ventricle. Pathological differences between Chiari I malformations (CIMs) and Chiari II malformations (CIIMs) can be explained with knowledge of the differences in the timing of the development of the hindbrain herniation.2
Chiari 0—These patients do not appear to have significant hindbrain hernias, although the posterior fossa may appear “crowded”; they have large syringes that resolve with posterior fossa decompression and have a unique position in our thinking about this subject.3,4 These malformations have been informally termed Chiari 0 because they behave as though they have fourth ventricular outlet obstruction, and at surgery they frequently do have physical barriers to CSF movement but do not have caudal displacement of the cerebellar tonsils beyond a point that could be considered pathological.
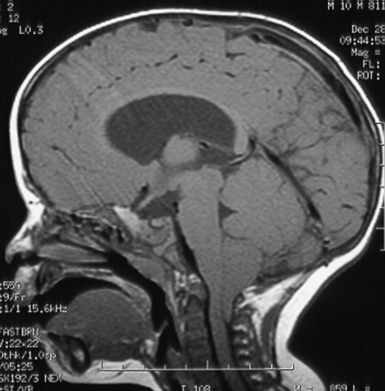
Chiari I—This common group of patients has been found to have caudal displacement of the cerebellar tonsils more than 5 mm below the foramen magnum. The brainstem is in a normal position and they may or may not have a syrinx. The 5 mm “rule” concerning the definition of pathological extent of caudal migration of the tonsils is arbitrary. Numerous patients have tonsils well below this point and are asymptomatic, especially young infants and children. When followed over time they frequently remain asymptomatic if their initial evaluation was performed for an unrelated reason. The extent of their caudal migration may progressively improve with time and become less impressive. This, however, is not assured and the patient should be followed for the development of symptoms. Syringomyelia is often associated with the CIM.
Chiari I.5—Although somewhat confusing, this term is applied to patients who bridge the gap between the CIM and CIIM. They have characteristics of both groups and are best considered separately. They are unassociated with neural tube defects and have caudal displacement of the cerebellar tonsils similar to that seen in the CIM patient. However, their brainstem and fourth ventricle are displaced inferiorly like CIIM patients. In our series of patients with a hindbrain hernia but without a neural tube defect, 17% had significant caudal displacement of the brainstem.5,6
Chiari II—This lesion almost always occurs in patients with neural tube defects (myelomeningoceles and encephaloceles). It consists of caudal migration of the cerebellar vermis, brainstem, and fourth ventricle. Syringomyelia is common in this group.
Chiari III—The Chiari III malformation is a rare and extreme form of hindbrain hernia that may be confused with an occipital encephalocele. It comprises less than 1% of all patients in this category. Patients have low occipital or high cervical sacs containing significant portions of the cerebellum and brainstem.7 Other intracranial anomalies such as are found with CIIM patients may be seen with this group. Hydrocephalus is common and severe neurological and developmental problems are almost always present.
Chiari IV—Type IV Chiari malformation patients have cerebellar hypoplasia or aplasia; this malformation is not a form of hindbrain hernia. For this reason, inclusion in a discussion of hindbrain hernias is debatable and will not be considered further.
Chiari I Malformation
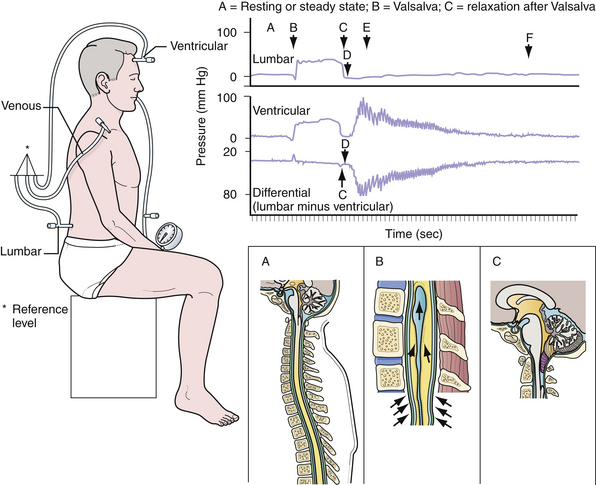
Many theories have been put forth to explain the development of Chiari malformations in the absence of hydrocephalus (Figs. 9.1 to 9.3). Currently, the most appealing theory emphasizes the difficulty in rapidly equilibrating the CSF pressure wave seen during the Valsalva maneuver.8–10 During this delay in achieving equilibrium, there is a vector of force out of the intracranial cavity (Fig. 9.4). The prolonged intracranial hypertension relative to the intraspinal compartment may result in the downward migration of the cerebellar tonsils, resulting in obstruction of normal CSF flow between the posterior cranial fossa and the cervical subarachnoid space. Conditions that impede the physiological flow of CSF at the foramen of Magendie enhance the formation of the malformation. These could include arachnoid veils or septations in this region as well as adhesions.11 Alternatively, conditions that artificially lower the intraspinal pressure relative to the intracranial pressure (lumboperitoneal shunts) have been observed to “cause” the CIM with the subsequent development of syringomyelia. Parenthetically, there is some evidence that the CIM may result from derailment on chromosomes 9 and 15.12
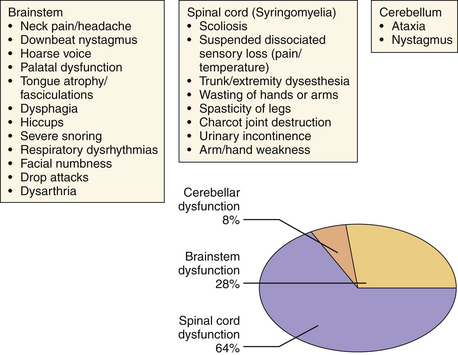
Initially considered an “adult” disease, the CIM is reported more frequently now in the pediatric population as a result of the widespread availability of detailed MRI. The clinical presentation of CIM essentially falls into three categories (Fig. 9.5):
1. Signs or symptoms related to brainstem compression
Most commonly, patients present with occipital or cervical pain that is nonradicular but frequently associated with dysesthesias in the C2 dermatome.13 The neck pain or headache is frequently brought on by exertion or by coughing or sneezing (Valsalva-induced). Nonverbal children may relate their pain via irritability, crying, failure to thrive, or the presence of opisthotonos.
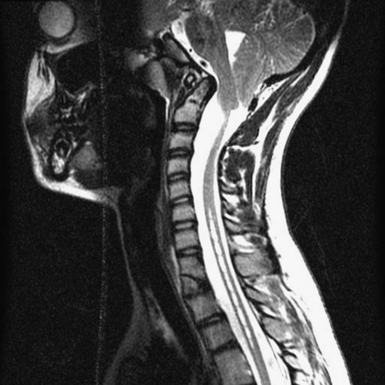
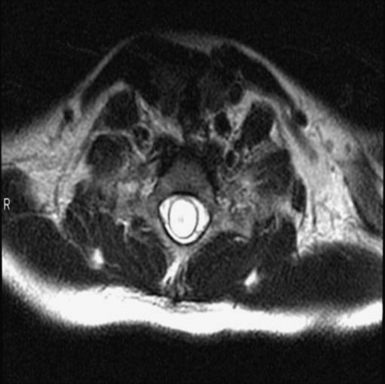
The diagnosis of CIM can easily be confirmed with MRI. The presence and extent of syringomyelia, seen in 50% to 75% of patients,5,14–16 should be determined with an MRI of the entire spinal cord. Computed tomography (CT) may further elucidate the bony abnormalities and plain films may assist in the evaluation of stability issues. Dynamic MRI (cine-MRI) has been used to evaluate CSF flow around the craniocervical junction. In a review of 800 MRI examinations, one study noted that “normal” or “asymptomatic” patients may have cerebellar tonsils that extend 3 mm below the foramen magnum.17 The tonsillar herniation was noted to be clearly pathological when it exceeded 5 mm. Similarly, Barkovich and associates18 studied 200 normal patients and 25 patients with a “firm” diagnosis of CIM. Tonsils 2 mm below the foramen magnum were considered as the lowest extent of the tonsils in normal patients (specificity of 98.5% [three false positive results] and a sensitivity of 100%). Tonsils 3 mm below the foramen magnum were considered the lowest extent in normal patients (sensitivity 96% and specificity 99.5%). An additional study has documented ascent of the tonsils with increasing age in some patients.19
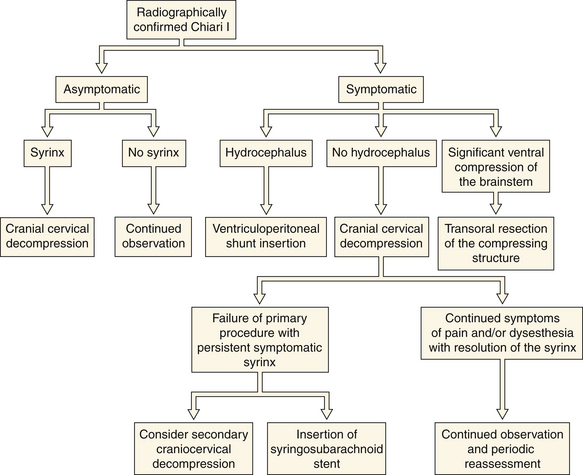
No medical treatment exists for the Chiari malformations. Numerous therapeutic paradigms exist (Fig. 9.6). The first decision that must be made is whether the lesion is truly symptomatic. Observation is warranted in asymptomatic patients without an associated syrinx. In symptomatic patients or asymptomatic patients with syringomyelia, surgical intervention is recommended. The degree of brainstem compression and tonsillar herniation is taken into account as well. In about 10% of patients hydrocephalus is associated with the CIM, in which case a CSF diversionary shunt or endoscopic third ventriculostomy should be the initial form of therapy. Similarly, symptomatic ventral compression out of proportion to dorsal compression may require a ventral decompression (i.e., transoral odontoid resection), especially in cases of myelopathy. The most common surgical procedure for the treatment of CIM is posterior fossa decompression. The goal of the operation is to enlarge the posterior fossa and to re-create the cisterna magna, thereby permitting normal flow of CSF from the posterior fossa to the cervical subarachnoid space. In the majority of CIM patients with a syrinx, the syrinx decreases in size and does not require direct treatment because the posterior fossa decompression treats the underlying pathological process causing the syringomyelia.
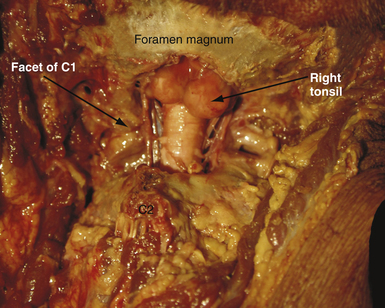
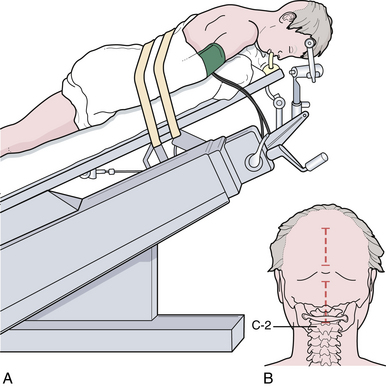
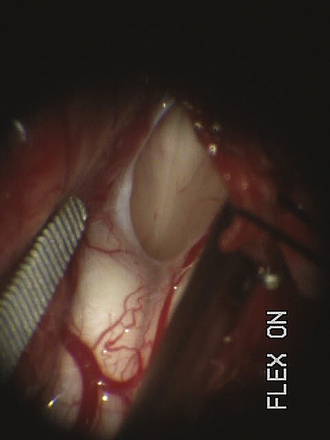
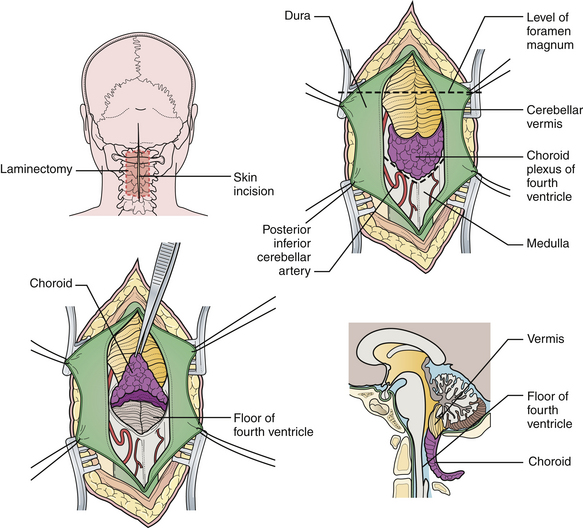
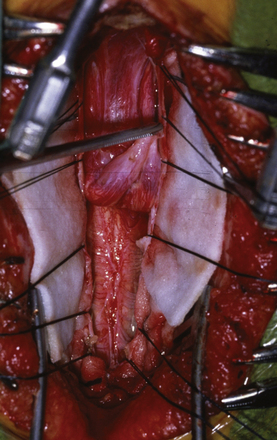
For decompression, the patient should be prone and the neck flexed (Fig. 9.7). The incision extends from a point just below the inion to the spinous process of C2. An avascular plane (nuchal ligament) between the paraspinous muscles is followed down to bone, and a subperiosteal dissection is then performed. A moderate suboccipital craniectomy, the width of the foramen magnum, is followed by the removal of the posterior arch of C1. Some techniques advocate either stopping here or opening the outer layer of the dura and then closing.20,21 We open all layers of the dura and clip the opened arachnoid to the dural edge. Any arachnoid adhesions potentially obstructing the outflow of CSF from the foramen of Magendie are removed and the floor of the fourth ventricle is examined (Fig. 9.8). A portion of the occipital pericranium is harvested through a separate incision and duraplasty performed. The wound is closed in anatomical layers.
The results of craniocervical decompression are encouraging in long-term follow-up. Postoperative outcome is not age-based and early treatment tends toward better outcomes. Nearly 85% of patients will have relief of their head and neck pain, especially if it was Valsalva-induced.5,20 Associated syringes decrease in size or collapse in the majority, albeit not all, of the patients who undergo a well-performed posterior fossa decompression. If decompression fails to improve the symptoms or decrease the size of the syrinx in a 6-month period, consideration should be given to either a reexploration of the posterior fossa with coagulation or resection of a cerebellar tonsil. Placement of a syringosubarachnoid shunt may be indicated in recalcitrant cases of syringomyelia that do not respond to an adequate posterior fossa decompression, although this lack of response is rare. Advanced symptoms of medullary dysfunction, muscle wasting, or dysesthesias in the trunk or extremities are unlikely to resolve but should not progress. Mild to moderate scoliosis has a high likelihood of improvement.
Chiari II Malformation
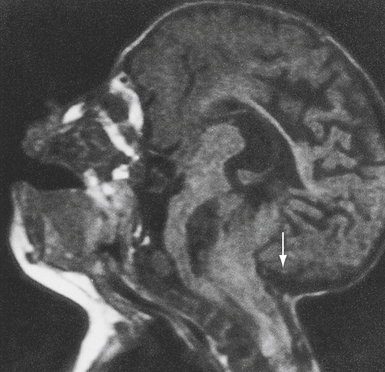
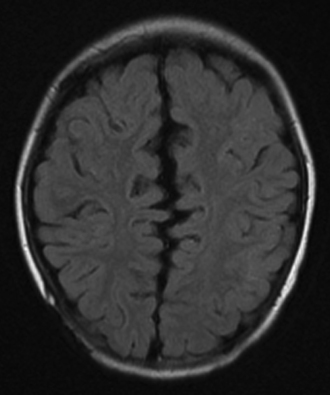
The caudal displacement of the cerebellar vermis, lower brainstem, and fourth ventricle is seen almost exclusively in patients with myelomeningoceles (Fig. 9.9). Numerous other anomalies of the nervous system and its support structure are seen in various combinations (Fig. 9.10). Of particular note is the vertical straight sinus resulting from the low-lying tentorium cerebelli, as well as the possibility of large venous lakes in the tentorium. Fenestrations may also be seen in the falx cerebri, which is often not well formed, and on occasion allow the gyri of the left and right cerebral hemispheres to interdigitate, giving the appearance of “Chinese lettering” on axial MRI (Fig. 9.11). Hydrocephalus occurs in approximately 90% of patients with CIIM.22 Split cord malformation is seen in up to 6% of patients with CIIM and syringomyelia has been reported to occur in between 20% and 95% of patients.2 Other associated anomalies include tectal beaking secondary to partial or complete fusion of the colliculi into a single backward pointed peak and kinking at the level of the cervicomedullary junction. The latter anomaly is caused by caudal displacement of a portion of the medulla in conjunction with a spinal cord that is held in relative immobility by the dentate ligaments. The tracts of the spinal cord then double back on themselves for a short distance.The cerebellum is smaller than usual; in addition, the vermis and adjacent medial cerebellar hemispheres are poorly differentiated from each other. The extent of the medial cerebellar protrusion and the character of tissues involved vary considerably from case to case. Abnormalities of the cerebral cortical pattern have frequently been described in Chiari II patients. Radiographic signs of CIIM evident on CT scans include lückenschädel (85% of cases), scalloping of the posterior surface of the petrous pyramid (80%), tentorial hypoplasia (95%) with a wide incisura and a small posterior fossa, enlargement of the foramen magnum (73%), and inversion (or transtentorial upward herniation) of the cerebellum. Most of these abnormalities may be observed with other conditions; however, their combination is highly suggestive of CIIM. In addition to hydrocephalus, the ventricular system displays multiple abnormalities: the third ventricle may be only mildly dilated and contains a large massa intermedia (75%); the fourth ventricle is typically small or nonvisualized (70%); it is often flattened and elongated, extending into the cervical canal; the lateral ventricles may be asymmetrically dilated, with prominence of the atria and occipital horns (colpocephaly), and the septum pellucidum is frequently absent. The frontal horns and the anterior portion of the third ventricle are frequently pointed and acutely angulated. This sharpness of the frontal horn (lemon sign) and the caudal displacement of the fourth ventricle (banana sign) are relatively easily seen on in utero ultrasound examinations. The upper cervical canal also displays several bony and spinal cord anomalies in association with the CMII. The C1 arch may be incomplete in up to 70% of these patients, with the missing bone being replaced by a fibrous band corresponding to the periosteal envelope. The dorsal arch of C2 is almost always intact. Klippel-Feil fusion anomalies of the cervical spine are present in a small group of patients.23 Basilar impression and C1 assimilation are quite uncommon in CIIM as opposed to CIM. Significant shortening and scalloping of the clivus can be seen.
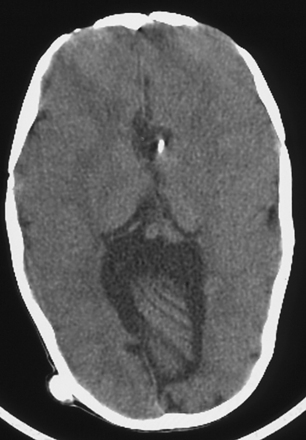
FIGURE 9.10 Axial CT image noting the towering cerebellum seen in patients with Chiari II malformation.
The pathophysiology of the malformation has been postulated to be similar to that of the CIM, that is, a difficulty in equilibrating the dynamic CSF pulse pressure induced by Valsalva.10,24 In the case of CIIM, the presence of CSF pooling in or leaking from the myelomeningocele sac lowers the intraspinal pressure and the result is the ectopia of not only the cerebellar vermis but the abovementioned portions of the brainstem. In support of this theory is the observation of reduced hindbrain herniation observed in some children who undergo fetal myelomeningocele closure.25
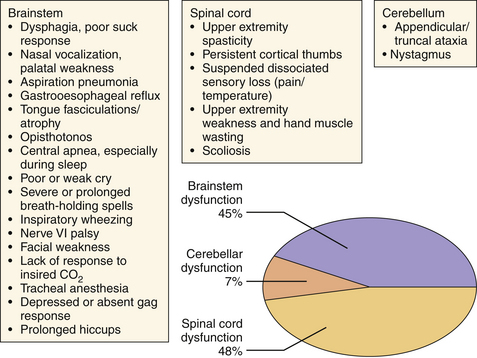
Similarly grouped as in the earlier section, the symptoms of CIIM are best considered according to the area demonstrating disturbed function: brainstem, cerebellar, or spinal cord dysfunction (Fig. 9.12). Approximately 33% of these patients will develop some symptom of hindbrain herniation prior to the age of 5 years and outcome significantly worsens for presentation of these symptoms before 3 months of age.26,27 Symptoms that may result in death if left untreated include stridor, apnea, and dysphagia resulting in aspiration, and are the leading cause of death in myelodysplastic patients. Nystagmus may be the earliest sign of cerebellar dysfunction and the initial spinal cord symptoms (weakness, bowel and bladder dysfunction) are secondary to the inadequate formation of the lower spinal cord. It is important to note that spinal cord function can worsen during development and that CSF diversionary shunt malfunction, syringomyelia formation, or spinal cord tethering must be ruled out and surgically dealt with if present. Scoliosis may also be present in this population and may be related to syringomyelia.
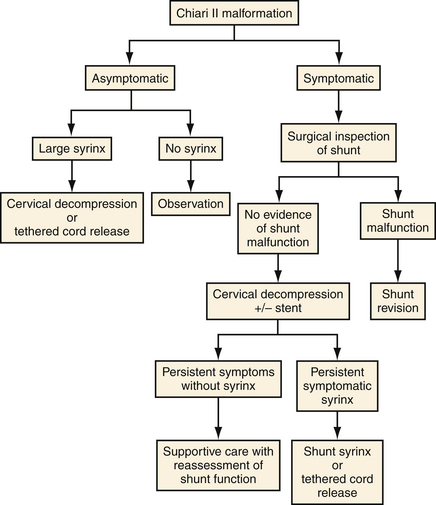
It is important to stress that adequate shunt function must be established prior to pursuing decompression of a symptomatic CIIM (Fig. 9.13).28 This examination includes surgical inspection of the shunt because the ventricular system may not change size in this group. The number of CIIM decompressions at our institution has decreased significantly following the establishment of this paradigm. Symptoms warranting intervention include inspiratory stridor, sleep apnea, recurrent aspiration pneumonia, opisthotonos, and progressive spasticity or ataxia.
When surgical decompression is elected, patients are positioned prone with the neck flexed. Unlike surgical decompression of a CIM, decompression of a CIIM rarely includes removal of the foramen magnum. Removal of the posterior elements of the vertebrae overlying the displaced cerebellar vermis must be performed (Figs. 9.14 and 9.15). The dura is opened widely and the fourth ventricle is identified. The choroid plexus is often in its embryonic extraventricular position. Following this frequently ectopically placed choroid plexus through the dense arachnoidal scarring will usually lead to the foramen of Magendie and the fourth ventricle. This is the crucial goal of the procedure. If any question exists as to the patency of the foramen, then a stent should be placed. The medullary kink, if present, must not be mistaken for the vermis, or the surgeon will end up dissecting into the brainstem. An expansion duraplasty is performed and the remainder of the wound is closed in anatomical layers.
Outcome following surgery is dependent on the severity of symptoms prior to intervention. Infants presenting with brainstem symptoms are less likely to have a significant improvement in their symptoms following decompression. Few studies exist regarding outcome; however, earlier intervention and fewer symptoms are thought to predict success.27
Syringohydromyelia
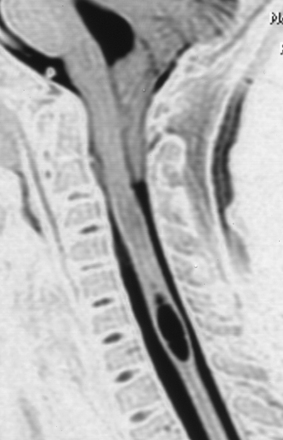
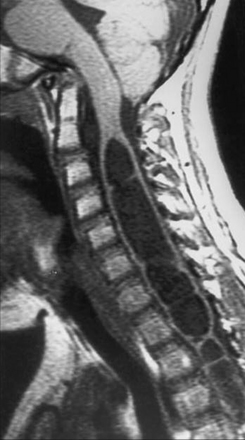
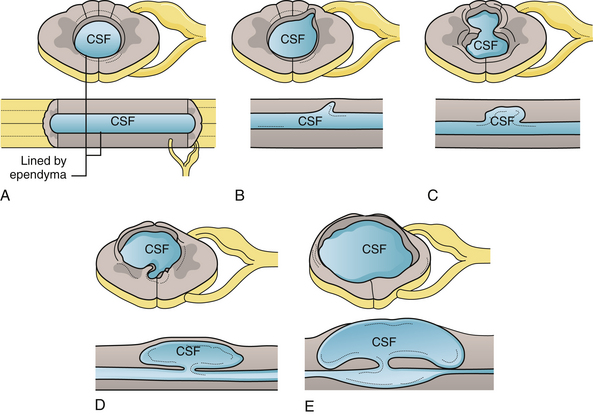
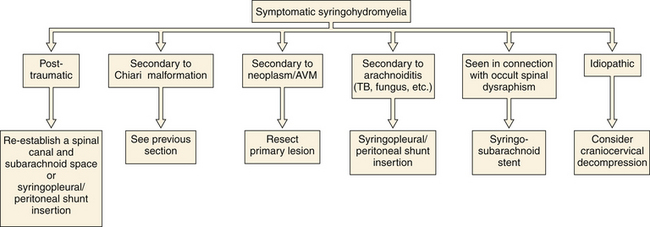
Syringohydromyelia is a secondary process with many causes. The term syringomyelia has been used to describe any longitudinal fluid collection within the spinal cord. This term, however, refers to fluid collections outside the central canal. Hydromyelia refers to fluid collections enlarging the central canal. A combination of these two words, syringohydromyelia better connotes this disease process. However, for the purposes of simplicity we have used the term syringomyelia or syrinx throughout this chapter to denote any fluid collection within the spinal cord. Known pathological situations in which a syrinx may form include post-traumatic circumstances or in association with Chiari malformations (Figs. 9.16 to 9.18), neoplasms, arteriovenous malformations, arachnoiditis, or occult spinal dysraphism. Idiopathic syringomyelia also exists. The mechanism by which the fluid accumulates varies significantly and a useful informal system to classify syringes divides them into either communicating (Chiari, occult spinal dysraphism) or noncommunicating (neoplasm, arteriovenous malformations, arachnoiditis, traumatic). A terminal syrinx (lower one third of the spinal cord) is normally not a result of hindbrain herniation.
Fluid collections that begin in the ependyma-lined central canal (hydromyelia) may cause an outpouching in weak areas that can grow into the white matter of the cord (Fig. 9.19). The absence of ependyma then enables the syrinx to enlarge with less constraint. Of note are the crossing fibers of the spinothalamic tract in the ventral white commissure that are particularly vulnerable to syrinx expansion.
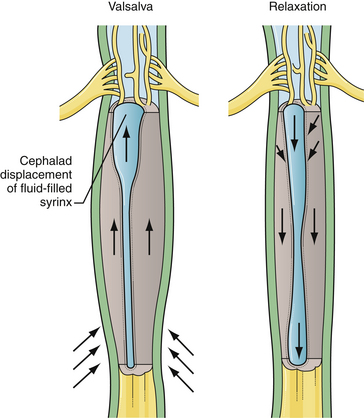
Symptoms include loss of pain and temperature sensation in the arm or chest, diminished or absent deep tendon reflexes, and spasticity of the lower extremities. Longer-term findings include wasting of the intrinsic muscles of the hand and scoliosis. Dysesthetic pain over the thorax and arms is a particularly poor prognostic indicator for postoperative relief of symptoms. Acute loss of neurological function may occur with coughing or straining. When the Valsalva maneuver causes the epidural venous complex to expand, the cavity may be forced to dissect through white matter within the cord (Fig. 9.20). This may result in symptomatic or asymptomatic enlargement of the syrinx.
MRI is the diagnostic procedure of choice for syringohydromyelia. Up to 75% of patients with CIM will have an associated syrinx identified on MRI, and between 20% and 95% of patients with CIIM will have a syrinx seen on imaging.2 An extreme form of syringomyelia, which has ruptured through the pia mater of the spinal cord and expanded, may be seen in this group and is referred to as an exophytic syrinx. These syringes may be confused with spinal arachnoid cysts in this population.
If symptoms are functionally significant (Fig. 9.21) or progressive, intervention should be considered, with the recommended procedure varying widely depending on the underlying condition (Fig. 9.22). No medical therapy exists for syringomyelia. If no alternative explanation for a surgically significant syrinx is identified, exploration of the craniocervical junction should be considered. Follow-up MRI can readily evaluate residual or recurrent fluid. In the setting of a residual syrinx following a decompressed CIM, the patient should return to the operating room and the posterior fossa should be explored for readhesion of the arachnoid at the outlet of the fourth ventricle.29–31 There is no consensus as to how long to wait and at our institution, we wait at least 6 months in asymptomatic patients. It is common for a cerebellar tonsil to be either partially coagulated or resected subpially during the secondary procedure. When operative therapy turns to stenting or shunting the syrinx, note that the arachnoid flow tends to be compromised in certain pathological states (i.e., arachnoiditis, post-traumatic) and therefore the distal end of the shunt should be placed either in the pleural space or the peritoneum. If the distal end of a syrinx stent is placed in the intrathecal space, it is imperative that it be placed in the subarachnoid space for absorption to take place properly. Reestablishment of a patent spinal canal and subarachnoid space may be undertaken in the setting of traumatic disruption through removal of bone fragments or bony realignment. This is made more difficult in the setting of a previous surgical fusion. Occasionally, syringes are multicompartmental and will require drainage at multiple sites. If the syrinx is the result of either arteriovenous malformations or tumor, primary treatment is directed at the appropriate pathology. Outcome is dependent on the degree of disability prior to intervention and the cause of the syrinx. Evaluation for any tethering elements (e.g., fatty filum terminale) should be made in patients with terminal syringes. If tethering is found, the offending element is addressed. If no tethering pathology is found, these patients can be watched unless there is unequivocal evidence for neurological decline (e.g., bladder function).
Hayhurst C., Richards O., Zaki H., et al. Hindbrain decompression for Chiari-syringomyelia complex: an outcome analysis comparing surgical techniques. Br J Neurosurg. 2008;22:86-91.
Milhorat T.H., Chou M.W., Trinidad E.M., et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017.
Oakes W.J., Tubbs R.S. Chiari malformations. In Winn H.R., editor: Youmans Neurological Surgery: A Comprehensive Guide to the Diagnosis and Management of Neurosurgical Problems, 5th ed, Philadelphia: WB Saunders, 1993.
Tubbs R.S., McGirt M.J., Oakes W.J. Surgical experience in 130 pediatric patients with Chiari I malformation. J Neurosurg. 2003;99:291-296.
Tubbs R.S., Shoja M.M., Ardalan M.R., et al. Hindbrain herniation: a review of embryological theories. Ital J Anat Embryol. 2008;113:37-46.
Please go to expertconsult.com to view the complete list of references.
1. Chiari H. Uber Veranderungen des Kleinhirns infolge von Hydrocephalie des Grosshirns. Dtsch Med Wochenshr. 1891;17:1172-1175.
2. Iskandar B., Oakes W. Chiari malformation and syringomyelia. In: Albright L., Pollack I., Adelson P., editors. Principles and Practice of Pediatric Neurosurgery. New York: Thieme; 1999:165-187.
3. Iskandar B.I., Hedlund G.L., Grabb P.A., Oakes W.J. The resolution of syringomyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg. 1998;89:212-216.
4. Tubbs R.S., Elton S., Grabb P., et al. Analysis of the posterior fossa in children with the Chiari 0 malformation. Neurosurgery. 2001;48:1050-1055.
5. Tubbs R.S., McGirt M.J., Oakes W.J. Surgical experience in 130 pediatric patients with Chiari I malformation. J Neurosurg. 2003;99:291-296.
6. Tubbs R.S., Iskandar B.J., Bartolucci A.A., Oakes W.J. A critical analysis of the Chiari 1.5 malformation. J Neurosurg. 2004;101:179-183.
7. Oakes W.J., Tubbs R.S. Chiari malformations. In Winn H.R., editor: Youmans Neurological Surgery: A Comprehensive Guide to the Diagnosis and Management of Neurosurgical Problems, 5th ed, Philadelphia: WB Saunders, 1993.
8. Williams B. Simultaneous cerebral and spinal fluid pressure recordings. II: cerebrospinal dissociation with lesions at the foramen magnum. Acta Neurochir. 1981;59:123-142.
9. Sansur C.A., Heiss J.D., DeVroom H.L., et al. Pathophysiology of headache associated with cough in patients with Chiari I malformation. J Neurosurg. 2003;98:453-458.
10. Tubbs R.S., Shoja M.M., Ardalan M.R., et al. Hindbrain herniation: a review of embryological theories. Ital J Anat Embryol. 2008;113:37-46.
11. Tubbs R.S., Smyth M.D., Wellons J.C.3rd, Oakes W.J. Arachnoid veils and the Chiari I malformation. J Neurosurg. 2004;100:485-487.
12. Boyles A.L., Enterline D.S., Hammock P.H., et al. Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am J Med Genet A. 2006;140:2776-2785.
13. Milhorat T.H., Chou M.W., Trinidad E.M., et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017.
14. Menezes A.H. Chiari I malformations and hydromyelia complications. Pediatr Neurosurg. 1991;17:146-154.
15. Logue V., Edwards M.R. Syringomyelia and its surgical treatment—an analysis of 75 patients. J Pediatr. 1991;118:567-569.
16. Batzdorf U. Chiari I malformation with syringomyelia. Evaluation of surgical therapy by magnetic resonance imaging. J Neurosurg. 1988;68:726-730.
17. Aboulezz A.O., Sartor K., Geyer C.A., Gado M.H. Position of cerebellar tonsils in the normal population and in patients with Chiari I malformation: a quantitative approach with MR imaging. J Comput Assist Tomogr. 1985;9:1033-1036.
18. Barkovich A.J., Wippold F.J., Sherman J.L., et al. Significance of cerebellar tonsillar position on MR. AJNR. 1986;7:795-799.
19. Mikulis D.J., Diaz O., Egglen T.K., Sanchez R. Variance of the cerebellar tonsils with age: preliminary report. Radiology. 1992;183:725-728.
20. Hayhurst C., Richards O., Zaki H., et al. Hindbrain decompression for Chiari-syringomyelia complex: an outcome analysis comparing surgical techniques. Br J Neurosurg. 2008;22:86-91.
21. Isu T., Sasaki H., Takamura H., Kobayashi N. Foramen magnum decompression with removal of the outer layer of the dura as treatment of syringomyelia occurring with Chiari I malformation. Neurosurgery. 1993;33:844-849. discussion 849-850
22. Rauzzino M., Oakes W.J. Chiari II malformation and syringomyelia. Neurosurg Clin North Am. 1995;6:293-309.
23. Curnes J.T., Oakes W.J., Boyko O.B. MR imaging of hindbrain deformity in Chiari II patients with and without symptoms of brainstem compression. Am J Neuroradiol. 1989;10:293-302.
24. Williams B. Cerebrospinal fluid pressure-gradients in spina bifida cystica, with special reference to the Arnold-Chiari malformation and aqueductal stenosis. Dev Med Child Neurol Suppl. 1975;17:138-150.
25. Tulipan N., Hernanz-Schulman M., Bruner J. Reduced hindbrain herniation after intrauterine myelomeningocele repair: a report of four cases. Pediatr Neurosurg. 1988;29:274-278.
26. Curnes J.T., Oakes W.J., Boyko O.B. MR imaging of hindbrain deformity in Chiari II patients with and without symptoms of brainstem compression. Am J Neuroradiol. 1989;10:293-302.
27. Pollack I.F., Pang D., Albright A.L., Krieger D. Outcome following hindbrain decompression of symptomatic Chiari malformations in children previously treated with myelomeningocele closure and shunts. J Neurosurg. 1992;77:881-888.
28. Tubbs R.S., Oakes W.J. Treatment and management of the Chiari II malformation: an evidence-based review of the literature. Childs Nerv Syst. 2004;20:375-381.
29. Tubbs R.S., Webb D.B., Oakes W.J. Persistent syringomyelia following pediatric Chiari I decompression: radiological and surgical findings. J Neurosurg. 2004;100:460-464.
30. Doughty K.E., Tubbs R.S., Webb D., Oakes W.J. Delayed resolution of Chiari I associated hydromyelia after posterior fossa decompression: case report and review of the literature. Neurosurgery. 2004;55:711.
31. Tubbs R.S., Lyerly M.J., Loukas M., et al. The pediatric Chiari I malformation: a review. Childs Nerv Syst. 2007;23:1239-1250.