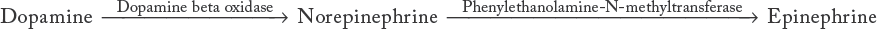11 The cardiovascular system
Adrenergic: A term that describes nerve fibers that liberate norepinephrine.
Afterload: The impedance to left ventricular ejection. The afterload is expressed as total peripheral resistance.
Angina Pectoris: Chest pain caused by myocardial ischemia.
Arrhythmia: An abnormal rhythm of the heart, also referred to as dysrhythmia.
Arteriosclerosis: Degenerative changes in the arterial walls that result in thickening and loss of elasticity.
Automaticity: The ability of the cardiac pacemaker cells to undergo depolarization spontaneously.
Bathmotropic: Affecting the response of cardiac muscle (or any tissue) to stimuli.
Bigeminy: A premature beat along with a normal heart beat.
Bradycardia: A heart rate of 60 beats/min or less.
Cardiac Arrest: Ventricular standstill.
Cardiac Index: A “corrected” cardiac output used to compare patients with different body sizes. The cardiac index equals the cardiac output divided by the body surface area.
Cardiac Output: The amount of blood pumped to the peripheral circulation per minute.
Cholinergic: Describes nerve fibers that liberate acetylcholine.
Chronotropic: Affecting the rate of the heart.
Conduction: Movement of cardiac impulses through specialized conduction systems of the heart that facilitate coordinated contraction of the heart.
Cor Pulmonale: Pulmonary hypertension as a result of obstruction of the pulmonary circulation that causes right ventricular hypertrophy.
Cyanosis: Bluish discoloration, seen especially on the skin and mucous membranes, as a result of a reduced amount of oxygen in the hemoglobin.
Diastole: The period of relaxation of the heart, especially of the ventricles.
Dromotropic: Affecting the conductivity of a nerve fiber, especially the cardiac nerve fibers.
Ectopic: Located away from a normal position; in the heart, a beat that arises from a focus outside the sinus node.
Ectopic Pacemaker: Focus of ectopic pacemaker shown as premature contractions of the heart that occur between normal beats.
Electrolyte: An ionic substance found in the blood.
Embolism: A blood clot or other substance, such as lipid material, in the blood stream.
Excitability: The ability of cardiac cells to respond to a stimulus with depolarization.
Exsanguinate: To deprive of blood.
Fibrillation: An ineffectual quiver of the atria or ventricles.
Flutter: A condition, usually atrial, in which the atria contract 200 to 400 beats/min.
Heart Block (Complete): A condition that results when conduction is blocked by a lesion at any level in the atrioventricular junction.
Hypertension: Persistently elevated blood pressure.
Hypervolemia: An abnormally large amount of blood in the circulatory system.
Infarction: A necrotic area resulting from an obstruction of a vessel.
Inotropic: Affecting the force of contraction of muscle fibers, especially those of the heart.
Ischemia: Local tissue hypoxia from decreased blood flow.
Leukocytosis: Increased number of white blood cells; a white blood cell count higher than 10,000 per mm3.
Leukopenia: Decreased number of white blood cells; a white blood cell count lower than 5000 per mm3.
Murmur: An abnormal heart sound heard during systole, diastole, or both.
Myocardium: The muscular middle layer of the heart between the inner endocardium and the outer epicardium.
Normotensive: With a normal blood pressure.
Occlusion: An obstruction of a blood vessel by a clot or foreign substance.
Pacemaker: The area in which the cardiac rate commences, normally at the sinoatrial node.
Palpitation: A patient’s abnormal rate, rhythm, or fluttering of the heart.
Paroxysmal Tachycardia: A period of rapid heart beats that begins and ends abruptly.
Pericarditis: An inflammation of the pericardium.
Peripheral Resistance: Resistance to blood flow in the microcirculation.
Polycythemia: An excessive number of red blood cells, which is reflected in an abnormally high hematocrit level.
Preexcitation Syndrome: When the atrial impulse bypasses the atrioventricular node to produce early excitation of the ventricle.
Preload: The left ventricular end-diastolic volume.
Pulse Deficit: The difference between the apical and radial pulses.
Reentry (Circus Movement): Reexcitation of cardiac tissue by the return of the same cardiac impulse via a circuitous pathway.
Syncope: Fainting, giddiness, and momentary unconsciousness, usually caused by cerebral anoxia.
Systole: The period of contraction of the heart, especially the ventricles.
Thrombosis: The formation of a clot (thrombus) inside a blood vessel or a chamber of the heart.
The cardiovascular system has a significant impact on the patient recovering from anesthesia. Fortunately, there are many ways available to monitor its status. The cardiovascular system also reflects the status of the patient in the postanesthesia care unit (PACU). More specifically, a return to normal values by the cardiovascular system is a good indicator of the progression of emergence of the patient from anesthesia.1
The basic anatomy of certain structures of the cardiovascular system is not covered completely in this chapter because basic nursing texts provide ample material on this subject. However, the clinical correlation between the physiology of the cardiovascular system and perianesthesia nursing care is provided throughout the chapter.2
The heart
The cardiac cycle
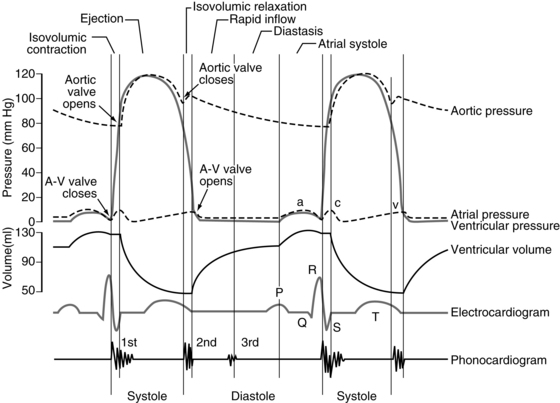
The heart is a four-chambered mass of muscle that pulsates rhythmically and pumps blood into the circulatory system. The chambers of the heart are the atria and the ventricles. The atria, which are pathways for blood into the ventricles, are thin walled, have myocardial muscle, and are divided into the right and left atria by a partition. During each cardiac cycle, approximately 70% of the blood flows from the great veins through the atria and into the ventricles before the atria contract. The other 30% is pumped into the ventricles when the atria contract. On contraction of the right atrium, the pressure in the heart is 4 to 6 mm Hg. The contraction of the left atrium produces a pressure of 6 to 8 mm Hg.1,2
Three pressure elevations are produced by the atria, as depicted on the atrial pressure curve. They are termed the a, c, and v waves (Fig. 11-1). The a wave is a result of atrial contraction. The c wave is produced by both the bulging of atrioventricular (AV) valves and the pulling of the atrial muscle when the ventricles contract. The v wave occurs near the end of the ventricular contraction as the amount of blood in the atria slowly increases and the AV valves close.1–3
The ventricles receive blood from the atria and then act as pumps to move blood through the circulatory system. During the initial third of diastole, the AV valves open and blood rushes into the ventricles. This phase is called the period of rapid filling of the ventricles. The middle third of diastole is referred to as diastasis, during which a small amount of blood moves into the ventricles. During the final third of diastole, the atria contract and the other 30% of the ventricles fills. As the ventricles contract, the AV valves contract and then close, thereby preventing blood from flowing into the ventricles from the atria.3,4
At the end of diastole, each ventricle usually contains approximately 120 mL of blood—the end-diastolic volume. During systole, each ventricle ejects 70 mL of blood, which is the stroke volume. The blood that remains in the ventricle at the end of systole is end-systolic volume and amounts to approximately 50 mL.3,4
Cardiac output
Cardiac output is the amount of blood ejected from the left or right ventricle in 1 minute. In the normal adult with a heart rate of 70 bpm, the cardiac output is approximately 4900 mL. This estimate can be derived with taking the rate of 70 times the stroke volume of 70 mL. User-friendly sophisticated equipment is available to monitor a patient’s cardiac output in the PACU. The information derived from serial measurements of the cardiac output can be helpful in assessing the general status of the cardiovascular system and determining the appropriate amount and type of fluid therapy for the patient.6
The cardiac output is measured with a variety of techniques. Kaplan suggests that the thermodilution method, which uses a pulmonary artery catheter, is the clinical method of choice. For a higher degree of reproducibility, Kaplan recommends a technique of standardization in which the injectate temperature and volume, and the speed of injection, are carefully controlled and duplicated. The most reproducible results have been obtained with injections of 10 mL of cold (1° to 2° C) 5% dextrose in water. It is important to remember that the thermodilution technique measures right-sided cardiac outputs; therefore measurements of cardiac output with the thermodilution technique are usually unreliable for patients with intracardiac shunts.6,7
Other methods of calculation of the cardiac output are the Fick and Stewart techniques. The Fick technique involves calculations of the amount of blood needed to carry oxygen taken up from the alveoli per unit of time. This technique is said to be accurate within a 10% margin of error. In the Stewart technique, a known quantity of dye is injected and its concentration is measured after the dye is dispersed per unit of time.7
Cardiac output can be influenced by venous return. If the heart receives an extra amount of blood from the veins (↑ preload), the cardiac muscle becomes stretched and the stretched muscle contracts with an increased force to pump the extra blood out of the heart. If the heart receives less blood than normal (↓ preload), according to the Frank-Starling law of the heart, it contracts with less force. This concept is important to the perianesthesia nurse. For example, if a patient is undergoing mechanical ventilation and too much positive end-expiratory pressure is overinflating the lungs, the increased pressure on the inferior vena cava impedes the venous return to the heart, thereby decreasing blood pressure. The blood pressure is derived from the following interacting factors: the force of the heart, the peripheral resistance, the volume of blood, the viscosity of blood, and the elasticity of the arteries. Thus, cardiac output can be seen to play a major role in the maintenance of a normal blood pressure.6–8
Arterial blood pressure
The arterial blood pressure consists of the systolic and diastolic arterial pressures. The systolic blood pressure is the highest pressure that occurs within an artery during each contraction of the heart. The diastolic blood pressure is the lowest pressure that occurs within an artery during each contraction of the heart. The mean arterial pressure is the average pressure that pushes blood through the systemic circulatory system. Methods of assessing and monitoring the arterial blood pressure in the PACU are discussed in Chapter 27.
Some factors that affect the arterial blood pressure are the vasomotor center, the renal system, vascular resistance, the endocrine system, and chemical regulation. The vasomotor center, located in the pons and the medulla, has the greatest control over the circulation. This center picks up impulses from all over the body and transmits them down the spinal cord and through vasoconstrictor fibers to most vessels of the body. These impulses can be excitatory or inhibitory. One type of pressoreceptor that sends impulses to the vasomotor center is the baroreceptor. The baroreceptors are located in the walls of the major thoracic and neck arteries, in particular, the arch of the aorta. When these vessels are stretched by an increased blood pressure, they send inhibitory impulses to the vasomotor center, which lowers the blood pressure. The aortic and carotid bodies located in the bifurcation of the carotid arteries and along the aortic arch can increase systemic pressure when stimulated by a low partial pressure of oxygen in arterial blood (PaO2).9,10
The renal regulation of arterial pressure occurs through the renin-angiotensin-aldosterone mechanism (see Chapter 13).
Historically, when the radial artery was to be cannulated for direct monitoring of blood pressure and sampling of arterial blood gases in the PACU, a modified Allen test was performed. This test was used for assessing the risk of hand ischemia if occlusion of the cannulated vessel should occur. The modified Allen test is performed with the patient making a tight fist, which partially exsanguinates the hand. The nurse then occludes both the radial and the ulnar arteries with digital pressure. The patient is asked to open the hand, and the compressed radial artery is then released. Blushing of the palm (postischemic hyperemia) should be observed. After approximately 1 minute, the test should be repeated on the same hand with the nurse now releasing the ulnar artery while continuing to compress the radial artery. If the release of pressure over the ulnar artery does not lead to postischemic hyperemia, the contralateral artery should be similarly evaluated. The results of the modified Allen test should be reported as “refill time” for each artery. The modified Allen test at most, is subjective, especially when hand blushing is slow and is rarely done in clinical practice.11
Valves of the heart
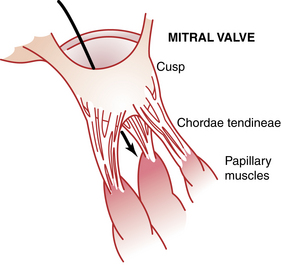
Attached to the valves are the chordae tendineae, which are attached to the papillary muscles, which in turn are attached to the endocardium of the ventricles. When the ventricles contract, so do the papillary muscles, thus pulling the valves toward the ventricles to prevent bulging of the valves into the atria (Fig. 11-2).1,2
Heart muscle
The heart muscle comprises three major muscle types: atrial muscle, ventricular muscle, and excitatory and conductive muscle fibers. The atrial and ventricular muscles act much like skeletal muscles. The excitatory and conductive muscles function primarily as an excitatory system for the heart and a transmission system for conducting impulses throughout the heart.
The cardiac muscle fibers are arranged in a latticework; they divide and then rejoin. The constriction of the cardiac muscle fibers facilitates action potential transmission. The muscle is striated, and the myofibrils contain myosin and actin filaments. Cardiac muscle cells are separated by intercalated disks, which are actually the cardiac cell membranes that separate the cardiac muscle cells from one another. The intercalated disks do not hinder conductivity or ionic transport between cardiac muscle cells to any great extent. When the cardiac muscle is stimulated, the action potential spreads to excite all the muscles, which is called a functional syncytium (Fig. 11-3). This functional syncytium can be divided into atrial and ventricular syncytia, which are separated by fibrous tissue. However, an impulse can be transmitted throughout the atrial syncytium and then via the AV bundle to the ventricular syncytium. The “all-or-none” principle is in effect: when one atrial muscle fiber is stimulated, all the atrial muscle fibers react if the action potential is met. This principle applies to the entire ventricular syncytium as well.1,2
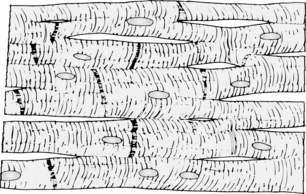
FIG. 11-3 Syncytial nature of cardiac muscle.
(From Hall J: Guyton and Hall textbook of medical physiology, ed 12, Philadelphia, 2011, Saunders.)
The main properties of cardiac muscle are excitability (bathmotropism), contractility (inotropism), rhythmicity and rate (chronotropism), and conductivity (dromotropism). When cardiac muscle is excited, its action potential is reached and the muscle contracts. Certain chemical factors alter the excitability and contractility of cardiac muscle (Box 11-1).
Conduction of impulses
The heart has a special system for generating rhythmic impulses. This system for providing rhythmicity and conductivity consists of the sinoatrial (SA) node, the AV node, the AV bundle, and the Purkinje fibers (Fig. 11-4). The SA node is situated at the posterior wall of the right atrium and just below the opening of the superior vena cava. The SA node generates impulses with self excitation, which is produced by the interaction of sodium and potassium ions. The SA node provides a rhythmic excitation approximately 72 times per minute in an adult at rest. The action potential then spreads throughout the atria to the AV node.1,2
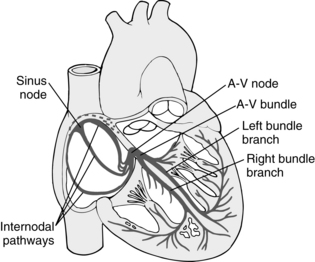
FIG. 11-4 Sinoatrial node and Purkinje’s system of the heart. A-V, Atrioventricular.
(From Hall J: Guyton and Hall textbook of medical physiology, ed 12, Philadelphia, 2011, Saunders.)
The AV node is located at the base of the wall between the atria. Its primary function is to delay the transmission of the impulses to the ventricles, which allows time for the atria to empty before the ventricles contract. The impulses then travel through the AV bundle, sometimes called the bundle of His. The AV node is able to discharge impulses 40 to 60 times per minute if not stimulated by an outside source.
The parasympathetic nerve endings are distributed mostly at the SA and AV nodes, over the atria, and to a lesser extent over the ventricles. If stimulated, they produce a decrease in the rate of rhythm of the SA node and slow the excitability at the AV node. The sympathetic nerves are distributed at the SA and AV nodes and all over the heart, especially the ventricles. Sympathetic stimulation increases the SA node rate of discharge, increases cardiac excitability, and increases the force of contraction.1,2
Coronary circulation
The regulation of coronary blood flow is determined primarily with the oxygen tension of the cardiac tissues. The most powerful vasodilator of the coronary circulation is hypoxemia. Other factors that can affect coronary blood flow are carbon dioxide, lactate, pyruvate, and potassium, all of which are released from the cardiac muscle. Coronary artery steal occurs when collateral perfusion of the myocardium is significantly reduced by an increase in blood flow to a portion of the myocardium that is normally perfused. More specifically, drug-induced vasodilatation of normal coronary arterioles can then divert or steal blood flow from potentially ischemic areas of the myocardium perfused by the vessels that have increased resistance (atherosclerotic vessels). Coronary artery steal can occur when arteriolar-vasodilating drugs, such as nitroprusside and isoflurane (Forane), are administered. This situation is especially likely to occur in people who are “stealprone”; they constitute approximately 23% of the patients with coronary artery disease, especially patients who have significant stenosis and occlusions to one or more coronary arteries.1,2
Stimulation of the parasympathetic nervous system causes an indirect decrease in coronary blood flow. Direct stimulation is slight because of the sparse amount of parasympathetic nerve fibers to the coronary arteries. The sympathetic nervous system serves to increase coronary blood flow both directly (as a result of the action of acetylcholine and norepinephrine) and indirectly (caused by a change in the activity level of the heart). The coronary arteries have both alpha and beta receptors in their walls (see the section on adrenergic and cholinergic receptors).12
Because so much cardiac disease involves the coronary arteries, the anesthetic risk rate increases in patients with cardiac disease. A functional classification of cardiac cases is based on the ability to perform physical activities (Box 11-2). Patients in classes III and IV have a significant risk for surgery and anesthesia and should undergo complete monitoring when they receive care in the PACU.13
BOX 11-2 Functional Classification of Cardiac Cases
Class I: No limitation. Ordinary physical activity does not cause undue fatigue, dyspnea, palpitation, or angina.
Class II: Slight limitation of physical activity. Such patients are comfortable at rest. Ordinary physical activity results in fatigue, palpitation, dyspnea, or angina.
Class III: Marked limitation of physical activity. Less than ordinary activity leads to symptoms. Patients are comfortable at rest.
Class IV: Inability to carry on any physical activity without discomfort. Symptoms of congestive failure or angina are present even at rest. With any physical activity, increased discomfort is experienced.
Modified from Hall J: Guyton and Hall textbook of medical physiology, ed 12, Philadelphia, 2010, Saunders.
Effect of anesthesia on the heart
Cardiac dysrhythmias are observed in approximately 60% of all patients who undergo anesthesia.14–15 The inhalation anesthetics, such as isoflurane, sevoflurane, and desflurane, can evoke junctional rhythms or increase ventricular automaticity or both. These anesthetics also slow the rate of SA node discharge and prolong the bundle of His-Purkinje and ventricular conduction times. Along with these changes in rhythm, alterations in the balance of the autonomic nervous system between the parasympathetic and sympathetic systems caused by drugs such as anticholinergics and catecholamines or by light anesthesia can initiate cardiac dysrhythmias. Therefore, in the immediate postoperative period, cardiac dysrhythmias are likely because of light anesthesia during emergence or because of the administration of drugs that alter sympathetic activity. Consequently, continuous monitoring of cardiac rate and rhythm is mandated in the PACU.14,15
Myocardial infarction
Acute myocardial infarction is a commonly encountered medical emergency that can occur in the PACU. More than 90% of myocardial infarctions result from disruption of an atherosclerotic plaque with subsequent platelet aggregation and formation of an intracoronary thrombus. The form of myocardial infarction that results depends on the degree of coronary obstruction and associated ischemia. A partially occlusive thrombus is the typical cause of non-ST elevation myocardial infarction. At the other end of the spectrum, if the thrombus completely obstructs the coronary artery, the results are more severe ischemia and a larger amount of necrosis, manifesting as an ST-elevation myocardial infarction.15,16 The objectives in the management of a patient with an acute myocardial infarction are pain relief, control of complications, salvage of ischemic myocardium, and a return to a productive life. The diagnosis of myocardial infarction is made on the basis of clinical findings, and therapy should be instituted immediately when suspected. (Cardiopulmonary resuscitation is discussed in Chapter 57.) An electrocardiogram performed in the PACU may reveal an injury pattern, but normal electrocardiographic results certainly do not exclude a diagnosis of myocardial infarction.16
Physical assessment for a suspected myocardial infarction can include the following subjective findings: (1) pain or pressure, which is usually substernal but may be manifested in the neck, shoulder, jaws, arms, or other areas; (2) nausea; (3) vomiting; (4) diaphoresis; (5) dyspnea; and (6) syncope. The onset of pain can occur with activity but can also occur at rest. The duration may be prolonged, from 30 minutes to several hours. Objective findings can include hypotension, pallor, and anxiety. The blood pressure, pulse, and heart sounds may be normal with an acute myocardial infarction. On auscultation of the chest, the abnormal cardiac findings may include atrial gallop, ventricular gallop, paradoxic second heart sound, friction rub, and abnormal precordial pulsations.16
Serum markers of infarction
Necrosis of myocardial tissue causes disruption of sarcolemma, so that the intracellular macromolecules leak into the bloodstream. Detection of such molecules in the serum, particularly cardiac-specific troponin and creatine kinase MB isoenzyme, serves important diagnostic and prognostic roles.8–11
Troponin is a cardiac specific regulatory protein in muscle cells that controls interactions between myosin and actin. Although found in both skeletal and cardiac muscles, the cardiac forms of troponin I and troponin T are structurally unique, and highly specific assays for their detection in the serum have been developed. Cardiac troponin serum levels begin to rise 3 to 4 hours after the onset of chest discomfort, peak between 18 and 36 hours, and then decline slowly, allowing for detection for up to 10 to 14 days after a large infarction.8–11
In the absence of trauma, the elevation of CK-MB is highly suggestive of myocardial injury. To facilitate the diagnosis of infarction using this marker, it is common to calculate the ratio of CK-MB to total CK. The ratio is usually greater than 2.5% in the setting of myocardial injury and less than that when CK-MB is from another source. The serum level of CK-MB starts to rise 3 to 8 hours after infarction, peaks at 24 hours, and returns to normal within 48 to 72 hours.8–11
Research studies have shown that patients who have had a myocardial infarction within 6 months before surgery have a recurrence rate of 54.5% for a myocardial infarction that could occur during or after the surgical procedure. If the myocardial infarction occurred between 6 months and 2 years before surgery, the rate of recurrence of infarction is between 20% and 25%. Between the second and third years, the incidence rate of reinfarction is approximately 5%. Most studies indicate that 3 years after the original myocardial infarction, the recurrence rate is approximately 1%, which equals the normal rate of myocardial infarction in the general population. Therefore the chance of a patient having an acute myocardial infarction in the PACU can be considered significant. This chance is especially true for patients in the PACU who have had a myocardial infarction within the last 3 years, who have a documented myocardial infarction risk factor (e.g., angina, hypertension, diabetes), or who have some combination of the previous factors.16–17
Perianesthesia nursing care
The perianesthesia nurse should be constantly alert for complications such as anxiety, arrhythmias, shock, left ventricular failure, and pulmonary and systemic embolisms. Pain and apprehension can be relieved with treatment with morphine sulfate, fentanyl, or hydromorphone (Dilaudid). Oxygen should be administered with nasal prongs because a face mask may increase the patient’s apprehension. Continuous cardiac monitoring should be instituted, and the patient should be kept in a quiet area. Drugs such as atropine, lidocaine, digitalis, quinidine, sodium nitroprusside (SNP), phentolamine, and nitroglycerin should be available. A machine for countershock also should be immediately available. Fluid therapy and urine output should be monitored completely for prevention of fluid overload. A pulmonary artery catheter or central venous pressure (CVP) monitor may be used for determining fluid replacement in patients with reduced intravascular volume and hypotension (see the discussion of CVP catheters in the following section). A benign myocardial infarction does not exist; all patients with a diagnosed myocardial infarction need constant competent perianesthesia care.9,18
Central venous pressure monitor
In the immediate postoperative setting, the CVP remains an excellent parameter indicating the adequacy of blood volume. In the hypovolemic state, the CVP is decreased. The administration of appropriate fluids and blood to expand the intravascular space increases the CVP toward the patient’s baseline reading. In the clinical setting, no absolute predetermined normal value for a CVP reading exists. The best use of this particular monitoring mode is for serial measurements for assessment of cardiovascular performance. See Chapter 27 for a discussion of the CVP monitor.4,11,13
Pulmonary artery catheter
The pulmonary artery catheter is used to monitor the central venous, pulmonary artery, and pulmonary capillary wedge pressures. This balloon-tipped catheter with four or five ports is discussed in detail in Chapter 27. Pulmonary artery catheters are used predominantly for cardiothoracic surgical patients and surgical patients with large volume shifts.
In the immediate postoperative period, the pulmonary artery catheter is usually used for patients with clinical shock, compromised ventricular function, and severe cardiac or pulmonary disease. In addition, patients who have had extensive surgical procedures or major cardiovascular surgery can benefit from this monitor. Accurate monitoring of left-sided and right-sided preload along with the rapid determination of cardiac output makes this monitor an excellent parameter for determining mechanical and pharmacologic therapy, with the intended outcome of enhanced cardiac performance and tissue perfusion.11,13
Circulatory system
Red blood cells
RBCs are produced by the bone marrow. The normal rate of production is sufficient to form approximately 1250 mL of new blood per month. This rate is also the normal rate of destruction. The average life span of an RBC is 120 days. The hematocrit value is the percentage of RBCs in the blood. The optimal range in adults is between 30% and 42%. When the hematocrit level is reduced to less than 30%, the oxygen-carrying capacity declines steeply. Moreover, when the hematocrit level rises to greater than 55%, the oxygen-carrying capacity declines because the increase in blood viscosity causes increased work for the heart and decreased cardiac output. The normal amount of hemoglobin in the RBC ranges from 10 to 13.5 g. In fact, the amount and type of hemoglobin determine the oxygen-carrying capacity. Recent evidence indicates that the cutoff value for risk of reduced oxygen-carrying capacity and blood volume is a hemoglobin level of 11 g, a hematocrit of 27%, or both. Transfusion with blood or blood products to raise the level of hemoglobin should be strongly considered for any patient with values lower than the cutoff values.2
White blood cells
White blood cells (WBCs), or leukocytes, are the body’s major defense against infection. The two primary types of circulating leukocytes are polymorphonuclear leukocytes (PMNs) and lymphocytes. The role of the PMNs in combating infection is to migrate to the infectious site in large numbers and phagocytize the invading microbe. The role of the lymphocytes is to mediate immunoglobulin production and act in the delayed hypersensitivity in the type IV reaction (see Chapter 18). Evaluations of the WBC count should focus on the number of PMNs. When the PMN level is less than 1000 per mm3, the incidence rate of infections is increased. Postanesthesia patients with a PMN level of 500 to 100 per mm3 are at great risk of infection.2
Blood platelets
Normal hemostasis requires a proper interaction between blood vessels, platelets, and coagulation proteins. Any dysfunction in any one of the three components has a profound effect on hemostasis. When a tissue injury occurs, the vessel wall undergoes vasoconstriction and activates the extrinsic pathway for coagulation proteins. Platelet adhesion and aggregation occur along with the activation of the intrinsic and extrinsic pathways for the coagulation proteins. The result of this interaction is a hemostatic plug.2
A prolongation of the BT and surgically related hemorrhage seem to be correlated. The normal BT is between 2 and 11 minutes. The test results are considered abnormal when the BT is longer than 12 minutes. The template procedure should be used when the BT is performed because it is more sensitive than older methods. The normal platelet count is between 200,000 and 450,000 per mm3. More specifically, the patient usually tolerates surgery and the postanesthesia phase well in regard to hemostasis with a platelet count of 100,000 per mm3 or higher. Patients with a platelet count of 50,000 to 100,000 per mm3 may have ecchymoses from tissue trauma. If the platelet count is less than 50,000 per mm3, many alterations in bleeding may occur. These patients need constant evaluation and therapy in the postoperative period.5
The PTT is a test for evaluation of the intrinsic and common coagulation pathways of the coagulation system. It is most commonly used for monitoring heparin therapy. Normal results are considered to be 25 to 32 seconds, depending on the reagent. Abnormal results are considered to be longer than 35 seconds. The PT is used to examine the extrinsic coagulation system for evaluation of oral anticoagulant therapy. Normal results are based on laboratory control for interpretation. Usually, the control is normal in patients with an appropriately functioning extrinsic coagulation system. When the value is more than 3 seconds greater than the control, the test results are considered to be abnormal. The international normalized ratio (INR) evaluates the extrinsic and common pathway independently of various reagents used in different laboratory setting and in different areas of the world. The normal INR is 1.5 to 2.5. Many institutions report both the PT and INR values.8 Postoperative bleeding can occur when the patient’s preoperative or intraoperative coagulation study results are abnormal. Bleeding tendencies are enhanced by the presence of postoperative hypertension. In addition, when hemostasis is lacking at the suture line or extensive surgical tissue trauma exists, the likelihood of postoperative bleeding is increased. Finally, the use of antibiotics during and after surgery can also increase bleeding tendencies. Therefore the perianesthesia nurse should evaluate the patient’s preoperative and intraoperative coagulation study results and examine the surgical incision for bleeding during initial assessment. Certainly, the postoperative trauma patient who has undergone extensive surgical trauma should be constantly monitored for bleeding tendencies, especially if intraoperative antibiotics were administered. If the patient is undergoing anticoagulant therapy, continued monitoring of the anticoagulant activity is mandated. Finally, in the patient with a demonstrated bleeding tendency, maintenance of a normal arterial blood pressure must be ensured. For a complete review of the fluid and electrolyte administration see Chapter 14.19
Blood vessels
The circulatory system can be divided into the systemic and the pulmonary circulation. The systemic or peripheral circulation comprises arteries, arterioles, capillaries, venules, and veins. The walls of the blood vessels, except the capillaries, are composed of three distinct coats: the tunica adventitia, the tunica media, and the tunica intima. The outer layer, the tunica adventitia, consists of white fibrous connective tissue, which gives strength to and limits the distensibility of the vessel. The vasa vasorum, which supplies nourishment to the larger vessels, is in this layer. The middle layer, the tunica media, consists of mostly circularly arranged smooth muscle fibers and yellow elastic fibers. The innermost layer, the tunica intima, is a fine transparent lining that serves to reduce resistance to the flow of blood. The valves of the veins are formed by the foldings of this layer. The capillaries consist of a single layer of squamous epithelial cells, which is a continuation of tunica intima.2
Microcirculation
Microcirculation is the flow of blood in the finer vessels of the body. It involves the arterioles, capillaries, and venules. The arteries subdivide to the last segment of the arterial system, the arteriole. The arteriole consists of a single layer of smooth muscle in the shape of a tube for conducting blood to the capillaries. As the arterioles approach the capillaries, they lack the coating of smooth muscle and are termed metarterioles. At the point at which the capillaries originate from the metarterioles, a smooth muscle fiber (the precapillary sphincter) encircles the capillary. At the other end of the capillary is the venule, which is larger but has a much weaker muscular coat than the arteriole.2
The microcirculation serves three major functions: (1) transcapillary exchange of nutrients and fluids; (2) maintenance of blood pressure and volume flow; and (3) return of blood to the heart and regulation of active blood volume.2
Adrenergic and cholinergic receptors
The cardiovascular system and the concept of adrenergic and cholinergic receptors are closely related. The perianesthesia nurse must understand the pharmacodynamics of these receptors.20,21
Functional anatomy: the mediators
Cholinergic is a term used to describe the nerve endings that liberate acetylcholine. The cholinergic neurotransmitter, acetylcholine, is present in all preganglionic parasympathetic fibers, all preganglionic sympathetic fibers, all postganglionic parasympathetic fibers, and all somatic motor neurons. Two exceptions to the general rule are postganglionic sympathetic fibers to the sweat glands and to the vasculature of skeletal muscle. These fibers are considered sympathetic anatomically but cholinergic in terms of their neurotransmitter (i.e., they release acetylcholine as their neurotransmitter).20,21
The term adrenergic is used to describe nerves that release norepinephrine as their neurotransmitter. Epinephrine may be present in the adrenergic fibers in small quantities, usually representing less than 5% of the total amount of both epinephrine and norepinephrine. The adrenergic fibers are the postganglionic sympathetic fibers, with the exception of the postganglionic sympathetic fibers to the sweat glands and to the efferent fibers to the skeletal muscle (Box 11-3).20,21
BOX 11-3 Cholinergic and Adrenergic Nerves
Modified from Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2009, Saunders.
The adrenal medulla should be considered separately because it is innervated by a preganglionic sympathetic fiber that liberates the neurotransmitter acetylcholine and because the postganglionic portion is the adrenal medulla, which behaves much like a postganglionic sympathetic fiber. The adrenal medulla is therefore stimulated by acetylcholine, which causes the release of both epinephrine and norepinephrine from its chromaffin cells. As opposed to the usual finding of a preponderance of norepinephrine at the postganglionic nerve fiber terminals, the distribution in the adrenal medulla is 80% epinephrine and 20% norepinephrine. Therefore the neurotransmitter of the adrenal medulla is epinephrine.2,20,21
Cholinergic neurotransmitter: biochemistry
The neurotransmitter acetylcholine is synthesized from choline and acetate through the enzymatic activity of choline acetylase to form acetylcholine (Box 11-4); it is then stored in vesicles. When acetylcholine is released from a preganglionic fiber, it may then act on the membrane of the preganglionic fiber with a positive feedback mechanism, thus enhancing the release of acetylcholine. The calcium ion facilitates this additional release of acetylcholine. This process is called excitation-secretion coupling through calcium.2,20,21
Adrenergic neurotransmitter: biochemistry
The adrenergic neurotransmitter, epinephrine, begins in the body as phenylalanine, which is hydroxylated to tyrosine, which is again hydroxylated to form L-dopa, an amino acid. This process is probably the weakest step in the biosynthetic chain and may be a possible site of action of an autonomic drug. A soluble enzyme, L-dopa decarboxylase, acts on L-dopa to form dopamine, which in turn is synthesized to norepinephrine. In the adrenal medulla, norepinephrine can be methylated in the cell to form the final product, epinephrine. This reaction is catalyzed by the enzyme phenylethanolamine-N-methyltransferase (see Box 11-4).20,21
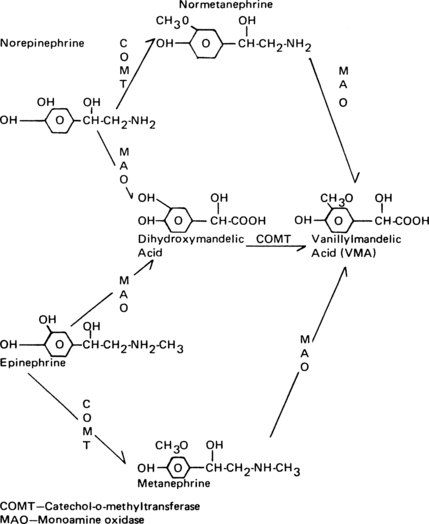
The norepinephrine that is not recaptured is metabolized eventually to vanillylmandelic acid. Epinephrine also undergoes a number of steps in its biodegradation to vanillylmandelic acid. An increase in vanillylmandelic acid concentration in the urine is useful in the diagnosis of conditions such as pheochromocytoma and neuroblastoma (Fig. 11-5).2,20,21
Cholinergic receptors
The pharmacologic and physiologic actions of acetylcholine are apparently mediated by its combination with specific cholinergic receptors. The actions of acetylcholine and drugs that mimic acetylcholine are mediated through two types of cholinergic receptors: nicotinic and muscarinic (see Chapter 23).2,20,21
When the nicotinic receptors are stimulated, the following responses are observed:
• Stimulation of autonomic ganglia, both parasympathetic and sympathetic.
• Stimulation of the adrenal medulla, which results in the release of both epinephrine and norepinephrine.
The muscarinic responses elicited by muscarine and acetylcholine are the following:
• Stimulation or inhibition of smooth muscle in various organs or tissues.
• Stimulation of exocrine glands.
Nicotinic responses in terms of antagonism can be blocked with drugs such as ganglionic or neuromuscular blocking agents, or both, whereas muscarinic responses are blocked with the class of drugs best typified by atropine.2,20,21
Muscarine is a specific agonist at muscarinic receptors, whereas nicotine is a specific agonist at nicotinic receptors. However, acetylcholine is capable of stimulating both receptor types (Table 11-1).2,20,21
| ORGAN STIMULATED BY CHOLINERGIC AGONIST | RESPONSE | TYPE OF CHOLINERGIC RECEPTOR RESPONSE |
|---|---|---|
| Heart | ||
| Sinoatrial node | Negative chronotropic effect | Muscarinic |
| Atria | Decreased contractility and increased conduction velocity | Muscarinic |
| AV node and conduction system | Decrease in conduction velocity—AV block | Muscarinic |
| Eye | ||
| Sphincter muscle of iris | Contraction (miosis) | Muscarinic |
| Lung | ||
| Bronchial muscle | Contraction | Muscarinic |
| Bronchial glands | Stimulation | Muscarinic |
| Exocrine Glands | ||
| Salivary glands | Profuse watery secretion | Muscarinic |
| Lacrimal glands | Secretion | Muscarinic |
| Nasopharyngeal glands | Secretion | Muscarinic |
| Adrenal medulla | Catecholamine secretion | Nicotinic |
| Autonomic ganglia | Ganglion stimulation | Nicotinic |
| Muscarinic | ||
| Skeletal Muscle | ||
| Motor endplate | Stimulation | Nicotinic (motor endplate receptor) |
AV, Atrioventricular.
Modified from Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2009, Saunders.
Belladonna alkaloids such as atropine have adverse effects that are peculiar to the PACU phase of the surgical experience. More specifically, belladonna alkaloids that cross the blood-brain barrier can cause disorientation, violent behavior, or somnolence. Physostigmine salicylate, an anticholinesterase that is capable of penetrating the blood-brain barrier, has been shown to be useful in reversing the adverse effects of belladonna alkaloids on the central nervous system. Physostigmine salicylate is also useful in reversing the disorientation or somnolence caused by drugs such as diazepam, phenothiazines, tricyclic antidepressants, antiparkinsonian drugs, promethazine, and droperidol. Patients in the PACU who may benefit from treatment with physostigmine are those who have received a belladonna alkaloid or neuroleptic type of agent either before or during surgery, who have had disorientation or restlessness or both for more than 30 minutes after anesthesia, and who are difficult to arouse over an appropriate period. Patients with any one of these dysfunctions qualify for treatment and can be given 1-mg increments of physostigmine intravenously at 15-minute intervals until they are conscious and oriented to time, place, and person. After treatment has begun, the perianesthesia nurse should monitor the blood pressure and pulse immediately before and 5 minutes after the administration of physostigmine. In addition, some patients may have side effects from physostigmine, such as nausea, pallor, sweating, and bradycardia. Because glycopyrrolate (Robinul) does not cross the blood-brain barrier, treatment of the side effects of physostigmine is especially helpful. Finally, patients who have undergone treatment with physostigmine probably should remain in the PACU for approximately 1 hour after the administration of the anticholinesterase.20–23
Adrenergic receptors
The stimulation of the sympathetic nervous system can be both inhibitory and excitatory, which has caused considerable confusion. Originally, theories were postulated that this phenomenon handled the release of two different compounds. The variation in the effects of stimulation was later found to be related not to the differences in chemical release but rather to a difference in the receptors’ responses to the transmitter.2
The adrenergic receptors, which respond to catecholamines, can be subdivided into three main types: dopaminergic, alpha, and beta. The dopaminergic receptors are primarily in the central nervous system and the mesenteric and renal blood vessels. The agonist for these receptors is dopamine. The alpha receptors can be further divided into alpha1 and alpha2 receptors. The postsynaptic alpha1 receptors are excitatory in action, except in the intestine. Stimulation of the alpha1 receptors causes smooth muscle contraction, which results in a vasoconstriction or pressor response. Hence, the alpha1 receptor is activated by the release of norepinephrine, and this released norepinephrine also activates the presynaptic alpha2 receptors to inhibit the further release of norepinephrine. As a result, the alpha1 receptor is activated by the release of norepinephrine, and the released norepinephrine in turn stimulates the alpha2 receptor, thus producing inhibition of the release of norepinephrine and resulting in a negative feedback loop (Fig. 11-6).24
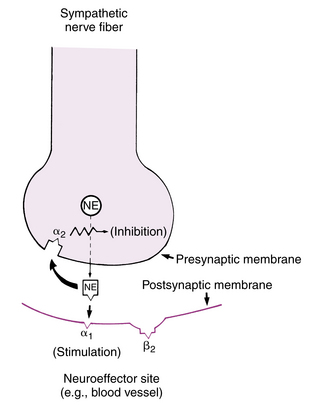
FIG. 11-6 Presynaptic and postsynaptic alpha receptors at ending of norepinephrine (NE)-secreting neuron.
(From Gardenhire DS: Rau’s respiratory care pharmacology, ed 7, St. Louis, 2008, Mosby.)
The drug clonidine (Catapres) is believed to stimulate the alpha2 receptors, which lower the sympathetic outflow of norepinephrine and ultimately lead to a hypotensive effect. In addition to lowering catecholamine levels, clonidine can reduce the plasma renin activity. This antihypertensive drug enjoys a significant degree of popularity, but it can have a negative effect on the patient in the PACU. More specifically, the clonidine withdrawal syndrome has been reported when the drug has been stopped abruptly. The sequelae of the syndrome resemble pheochromocytoma in that shortly after the withdrawal of the clonidine the patient can have hypertension, tachycardia, and increased blood levels of catecholamines. Treatment of this syndrome usually involves a reinstitution of the clonidine therapy and alpha-adrenergic blocking agents such as phentolamine.24
Stimulation of the beta receptor causes vascular smooth muscle relaxation, which then leads to a decrease in blood pressure through a decrease in peripheral resistance. The beta receptor can be divided into two types: beta1 and beta2. Beta1-subtype receptors are found in all cardiac tissue except the coronary vasculature and are responsible for characteristic effects noted after stimulation of the heart with epinephrine, including the following effects: (1) increase in heart rate; (2) increase in contractile force; (3) increase in conduction velocity; and (4) shortening of the refractory period. Beta1-subtype receptors mediate effects elicited by catecholamines (Table 11-2).21–24
| RESPONSE | TYPE OF ADRENERGIC RECEPTOR |
|---|---|
| Heart | |
| Positive inotropic effect | Beta1 |
| Positive chronotropic effect | Beta1 |
| Cardiac arrhythmias | Beta1 |
| Positive dromotropic effect | Beta1 |
| Vascular | |
| Arterial and arteriolar constriction | Alpha1 |
| Coronary artery constriction | Alpha1 |
| Coronary artery dilatation | Beta1 |
| Arteriolar relaxation | Beta2 |
| Gastrointestinal Tract | |
| Intestinal relaxation | Alpha1, beta1 |
| Sphincter contraction (usually) | Alpha1 |
| Urinary Bladder | |
| Bladder relaxation (detrusor) | Beta2 |
| Bladder contraction (trigone and sphincter) | Alpha1 |
| Eye | |
| Contraction (mydriasis) | Alpha1 |
| Ciliary muscle of iris | Beta2 |
| Metabolic | |
| Liver glycogenolysis (hyperglycemia) | Alpha1, beta2 |
| Muscle glycogenolysis | Beta1 |
| Lipolysis | Beta1 |
| Oxygen consumption (increases) | Beta1, beta2 |
| Other Smooth Muscle | |
| Bronchial (relaxation) | Beta2 |
| Spleen (contraction) | Alpha1 |
| Ureter (contraction) | Alpha1 |
| Uterus (contraction) | Alpha1 |
| Uterus (relaxation)—nonpregnant condition | Beta2 |
Modified from Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2009, Saunders.
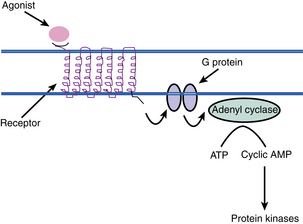
The physiology of the beta receptor has many implications for the care of the PACU patient. Once the beta receptor has been activated by first messengers, endogenous catecholamines, or exogenous beta agonists such as isoproterenol, certain biochemical events occur (Fig. 11-7). The enzyme adenylate cyclase, which is located on the plasma membrane, is stimulated with beta-receptor activation. Next, within the cell, adenosine triphosphate is broken down to 3′,5′-adenosine monophosphate (cyclic AMP). The cyclic AMP is then released into the cytoplasm of the cell and acts to modulate cellular activities. Hence, the cyclic AMP is considered to be the second messenger. Cyclic AMP is inactivated to 5-AMP by the enzyme phosphodiesterase.21–24
Clinically, isoproterenol or terbutaline can be administered to increase the cyclic AMP levels in the beta2 receptors in the bronchial airways with the intended result of bronchodilatation. Another way to increase the cyclic AMP levels is inhibition of the action of phosphodiesterase. Caffeine and methylxanthines, such as aminophylline, are inhibitors of the enzyme phosphodiesterase and can be used alone or in combination (for synergistic effects) with the beta agonists to produce the desired bronchodilatation in the patient. It is important to remember that other catecholamine effects are produced by the increase in cyclic AMP levels. Consequently, although aminophylline is considered a bronchodilator, it increases the myocardial contractility and heart rate of the patient, thus mandating that the perianesthesia nurse monitor both respiratory and cardiac function when methylxanthines are administered.
The coronary arteries contain alpha1 and beta1 receptors and therefore also have the ability to vasoconstrict and vasodilate (see Table 11-2). The endogenous catecholamines, norepinephrine and epinephrine, are capable of stimulating both the alpha and the beta receptors.21–24
Site of action of autonomic drugs
Methyldopa (Aldomet; the alpha-methylated analogue of L-dopa) is an antihypertensive drug. Methyldopa reduces the sympathetic nerve stimulation through the production of a selective agonist, alpha methylnorepinephrine.21–24
Guanethidine has the ability to prevent nerve stimulation and thus inhibit norepinephrine release. Guanethidine interferes with the storage of norepinephrine and, if given chronically, results in a decrease in the amount of norepinephrine stored in adrenergic nerves. Reserpine also shares this latter action with guanethidine. Therefore chronic use of guanethidine and reserpine results in a relative depletion of the norepinephrine content from sympathetic nerves (Table 11-3).
Table 11-3 Drugs that Interfere with Specific Steps in Process of Chemical (Neurohumoral) Transmission
| CHEMICAL TRANSMISSION | ADRENERGIC NERVES | CHOLINERGIC NERVES |
|---|---|---|
| Synthesis of mediator | Methyldopa | Hemicholinium |
| Storage of mediator | Reserpine | — |
| Release of mediator | Guanethidine | Botulinus toxin |
| Combination of mediator with its receptor | Phenoxybenzamine (alpha receptor)Propranolol (beta receptor) | Atropine (muscarinic)Nicotine (nicotinic) |
| Enzymatic destruction of mediator | Pyrogallol (COMT inhibitor)Tranylcypromine (MAOI) | Physostigmine (cholinesterase inhibitor) |
| Prevention of inactivation of mediator (blocks uptake) | Cocaine | — |
| Repolarization of postsynaptic membrane (persistent depolarization) | — | Succinylcholine |
COMT, Catechol-O-methyltransferase; MAOI, Monoamine oxidase inhibitor.
From Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2009, Saunders.
The calcium channel blockers have been found to have considerable value in the treatment of supraventricular tachycardias, angina pectoris, and myocardial infarction. The prototype calcium channel blockers are verapamil (Isoptin), nifedipine (Procardia, Adalat), and diltiazem (Cardizem). All three drugs depress calcium entry into conduction tissue and cardiac muscle, which results in a depression of conduction and leads to a reduction of the circus movements. These calcium entry blockers produce hypotension with different mechanisms. Nifedipine, like SNP, decreases the systemic vascular resistance with a compensatory tachycardia, and verapamil and diltiazem lower the cardiac output by exerting a negative dromotropic effect. The effects of the calcium channel blockers can be enhanced with inhalation anesthesia agents such as halothane. Consequently, in the PACU, patients who received an inhalation of anesthetic and are undergoing treatment with a calcium channel blocker may have some hypotension. Therefore the perianesthesia nurse should vigorously monitor the cardiovascular parameters of these patients and report any confirmed hypotension to the attending physician.20–24
Dopamine is a naturally occurring biochemical catecholamine precursor of norepinephrine. It exerts a positive inotropic effect and a minimal chronotropic effect on the heart. Therefore, the contractility of the heart is increased without a change in the afterload (total peripheral resistance), which leads to an increase in cardiac output. The increase is in the systolic and pulse pressures, with virtually no effect on the diastolic pressure. Dopamine is not associated with tachyarrhythmias and produces less of an increase in myocardial oxygen consumption than does isoproterenol. Blood flow to peripheral vascular beds may decrease while mesenteric flow increases. One of the major reasons for the increase in the use of dopamine clinically is its dilatation of the renal vasculature. This action is the result of the inotropic effect and decreased peripheral resistance. Therefore the glomerular filtration rate is increased along with the renal blood flow and sodium excretion.20–24
Dobutamine (Dobutrex) is synthetically derived from the catecholamine isoproterenol. Consequently, it produces a positive inotropic effect with specificity to the beta1 receptors, thus resulting in an increase in cardiac output with minimal effects on blood pressure, heart rate, and systemic vascular resistance. The drug is usually administered intravenously in a dose range of 2 to 10 mcg/kg/min and is especially useful for patients who are recovering from cardiopulmonary bypass surgery. Dobutamine is sometimes combined with a vasodilator to reduce afterload in an effort to optimize the cardiac output.20–24
Hypotension therapy
Hypotension in the immediate postoperative period is of great concern and deserves the prompt attention of the perianesthesia nurse. When hypotension is detected in the postanesthesia patient, the nurse should first reaffirm the measurements. An incorrectly placed or sized blood pressure cuff or malfunction of the stethoscope can yield incorrect measurements (see Chapter 27). If an arterial catheter transducer system is used, it should be appropriately zeroed and calibrated and the air bubbles removed to ensure that artificially low readings are not observed. In addition, if the patient is hypothermic or receiving alpha-adrenergic agonists such as phenylephrine (Neosynephrine), the patient may have low blood pressures in the radial and brachial arteries, whereas the central blood pressure may be higher. This difference is because of the peripheral vasoconstriction produced by the alpha-adrenergic drugs.25
If the hypotension is confirmed, hypovolemia should be considered as a possible cause. The clinical signs of hypotension from hypovolemia include cold, pale, clammy, or diaphoretic skin; rapid, thready pulse; shallow, rapid respirations; disorientation, restlessness, or anxiety; decreased CVP; and oliguria. The nursing assessment of the patient with hypotension should include an inspection of the dressings for excessive bleeding and an evaluation for the clinical signs of hypovolemia. If the patient’s circulating blood volume is reduced by more than 15% to 20%, hypotension can ensue. This condition usually happens when the patient has not received appropriate fluid volume replacement during surgery. Other factors in the development of postoperative hypovolemia are ongoing internal or external hemorrhage, sweating, insensible losses, and third-space losses. Third-space losses occur when an exudation of fluid into the tissues occurs (see Chapter 14). Other causes of hypotension include a high alveolar-inflating pressure when a patient is undergoing mechanical ventilation, ventricular dysfunction, myocardial ischemia, and cardiac dysrhythmias. If the hypotension is 30% less than the preoperative baseline blood pressure readings or one or more of the clinical signs of hypovolemia is present, then the attending physician should be notified.25
Usual therapy for hypotension in the PACU includes the administration of a high fractional concentration of oxygen, fluid infusion, reversal of residual anesthetic depressant effects, repositioning of the patient to facilitate venous return, reduction in ventilator airway pressures, and administration of vasopressors or anticholinergics or both, such as glycopyrrolate or atropine, as indicated. More specifically, the first line of defense is to return the patient’s condition to normovolemia and, in this instance, administer a bolus of crystalloid solution of 300 to 500 mL. The anticholinergics are indicated if sinus bradycardia accompanies the hypotension. The vasopressors exert their effect either directly or indirectly. The direct-acting vasopressor exerts its effect directly on the receptor. Conversely, the pharmacologic action of an indirect vasopressor facilitates the release of norepinephrine from its storage vesicles (primarily the terminal sympathetic nerve fibers), which stimulates the adrenergic receptor to achieve the desired effect. Therefore a direct-acting vasopressor is probably necessary to achieve a response in patients who are depleted of catecholamines by drugs such as reserpine and guanethidine (Table 11-4).20–25
| GENERIC NAME | TRADE NAME |
|---|---|
| Direct-Acting Adrenergic Amines | |
| Epinephrine | Adrenalin |
| Norepinephrine | Levophed |
| Dopamine | Intropin |
| Dobutamine | Dobutrex |
| Isoproterenol | Isuprel |
| Methoxamine | Vasoxyl |
| Phenylephrine | Neosynephrine |
| Indirect-Acting Adrenergic Amines | |
| Metaraminol | Aramine |
| Mephentermine | Wyamine |
| Ephedrine | Ephedrine |
Another area of consideration in selection of a vasopressor is the cardiotonic action desired. Metaraminol (Aramine), with its action of norepinephrine release, causes improved cardiac function as a result of its beta-receptor activity. Conversely, phenylephrine and methoxamine (Vasoxyl) possess little or no cardiac effect and exert a pressor action with pure alpha stimulation. The alpha-adrenergic agonists are useful especially for patients who have received a “high” spinal or epidural anesthetic. High levels of regional anesthetics are associated with peripheral vasodilatation and bradycardia because of a sympathetic blockade. Consequently, an alpha-adrenergic agonist produces peripheral vascular vasoconstriction, or a mixed-action alpha and beta drug such as ephedrine can be administered.20–25
Drugs that combat hypotension include the cardiac inotropic agents. This class of drugs produces positive inotropic and vasodilating effects and can be considered to be related to digitalis in regard to pharmacologic effects. The major pharmacologic actions of these drugs include increased cardiac output and decreased LVEDP. These drugs are of benefit for the short-term management of congestive heart failure, especially in patients with congestive heart failure who do not have adequate responses to digitalis, diuretics, or vasodilators. Also, the inotropic agents may be valuable in the treatment of cardiogenic shock. This class of drugs can be considered as an alternative to catecholamines for the treatment of low cardiac output in the postoperative period. Drugs in this category include amrinone (Inocor) and milrinone (Primacor). As with other vasopressors, constant monitoring of the patient’s vital signs is warranted when inotropic agents are administered.20–25
Hypertension therapy
Increased sympathetic nervous system activity causes postoperative hypertension. More specifically, pain, stimulation with an endotracheal tube, bladder distention, and preeclampsia are some of the clinical phenomena that may lead to hypertension. Postoperative pain should be assessed because it can cause a significant degree of hypertension. Pain can be eliminated as a causative factor with determination of whether adequate analgesia exists. If the patient has a significant amount of pain, an analgesic should be administered immediately. In addition, if the hypertension is caused by acute anxiety, the use of sedatives may dramatically reduce the blood pressure. Hypoxemia with hypercarbia from hypoventilation is also a common cause of postoperative hypertension. Therefore, during the evaluation of the patient, the patient’s rate and depth of ventilation should be assessed. If the patient has hypoventilation, prompt use of the stir-up regimen is mandated. Another assessment tool in the evaluation of postoperative hypertension is the amount and degree of hypothermia. More specifically, if the patient is shivering, an accompanying increase in blood pressure is seen. Prompt interventions to increase the patient’s core temperature to reduce shivering is warranted (see Chapter 53). An assessment of the patient’s fluid volume status should be made to determine whether hypervolemia exists because fluid overload can cause postoperative hypertension. In addition, if the patient has acute pulmonary edema caused by hypertensive heart disease, correction of the pulmonary edema usually reduces the blood pressure to acceptable limits. Certainly, whether a hypertensive emergency exists should be determined; if it does, treatment must be started promptly.20–25
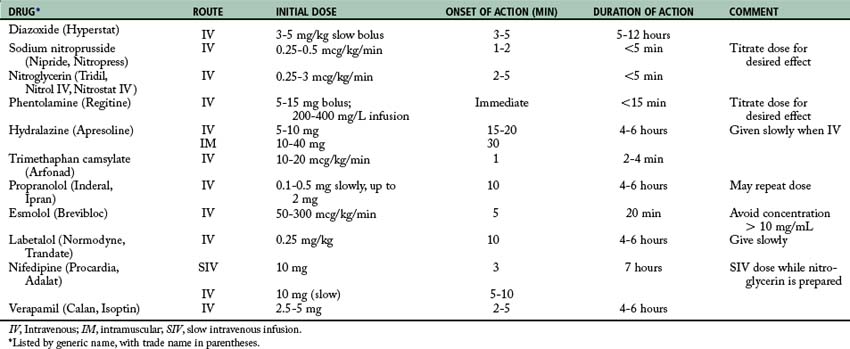
If pharmacologic antihypertensive therapy is deemed necessary by the physician, the drugs listed in Table 11-5 usually are instituted. For severe postoperative hypertension, SNP is probably the drug of choice. While the SNP is prepared, nifedipine or diltiazem can be given intravenously. Calcium channel blockers decrease blood pressure, cardiac workload, and myocardial oxygen consumption especially in the presence of ischemic heart disease. In addition, the use of nifedipine or diltiazem may preclude the use of a central venous catheter that is required when SNP is given. Once the patient’s condition is stabilized, hydralazine (5 to 10 mg) and propranolol (0.2 to 0.5 mg) can be given in repeated intravenous doses to wean the patient off SNP. Propranolol should be titrated to maintain the heart rate at approximately 100 beats/min. Other beta blockers, such as labetalol, metoprolol, and esmolol, can be used intravenously. Esmolol may be the drug of choice because of its short duration of action and rapid onset. The hydralazine can be given as intravenous boluses every 20 to 30 minutes to keep the patient’s condition normotensive. Because these drugs are extremely potent and have their own complications, they are discussed briefly in the following sections.20–25
Recent research has shown that beta-blockers are able to aid in controlling the sympathetic responses of patients in the perioperative phase of recovery. Controlling sympathetic responses reduces perioperative myocardial ischemia and improves long-term survival in vascular and cardiovascular surgical patients. Research has also shown that the use of alpha-2-agonists such as clonidine and dexmedetomidine in the postanesthesia phase decreases oxygen consumption and improves hemodynamic outcomes.24
Dexmedetomidine
Dexmedetomidine is indicated for sedation of patients requiring postoperative mechanical ventilation in the PACU setting. Its indication in the United States was recently expanded to include nonintubated patients requiring sedation for surgery or procedures. Dexmedetomidine is also useful as an adjunct for sedation and general anesthesia in the setting of certain operations and invasive medical procedures, such as colonoscopy. There are no absolute contraindications to the use of dexmedetomidine; its usefulness is limited because the drug cannot be given as a bolus owing to concerns about peripheral alpha-2 receptor stimulation with resulting hypotension. Compared to midazolam, dexmedetomidine was similarly effective for sedation, but shortened the time to extubation. It was associated with less delirium, tachycardia, and hypertension, but more bradycardia. Dexmedetomidine has sedative, analgesic, sympatholytic, and anxiolytic effects that blunt many of the cardiovascular responses in the perioperative period. It reduces the requirements for volatile anesthetics, sedatives, and analgesics without causing significant respiratory depression.24
Diazoxide
Diazoxide (Hyperstat) is avidly bound to and inactivated by serum proteins and thus must be given as a rapid intravenous bolus (within 15 seconds) of 3 to 5 mg/kg every 5 minutes. If the desired response is still not obtained after three bolus administrations, the use of SNP should be considered. The major disadvantage of diazoxide as compared with SNP is that diazoxide cannot be titrated in accordance with the patient’s response. The onset of action of this drug is within 3 to 5 minutes, and its duration is 5 to 12 hours. Its action is immediate and is achieved through its direct vasodilating effects. Because the drug has more effect on the resistance vessels than the capacitance vessels, it decreases the afterload and has no effect on the preload. Concurrent administration of a loop diuretic, such as furosemide (40 to 80 mg intravenously), is usually advantageous especially if the patient’s condition is edematous as a result of either cardiac or renal failure.22–24
Clonidine
Clonidine treats high blood pressure by stimulating α2 receptors in the brain, which decreases cardiac output and peripheral vascular resistance, lowering blood pressure. It has specificity toward the presynaptic α2 receptors in the vasomotor center in the brainstem. This binding decreases presynaptic calcium levels and inhibits the release of norepinephrine. The net effect is a decrease in sympathetic tone. The antihypertensive effect of clonidine is in fact due to its agonistic effect on the I1-receptor (imidazoline receptor), which mediates the sympatho-inhibitory actions of imidazoline to lower blood pressure.22–24
Sodium nitroprusside
A compound of unusual chemical structure, SNP (Nipride) is immediately effective in all cases of severe hypertensive crises, including those resistant to diazoxide. Its action is thought to result from the peripheral arteriolar dilatory effect of the drug. Because the drug can lower blood pressure rapidly, careful intravenous administration with constant bedside arterial pressure monitoring is required. The drug is extremely sensitive to light and must be administered through bottles and tubing that are wrapped and protected from the light. Only fresh solutions should be used. Solutions that are more than 4 hours old should be discarded because they may form thiocyanates. Treatment is started with a solution of 250 mL of 5% dextrose in water and 50 mg of SNP (200 mcg/mL) with use of an infusion pump to ensure a precise flow rate. A dose of 1 to 2 mcg/kg/min usually produces a prompt decrease in blood pressure, which returns to control levels within 5 minutes after the drug is stopped. Acute postoperative hypertension can be treated with a one-time single intravenous injection of 50 to 100 mcg of SNP. The onset of action of this drug is 1 or 2 minutes, and its duration of action is 2 to 5 minutes. Because of its unique chemical structure, cyanide is released into the blood stream when the drug is used. The cyanide is quickly converted to thiocyanate by the liver. Thiocyanate toxicity (fatigue, nausea, anorexia, muscle spasms, and disorientation) may result from prolonged use or from high dosages; therefore monitoring of serum thiocyanate levels is advised when the drug is used longer than 24 hours. Toxic symptoms appear with serum thiocyanate levels of 5 to 10 mg/dL, and the compound can be removed rapidly with peritoneal dialysis. As with diazoxide, once blood pressure has been brought to control levels, concomitant use of an oral medication such as guanethidine or methyldopa allows the gradual tapering and discontinuance of SNP.22–24
Phentolamine
Phentolamine mesylate (Regitine), an alpha-receptor blocker, is specifically indicated for management of hypertensive crises associated with increased circulating catecholamines. These crises can result from pheochromocytoma or the sudden release of tissue catecholamine stores caused by certain drugs or foods that contain tyramine in patients receiving monoamine oxidase inhibitors (pargyline derivatives, primarily Eutonyl). The antipressor effect of a single intravenous injection is short lived, usually lasting less than 15 minutes; therefore administration of phentolamine with intravenous infusion (200 to 400 g/L) is desirable, along with titration of the dose to achieve the desired pressure level after the blood pressure has been controlled initially with a rapid intravenous dose of 2 to 15 mg. Because the drug blocks only alpha receptors, beta-mediated effects of the circulating catecholamine on the heart must be controlled with the specific beta blocker, propranolol hydrochloride.22–24
With rare exceptions, these three drugs (diazoxide, SNP, and phentolamine) can be considered the mainstays of modern therapy in acute hypertensive crises. The other drugs discussed here should be considered second-line drugs. Their primary disadvantages include slower onset of action, rapid development of tachyphylaxis, and marked central nervous system depressant effects. In most instances, the drugs should be used to supplement and initiate long-term control once the acute crisis is resolved with the primary drugs.22–24
Hydralazine
Hydralazine (Apresoline) is not effective in hypertensive encephalopathy—complicating acute or chronic glomerulonephropathy; it is used in encephalopathy that has chronic essential hypertension as an underlying cause. Blood pressure is reduced through vasodilatation, which reduces vascular resistance and results in a marked increase in cardiac output and heart rate that can aggravate underlying angina and cardiac failure. The determining factor in this situation is the net change in myocardial oxygen consumption achieved with lowering the elevated afterload; however a decrease in blood pressure produced with hydralazine is not accompanied by a commensurate decrease in renal blood flow; therefore the drug is especially suited for management of hypertensive emergencies associated with renal insufficiency. The initial intravenous dose of 5 to 10 mg should be given. The onset of action of this drug is 15 to 20 minutes, and the duration is 4 to 6 hours. Alternatively, the drug dosage may be increased in 5-mg increments up to 20 mg. The maintenance dose depends on patient response, but is generally 5 to 10 mg intravenously every 4 to 6 hours.22–24
Trimethaphan camsylate
Trimethaphan (Arfonad) is a ganglionic vasodepressor that blocks both the sympathetic and parasympathetic systems at the autonomic ganglia. The effect is primarily orthostatic; therefore large doses must be used to reduce blood pressure in supine patients. The head of the bed should be elevated (reverse Trendelenburg position), if possible, to augment the antipressor action. The dose of this drug is 10 to 20 mcg/kg/min. The onset of action is approximately 1 minute, and the duration of action is 2 to 4 minutes. The 500-mg ampule of trimethaphan is mixed in 250 mL of normal saline solution, which results in a strength of 2 mg/mL. Complications of such ganglionic blockade include atony of the bowel and bladder and paralytic ileus, especially when the drug is used longer than 24 hours. Because of the commensurate decrease in the glomerular filtration rate when the blood pressure is lowered with the use of this agent, use is not recommended in patients for whom renal insufficiency complicates the hypertensive crisis. The drug’s major disadvantage is that it rapidly loses effectiveness after 24 to 72 hours and another agent must be substituted. The drug requires extremely close monitoring by the perianesthesia nurse.22–24
Nitroglycerin
Nitroglycerin is a potent vasodilator that produces relaxation of both arterial and venous smooth muscles. The pharmacologic effects of nitroglycerin are mainly on the venous circulation. It produces an increase in venous capacitance, which leads to a reduction in venous return and a decrease in right atrial and pulmonary capillary wedge pressures; therefore the main effect of nitroglycerin is a reduction in the preload. In addition, the myocardial oxygen demand is decreased because of the decrease in myocardial wall tension.22–24
When intravenous nitroglycerin is administered in the PACU, an automated infusion pump should be used. The usual dose is between 0.25 and 3 mcg/kg/min. The onset of action for this drug is 2 to 5 minutes, and the duration of action is 3 to 5 minutes. The patient should be continuously monitored for hypotension. Should hypotension occur, an alpha agonist, such as methoxamine, may be used to ensure that the patient’s coronary perfusion pressure is maintained. Nitroglycerin migrates into plastic; therefore the perianesthesia nurse should periodically change the plastic tubing on the automated infusion pump and ensure that only glass bottles are used for dilution.22–24
Propranolol
Propranolol (Inderal, Ipran) is the prototype beta-blocking drug; consequently, all drugs in this class are compared with propranolol. This drug is known to be nonselective because it blocks both beta1 and beta2 receptors. After administration of this drug, decreased heart rate, contractility, and cardiac output occur. The drug can be administered in single intravenous doses of 0.1 to 0.5 mg, with a maximum dose of approximately 2 mg.22–24
Esmolol
Esmolol (Brevibloc) is a cardioselective ultrashort-acting beta-blocking agent with a rapid onset and short duration of action. Because it is cardioselective, esmolol does not appear to affect bronchial or vascular tone at the doses required to reduce the heart rate. This drug has also been shown to blunt the response to endotracheal intubation and can be effective in treatment of postoperative hypertension. In the treatment of postoperative hypertension, a loading dose of 500 mcg/kg should be administered over a 1-minute period. Next, a continuous infusion of 50 to 300 mcg/kg/min should be started. The peak response of esmolol occurs in 5 minutes, with a duration of action of approximately 20 minutes.22–24
Labetalol
Labetalol (Normodyne, Trandate) is a drug that possesses antagonist activity at both the alpha and beta receptors. With intravenous administration, it is approximately sevenfold more potent on the beta receptors than on the alpha receptors. More specifically, this drug is an alpha1 antagonist and has antagonist activities on both the beta1 and beta2 receptors. For treatment of postoperative hypertension, a loading dose of 0.25 mg/kg should be administered over a 2-minute period. After this initial dose, intravenous titration to effect should be done at 10-minute intervals to a total of 300 mg. If a continuous infusion is needed, a dose of 2 mg/min can be used.22–24
Metoprolol
Metoprolol (Lopressor) is a beta blocker that can be used in patients with reactive and obstructive lung disease because this drug selectively blocks the beta1 effects and consequently blocks the inotropic and chronotropic responses. This selective beta-adrenergic effect is dose related; at high doses, beta1 and beta2 receptors become blocked and airway resistance may increase. For treatment of postoperative hypertension, an intravenous dose of 2 to 5 mg should be used.22–24
Summary
The anatomy and physiology of the cardiovascular system has been presented in depth. The electrocardiogram and the electrical activity of the heart were presented, in addition to invasive and noninvasive methods to monitor the cardiovascular system. In addition, myocardial infarction was presented and the various cardiovascular drugs that are currently used were described in detail. One area of medical research is in discovery of new drugs to enhance cardiovascular function. The reader advised to seek the current literature in this area as new methods and drugs continue to be introduced.
1. Drake R, et al. Gray’s anatomy for students, ed 2. Philadelphia: Churchill Livingstone; 2009.
2. Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2010.
3. Estafanous F, et al. Cardiac anesthesia: principles and clinical practice. ed 2. Philadelphia: Lippincott Williams & Wilkins; 2001.
4. Barash P, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
5. Aitkenhead A, et al. Textbook of anaesthesia, ed 5. Philadelphia: Churchill Livingstone; 2007.
6. Lake C, et al. Clinical monitoring: practical applications for anesthesia and critical care. Philadelphia: Saunders; 2001.
7. Longnecker D, Murphy F, et al. Introduction to anesthesia. In Dripps, ed.: Introduction to Anesthesia, ed 9, Philadelphia: Saunders, 1997.
8. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2009.
9. Alspach J. Core curriculum for critical care nursing, ed 6. Philadelphia: Saunders; 2005.
10. Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
11. Smartt S. The pulmonary artery catheter: gold standard or redundant relic. J Perianesth Nurs.2005;20(6):373–379.
12. Gallager C, Issenberg B. Simulation in anesthesia. Philadelphia: Saunders; 2007.
13. Ganong W. Review of medical physiology, ed 22. New York: McGraw-Hill Medical; 2005.
14. Stoelting R, Miller R. Basics of anesthesia, ed 6. Philadelphia: Churchill Livingstone; 2011.
15. Miller R, et al. Anesthesia, ed 7. Philadelphia: Churchill Livingstone; 2009.
16. Benumof J, Saidman L. Anesthesia & perioperative complications, ed 2. St. Louis: Mosby; 1999.
17. Fisher L. Benumof’s anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
18. Murray J, Nadel J. Textbook of respiratory medicine, ed 2. Philadelphia: Saunders; 1994.
19. Townsend C, et al. Sabiston textbook of surgery: the biological basis of modern surgical practice. ed 17. Philadelphia: Saunders; 2004.
20. Stoelting R. Pharmacology and physiology in anesthetic practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
21. Evers A, Maze M. Anesthetic pharmacology: physiologic principles and clinical practice. Philadelphia: Churchill Livingstone; 2004.
22. Kier L, Dowd C. The chemistry of drugs for nurse anesthetists. Chicago: AANA Publishing, Inc; 2004.
23. Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill Professional; 2010.
24. Pandharipande P, Ely EW. Alpha-2 agonists: can they modify the outcomes in postanesthesia care unit. Current Drug Targets.2005;6:749–754.
25. Longnecker D, et al. Principles and practice of anesthesiology, ed 2. St. Louis: Mosby; 1998.