The Chest Wall
The chest wall provides support and protection to the various thoracic vascular and nonvascular structures. In addition, it allows the important physiologic motion of the lungs and airways. The chest wall is less rigid and more cartilaginous in children than in adults and consists of several fundamental structural components, including bones (ribs, sternum, and vertebrae), nerves, muscles, vessels, and subcutaneous soft tissues.1 Within these structural components, a wide variety of localized or systemic processes (e.g., congenital and developmental anomalies, inflammatory and infectious diseases, benign and malignant neoplasms, and traumatic lesions) can occur in pediatric patients.1–3
Congenital and Developmental Anomalies
During childhood, congenital and developmental anomalies of the thorax typically manifest as deformities or anterior chest wall defects that can be isolated or part of a syndrome. Most of these lesions do not have serious physiologic consequences but rather cause cosmetic problems. However, pediatric patients with severe degrees of congenital or developmental anomalies of the chest wall may present with pain, respiratory distress, or cardiovascular compromise. Reconstructive surgery can be offered to correct the chest wall deformities or defects in both asymptomatic and symptomatic pediatric patients. Imaging can provide a road map for the surgeon in evaluating the anatomy and associated anomalies that can affect the reconstructive options, as well as the timing of surgery. Furthermore, accurate recognition of the radiologic manifestation of congenital and developmental conditions that affect the chest wall often provides important clues that point to the correct diagnosis.4
Deformities of the Anterior Chest Wall
Etiology: Most asymptomatic palpable anterior chest wall lesions in pediatric patients are normal anatomic osseous or cartilaginous variants of the shape of the costal cartilage, ribs, and sternum.5,6 These normal anatomic variations occur in approximately one third of children.5 The most common cause of an asymptomatic, palpable anterior chest wall mass on physical examination is prominent anterior convexity of a solitary rib, costal cartilage, or sternal tilt (Fig. 59-1).6 Symptoms that may raise concern and require further evaluation are focal pain and rapid growth of the mass.3,5
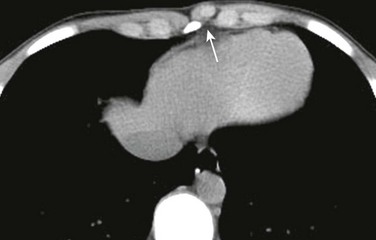
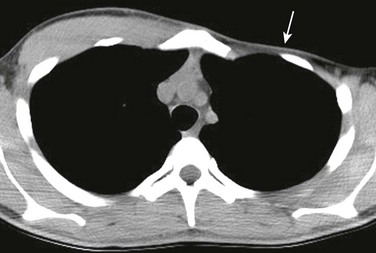
Figure 59-1 A chondral nodule in an adolescent boy who presented with a focal, palpable, painless lump in the anterior chest wall.
An axial computed tomography soft tissue window image shows a small, round, well-defined structure (arrow) protruding anteriorly in the chest wall to the left of the sternum with the same density as the adjacent costochondral junctions.
Imaging: In the case of a palpable but otherwise asymptomatic anterior chest wall lesion in children, a chest radiograph with a BB marker is usually adequate to exclude a potentially aggressive or malignant underlying condition. When chest radiographic findings are equivocal, ultrasound can be performed.7 To minimize ionizing radiation exposure, computed tomography (CT) should be reserved for situations in which further characterization is still necessary after radiographic and ultrasound evaluation.3,6 Although it often is less available than CT, magnetic resonance imaging (MRI), which is not associated with ionizing radiation exposure, is a particularly helpful imaging modality for confirming and characterizing congenital and developmental chest wall lesions.
Pectus Deformity
Etiology: Two of the most common abnormalities of the chest wall are pectus excavatum and pectus carinatum, with the former being the most common congenital deformity of the sternum and the most common sternal deformity requiring surgery. The current leading theory for the development of pectus excavatum is a misdirected, rapid growth of the lower costal cartilages. Such abnormal growth of the lower costal cartilages, which often intensifies during growth spurts, displaces the sternum either inward or outward, resulting in pectus deformity.8 Pectus excavatum occurs in between 1 in 400 and 1 in 1000 live births.9 In persons with pectus excavatum, the inferior aspect of the sternum is depressed inward, resulting in varying degrees of anterior chest wall concavity and anterior protrusion of the costochondral junctions. Although most cases of pectus excavatum are isolated, approximately 45% of cases are familial.10,11
Pectus carinatum is a less common chest wall deformity that occurs in approximately 1 in 1500 live births and has a familial occurrence of approximately 25%.9 In persons with pectus carinatum, outward displacement of the sternum occurs, with secondary abnormal protrusion of the ribs.
Pectus deformities may occur in association with scoliosis (more frequently with pectus excavatum), Marfan syndrome, Ehlers-Danlos syndrome, Poland syndrome, and congenital heart disease. In addition to cosmetic issues, severe pectus deformities also can cause chest pain, dyspnea, palpitations, and restrictive lung disease.3
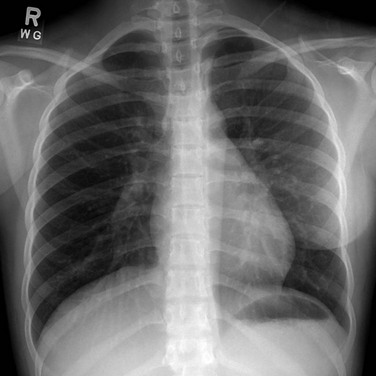
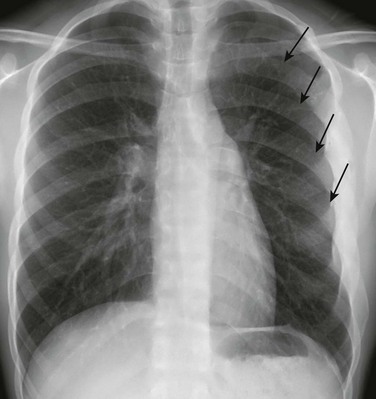
Imaging: On frontal chest radiographs, the sternal depression of pectus excavatum may cause a pseudo-infiltrate over the right side of the heart and various degrees of leftward cardiac shift. On the lateral chest radiograph, the sternal depression causes narrowing of thoracic anteroposterior (AP) diameter (Fig. 59-2). Pectus carinatum usually is diagnosed on a lateral chest radiograph, which shows an increased AP diameter of the thoracic cavity and an extended retrosternal space (Fig. 59-3).3,8
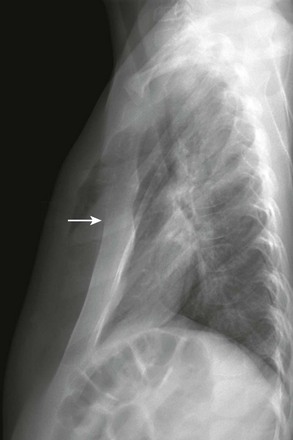
Figure 59-2 Pectus excavatum in an 8-year-old girl.
A lateral chest radiograph shows the sternal depression (arrow) causing narrowing of the anteroposterior diameter of the thorax.
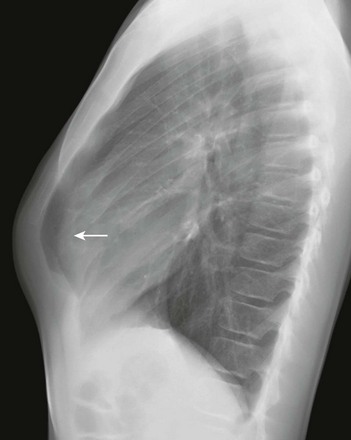
Figure 59-3 Pectus carinatum in an adolescent boy.
A lateral chest radiograph demonstrates an increased anteroposterior diameter of the thoracic cavity and extended retrosternal space (arrow).
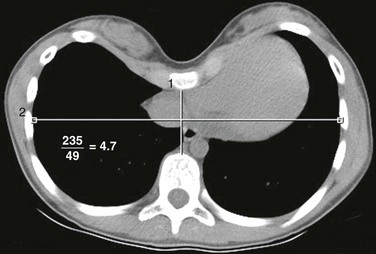
The Haller or pectus index is used to estimate the severity of pectus excavatum. It is calculated from an axial CT image by dividing the maximum transverse dimension of the thorax from the inner aspect of the ribs by the AP dimension of the thoracic cavity at its narrowest point (Fig. 59-4). A pectus index greater than 3.25 in symptomatic pediatric patients typically initiates corrective surgery, whereas an index less than 2.56 is considered normal.12 Recent studies have advocated the use of chest radiographs instead of CT to preoperatively evaluate the severity of the deformity.13 In more severe cases, the use of a limited, low-dose CT scan with selective 5-7 axial CT images through the deformity can be obtained to generate the pectus index.14 When the primary purpose of imaging is to assess the Haller index, axial MRI can be used to avoid ionizing radiation.15
Treatment and Follow-up: Severe pectus deformity requires surgical correction, particularly in symptomatic pediatric patients. The Ravitch procedure for pectus excavatum includes resection of deformed cartilages and correction of the sternum by a wedge osteotomy in the upper sternal cortex. In the Nuss procedure, a convex metal bar is placed behind the sternum, pulling the sternum anteriorly. Corrective surgery for pectus carinatum also requires costochondral resection and a wedge osteotomy, but the sternum is pushed inward. The Nuss procedure has a 4% to 11% complication rate,16 and thus follow-up imaging is necessary to monitor the implanted bar and its complications. Infection and dislocation of the metallic bar are the two most common complications following the Nuss procedure.16–18
Cleidocranial Dysostosis
Etiology: Cleidocranial dysostosis is a rare syndrome usually caused by an autosomal dominant gene with high degree of penetrance and variable expression. In this condition, a mutation is present in the CBFA1 gene located in the chromosome 6p21, which encodes a protein necessary to activate osteoblast differentiation. The triad of partial or complete absence of the clavicles, supernumerary or impacted teeth, and delayed fontanel closure is highly suggestive of this syndrome. Other features of the syndrome include midface hypoplasia, short stature, and pelvic and distal phalangeal hypoplasia that causes brachydactyly.19–21
Imaging: Typical chest radiographic findings include a bell-shaped thorax, short ribs, and either complete or partial absence of the clavicles (e-Fig. 59-5). When the clavicle is partially absent, the distal portion of the clavicle usually is involved. Scoliosis may be present. Because many of the characteristic manifestations only become evident during adolescence, early clinical diagnosis may be difficult and often requires a skeletal survey. Other features of the syndrome can support the diagnosis.19–21
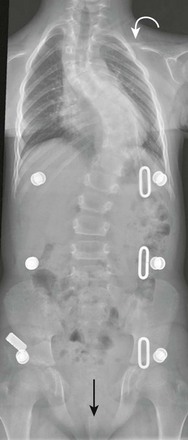
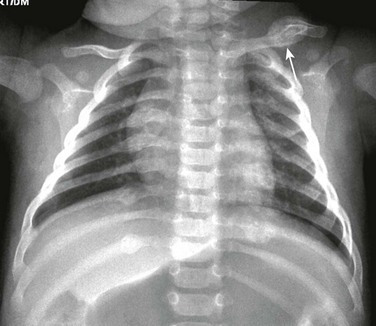
e-Figure 59-5 Cleidocranial dysostosis in an adolescent boy.
A chest and abdominal radiograph shows absence of the right clavicle and hypoplasia of the distal half of the left clavicle (curved arrow). Diastases (straight arrow) of the symphysis pubis also is present. The patient has scoliosis and is wearing a brace.
Treatment and Follow-up: Multidisciplinary management by a team of specialists including pediatricians, geneticists, and orthodontists is the optimal approach for patients with cleidocranial dysostosis. Genetic counseling is recommended. No imaging follow-up is required once the diagnosis has been made.19
Poland Syndrome
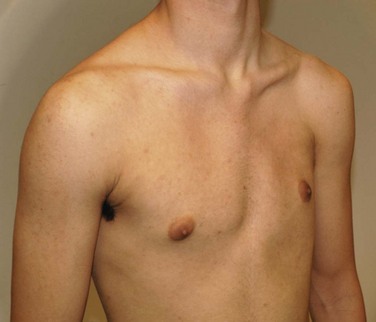
Etiology: Poland syndrome is a form of chest wall and breast hypoplasia that occurs in 1 in every 20,000 to 30,000 live births.22 The syndrome includes absence of the pectoralis muscles and ipsilateral syndactyly. Other associations include rib aplasia, amastia/athelia, and absence of axillary hair. Poland syndrome typically is unilateral and (more often) sporadic and occurs more frequently in boys than in girls, with a ratio of 2-3 : 1.23 The tendency toward unilaterality (with the right side affected more frequently than the left side) has raised the hypothesis that this anomaly is perhaps a result of interrupted or insufficient blood supply during limb bud development in the sixth week of gestation.23 Poland syndrome is diagnosed clinically by the apparent asymmetry of the chest wall (e-Fig. 59-6) and syndactyly. Cardiopulmonary impairment and functional deficiency of the shoulder, although rare, also can be present.3,22,23
Imaging: On chest radiographs, hyperlucency of the affected hemithorax is usually apparent in unilateral cases because of the absent pectoralis muscle and breast (Fig. 59-7). Because the extent of involvement is difficult to assess with clinical examination or chest radiographs, cross-sectional imaging with CT or MRI is useful (Fig. 59-8). Furthermore, it is imperative to preoperatively assess the presence of all of the chest wall and anterior abdominal wall muscles (e.g., the latissimus dorsi and rectus abdominis muscles), because muscle flaps are used in surgical reconstruction.2,22
Treatment and Follow-up: Corrective surgery often is performed for cosmetic reasons, particularly in girls. Such surgery includes (1) restoration of the structural integrity of the rib cage and (2) improvement in the appearance of the chest by performing transposition of musculocutaneous flaps (most commonly with latissimus dorsi and rectus abdominis muscles), as well as concomitant breast implants in female patients.8,22,23
Infectious Diseases
Chest wall infections are relatively rare in children. Chest wall infections occur either by direct extension or, less frequently, by hematogenous spread. Such infectious processes can involve the soft tissues, cartilage, and osseous structures. Chest wall infections can be the result of bacterial, mycobacterial, or fungal infections. They range from superficial cellulitis to deeper infections, which include myositis, abscess, necrotizing fasciitis, and osteomyelitis.24,25
Bacterial Infection
Etiology: Bacterial infections of the chest wall are rare in children. The common organisms that cause bacterial infections of the chest wall are Staphylococcus aureus and Salmonella species in persons with sickle cell disease. Sternal osteomyelitis may occur in patients with an underlying disease, such as immunodeficiency and hemoglobinopathies.11 Rib osteomyelitis is most commonly acquired by direct spread from an adjacent pleuropulmonary infection, such as severe pneumonia or empyema. Empyema necessitatis is a rare complication of empyema characterized by the extension of the infection from the pleural space into the chest wall. Pediatric patients with a bacterial chest wall infection often present with focal soft tissue erythema, edema, and pain. Additionally, other signs of infection, such as leukocytosis and elevated erythrocyte sedimentation rate, often are present.3,11,26,27
Imaging: Chest radiographs may reveal localized soft tissue edema in cases of cellulitis or fasciitis. Bone changes such as periosteal reaction or lytic or sclerotic lesions can be present, particularly in the late stage of disease. Concomitant pulmonary consolidation, empyema, and subcutaneous emphysema also may be present. Ultrasound, CT, and MRI are more sensitive than chest radiographs in detecting and characterizing chest wall infections. Ultrasound should be reserved for superficial and more localized chest wall infections because of the limited field of view (Fig. 59-9, A). The extent of the chest wall infection is best demonstrated on CT or MRI. For evaluation of chest wall abscess and bone marrow edema in the earlier stage of osteomyelitis, MRI is the most sensitive imaging modality (Fig. 59-9, B). CT is the imaging modality of choice for evaluating concomitant pulmonary parenchymal infection.2,11,26–28
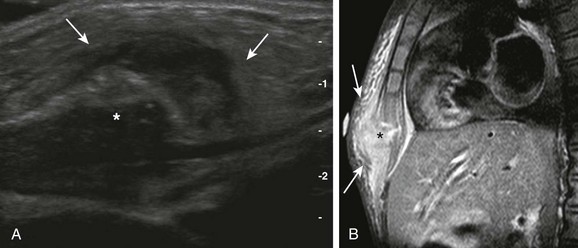
Figure 59-9 Sternal osteomyelitis in a 6-year-old girl who presented with prolonged fever and a painful, palpable, and erythematous sternal lump.
A, A longitudinal ultrasound view of the inferior aspect of the sternum (asterisk) shows marked surrounding soft tissue edema and swelling (arrows).
B, A sagittal contrast T1-weighted image of the chest with fat saturation demonstrates focal destruction of the distal ossification center of the sternum (asterisk) with contrast enhancement and edema of the adjacent soft tissues (arrows).
Treatment and Follow-up: Antibiotics are administered to treat bacterial chest wall infections. In cases of abscesses localized to the chest wall or empyema, drainage may be necessary to relieve the focus of infection and avoid further spread. Prolonged antibiotic therapy is necessary for cases of osteomyelitis.27,29 Evaluation of response to treatment by monitoring C-reactive protein in pediatric patients with osteomyelitis has dramatically decreased both the average duration of therapy (now 3 to 4 weeks) and the need for follow-up imaging.30
Tuberculosis
Etiology: Tuberculosis (TB) is a potentially deadly infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis in humans. Tuberculosis infection of the chest wall often is a result of the hematogenous spread of pulmonary TB infection.31 However, tuberculosis of the chest wall also can occur as an isolated primary infection with no evidence of pulmonary disease, although this presentation is rare.32 Skeletal TB infection accounts for 10% to 20% of extrapulmonary cases and 1% to 2% of all TB infection cases.33 Affected patients present with a slow-growing soft tissue chest wall mass, often without associated pain or fever. Respiratory symptoms may be present in cases of lung involvement.32,34
Imaging: TB infection can involve any bone of the chest wall; however, the ribs are most commonly affected. On radiographs, osseous TB infections are usually lytic and sharply marginated. Associated periosteal reaction is a relatively rare finding.35 On CT or MRI, TB chest wall infections usually present as a rim-enhancing chest wall mass. CT and MRI can play an important role in demonstrating bone involvement from a chest wall TB infection that may not be seen on radiographs.32 Expansion and destruction of the bone with an adjacent soft tissue mass from a TB infection of the chest wall may mimic an underlying malignancy. Rim-enhancing masses with central necrosis and sequestrum indicating abscess formation, regional hypodense adenopathy, and vertebral body involvement also may occur in affected patients.31,33,34
Treatment and Follow-up: Treatment of chest wall TB infection currently is not well established. Some persons advocate the use of an antituberculous medication regimen alone for at least 6 months. Others suggest more aggressive management consisting of surgical excision of affected bones and soft tissues, in addition to preoperative and postoperative antituberculous medication regimens.32,36
Actinomycosis
Etiology: In humans, actinomycosis is most commonly caused by Actinomyces israelii, a branching gram-positive, facultative anaerobe. Actinomycosis infection typically affects the cervicofacial region in patients with dental caries. Pulmonary and chest wall involvement of actinomycosis in children is less frequent without underlying predisposing pulmonary conditions. Actinomycosis infection initially involves the lungs and then usually spreads to the adjacent soft tissues. Clinically, affected patients present with cough, intermittent fever, and weight loss. Pain develops once the microorganism invades the pleura and chest wall.37,38
Imaging: On a chest radiograph, the classic radiologic appearance of actinomycosis infection is a chronic lung consolidation that crosses the fissures with a pleural effusion. Because actinomycosis infection does not respect tissue boundaries, it tends to invade the surrounding tissues with fistulous tracts and abscess formation that are best seen on CT or MRI. Rib periostitis, pulmonary cavitation, and diaphragmatic invasion also may occur in more advanced cases and are very suggestive of actinomycosis infection.38,39Advanced actinomycosis infection of the chest wall may mimic TB or a malignancy of the Ewing sarcoma family of tumors (ESFT).37
Treatment and Follow-up: A definite diagnosis of actinomycosis of the chest wall requires either microscopic visualization of the typical sulfur granules representing the colonies of the microorganism or recovery of the Actinomyces organisms in an anaerobic culture. The standard treatment of actinomycosis is long-term, high-dose penicillin. In cases with an abscess, image-guided percutaneous drainage may help expedite recovery and isolate the organism. Rarely, surgical resection of the area involved is required in cases that are unresponsive to medical treatments.37,38
Tumors of the Chest Wall
Because the chest wall is composed of several different types of tissues, a wide variety of both benign and malignant tumors may occur, including vascular, cartilaginous, osseous, muscular, or adipose tumors. Imaging evaluation plays a pivotal role in (1) initial detection, (2) assessment of the extent of disease, (3) guidance for tissue sampling, (4) evaluation of the response to treatment, and (5) surveillance for recurrence.7,25
Benign Tumors
Etiology: Infantile hemangiomas are benign vascular tumors of abnormally proliferating endothelial cells that follow a predictable course. Infantile hemangiomas are the most common tumor of childhood, with a reported incidence of approximately 10% in the general population.40,41 The etiology of hemangiomas currently is unknown. Several proposed theories include placental emboli, somatic mutation, and clonal expansion of progenitor stem cells.42,43 Infantile hemangiomas are more common in premature white children, with female predominance ratios ranging from 1.4 : 1 to 3 : 1.41 Hemangiomas can involve any part of the body, including the chest wall and breast bud. At birth, these lesions frequently are underdeveloped, with only a macule or discoloration representing a “precursor” lesion. Soon after birth, infantile hemangiomas grow during the so-called proliferative phase and become raised and red in color. Around the first year of life, they involute, leaving a fibro-fatty scar. Multiple lesions are found in 15% to 30% of patients.41,43
Imaging: Ultrasound is usually the first imaging modality of choice, especially for evaluating localized, superficial hemangiomas. For large and extensive lesions with a deep soft tissue component, MRI often is necessary for a complete evaluation. Although CT can be used when MRI is not available, it is less preferable because of the associated radiation and inferior soft tissue characterization. The imaging appearance of infantile hemangiomas may change according to the stage of disease. On ultrasound, hemangiomas are well-defined, lobulated, hyperechoic masses that become more heterogeneous during the involuting phase. Increased vascularity, containing arteries, veins, and even evidence of shunting, often is seen on color Doppler, especially during the proliferative phase (e-Fig. 59-10). On MRI, infantile hemangiomas are isointense or slightly hyperintense compared with muscle on T1-weighted images, and they are hyperintense on T2-weighted images, containing flow voids that represent arteries or rapid venous flow. Hyperintense fatty elements may be seen on T1-weighted images. The pattern of contrast enhancement also varies according to the stage of disease. Marked contrast enhancement usually is seen during the proliferative phase, whereas heterogeneous contrast enhancement reflecting areas of necrosis often is observed during the involuting phase. On CT, hemangiomas present as well-defined soft tissue masses that are isodense to muscle with marked postcontrast enhancement.43,44
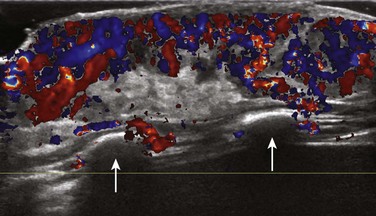
e-Figure 59-10 An infantile hemangioma in a 6-week-old girl in whom a raised red cutaneous lesion developed in the anterior chest wall 3 weeks after birth.
Color Doppler ultrasound of the lesion shows a well-defined soft tissue mass confined to the subcutaneous soft tissues that insinuates along the ribs (arrows) and involves the dermis. The lesion has a hyperechoic and markedly vascular soft tissue component containing arteries and veins.
Treatment and Follow-up: Most infantile hemangiomas require no treatment because they typically involute. A pulsed dye laser can be used for the cosmetic management of superficial lesions. In the past, corticosteroids were the cornerstone of management of hemangiomas; however, secondary effects, such as cushingoid features, were commonly seen.30 Propranolol has been used recently in the treatment of hemangiomas with promising results and minor complications, such as hypoglycemia and hypotension.45,46
Etiology: Lymphatic malformations are a subtype of slow-flow vascular malformations. These lesions are fully developed at birth and grow commensurate to the patient’s growth. Lymphatic malformations consist of dilated lymphatic channels and spaces, with walls lined by mature endothelium that have normal rates of endothelial cell turnover. These lesions occur anywhere in the body, but the cervicofacial, axillary, and chest regions are the most common locations. Lymphatic malformations have no sex predilection. Except for very small lesions, these lesions present as a mass that usually is discovered at birth. The lesions have normal overlying skin and often display positive transillumination. With superimposed hemorrhage or infection, a sudden increase in the lesion size is typical.3,47
Imaging: Plain radiography plays no role in the evaluation of lymphatic malformations in the chest wall of pediatric patients. The imaging workup of lymphatic malformations includes ultrasound and MRI, with the former more commonly used for localized and superficial lesions. More extensive lesions are better evaluated with contrast-enhanced MRI, with CT as an alternative imaging modality. On ultrasound, lymphatic malformations appear as multiloculated cystic lesions that are macrocystic or microcystic, depending on the size of the locules. No internal blood flow is seen on color Doppler imaging. With associated bleeding or superimposed infection, echogenic material, low-level echoes, or fluid-fluid levels can be observed (Fig. 59-11, A). On MRI, the cystic lesions are hyperintense on T2-weighted images (Fig. 59-11, B) and hypointense on T1-weighted images. Lymphatic malformations display no solid components and have minimal, if any, peripheral and septal enhancement, unless superimposed infection is present. Hyperintense elements on T1-weighted images suggest proteinaceous material, bleeding, or infection. Because of their pliable nature, lymphatic malformations typically insinuate and displace, rather than invade, adjacent structures.3,44,48
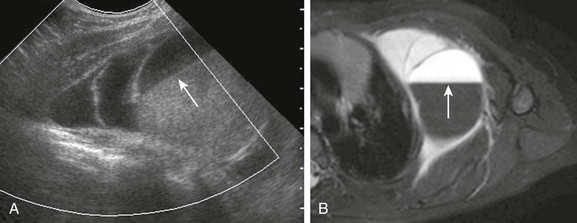
Figure 59-11 Lymphatic malformation in a 2-year-old boy who presented with an enlarging chest wall lump after a fall.
A, A longitudinal view of color Doppler ultrasound shows a multiloculated avascular cystic mass in the anterolateral chest wall. Some of the locules are anechoic, but a fluid-fluid level (arrow) is present in the most dominant one, likely indicating recent bleeding. B, An axial T2-weighted image of the chest demonstrates the multiseptated mass with hyperintense elements representing lymphatic fluid and hypodense elements with a fluid-fluid level (arrow) indicating recent bleeding. The patient has minimal, if any, adjacent soft tissue edema, and no soft tissue component is seen in the lesion.
Treatment and Follow-up: The treatment for lymphatic malformation is percutaneous sclerosis, surgical resection, or frequently a combination of both. Sclerotherapy is the preferred method for macrocystic lymphatic malformations. The treatment of microcystic lesions is more difficult and can include observation, sclerotherapy, and/or surgical resection. Multiple agents, including doxycycline, sodium tetradecyl sulfate, and alcohol currently are used for sclerotherapy. Sclerotherapy usually requires several interventions.44,47,48
Etiology: An infantile fibrous hamartoma is a tumor of neonates, infants, and young children, with approximately 90% occurring in the first year of life.49 They are more common in boys than in girls. The most common location is around the shoulder girdle. The tumor usually presents as a painless, mobile soft tissue mass. Histologically, an infantile fibrous hamartoma contains fibrocollagenous tissue, primitive mesenchyma, and mature fat.49–51
Imaging: An infantile fibrous hamartoma is a well-defined lesion with a heterogeneous echotexture on ultrasound, attenuation on CT, and signal intensity on MRI because of different underlying tissue components. Hyperechoic elements seen on ultrasound and T1 hyperintensities seen on MRI represent the fatty elements, which is an important clue in the diagnosis. On MRI, the fibrous component of infantile fibrous hamartoma is seen as hypointense areas on T1- and T2-weighted images.49
Osseous Tumors
Etiology: Fibrous dysplasia is a benign, intramedullary, fibroosseous lesion thought to occur as a result of a developmental failure in the remodeling of primitive bone to mature lamellar bone and failure of the bone to realign in response to mechanical stress. The etiology of fibrous dysplasia has been linked to an activating mutation in the gene that encodes the alpha subunit of stimulatory G-protein located at 20q133.2-13.3.53 The true incidence of fibrous dysplasia is difficult to estimate, but the lesions are not rare and typically are discovered before the age of 30 years. Fibrous dysplasia has been reported to represent 20% to 30% of chest wall masses, with the rib being the most commonly affected bone.3,54 Fibrous dysplasia can be monostotic or polyostotic; however, monostotic disease does not progress to polyostotic disease. Although a focal mass or chest wall deformity can be seen with fibrous dysplasia, most cases of monostotic fibrous dysplasia are discovered incidentally when radiographs of the area are obtained for different reasons. Localized pain may be the presenting symptom in cases with pathologic fractures or effects of the mass upon adjacent thoracic structures.53,54
Imaging: The diagnosis of fibrous dysplasia usually is made with plain radiographs. Radiographic characteristics of fibrous dysplasia include a focal, well-defined, expansile, intramedullary lytic lesion with a lucent, ground glass, or sclerotic matrix, causing cortical thinning and loss of trabecular definition (e-Fig. 59-12). Rarely, fibrous dysplasia can produce adjacent soft tissue proliferation, or it can be cystic with fluid-fluid levels. Fibrous dysplasia is usually a radiographic diagnosis that requires no further imaging.3,55 When seen on CT, amorphous calcification inside the lesion with bone expansion is usually present. Pathologic fractures also can be confirmed on CT if they are not detected on radiographs. MRI is helpful in assessing the extent of the disease. Despite its name, fibrous dysplasia does not follow the same signal characteristics of pure fibrous tissue. The lesions are isointense to muscle on T1-weighted images. On fluid-sensitive sequences, the lesions are predominantly hyperintense, with focal hypointense, isointense, or markedly hyperintense foci. The heterogeneous signal of fibrous dysplasia on MRI is due to the presence of calcifications, fat, cystic changes, or septations. Fibrous dysplasia displays various contrast enhancement patterns that can be patchy, peripheral, or homogeneous.55,56
Treatment and Follow-up: Fibrous dysplasia of the chest wall usually requires no treatment unless symptoms such as nerve or vascular compression develop. In such cases, resection of the affected bone, usually a rib, can alleviate the symptoms. Impending bone collapse also is an indication for preventive surgical excision and bone grafting.57,58 In symptomatic polyostotic cases, bisphosphonates can be used because of their inhibitory effect on bone resorption. When the lesions are discovered incidentally and the radiographic features are characteristic, no biopsy is indicated; a follow-up study every 6 months to ensure stability currently is advised.53
Etiology: An osteochondroma is a benign cartilage-capped developmental lesion rather than a true neoplasm. It affects bones with epiphyses and apophyses. These lesions result from the separation of a fragment of epiphyseal growth plate cartilage, which subsequently herniates through the periosteal bone cuff normally surrounding the physis. An osteochondroma usually is diagnosed in the first three decades of life and may be inherited as a multifocal, familial, autosomal-dominant disease. Solitary lesions have a male predilection; they occur in approximately 1% to 2% of persons undergoing radiographic evaluations and constitute 10% to 15% of all bone tumors.59 Osteochondromas are the most common benign osseous neoplasm involving the chest wall.3 Most solitary osteochondromas are asymptomatic. Symptomatic lesions tend to occur in younger patients, with 75% to 80% of those cases discovered before the age of 20 years.59 Lesions in the chest wall usually present with a palpable, painless mass or focal deformity. Pain may occur as a result of mechanical irritation, fracture, and nerve or vascular compression.3,55,60
Imaging: Osteochondromas usually can be diagnosed on the basis of plain radiographs. In patients with multiple osteochondromatosis, a skeletal survey usually is obtained to assess the overall extent of disease. In the chest wall, the lesions arise most frequently from the ribs or scapula (Fig. 59-13). They can be sessile or pedunculated, depending on whether a narrow or broad pedicle is located at the base. Corticomedullary continuity is the characteristic imaging finding of an osteochondroma, but this finding may be difficult to completely assess on radiographs. Although both CT and MRI demonstrate the corticomedullary continuity, MRI has an advantage over CT in evaluating the thickness of the cartilaginous cap that should be thin, uniform, and hyperintense on fluid-sensitive sequences. Additionally, MRI can be helpful in assessing the adjacent soft tissue edema and bursa formation that are indicative of the irritation often resulting from osteochondromas. It has been reported that CT with three-dimensional reconstructions can be valuable for the surgeon in preoperative planning.27,55,59,60
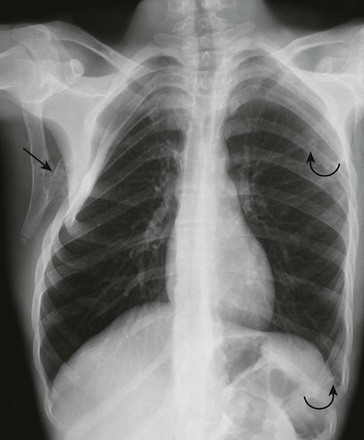
Figure 59-13 Multiple osteochondromas in an adolescent boy with hereditary exostosis.
A frontal chest radiograph shows a large pedunculated osteochondroma (straight arrow) arising from the undersurface of the scapula that is causing remodeling of the underlying right ribs and chest wall deformity. Smaller lesions (curved arrows) are seen in the left scapula and the tenth left rib at the mid axillary line, producing focal rib deformity.
Treatment and Follow-up: Indications for surgical resection of solitary osteochondromas include pain, cosmetic deformity, and nerve impingement. The treatment choice for multiple hereditary exostoses is more complicated and often is directed to correct bone deformity.59,60 Malignant transformation of the cartilaginous cap into a chondrosarcoma occurs in fewer than 1% of solitary cases and in up to 25% of multiple hereditary exostoses.59 The risk of malignant transformation is proportional to the size and number of lesions and is only found in adulthood. Solitary osteochondromas in children do not require routine follow-up unless symptoms or rapid growth occur.60 In cases of multiple lesions, close clinical and radiographic monitoring is required to evaluate the progression of deformities and the development of complications.59
Etiology: A mesenchymal hamartoma of the chest wall is a rare, benign lesion. It typically presents at birth as a large, extrapleural mass arising from a rib that causes chest wall deformity or respiratory distress because of mass effect. Mesenchymal hamartomas, which are not considered true neoplasms, are composed of maturing, proliferating normal skeletal elements with a prominent cartilaginous component and hemorrhagic cavities as a result of aneurysmal bone cyst (ABC) formation. To date, no instances of malignant degeneration of mesenchymal hamartoma of the chest wall have been reported.61–63
Imaging: A mesenchymal hamartoma may have an ominous imaging appearance, despite its benign nature. On chest radiographs, it usually presents as a partially calcified extrapleural mass causing erosion of one or more ribs. A mass effect on the adjacent lungs and mediastinum often is present.64 CT or MRI scans show that a mesenchymal hamartoma is a well-circumscribed lesion with solid and cystic components, the latter representing ABC, a feature specific to this lesion (Fig. 59-14). Cortical bone partially surrounding the lesion and mineralization also can be seen. In one series, mineralization was present in 100% of cases and hemorrhagic cystic areas of aneurysmal bone cyst were present in more than half of patients with a mesenchymal hamartoma.61 On MRI, the signal intensity of mesenchymal hamartoma is variable. Hyperintense areas on fluid-sensitive sequences represent ABC formation and chondroid elements, whereas areas of low signal on T1 and fluid-sensitive sequences represent areas of mineralization.61,65 Multifocal cases of mesenchymal hamartoma also have been reported.61,65
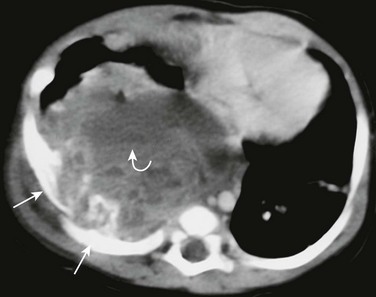
Figure 59-14 A mesenchymal hamartoma in a newborn girl with respiratory distress and a palpable mass in the right chest wall.
A postcontrast axial computed tomography soft tissue window image shows a very large, round mass in the posterior right hemithorax with erosion of two consecutive ribs (straight arrows). The lesion has a heterogeneous parenchyma with a fluid-fluid level (curved arrow) corresponding to the aneurysmal bone cyst component.
Treatment and Follow-up: Surgical excision of a mesenchymal hamartoma with rib resection and chest wall reconstruction is recommended if cardiorespiratory symptoms from mass effect or chest wall deformity are present. Mesenchymal hamartomas, which are benign lesions, generally are cured with en bloc resection. Smaller lesions can be monitored with chest radiographs and ultrasound because spontaneous regression of the lesion has been reported, and the lesions typically stop growing after age 1 year.61,62,66
Other Osseous Tumors (Langerhans Cell Histiocytosis, Enchondroma, and Aneurysmal Bone Cyst):
Etiology: Several other benign tumors can involve the chest wall in the pediatric population, including eosinophilic granuloma (EG), enchondroma, and ABC. These tumors can arise from any bone in the chest wall, including the ribs, scapula, and (less frequently) the sternum.
An EG that is localized to bone is the most common form of Langerhans cell histiocytosis. An EG is characterized by a proliferation of Langerhans cells, which is a type of histiocyte that originates from the bone marrow and plays a role in the immune system. In the chest wall, an EG most commonly affects the thoracic vertebral bodies, causing vertebrae plana, but it can involve any bone. Bone destruction, pain, localized swelling, and a palpable mass often are present in pediatric patients with EG involving the chest wall.67,68
Enchondromas occur less frequently in children than osteochondromas and represent benign, mainly hyaline cartilage tumors arising from the medullary canal of a bone. Most enchondromas are solitary and usually are found incidentally; they also may be multiple, such as in patients with Ollier or Maffucci syndromes.3,26,55,69
ABCs are benign, expansile, osteolytic lesions that contain multiple blood-filled spaces of different sizes and are lined by fibroblasts and giant cells of the osteoclast type. An ABC is a hyperplastic lesion reactive to a subperiosteal or intraosseous hemorrhage. The pathogenesis of the hemorrhage of an ABC currently is unclear. Although they are benign, these lesions can grow rapidly, causing bone destruction and pathologic fractures that produce pain and a palpable mass. ABCs may be found either as primary osteolytic lesions or as a reactive process as part of another lesion. The primary ABC is found mostly in the second decade of life and more frequently in females, with a ratio of 2 : 1.55,70 In the chest wall, the most common sites of involvement from ABCs are the posterior elements of the spine and the ribs.55
Imaging: The classic imaging finding of an EG when it involves the thoracic spine is vertebrae plana, characterized by flattening of vertebral bodies. When it involves other bones of the chest wall, the radiographic appearance of EG varies. In the acute phase of the disease, the lesion develops rapidly and has a more aggressive appearance. In this phase, the lesions typically are lytic with poorly defined borders (Fig. 59-15). Periosteal reaction occurs when the lesion involves the cortex. A soft tissue component of an EG often is present and is best seen on CT or MRI. In the later phase of disease, the lesions can be lytic and sclerotic, and they may adopt a bubbly appearance and have sharp, sclerotic borders.3,67,68
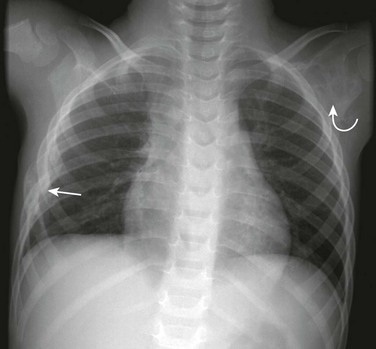
Figure 59-15 Eosinophilic granuloma of a rib and left scapula in a young boy who presented with chest pain.
A frontal chest radiograph shows a lytic, slightly expansile lesion involving a segment of the right sixth rib (straight arrow) with cortical disruption. A second lytic lesion (curved arrow) with rather defined borders involves the left scapular neck.
Enchondromas are osseous lesions arising from the medullary portion of bone with well-defined, lobulated borders and endosteal scalloping. Localized bone expansion and a chondroid matrix frequently are present in enchondromas (Fig. 59-16).3,55,69 ABCs are well-defined, lytic, expansile, intramedullary lesions. In the short and flat bones, such as the ribs, the cyst usually appears as an eccentric osteolytic area extending into the cancellous bone with overlying cortical thinning. Because of the rapid growth, a more aggressive appearance with cortical disruption also can be seen (Fig. 59-17, A). On CT or MRI, ABCs are typically cystic, with multiple locules and fluid-fluid levels representing the underlying hemorrhagic nature of the lesion (Fig. 59-17, B). Fluid-fluid levels, which frequently are seen within the lesions, are not pathognomonic of ABCs. ABCs do not have an associated soft tissue component.55,70
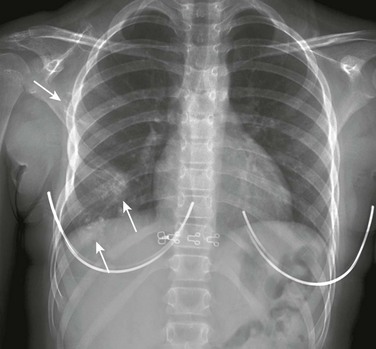
Figure 59-16 Chest wall enchondromas in an adolescent girl with Ollier disease.
A frontal chest radiograph shows several well-defined lytic lesions involving the costochondral junction of the right sixth and seventh ribs and the mid aspect and superior border of the right scapular wing (arrows). Chondroid matrix is better identified in the rib lesions.
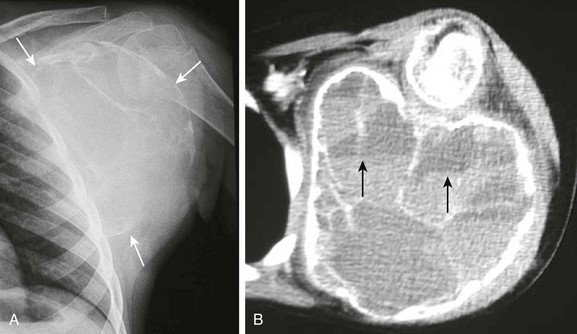
Figure 59-17 A large aneurysmal bone cyst in a patient with a slowly growing left shoulder mass.
A, A frontal view of the left shoulder demonstrates a very large expansile lytic lesion (arrows) arising from the left scapular wing. Thin bony septations are seen inside the lesion. The inferior margin of the lesion is indistinct. B, An axial contrast computed tomography soft tissue image clearly shows the expansile lytic lesion containing multiple bony septae arising from the left scapular wing and its relationship with the rib cage and humerus. Several slightly hyperintense fluid-fluid levels are seen in some of the internal locules (arrows).
Treatment and Follow-up: The treatment for an EG currently is not standardized. Solitary EG lesions initially can be managed conservatively with close radiographic follow-up, because some of these lesions may heal spontaneously with bone remodeling. Alternatively, the lesion can be treated with curettage and bone graft implantation. More disseminated disease may require systemic chemotherapy.67,68 Enchondromas, especially the solitary ones, can be incidental findings and, as such, require no treatment. For symptomatic lesions that often are associated with pathologic fractures, curettage and bone grafting usually are curative.71 The progression of ABCs is variable. Some of the lesions display an aggressive growth pattern, whereas other lesions grow slowly, mature, and may regress spontaneously. ABCs can be treated with selective arterial embolization, percutaneous sclerotherapy, or intralesional excision with bone grafting.72,73 En bloc resection of ABC is recommended for small lesions located in the ribs. For large lesions, excision with bone grafting may be necessary.70
Malignant Tumors
Etiology: Rhabdomyosarcoma (RMS) tumors are the second most common primary malignancy that occurs in the chest wall in children.74 An RMS arises from primitive mesenchymal cells committed to develop into striated muscles. It can arise from any tissue anywhere in the body, except from cortical bone.74 In children, the age-standardized annual incidence rate for RMS is 4 to 7 per million. Tumors of the trunk account for 7% of all RMS tumors.75 In a study of 15 chest wall tumors out of 303 RMS tumors, the mean age of diagnosis was 16 years.76 Affected children typically present with a painful and rapidly enlarging chest wall mass. Respiratory distress can be the presenting symptom in children with large RMS tumors because of mass effect on the adjacent lung or when an associated pleural effusion is present.76,77
Imaging: On ultrasound, an RMS presents as a heterogeneous soft tissue mass with increased internal vascularity. The outer margin of the tumor may be either well defined or ill defined. For a complete assessment of tumor extension, CT or MRI is necessary. Although CT is more sensitive than MRI for the evaluation of osseous abnormality and pulmonary metastatic disease in pediatric patients with an RMS mass, MRI is better than CT for assessment of the local extension of the tumor because of its higher soft tissue characterization capability. On CT and MRI, chest wall RMS usually presents as a large, heterogeneous, soft tissue mass with intrathoracic extension. The tumor tends to show a heterogeneously increased signal on T1 and fluid-sensitive sequences on MRI. After administration of intravenous contrast material, an RMS mass typically displays enhancement that is heterogeneous in cases of underlying tumor necrosis.5,78–80 In comparison with ESFT, bone involvement is much less frequent in children with an RMS, and if present, it tends to occur later in the course of the disease (Fig. 59-18).78
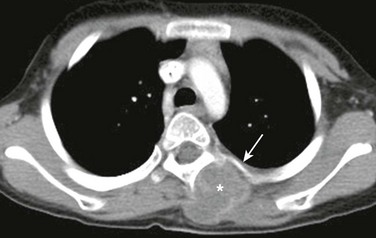
Figure 59-18 A rhabdomyosarcoma of the left paraspinal musculature in a 14-year-old girl who presented with a painful, palpable, soft tissue lump.
A postcontrast axial computed tomography soft tissue window image shows a well-defined, slightly hypodense mass in the left paraspinal region (asterisk). The mass causes mild remodeling of the underlying bone but no destruction (arrow).
Treatment and Follow-up: The current treatment of RMS tumors of the chest wall is chemotherapy to control local disease and reduce the size of the mas before surgical resection. Radiotherapy also often is used if surgical resection margins are positive.74,77 Because of its higher sensitivity in detecting residual, recurrent, or metastatic disease, CT positron emission tomography (PET) in conjunction with MRI currently is recommended as the follow-up imaging modality of choice.81–83
Osseous Tumors
Ewing Sarcoma Family of Tumors:
Etiology: The ESFT represents the most common primary chest wall malignancies in children and young adults.84 They comprise the classic Ewing sarcoma, atypical Ewing sarcoma, primitive neuroectodermal tumor, and Askin tumor. These tumors belong to the small, round, blue cell tumors that originate from unique mesenchymal cells capable of multilineage differentiation. Based on their varied but similar underlying neural differentiation, their immunohistochemical, cytogenetic, and molecular uniformity, and their identical response to Ewing-based chemotherapy regimens, tumors such as ESFT are regarded as being related to sarcomas. A common chromosomal translocation (t11; 22) (q24; q12) has been implicated in 80% to 95% of cases. ESFT lesions have been reported in all age groups, but most cases are diagnosed in the second decade of life, with a median age of 15 years.85,86 The occurrence of an ESFT lesion is more common in males, with a ratio of 1.3 to 1.5 : 1; in addition, a predilection for white persons has been noted.85 Affected children usually present with a rapidly growing palpable chest wall mass that often is painful. In cases with pleural effusions and substantial mass effect on the lung, dyspnea can be the predominant symptom. Pediatric patients with an ESFT lesion in a paraspinal location may present with symptoms related to neurologic impairment. Systemic symptoms, such as fever, and laboratory test abnormalities, including leukocytosis and an elevated erythrocyte sedimentation rate, may simulate infectious processes such as osteomyelitis.77,85
Imaging: On chest radiographs, an ESFT lesion usually presents as an extraparenchymal and lytic osseous mass with associated bone destruction and aggressive periosteal reaction. An associated soft tissue component can be suggested on plain radiographs but is best seen on CT or MRI (Fig. 59-19).85,87,88 Calcifications within the mass may be present in approximately 10% of cases.88 Because of its higher intrinsic contrast resolution and soft tissue characterization, MRI can better depict soft tissue and marrow involvement; local spread, especially into the spinal canal when the lesion has a paraspinal location; and the relationship with adjacent organs or neurovascular structures. On CT and MRI, an ESFT mass typically presents as a lytic and destructive osseous lesion associated with aggressive periosteal reaction and heterogeneous soft tissue components (Fig. 59-20). On MRI, the soft tissue component of an ESFT lesion is isointense or slightly hyperintense to muscle and hyperintense on fluid-sensitive sequences. Heterogeneous contrast enhancement is seen frequently, except in areas of necrosis. Pleural effusions that obscure the soft tissue mass can be present. Regional lymphadenopathy in patients with an ESFT lesion is unusual.85,87,88
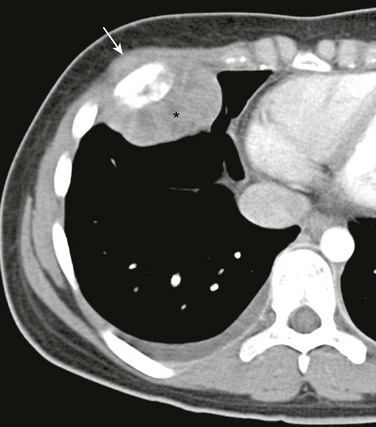
Figure 59-19 A Ewing sarcoma of a rib in an 11-year-old boy who presented with a painful, palpable lump in the anterior chest wall.
A postcontrast axial computed tomography soft tissue window image shows focal thickening of the anterior aspect of a right rib (arrow) surrounded by a soft tissue mass (asterisk).
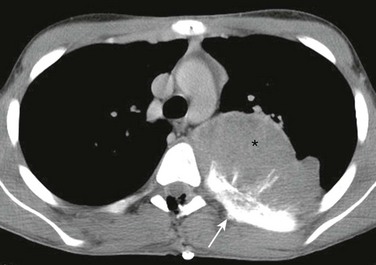
Figure 59-20 A primitive neuroectodermal tumor of a rib in a 10-year-old boy with back pain.
A contrast axial computed tomography soft tissue window image shows a large, extrapleural, soft tissue mass arising from the medial aspect of a left rib (asterisk). The patient has aggressive associated periosteal reaction, sclerosis, and thickening of the rib (arrow).
Treatment and Follow-up: An ESFT lesion of the chest wall currently is treated with chemotherapy to reduce the size of the tumor and achieve optimal local control and control of distant microscopic metastatic lesions, followed by surgical resection.74,77,84,85 Currently, CT-PET is used to stage the tumor and monitor patients with an ESFT mass. CT in the follow-up of these patients plays an important role in detecting pulmonary metastasis, because surgical resection of the pulmonary metastasis and radiotherapy may be curative. Fluorine deoxyglucose PET has a limited sensitivity in the detection of small pulmonary metastasis.81,89
Etiology: A chest wall osteosarcoma is rare. Osteosarcomas that do present in this location occur more frequently in younger children. A chest wall osteosarcoma can arise from a rib, scapula, or clavicle or can be a focus of metastatic disease.77 The incidence of osteosarcomas is 4.8 per 1 million children, with about 1% located in the chest wall and spine.77 The peak incidence of osteosarcoma lesions occurs in the second decade of life.90 Much like the other malignant chest wall tumors, osteosarcomas typically present as rapidly growing and usually painful chest wall masses.2
Imaging: An osteosarcoma typically demonstrates prominent new bone formation on all imaging modalities. Chest radiographs show a lytic or blastic osseous lesion with a soft tissue mass containing calcifications. On CT, areas of bone formation characteristically are seen at the center, rather than the periphery, of the lesion (Fig. 59-21). On MRI, the areas of bone formation are seen as hypointense relative to muscle on T1-weighted images. A mixed but predominantly hyperintense mass is seen on fluid-sensitive sequences. Heterogeneous contrast enhancement of the soft tissue component of an osteosarcoma indicates necrosis.80
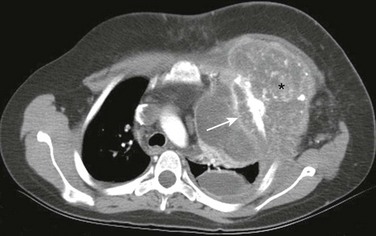
Figure 59-21 An osteosarcoma lesion in an adolescent boy who presented with a painful asymmetry of the anterior chest wall.
A postcontrast axial computed tomography soft tissue image shows destruction of a left rib with aggressive periosteal reaction (arrow) and a large, partially calcified soft tissue mass (asterisk) that is causing deformity of the anterior left chest wall.
Treatment and Follow-up: Chemotherapy and surgical resection constitute the current treatment of choice for a chest wall osteosarcoma; this lesion is relative insensitivity to radiotherapy. The possibility of complete surgical resection is the primary factor in determining the ultimate outcome of affected patients.74
Trauma of the Chest Wall
The chest wall in children is more elastic and flexible than in adults because of its larger cartilaginous component, and thus less force is absorbed by the chest wall with impact and proportionally more force is transmitted to intrathoracic organs. Thus intrathoracic injury may occur without visible damage to the chest wall. Trauma is the leading cause of mortality in children. Although death from a chest injury is uncommon, after head injury, chest trauma is the second most common cause of death in pediatric patients. In children younger than 12 years, 60% to 80% of chest injuries are the result of blunt trauma, and more than half are accounted for by impact with motor vehicles.91–93 However, among infants and young children, it is important to recognize that rib fractures occur most commonly as the result of nonaccidental trauma.94–97
Etiology: Rib fractures constitute the majority of chest wall injuries in children. In a prospective study of 80 children after thoracic trauma, 28 (35%) had rib fractures, and one (1%) had a sternal fracture.94 Rib fractures occur as a result of blunt trauma, such as falls or motor vehicle accidents. In pediatric trauma, the location of rib fractures is more a function of the mechanism of injury than the magnitude of the force that is transferred, although the number of fractured ribs is proportional to the severity of the trauma and the likelihood of associated multisystemic injury. This situation occurs because a greater force is necessary to deform and fracture multiple ribs in pediatric patients.97
In infants or young children (<3 years) without underlying metabolic bone disease, rib fractures are very unusual and highly suggest nonaccidental trauma as a result of abuse.98 Although fractures related to abuse can occur along any part of the ribs, when they are posterior in location, they are highly specific for nonaccidental trauma. This specificity occurs because of the substantial posterior levering force that the transverse processes exert over the posterior aspect of the ribs, resulting in fracture during a tight squeeze by the perpetrator.26,98 In addition to abuse, birth trauma and diseases associated with underlying bone fragility, such as rickets and osteogenesis imperfecta, are other rare causes of rib fractures in infants and young children.99
Imaging: In the acute setting, the initial assessment of possible chest wall injury in pediatric patients with trauma usually requires chest radiographs. Radiographs can be obtained portably and rapidly, and they provide not only an overview of the severity of the trauma and evaluation of the bones but also the location of the support tubes and lines. Once the diagnosis of rib fractures is established, careful attention to exclude associated intrathoracic injury must be provided. The most commonly associated nonosseous abnormality with chest wall injury is pulmonary contusion (Fig. 59-22), followed by pneumothorax. However, other injuries such as hemothorax and fractures of other bones also may be present and can be detected on chest radiographs. In the case of unconscious and hemodynamically unstable pediatric patients, a CT of the chest with contrast using the CT angiography technique can be performed to evaluate the intrathoracic vascular and nonvascular structures.94,93 First rib fractures are uncommon but suggest severe trauma and increased risk of intrathoracic vascular injury.100 Likewise, flail chest is rare in the pediatric population compared with adults. Flail chest occurs when a segment of the rib cage fractures and becomes detached from the rest of the chest wall.101 Lower rib fractures can be associated with traumatic injury to the upper abdominal organs, such as the liver, spleen, and kidneys.102
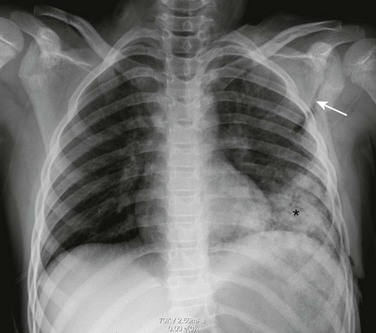
Figure 59-22 Multiple fractures of the left ribs and a left scapular fracture in a young boy involved in a high-speed jet ski accident.
A frontal chest radiograph shows fractures of the seventh through ninth left ribs posterolaterally and more subtle fractures of the left tenth and eleventh ribs. A minimally diastatic fracture (arrow) is seen through the neck and body of the left scapula. Subcutaneous emphysema is seen along the lateral side of the left chest wall, as well as a fluffy consolidation (asterisk) in the left lower lobe consistent with a pulmonary contusion.
In cases of suspected child abuse, a skeletal survey must be obtained. Acute nondisplaced rib fractures can be difficult to detect on radiographs in infants and young children. These fractures become more apparent during healing, when callus is present (Fig. 59-23). Fractures involving the anterior and posterior ends of a rib can be seen better on oblique radiographic views. The presence of acute and healing rib fractures is highly suggestive of abuse.26,98,99 First rib fractures can be seen in the setting of child abuse.103 Because not all chest wall fractures are detected on radiographs, a bone scintigraphy or follow-up radiographs may be necessary.26,98 Assessment of the chest wall musculature and tendon injury can be performed with ultrasound or MRI. Ultrasound also allows dynamic evaluation of the muscle function.104
Treatment and Follow-up: The treatment of rib fractures is supportive with pain medications and aggressive respiratory therapy to prevent atelectasis and pneumonia. In cases of flail chest, ventilatory support during the healing phase may be necessary.93,96 Because nondisplaced acute rib fractures can be difficult to identify on plain radiographs, follow-up chest radiographs or bone scintigraphy can be obtained approximately 1 week later to increase the detection of fractures in cases of suspected child abuse.26,98 Nonoperative, conservative treatment currently is advocated for the low-grade muscular and musculotendinous injury of the chest wall. Early surgical intervention is recommended for patients who have complete tears or avulsion of tendon from the bone.104
Donnelly, LF, Taylor, CN, Emery, K, et al. Asymptomatic, palpable, anterior chest wall lesions in children: is cross-sectional imaging necessary? Radiology. 1997;202:829–831.
Fefferman, NR, Pinkney, LP. Evaluation of chest wall disorders in children. Radiol Clin North Am. 2005;43:355–370.
Glass, RB, Norton, KI, Mitre, SA, et al. Pediatric ribs: a spectrum of abnormalities. Radiographics. 2002;22:87–104.
Groom, KR, Murphey, MD, Lonergan, GJ, et al. Mesenchymal hamartoma of the chest wall: radiologic manifestations with emphasis on cross-sectional imaging and histopathologic comparison. Radiology. 2002;222:205–211.
La Quaglia, MP. Chest wall tumors in childhood and adolescence. Semin Pediatr Surg. 2008;17:173–180.
Laffan, EE, Ngan, BY, Navarro, OM. Pediatric soft tissue tumors and pseudotumors: MR imaging features with pathologic correlation. Part 2. Tumors of fibroblastic/myofibroblastic, so called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix and uncertain origin. Radiographics. 2009;29(4):e36.
References
1. Netscher, DT, Peterson, R. Normal and abnormal development of the extremities and trunk. Clin Plast Surg. 1990;17:13–21.
2. Donnelly, LF, Frush, DP. Abnormalities of the chest wall in pediatric patients. AJR Am J Roentgenol. 1999;173:1595–1601.
3. Fefferman, NR, Pinkney, LP. Evaluation of chest wall disorders in children. Radiol Clin North Am. 2005;43:355–370.
4. Williams, AM, Crabbe, DCG. Pectus deformities of the anterior chest wall. Pediatr Respir Rev. 2003;4:237–242.
5. Donnelly, LF, Frush, DP, Foss, JN, et al. Anterior chest wall: frequency of anatomic variations in children. Radiology. 1999;212:837–840.
6. Donnelly, LF, Taylor, CN, Emery, K, et al. Asymptomatic, palpable, anterior chest wall lesions in children: is cross-sectional imaging necessary? Radiology. 1997;202:829–831.
7. Coley, BD. Pediatric chest ultrasound. Radiol Clin North Am. 2005;43:405–418.
8. Fokin, AA, Steuerwald, NM, Ahrens, WA, et al. Anatomical, histologic and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg. 2009;21:44–57.
9. Creswick, HA, Stacey, MW, Kelly, RE, Jr., et al. Familial study of the inheritance of pectus excavatum. J Pediatr Surg. 2006;41:1699–1703.
10. Goretsky, MJ, Kelly, RE, Jr., Croitoru, D, et al. Chest wall anomalies: pectus excavatum and pectus carinatum. Adolesc Med Clin. 2004;15:455–471.
11. Restrepo, CS, Martinez, S, Lemos, DF, et al. Imaging appearances of the sternum and sternoclavicular joints. Radiographics. 2009;29(3):839–859.
12. Haller, JA, Kramer, SS, Letman, SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg. 1987;10:904–906.
13. Mueller, C, Saint-Vil, D, Bouchard, S. Chest x-ray as a primary modality for preoperative imaging of pectus excavatum. J Pediatr Surg. 2008;43:71–73.
14. Rattan, AS, Laor, T, Ryckman, FC, et al. Pectus excavatum imaging: enough but not too much. Pediatr Radiol. 2010;40:168–172.
15. Marcovici, PA, LoSasso, BE, Kruk, P, et al. MR for the evaluation of pectus excavatum. Pediatr Radiol. 2011;41(6):757–758.
16. Iida, H. Surgical repair of pectus excavatum. Gen Thorac Cardiovasc Surg. 2010;58:55–61.
17. Robicsek, F, Watts, LT, Fokin, AA. Surgical repair of pectus excavatum and carinatum. Semin Thorac Cardiovasc Surg. 2009;21:64–75.
18. Fonkalsrud, EW, Beanes, S. Surgical management of pectus carinatum: 30 years’ experience. World J Surg. 2001;25:898–903.
19. Cooper, S, Flaitz, CM, Johnston, DA, et al. A natural history of cleidocranial dysplasia. Am J Med Genet. 2001;104:1–6.
20. Tanaka, JLO, Ono, E, Medici, E, et al. Cleidocranial dysplasia: importance of radiographic imaging in diagnosis of the condition. J Oral Sci. 2006;48:161–166.
21. Mundlos, S. Cleidocranial dysplasia: clinical and molecular genetics. Am J Med Genet. 1999;36:177–182.
22. van Aalst, JA, Phillips, JD, Sadove, AM. Pediatric chest wall and breast deformities. Plast Reconst Surg. 2009;124(suppl):38e–49e.
23. Borschel, GH, Costantino, DA, Cerdena, PS. Individualized implant-based reconstruction of Poland syndrome breast and soft tissue deformities. Ann Plast Surg. 2007;59:507–514.
24. Garcia Pena, P, Barber, I. Pathology of the thoracic wall: congenital and acquired. Pediatr Radiol. 2010;40:859–886.
25. Lee, TJ, Collins, J. MR imaging evaluation of disorders of the chest wall. Magn Reson Imaging Clin North Am. 2008;16(2):355–379.
26. Glass, RB, Norton, KI, Mitre, SA, et al. Pediatric ribs: a spectrum of abnormalities. Radiographics. 2002;22:87–104.
27. Bishara, J, Gartman-Israel, D, Weinberger, M, et al. Osteomyelitis of the ribs in the antibiotic era. Scand J Infect Dis. 2000;32:223–227.
28. Komolafe, F. Pyogenic osteomyelitis of the rib in children. Pediatr Radiol. 1982;12:245–248.
29. Basa, NR, Ming, S, Ndiforchu, F. Staphylococcal rib osteomyelitis in a pediatric patient. J Pediatr Surg. 2004;39:1576–1577.
30. Song, KM, Sloboda, JF. Acute hematogenous osteomyelitis in children. J Am Acad Orthop Surg. 2001;9:166–175.
31. De Vuyst, D, Vanhoenacker, F, Gielen, J, et al. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809–1819.
32. Teo, HE, Peh, WC. Skeletal tuberculosis in children. Pediatr Radiol. 2004;34:853–860.
33. Gaude, GS, Reyas, AK. Tuberculosis of the chest wall without pulmonary involvement. Lung India. 2008;25:135–137.
34. Watt, AJ. Chest wall lesions. Pediatr Respir Rev. 2002;3:328–338.
35. Lee, G, Im, JG, Kim, JS, et al. Tuberculosis of the ribs. J Comput Assist Tomogr. 1993;17:363–367.
36. Hsu, HS, Wang, LS, Wu, YC, et al. Management of primary chest wall tuberculosis. Scand J Thorac Cardiovasc Surg. 1995;29:119–123.
37. Yeung, VHW, Wong, QHY, Chao, NSY, et al. Thoracic actinomycosis in an adolescent mimicking chest wall tumor or pulmonary tuberculosis. Pediatr Surg Int. 2008;24:251–254.
38. Snape, PS. Thoracic actinomycosis: an unusual childhood infection. South Med J. 1993;86:222–224.
39. Thompson, AJ, Carty, H. Pulmonary actinomycosis in children. Pediatr Radiol. 1979;8:7–9.
40. Bruckner, AL, Frieden, I. Infantile hemangiomas. J Am Acad Dermatol. 2006;55:671–682.
41. The Hemangioma Instigator GroupHaggstrom, A, Drolet, BA, Baselga, E, et al. Prospective study of infantile hemangiomas: demographic, prenatal and perinatal characteristics. J Pediatr. 2007;150:291–294.
42. Ritter, MR, Butscheck, RA, Friedlander, M, et al. Pathogenesis of infantile haemangioma: new molecular and cellular insights. Expert Rev Mol Med. 2007;9:1–19.
43. Restrepo, R, Palani, R, Cervantes, LF, et al. Hemangiomas revisited: the useful, the unusual and the new. Part 1: overview and clinical and imaging characteristics. Pediatr Radiol. 2011;41(7):895–904.
44. Burrows, PE, Laor, T, Paltiel, H, et al. Diagnostic imaging in the evaluation of vascular birth marks. Pediatr Dermatol. 1998;16:455–488.
45. Leaute-Labreze, C, Dumas de la Roque, E, Hubiche, T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651.
46. Restrepo, R, Palani, R, Cervantes, LF, et al. Hemangiomas revisited: the useful, the unusual and the new. Part 2: endangering hemangiomas and treatment. Pediatr Radiol. 2011;41(7):910–915.
47. Garzon, MC, Huang, JT, Enjolras, O, et al. Vascular malformations. Part I. J Am Acad Dermatol. 2007;56:353–370.
48. Dubois, J, Alison, M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol. 2010;40:895–905.
49. Laffan, EE, Ngan, BY, Navarro, OM. Pediatric soft tissue tumors and pseudotumors: MR imaging features with pathologic correlation. Part 2. Tumors of fibroblastic/myofibroblastic, so called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix and uncertain origin. Radiographics. 2009;29(4):e36.
50. Dickey, GE, Sotelo-Avila, C. Fibrous hamartoma of infancy: current review. Pediatr Dev Pathol. 1999;2:236–243.
51. Eich, GF, Heffel, JC, Tschappeler, H, et al. Fibrous tumors in children: imaging features of a heterogeneous group of disorders. Pediatr Radiol. 1998;28:500–509.
52. Seguier-Lipszyc, E, Hermann, G, Kaplinski, C, et al. Fibrous hamartoma of infancy. J Pediatr Surg. 2011;46:753–755.
53. DiCaprio, MR, Enneking, WF. Fibrous dysplasia. Pathophysiology, evaluation and treatment. J Bone Joint Surg. 2005;87A:1848–1864.
54. Stelzer, P, Gay, WA. Tumors of the chest wall. Surg Clin North Am. 1980;60:779–791.
55. Tateishi, U, Gladish, GW, Kusumoto, M, et al. Chest wall tumors: radiologic findings and pathologic correlation. Part 1. Benign tumors. Radiographics. 2003;23:1477–1490.
56. Shah, ZK, Peh, WCG, Hoh, WL, et al. Magnetic resonance imaging appearances of fibrous dysplasia. Br J Radiol. 2005;78:1104–1115.
57. Payne-James, JJ, Walesby, RK. Symptomatic fibrous dysplasia of the right first rib excised via a posterior thoracotomy. Thorax. 1986;41:575–576.
58. Andrisano, A, Soncini, G, Calderoni, PP, et al. Critical review of infantile fibrous dysplasia: surgical treatment. J Pediatr Orthop. 1991;11:478–481.
59. Murphey, MD, Choi, JJ, Kransdorf, MJ, et al. From the Archives of the AFIP. Imaging of osteochondroma: variants and complications with radiologic-pathologic correlation. Radiographics. 2000;20:1407–1434.
60. Brien, EW, Mirra, JM, Luck, JV, Jr. Benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. II. Juxtacortical cartilage tumors. Skeletal Radiol. 1999;28:1–20.
61. Groom, KR, Murphey, MD, Lonergan, GJ, et al. Mesenchymal hamartoma of the chest wall: radiologic manifestations with emphasis on cross-sectional imaging and histopathologic comparison. Radiology. 2002;222:205–211.
62. Jung, AL, Johnson, DG, Condon, VR, et al. Congenital chest wall mesenchymal hamartoma. J Perinatol. 1994;14:487–491.
63. Schamberger, RC, Grier, HE. Chest wall tumors in infants and children. Semin Pediatr Surg. 1994;3:267–276.
64. Cohen, MC, Drut, R, Garcia, C, et al. Mesenchymal hamartoma of the chest wall: a cooperative study with review of the literature. Pediatr Pathol. 1992;12:525–534.
65. Sodhi, KS, Aiyappan, SK, Menon, P, et al. Unilateral multifocal mesenchymal hamartoma of the chest wall: report of a case and review of the literature. J Pediatr Surg. 2009;44:464–467.
66. Blumenthal, BI, Capitanio, MA, Queloz, JM, et al. Intrathoracic mesenchymal hamartoma. Pediatr Radiol. 1972;104:107–109.
67. Azouz, EM, Saigal, G, Rodriguez, MM, et al. Langerhans’ cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol. 2005;35:103–115.
68. Hoover, KB, Rosenthal, DI, Mankin, H. Langerhans cell histiocytosis. Skeletal Radiol. 2007;36:95–104.
69. Brien, EW, Mirra, JM, Luck, JV, Jr. Benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. II. The intramedullary cartilage tumors. Skeletal Radiol. 1997;26:325–353.
70. Capana, R, Campanacci, DA, Manfrini, M. Unicameral and aneurysmal bone cysts. Pediatr Orthop Oncol. 1996;27:605–614.
71. Matysiakiewicz, J, Tomasik, P, Miszczyk, L, et al. Manifestations, diagnosis and surgical treatment of enchondroma—own experience. Orthop Traumatol Rehabil. 2010;12:155–159.
72. De Cristofaro, R, Biagini, R, Boriani, S, et al. Selective arterial embolization in the treatment of aneurismal bone cysts and angioma of bone. Skeletal Radiol. 1992;21:523–527.
73. Varshney, MK, Rastogi, S, Khan, SA, et al. Is sclerotherapy better than intralesional excision for treating anerysmal bone cysts? Clin Orthop Relat Res. 2010;468:1649–1659.
74. van den Berg, H, van Rijn, RR, Merks, JH. Management of tumors of the chest wall in childhood: a review. J Pediatr Hematol Oncol. 2008;30:214–221.
75. Maurer, HM, Beltangady, M, Gehan, EA, et al. The intergroup rhabdomyosarcoma study I. A final report. Cancer. 1988;61:209–220.
76. Saenz, NC, Ghavimi, F, Gerald, W, et al. Chest wall rhabdomyosarcoma. Cancer. 1997;80:1513–1517.
77. La Quaglia, MP. Chest wall tumors in childhood and adolescence. Semin Pediatr Surg. 2008;17:173–180.
78. Wyttenbach, R, Vock, P, Tschappeler, H. Cross-sectional Imaging with CT and/or MRI of pediatric chest wall tumors. Eur Radiol. 1998;8:1040–1046.
79. Kim, EE, Valenzuela, RF, Kumar, AJ, et al. Imaging and clinical spectrum of rhabdomyosarcoma in children. Clin Imaging. 2000;24:257–262.
80. Tateishi, U, Gladish, GW, Kusumoto, M, et al. Chest wall tumors: radiologic findings and pathologic correlation. Part 2. Malignant tumors. Radiographics. 2003;23:1491–1508.
81. Daldrup-Link, HE, Franzius, C, Link, TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. Am J Roentgenol. 2001;177:229–236.
82. Baum, S, Fruedhwald, M, Rahbar, K, et al. PET/CT and outcome in children and young adults with rhabdomyosarcoma. J Nucl Med. 2010;51(suppl):511.
83. Bagheritoolabi, B, Magram, M, Chirindel, A, et al. The role of PET/CT in the management of rhabdomyosarcoma. J Nucl Med. 2009;50(suppl):1096.
84. Dang, NC, Siegel, SE, Phillips, D. Malignant chest wall tumors in children and young adults. J Pediatr Surg. 1999;34:1773–1778.
85. Maheshwari, AV, Cheng, EY. Ewing sarcoma family of tumors. J Am Acad Orthop Surg. 2010;18:94–107.
86. Gurney, J, Swensen, A, Bulterys, M. Malignant bone tumors. SEER pediatric monograph. Bethesda, Md: National Cancer Institute; 2006.
87. Sallustion, G, Pirronti, T, Lasorella, A, et al. Diagnostic imaging of primitive neuroectodermal tumour of the chest wall (Askin tumor). Pediatr Radiol. 1998;28:697–702.
88. Dick, EA, McHugh, K, Kimber, C, et al. Imaging of non-central nervous system primitive neuroectodermal tumors: diagnostic features and correlation with outcome. Clin Radiol. 2001;56:206–215.
89. Bestic, JM, Peterson, JJ, Bancroft, LW, et al. Use of FDG PET in staging, restaging, and assessment of therapy response on Ewing sarcoma. Radiographics. 2009;29:1487–1501.
90. Lim, WY, Sarji, AS, Yik, YI. Osteosarcoma of a rib. Biomed Imaging Interv J. 2008;4(1):90.
91. Samarasekera, SP, Mikocka-Wlaus, A, Butt, W, et al. Epidemiology of major pediatric chest trauma. J Pediatr Child Health. 2009;45:676–680.
92. Peterson, RJ, Tepas, JJ, III., Edwards, FH, et al. Pediatric and adult thoracic trauma: age related impact on presentation and outcome. Ann Thorac Surg. 1994;58:14–18.
93. Bliss, D, Silen, M. Pediatric thoracic trauma. Crit Care Med. 2002;30(suppl):s409–s415.
94. Holmes, JF, Sokolove, PE, Brant, WE, et al. A clinical decision rule for identifying children with thoracic injuries after blunt torso trauma. Ann Emerg Med. 2002;39:492–499.
95. Peclet, MH, Newman, KD, Eichelberger, MR, et al. Thoracic trauma in children: an indicator of increased mortality. J Pediatr Surg. 1990;25:961–965.
96. Schweich, P, Flesher, G. Rib fractures in children. Pediatr Emerg Care. 1985;1:187–189.
97. Garcia, VF, Gotschall, CS, Eichelberger, MR, et al. Rib fractures in children: a marker of severe trauma. J Trauma. 1990;30:695–700.
98. Lonergan, GJ, Baker, AM, Morey, MK, et al. From the archives of the AFIP. Child abuse: radiologic-pathologic correlation. Radiographics. 2003;23:811–845.
99. Bulloch, B, Schubert, CJ, Brophy, PD, et al. Cause and clinical characteristics of rib fractures in infants. Pediatrics. 2000;105:48e.
100. Hamilton, NA, Bucher, BT, Keller, MS. The significance of first rib fractures in children. J Pediatr Surg. 2011;46:169–172.
101. Moore, MA, Wallace, EC, Westra, SJ. The imaging of pediatric thoracic trauma. Pediatr Radiol. 2009;39:485–496.
102. Nakayama, DK, Ramenofsky, ML, Rowe, MI. Chest injuries in childhood. Ann Surg. 1989;210:770–775.
103. Strous, PJ, Owings, CL. Fractures of the first rib in child abuse. Radiology. 1995;197:763–765.
104. Hopper, MA, Tirman, P, Robinson, P. Injury of the chest wall and upper extremity. Semin Musculoskelet Radiol. 2010;14:122–130.