The biological effects associated with X-rays, risk and practical radiation protection
Radiation-induced tissue damage
The action of radiation on cells and the resultant damage are classified as:
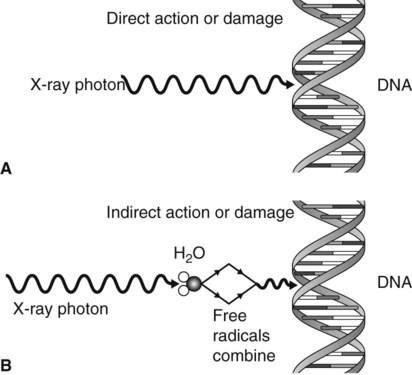
• Direct action or damage as a result of ionization of macromolecules
• Indirect action or damage as a result of the free radicals produced by the ionization of water.
Direct action or damage
The X-ray photons, or high-energy ejected electrons, interact directly with, and ionize, vital biologic macromolecules such as DNA, RNA, proteins and enzymes, as shown in Fig. 7.1A. This ionization results in the breakage of the macromolecule’s chemical bonds, causing them to become abnormal structures, which may in turn lead to inappropriate chemical reactions. Rupture of one of the chemical bonds in a DNA macromolecule may sever one of the side chains of the ladder-like structure. This type of injury to DNA is called a point mutation. The subsequent chromosomal effects from direct damage could include:
• Inability to pass on information
• Only temporary damage – the DNA being repaired successfully before further cell division.
If the radiation directly affects somatic cells, the effects on the DNA (and hence the chromosomes) could result in a radiation-induced malignancy. If the damage is to reproductive stem cells, the result could be a radiation-induced congenital abnormality.
What actually happens in the cell depends on several factors, including:
Indirect action or damage
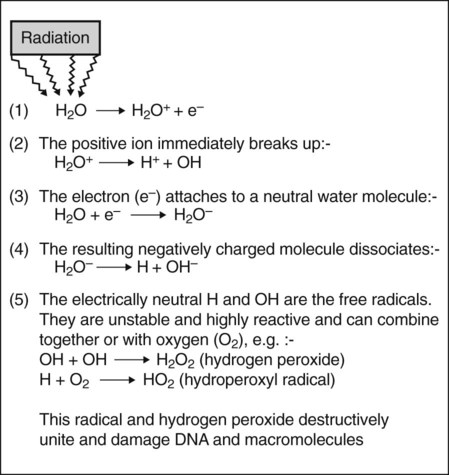
This process, which is shown in Fig. 7.1B, involves the ionization of the water molecule producing both ions and free radicals which can combine to damage the vital biologic macromolecules such as DNA. The sequence of events involved is summarized in Fig. 7.2. The free radicals can recombine to form hydrogen peroxide, a cellular poison, and a hydroperoxyl radical, another toxic substance. Both of these substances are highly reactive and produce biological damage. By themselves, free radicals may transfer excess energy to other molecules, thereby breaking their chemical bonds and having an even greater effect. As about 80% of the body consists of water, the vast majority of the interactions with ionizing radiation are indirect.
Classification of the biological effects
Stochastic effects
Cancer induction
• The survivors of the atomic explosions at Hiroshima and Nagasaki
• Patients receiving radiotherapy
• Radiation workers – people exposed to radiation in the course of their work
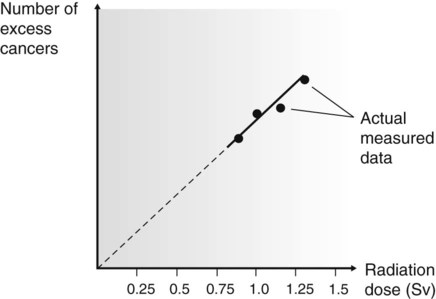
The problem of quantifying the risk is compounded because cancer is a common disease, so in any group of individuals studied there is likely to be some incidence of cancer. In the groups listed above, that have been exposed to high doses of radiation, the incidence of cancer is likely to be increased and is referred to as the excess cancer incidence. From the data collected, it has been possible to construct dose–response curves (Fig. 7.3), showing the relationship between excess cancers and radiation dose. The graphs can be extrapolated to zero (the controversy on risk assessment revolves around exactly how this extrapolation should be done), and a risk factor for induction of cancer by low doses of radiation can be calculated.
After reviewing all the available evidence, the International Commission on Radiological Protection suggest there is a 1 in 20,000 chance of developing a fatal cancer for every 1 mSv of effective dose. Using this estimate, a broad estimate of risk from various X-ray examinations may be calculated and these are shown in Table 7.1.
Table 7.1
| X-ray examination | Estimated risk of fatal cancer |
| Bitewing/periapical radiograph (70 kV, round collimation, D-speed film) | 1 in 1,000,000 |
| Bitewing/periapical radiograph (70 kV, rectangular collimation, F-speed film) | 1 in 10,000,000 |
| Panoramic radiograph (average) | 1 in 1,000,000 |
| Upper standard occlusal | 1 in 2,500,000 |
| Lateral cephalometric radiograph | 1 in 5,000,000 |
| Skull radiograph (PA) | 1 in 1,000,000 |
| Skull radiograph (lateral) | 1 in 1,250,000 |
| Chest (PA) | 1 in 1,430,000 |
| Chest (lateral) | 1 in 540,000 |
| CT head | 1 in 14,300 |
| CT chest | 1 in 3000 |
| CT abdomen | 1 in 3500 |
| CT mandible and maxilla | 1 in 80,000 to 1 in 14,300 |
| Barium swallow | 1 in 13,300 |
| Barium enema | 1 in 9100 |
| Dento-alveolar cone beam CT | 1 in 2,000,000 to 1 in 30,000 |
| Craniofacial cone beam CT | 1 in 670,000 to 1 in 18,200 |
Risk is age-dependent, being highest for the young and lowest for the elderly. The risks shown in Table 7.1 are for a 30-year-old adult. The 2004 European Guidelines on Radiation Protection in Dental Radiology recommend that these should be modified by the multiplication factors shown in Table 7.2, which represent averages for the two sexes. In fact, at all ages risks for females are slightly higher and risks for males slightly lower.
Table 7.2
| Age group (yr) | Multiplication factor for risk |
| <10 | ×3 |
| 10–20 | ×2 |
| 20–30 | ×1.5 |
| 30–50 | ×0.5 |
| 50–80 | ×0.3 |
| 80+ | Negligible risk |
Effects on the unborn child
• Congenital abnormalities or death associated with large doses of radiation
• Mental retardation and reduction in Intelligence Quotient (IQ)
As a result, there is a maximum permissible dose allowable to the abdomen of a woman who is pregnant. The implications for radiation protection for pregnant women during dental radiography are described later.
Summary of the harmful effects important in dental radiology
In dentistry, the size of the doses used routinely are relatively small (see Ch. 6) and well below the threshold doses required to produce tissue reactions (deterministic effects). However, the stochastic effects can develop with any dose of ionizing radiation. Dental radiology does not usually involve irradiating the reproductive organs, thus in dentistry the heritable effects are of limited importance and the main concern is that of cancer induction.
Practical radiation protection
As a result of these damaging effects, ionizing radiation is the subject of considerable safety legislation designed to minimize the risks to radiation workers and to patients. As described in Chapter 6, the International Commission on Radiological Protection (ICRP) regularly publishes general radiation protection recommendations based on the general principles of justification, optimization and limitation. Their main aims of radiation protection are to:
• Prevent the detrimental tissue reaction (deterministic effects) by having rules and guidelines based on scientific evidence to ensure known threshold doses are not exceeded.
• Limit the probability of stochastic effects to acceptable levels by determining the level of risk involved.
Their recommendations to try to achieve these aims are usually incorporated eventually into national legislation and guidelines, although the precise details may vary from one country to another. A summary of the current UK legislation and recommendations and guidelines can be found at www.whaitesessentialsdentalradiography.com. This section summarizes generic practical radiation protection measures and good practice appropriate for patients, the general public and dental staff.
Practical radiation protection of patients
The main radiation dose to patients comes from:
• Being irradiated in the first place, which is totally dependent on the decision and clinical judgement of their dentist
The main practical radiation protection measures can therefore be considered under three headings:
Clinical judgement
• All dentists must have received adequate training in dental radiology and should undertake continuing education and training after qualification to keep their knowledge and skills up to date, particularly in relation to the clinical applications of new technology, e.g. cone beam CT (CBCT) (see Ch. 16). This seems reasonable as it is the dentist who decides on the acceptability of the risk to which the patient is being subjected.
• Before an exposure can take place, it must be clinically justified by a dentist (i.e. assessed to ensure that it will lead to a change in the patient’s management and prognosis). Every exposure should be justified on the grounds of:
– The availability and/or findings of previous radiographs
– The specific objectives of the exposure in relation to the history and following clinical examination of the patient
– The total potential diagnostic benefit to the patient
– The radiation risk associated with the radiographic examination
– The efficacy, benefits and risks of alternative techniques having the same objective, but involving no or less exposure to ionizing radiation.
• To assist with the justification process dentists should make use of published evidence-based selection criteria. For example, in 2013 the Faculty of Dental Practice (UK) of the Royal College of Surgeons of England published the 3rd Edition of their booklet Selection Criteria for Dental Radiography. Their recommendations are graded on the quality of the evidence available using the following scale:
• A = based on evidence from at least one study with in vitro validation as part of the body of literature of overall good quality and consistency.
• B = based on evidence from well conducted clinical trials but with no specific in vitro validation studies.
• C = based on evidence from expert committee reports or opinions and/or clinical experience of respected authorities and indicates an absence of directly applicable studies of good quality.
• NSR = based on evidence from high-quality, non-systematic literature review.
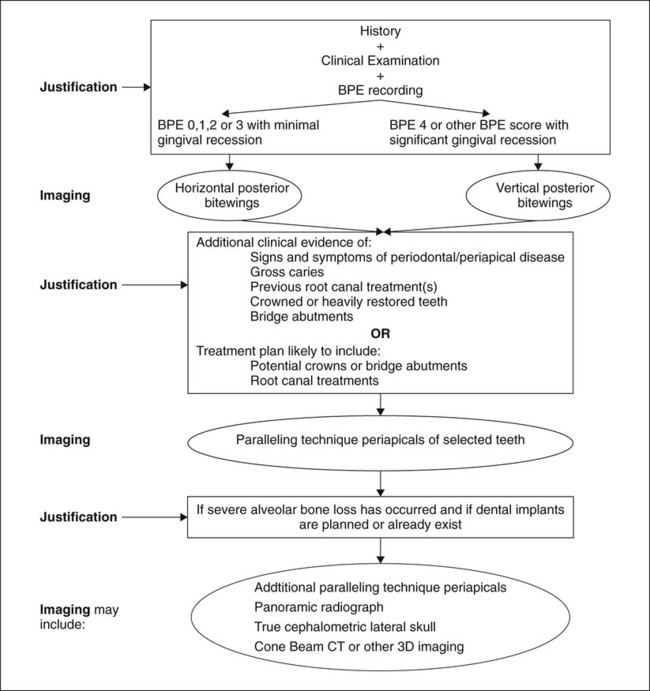
Several of their recommendations are included later in Chapter 20, 21 and 22. A flow diagram showing how imaging is justified on the basis of history, clinical examination, different clinical signs and symptoms of disease and possible treatment plans – based broadly on the recommendations on the 2013 Selection Criteria is shown in Figure 7.4.
Equipment
• All dental X-ray generating equipment should:
– Be installed correctly and tested by a medical physicist for safety and output before being used on a patient
– Be checked regularly by a medical physicist, for example every 1–3 years
– Function within agreed parameters – typically 60–70 kV, 7–12 mA
– Contain adequate filtration (inherent and added) – typically 1.5 mm of aluminium for sets operating below 70 kV and 2.5 mm for sets operating above 70 kV (see Ch. 3)
– Have the main beam collimated to cover the rectangular image receptor (film packet or digital sensor) being used (see Ch. 3) and should not exceed 40 × 50 mm
– Have a focus to skin distance (fsd) of at least 100 mm if operating below 60 kV and at least 200 mm if operating above 60 kV
– Have an exposure switch (timer) that only functions when continuous pressure is maintained and that terminates if pressure is released
– Be provided with film speed controls and finely adjustable exposure time setting
– Be assessed as to the actual dose delivered to enable comparison with national Diagnostic Reference Levels (DRLs) – if available.
• All panoramic X-ray generating equipment should:
– Be installed correctly and tested by a medical physicist for safety and output before being used on a patient
– Be checked regularly by a medical physicist, for example every 1–3 years
– Function within agreed parameters – typically 60–90 kV
– Contain adequate filtration (inherent and added)
– Have the beam collimated to 125 mm or 150 mm (height) × 5 mm (width) at the image receptor
– Have an exposure switch (timer) that only functions when continuous pressure is maintained and that terminates if pressure is released
– Be provided with adequate patient-positioning aids incorporating light beam markers (see Ch. 15)
– Ideally be provided with facilities for field-limitation techniques (see Ch. 15).
Radiographic technique
• All staff involved in X-raying patients should:
– Have received adequate training and should undertake continuing education and training after qualification to keep their knowledge and skills up to date.
– Undertake radiography accurately to avoid retakes, for example by using image receptor holders and beam-aiming devices for intraoral radiography as described in Chapters 9 and 10, or by using patient positioning aids during panoramic radiography as described in Chapter 15
– Use the minimum number of projections
– Optimize all exposure settings to ensure that all doses are kept as low as reasonably practicable (ALARP) consistent with the intended purpose
– Ensure all image processing (chemical or computer) (see Ch. 5) is carried to the highest standards so that images do not have to be retaken
• There is no justification for the routine use of lead aprons for patients undergoing intraoral or panoramic radiography.
• Thyroid collars, as shown in Fig. 7.5, should be used in those few cases where the thyroid may be in the primary beam. (In the authors’ opinion, this can include maxillary occlusal radiography and CBCT, and thyroid protection is therefore shown in Chs 11 and 16.)
• Lead aprons do not protect against radiation scattered internally within the body.
• Protective aprons, having a lead equivalence of not less than 0.25 mm, should be provided for any adult who provides assistance by supporting a patient during radiography.
• When a lead apron is provided, it must be correctly stored (e.g. over a suitable hanger) and not folded. Its condition must be routinely checked including a visual inspection at annual intervals.
Practical radiation protection of the general public
• The siting of X-ray equipment to ensure that the primary beam is not aimed directly into occupied rooms or corridors
• The thickness/material of partitioning walls
• Advice from a medical physicist on the siting of all X-ray equipment, surgery design and the placement of radiation warning signs, as shown in Fig. 7.6.
Practical radiation protection of radiation workers
The radiation dose to dentists and their staff can come from:
• The primary beam, if they stand in its path
• Scattered radiation from the patient if they stand too close
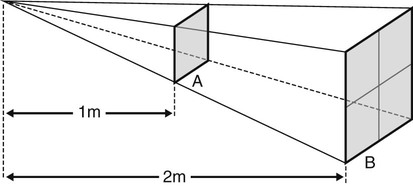
The main protective measures to limit the dose that workers might receive are therefore based mainly on a combination of common sense and the knowledge that ionizing radiation is attenuated by distance and obeys the inverse square law (see Fig. 7.7).
• The main practical radiation protection measures include:
– Ensuring all radiation workers (dental staff) know the risks to their own health created by exposure to X-rays and the safety precautions they need to take, including:
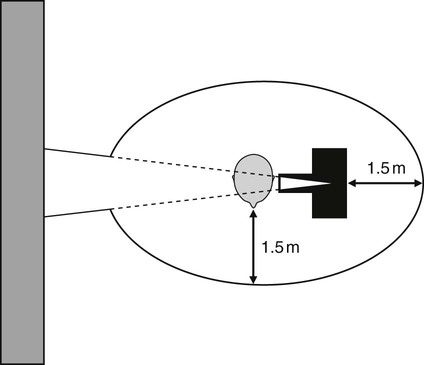
– Always standing outside the so-called controlled area – approximately 1.5 m from the X-ray machine and the patient (or behind appropriate lead screens/barriers) and never in the path of the main beam, as shown in Fig. 7.8
– Never holding an image receptor in a patient’s mouth
– Never holding the X-ray tubehead during an exposure
– Always using the X-ray equipment safely and in accordance with current guidance and good practice.
Monitoring
Dental staff are almost always designated as non-classified workers by the International Commission on Radiological Protection (ICRP). As explained in Chapter 6, the ICRP sets annual dose limits for different categories of radiation workers and for non-classified workers the current limit is 6 mSv per year. In the UK, the Health Protection Agency regards an annual limit of 1 mSv as more appropriate for dental staff working in general dental practice. The amount of radiation received by individuals can be monitored and measured using a variety of different monitoring devices and can include:
These devices do not protect against radiation. They merely provide data as to the amount of radiation that has been received over a period of time. More immediate information that the wearer is being irradiated can be provided by personal electronic dosimeters, including sophisticated systems such as the recently developed Unfors Alert dosimeter. A selection of various dosimeters is shown in Fig. 7.9.
Film badges
The main features of film badges are:
• They consist of a plastic frame (usually blue or white) containing a variety of different metal filters and a small radiographic film which reacts to radiation.
• They are worn on the outside of the clothes, usually at the level of the reproductive organs, for 1–3 months before being processed.
• They are the most common form of personal monitoring device currently in use.
Thermoluminescent dosimeters (TLDs)
The main features of TLDs are:
• They are used for personal monitoring of the whole body and/or the extremities, as well as measuring the skin dose from particular investigations.
• They contain materials such as lithium fluoride (LiF) which absorb radiation and then release the energy in the form of light when heated.
• The intensity of the emitted light is proportional to the radiation energy absorbed originally.
• Personal monitors consist of a yellow or orange plastic holder, worn like the film badge for 1–3 months.
Optically stimulated luminescence dosimeters (OSLDs)
The main features of OSLDs are:
• They are used for personal monitoring of the whole body.
• They consist of a badge containing an aluminium oxide detector and metal and plastic filters.
• The detector is read by exposing it to a light source, which releases the stored radiation energy in the aluminium oxide as blue light (luminescence).
• The radiation exposure can be calculated from the amount and intensity of blue light released.
Personal electronic dosimeters (PEDs)
The main features of PEDs are:
• They are battery operated devices normally worn in the operator’s pocket for personal monitoring of the whole body.
• They are usually based on an energy-activated silicon diode (a solid-state detector) to measure radiation dose.
• Some also measure the dose rate and have an audible alarm to indicate radiation as it is received.