Chapter 17 The central nervous system
However, for milder psychiatric conditions, phytotherapy can provide useful support. The prevalence of mental health problems, particularly depression and anxiety, in the general population is around one in six people, and around 40% of people with mental health problems will have symptoms of both anxiety and depression. Depression is more common in women than men; around one-half of women and one-quarter of men will be affected by depression at some time. However, other than in mild cases, these disorders are not suitable for self-treatment, and medical supervision is necessary. Sleep disturbances, such as insomnia and early morning awakening, are characteristic of depression and anxiety, although they can also occur independently of mental health problems. Around one-third of adults are thought to experience insomnia, and most do not seek treatment from a physician. Phytotherapy has a role to play in helping to re-establish a regular pattern of sleep. Valerian (Fig. 17.1), for example, has been advocated as a means of alleviating the symptoms of benzodiazepine withdrawal.
Hypnotics and sedatives
Hops, Humulus lupulus L. (Humuli lupuli strobuli) 
Constituents
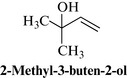
The main active constituents of hops are the bitter principles found in the oleo-resin. These include the α-acid humulone and the β-acid lupulone, and their degradation products, such as 2-methyl-3-buten-2-ol (Fig. 17.2). Other constituents include flavonoids, chalcones, tannins and volatile oils.
Therapeutic uses and available evidence
Modern pharmaceutical uses of hops include sleep disturbances and restlessness. Sedative and hypnotic activities have been documented in vivo (mice) for extract of hops, and for the bitter acid degradation product 2-methyl-3-buten-2-ol. Clinical studies provide some evidence of the hypnotic effects of hops given in combination with the herbal sedative, hypnotic valerian (Zanoli and Zavatti 2008). Antibacterial and antifungal activities have been documented in vitro for certain constituents of hops. Hops are non-toxic, as their use in beer would suggest. Cheers!
Lemon balm, Melissa officinalis L. (Melissae folium) 
Therapeutic uses and available evidence
Sedative and antispasmodic effects have been documented for melissa extracts using in vivo studies (mice, rats). It is used for nervous or sleeping disorders and functional gastrointestinal complaints. There has been no clinical investigation of the sedative effects of melissa alone in individuals with sleeping disorders. However, clinical trials have explored the effects of melissa in combination with other herbal sedatives (e. g. valerian and hops) and provide some evidence to support the sedative and hypnotic effects of such preparations. Antihormonal effects of balm, mainly antithyroid, have been documented and, recently, cholinergic activity has been found for extracts using human cerebral cortical cell membrane homogenates. Dried lemon balm is usually taken internally in the form of a herbal tea, at a dose of 2–4 g three times a day. Melissa extracts are also applied topically in cases of Herpes simplex labialis resulting from HSV-1 infection (see anti-infectives). Lemon balm is regarded as non-toxic, although it should not be used to excess because of the reputed antithyroid activity. For review, see Ulbricht et al 2005.
Kava, Piper methysticum Forst. (Piperis methystici rhizome)
Piper methysticum (Piperaceae), also known as kava-kava or kawa, has been used in the Pacific Islands, notably Fiji, for hundreds of years. It is a small shrub with heart-shaped leaves and thick, woody roots and rhizomes, which are ground or chewed to release the actives. These are then fermented to make the ceremonial drink Kava, which induces a relaxed sociable state, and is given to visiting dignitaries (including the Pope and the Queen of England). Kava is used medicinally for its tranquillizing properties and numerous other disparate complaints. Recent safety concerns have resulted in Kava products being withdrawn from sale at present (2011), but moves are being made to try to re-introduce it into the EU (Sarris et al 2011).
Therapeutic uses and available evidence
In vitro studies have previously provided some conflicting data on receptor interactions of kava extract and isolated kavalactones. Current thinking is that kavalactones potentiate GABAA receptor activity. Other receptor binding studies demonstrate no interaction with benzodiazepine receptors. The efficacy of kava extracts in relieving anxiety is supported by data from several randomized, placebo-controlled, clinical trials, for example an aqueous kava preparation produced significant anxiolytic and antidepressant activity and appeared equally effective in cases where anxiety is accompanied by depression (Sarris et al 2009). Overall, studies indicate reductions in anxiety after 4–12 weeks of treatment with kava extracts at dosages equivalent to 60–240 mg of kavalactones daily.
Kava extracts are generally well tolerated when used at recommended doses for limited periods. However, kava-induced liver injury has been demonstrated in several patients worldwide, and it has been suggested that this is due to inappropriate quality of the kava raw material (Teschke et al 2011). There may also be a pharmacogenomic component to the toxicity (Sarris et al 2011) and assessment of the causal role of kava is complicated by other factors, including concomitant drugs linked with liver toxicity, and alcohol. There is circumstantial evidence for the roles of toxic metabolites, inhibition of cyclooxygenase (COX) enzymes and depletion of liver glutathione, and pharmacogenomic effects are likely, particularly for cytochrome P450 genes. Experimental and clinical cases of hepatotoxicity show evidence of hepatitis, but the question remains whether this inflammation is caused by components of kava directly, or is due to downstream effects (Zhang et al 2011). A recent study in hepatocytes has shown that kavain has minimal cytotoxicity and methysticin moderate concentration-dependent toxicity, whereas yangonin displayed marked toxicity (Tang et al 2011).
Passion flower, Passiflora incarnata L. (Passiflorae herba) 
Therapeutic uses and available evidence
Animal studies (mice, rats) have documented CNS-sedative effects or reductions in motility for aqueous ethanolic extracts of passion flower and for the constituents maltol and ethylmaltol. Anxiolytic activity has been reported in mice (Sampath et al 2010) and modulation of the GABA system is known to be involved (Appel et al 2010, Elsas et al 2010). Sedative effects are attributed at least in part to the flavonoid, particularly chrysin, content. There are few clinical studies of passiflora; however, a preliminary double-blind randomized trial using 36 patients with generalized anxiety showed the extract to be as effective as oxazepam, but with a lower incidence of impairment of job performance. It has been advocated as an adjunctive therapy for opiate withdrawal symptoms (for review, Miyasaka et al 2007). Generally, passiflora is well tolerated with few side effects; however, isolated reactions involving nausea and tachycardia in one case, and vasculitis in another, have been reported.
Valerian, Valeriana officinalis L. (Valerianae radix) 
Constituents
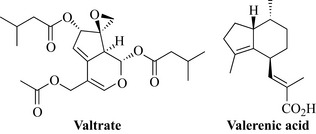
The main components of valerian are the volatile oil and the iridoid valepotriate constituents. The volatile oil contains monoterpenes and sesquiterpenes, such as β-bisabolene, caryophyllene, valeranone, valerianol, valerenol, valerenal, valerenic acid and derivatives (Fig. 17.1). The valepotriate compounds include valtrate, didrovaltrate and isovaltrate. The valepotriates readily decompose on storage and processing to form mainly baldrinal and homobaldrinal, which are also unstable. Valerian also contains alkaloids, including valerianine and valerine, and amino acids such as arginine, γ-aminobutyric acid (GABA), glutamine and tyrosine.
Therapeutic uses and available evidence
In Europe valerian and its various preparations (tablets, tinctures) have been approved for the temporary relief of symptoms of mild anxiety and to aid sleep, and are generally based on traditional use. The sedative effects of valerian root are well documented. In vivo studies (in mice) have demonstrated CNS-depressant activity for the volatile oil, the valepotriates and the valepotriate degradation products. The sedative effects of valerian root are thought to be due to the activities of these different components, particularly valerenal and valerenic acid (constituents of the volatile oil), and the valepotriate compounds. Therefore, the profile of these constituents, and their concentrations, in a specific valerian preparation will determine its activity. Biochemical studies have indicated that certain components of valerian, particularly valerenic acid, may lead to increased concentrations of the inhibitory neurotransmitter GABA in the brain by inhibiting its catabolism, inhibiting uptake and/or by inducing GABA release. Increased GABA concentrations are associated with decreased CNS activity, which may, at least partly, explain valerian’s sedative activity. It is not clear whether valerian root extracts have effects on the binding of benzodiazepines to receptors. Modern medicinal uses for valerian root preparations are for insomnia, stress and anxiety. Clinical trials have tested the effects of valerian preparations on subjective (e.g. sleep quality) and objective (e.g. sleep structure) sleep parameters, and on measures of stress. Some, but not all, of these studies provide evidence to support the traditional uses of valerian. Several preparations contain valerian root in combination with other herbs reputed to have hypnotic and/or sedative effects, such as hops (Humulus lupulus) and melissa (Melissa officinalis) (see Salter and Brownie 2010 for review). It is recommended that valerian preparations should not be taken for up to 2 hours before driving a car or operating machinery; also, the effect of valerian preparations may be enhanced by alcohol consumption. There are isolated reports of hepatotoxicity associated with valerian-containing products, although causality has not been established.
Antidepressants
St John’s wort, Hypericum perforatum L. (Hyperici herba) 
Constituents
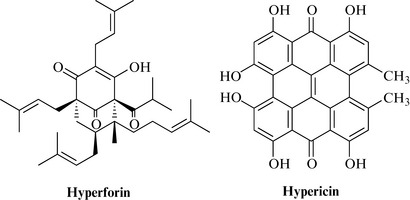
St John’s wort contains a series of naphthodianthrones, which include hypericin and pseudohypericin, and the prenylated phloroglucinols, such as hyperforin and adhyperforin. Initially, hypericin was considered to be the antidepressant constituent of St John’s wort, although evidence has now emerged that hyperforin (Fig. 17.3) is also a major constituent required for antidepressant activity. Further research is necessary to determine which other constituents contribute to the antidepressant effect. The Eur. Ph. states that the drug should contain not less than 0.08% of total hypericins, expressed as hypericin, calculated with reference to the dried drug. Most products containing standardized extracts of St John’s wort are still standardized on hypericin content, as hyperforin is fairly unstable. St John’s wort also contains other biologically active constituents, such as flavonoids. The leaves and flowers also contain an essential oil, of which the major components are β-caryophyllene, caryophyllene oxide spathulenol, tetradecanol, viridiflorol, α- and β-pinene, and α- and β-selinene.
Therapeutic uses and available evidence
The precise mechanism of action for the antidepressant effect of St John’s wort is unclear. Results of biochemical and pharmacological studies have suggested that St John’s wort extracts inhibit synaptosomal uptake of the neurotransmitters, serotonin (5-hydroxytryptamine, 5-HT), dopamine and noradrenaline (norepinephrine) and GABA. Studies involving small numbers of healthy male volunteers have indicated that St John’s wort extracts may have dopaminergic activity and effects on cortisol, which may influence concentrations of certain neurotransmitters. Previous in vitro studies suggested that St John’s wort inhibited monoamine oxidase, although other studies failed to confirm this. Experimental studies involving animal models of depression provide supporting evidence for the antidepressant effects of St John’s wort. Evidence from randomized controlled trials indicates that preparations of St John’s wort extracts are more effective than placebo, and possibly as effective as conventional antidepressants, in the treatment of mild to moderate depression (for overview, see Linde 2009, Nahrstedt and Butterweck 2010). Generally, a few weeks’ treatment is required before marked improvement is seen. St John’s wort is not recommended for the treatment of major depression.
Stimulants
Caffeine 
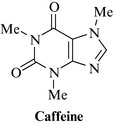
Caffeine is a methylxanthine derivative found in tea, coffee and cocoa (Fig. 17.4). It is a mild stimulant, and is added to many analgesic preparations to enhance activity, although there is no scientific basis for this practice. High doses may lead to insomnia and a feeling of anxiety, and can induce withdrawal syndrome in severe cases.
Cola nut, Cola spp. (Colae semen) 
Cola contains the xanthine derivative caffeine (Fig. 17.4), with traces of theobromine and theophylline. Tannins and phenolics, including catechin, epicatechin, kolatin, kolatein, kolanin, and amines, including dimethylamine, methylamine, ethylamine and isopentylamine, are also present, together with thiamine and other B vitamins.
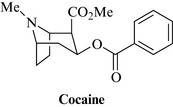
Cocaine 
Cocaine (Fig. 17.5) is a tropane alkaloid extracted from the leaf of coca [Erythroxylum coca Lam. and E. novogranatense (Morris) Hieron, Erythroxylaceae]. These are shrubs growing at high altitudes in the South-American Andes. The leaf is still chewed by the local people (along with lime to assist buccal absorption), in order to alleviate symptoms of altitude sickness and fatigue. Cocaine is rarely used medicinally, except as a local anaesthetic in eye surgery, but is now a major illicit drug responsible for many health problems and associated crime. The supply and use of cocaine is strictly regulated in most countries.
Analgesics
Two types of analgesics are usually recognized: those that act via the CNS (the opioids) and will be discussed briefly here; the non-opiate and non-steroidal antiinflammatory drugs, which include aspirin, and which will be covered in Chapter 20. It is very common for the two types to be used in combination, for example aspirin with codeine. The opioid analgesics and their derivatives have never been surpassed as painkillers in efficacy or patient acceptability despite their disadvantages. They are obtained from the opium poppy (Papaver somniferum) and the most important are still the alkaloids morphine and codeine. Numerous derivatives such as oxycodone, dihydrocodeine, fentanyl, buprenorphine and etorphine have been developed which have different therapeutic and pharmacokinetic profiles, or can be administered via a different route (buccal tablets such as those containing buprenorphine, or transdermal patches, such as with fentanyl). The pharmacology of the opiates is covered in depth in many textbooks and these should be referred to for further information.
Opium, Papaver somniferum L. (Opii crudum; opii pulvatus normatus) 
Constituents
Alkaloids represent about 10% of the dried latex. The major alkaloid is morphine (Fig. 17.6), with codeine and thebaine and lesser amounts of very many others including narceine, narcotine, papaverine, salutaridine, oripavine and sanguinarine.
Migraine
Feverfew, Tanacetum parthenium (L) Schultz Bip. (Tanaceti parthenii herba) 
Constituents
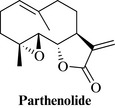
The sesquiterpene lactones are essential for the biological activity, the major one being parthenolide (Fig. 17.7), with numerous others reported from the species (e.g. santamarine). It also contains small amounts of essential oil (0.02–0.07%), with α-pinene and derivatives, camphor and others.
Therapeutic uses and available evidence
The main use of feverfew today is as a prophylactic and treatment for migraine. The fresh leaves may be eaten, usually with other foods to disguise the nauseous taste, or a standardized extract taken daily to prevent migraine attacks. Although some clinical studies have shown efficacy, others have not, and further work is needed to identify which extracts may be effective (Pittler and Ernst 2004).
Feverfew extracts inhibit secretion of serotonin from platelet granules and proteins from polymorphonuclear leukocytes (PMNs). Since serotonin is implicated in the aetiology of migraine and PMN secretion is increased in rheumatoid arthritis, these findings substantiate the use of feverfew in these conditions. Parthenolide is considered to be the main active constituent. It is a potent inhibitor of NF-kB. The sesquiterpene lactones as a class have an effect on a large number of other targets, including the inhibition of prostaglandin production and arachidonic acid release. This explains the antiplatelet and antifebrile actions to some extent, but in fact feverfew extract with the parthenolide removed also has antiinflammatory activity (Sur et al 2009). Feverfew may produce side effects such as dermatitis, and soreness or ulceration of the mouth. Also, contact dermatitis has been described, especially by workers handling material from this species, caused by the exposure to the allergenic sesquiterpene lactones.
Drugs used for cognitive enhancement and in dementia
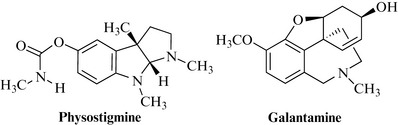
There are few effective treatments for improving memory, especially in dementia. Acetylcholinesterase-inhibiting drugs are available to treat Alzheimer’s disease with varying degrees of success. Rivastigmine is a reversible, non-competitive inhibitor of acetylcholinesterase. It is a semi-synthetic derivative of physostigmine, an alkaloid found in the Calabar bean (Physostigma venenosum), a highly poisonous plant indigenous to West Africa. Galantamine (= galanthamine), an alkaloid extracted from the snowdrop (Galanthus nivalis) was introduced around 2001 (Fig. 17.8, Heinrich and Teoh 2004). These drugs appear to slow down progression of the disease for a period of time, but do not cure it, and have side effects making them unacceptable to many patients.
Ginkgo, Ginkgo biloba L. (Ginkgo folium) 
Constituents
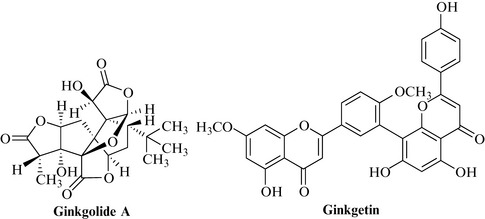
Ginkgo contains two major classes of actives, both of which contribute to the activity: ginkgolides A, B and C, and bilobalide, which are diterpene lactones; and the biflavone glycosides such as ginkgetin, isoginkgetin and bilobetin (Fig. 17.9). Ginkgolic acids are present in the fruit but normally only in very minor amounts in the leaf.
Therapeutic uses and available evidence
The most important use of ginkgo is to reduce or prevent memory deterioration, due to ageing and milder forms of dementia, including the early stages of Alzheimer’s disease (e.g. Ihl et al 2010). It’s enhancement of cognitive processes is thought to be by improving blood circulation to the brain and also due to its antiinflammatory and antioxidant effects. Many clinical studies have been carried out (not all of high quality), and the extract has been shown to improve the mental performance in healthy volunteers and geriatric patients where this was impaired (for review, see Weinmann et al 2010). The effects on the CNS are not yet well defined, but include effects on neurotransmitter uptake, neurotransmitter receptor changes during ageing, cerebral ischaemia and neuronal injury. Inhibition of nitric oxide may play a part. The usual dose of ginkgo (standardized) extracts is 120–240 mg daily. Ginkgo has been reported to cause dermatitis and gastrointestinal disturbances in large doses, although rarely. Allergic reactions in sensitive individuals are more likely to be due to ingestion of the fruits due to the ginkgolic acids, which are usually absent from leaf extracts and ginkgo products, or present only in very small amounts.
Appel K., Rose T., Fiebich B., Kammler T., Hoffmann C., Weiss G. Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytother. Res.. 2010. doi:10.1002/ptr.3352. [Epub ahead of print]
Elsas S.M., Rossi D.J., Raber J., et al. Passiflora incarnata L. (Passionflower) extracts elicit GABA currents in hippocampal neurons in vitro, and show anxiogenic and anticonvulsant effects in vivo, varying with extraction method. Phytomedicine. 2010;17:940-949.
Heinrich M., Lee Teoh H.L. Galanthamine from snowdrop – the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol.. 2004;92:147-162.
Ihl R., Bachinskaya N., Korczyn A.D., et al. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: a randomized controlled trial. Int. J. Geriatr. Psychiatry. 2010. [Epub ahead of print 2010 Dec 7]
Linde K. St. John’s wort – an overview. Forsch. Komplementarmed.. 2009;16:146-155.
Miyasaka L.S., Atallah A.N., Soares B.G. Passiflora for anxiety disorder. Cochrane Database Syst. Rev.. 24, 2007. CD004518
Nahrstedt A., Butterweck V. Lessons learned from herbal medicinal products: the example of St. John’s Wort (perpendicular). J. Nat. Prod.. 2010;73:1015-1021.
Pittler M.H., Ernst E. Feverfew for preventing migraine. Cochrane Database Syst. Rev.. 1, 2004. CD002286
Salter S., Brownie S. Treating primary insomnia – the efficacy of valerian and hops. Aust. Fam. Physician. 2010;39:433-437.
Sampath C., Holbik M., Krenn L., Butterweck V. Anxiolytic effects of fractions obtained from Passiflora incarnata L. in the elevated plus maze in mice. Phytother. Res.. 2010. doi:10.1002/ptr.3332. [Epub ahead of print 2010 Nov 12]
Sarris J., Kavanagh D.J., Byrne G., Bone K.M., Adams J., Deed G. The Kava Anxiety Depression Spectrum Study (KADSS): a randomized, placebo-controlled crossover trial using an aqueous extract of Piper methysticum. Psychopharmacology (Berl). 2009;205:399-407.
Sarris J., Teschke R., Stough C., Scholey A., Schweitzer I. Re-introduction of Kava (Piper methysticum) to the EU: is there a way forward? Planta Med.. 2011;77:107-710.
Sur R., Martin K., Liebel F., Lyte P., Shapiro S., Southall M. Anti-inflammatory activity of parthenolide-depleted Feverfew (Tanacetum parthenium). Inflammopharmacol. 2009;17:42-49.
Tang J., Tang J., Dunlop R.A., Rowe A., Rodgers K.J., Ramzan I. Kavalactones Yangonin and Methysticin induce apoptosis in human hepatocytes (HepG2) in vitro. Phytother. Res.. 2011;25:417-423.
Teschke R., Sarris J., Lebot V. Kava hepatotoxicity solution: A six-point plan for new kava standardization. Phytomedicine. 2011;18:96-103.
Ulbricht C., Brendler T., Gruenwald J., et al. Lemon balm (Melissa officinalis L.): an evidence-based systematic review by the Natural Standard Research Collaboration. J. Herb. Pharmacotherapy. 2005;5:71-114.
Weinmann S., Roll S., Schwarzbach C., Vauth C., Willich S.N. Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatr.. 2010;10:14.
Zanoli P., Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol.. 2008;116:383-396.
Zhang L.Y., Rowe A., Ramzan I. Does inflammation play a role in kava hepatotoxicity? Phytother. Res.. 2011;25:629-630. doi:10.1002/ptr.3301 [Epub ahead of print 2010 Sep 14]