70 The Biochemistry of Spinal Implants
Short- and Long-Term Considerations
KEY POINTS
Historical Background
In 1892, Sir William Aruthnot Lane began to fix tibia fractures with ordinary steel (Figure 70-1). He was successful in treating a large number of patients, but noted that the steel plates he used became corroded after time. Fortunately, and unbeknown to him, the rust that formed acted as a pseudoinsulator (oxide layer), and prevented further degradation and, likely, failure of the plate. If he had used a dissimilar metal, this layer would not have formed and a severe electrolyte reaction would have ensued, leading to the destruction of the metal and inflammation of the tissues. Though metals had previously been implanted in patients, it was with this advancement that the use of metal implants for fracture stabilization became a practical procedure.1
Tissue Response to Biomaterials
The biocompatibility of a material is directly related to the tissue response generated by the material. These are time-dependent processes and can be viewed in two different but interconnected ways: first, the bulk properties of a material, and, second, the physiochemical surface properties of the material, both of which contribute to the initial incorporation and long-term survival of biologic prostheses (Table 70-1).
| Implant-Tissue Reaction | Consequence |
|---|---|
| Toxic | Tissue necrosis |
| Biologically inert—smooth surface | Implant is encapsulated without bonding |
| Biologically inert—porous surface | Tissue grows into pores and forms mechanical bonds |
| Bioactive | Tissue forms interfacial bond with implant (bioactive fixation) |
| Dissolution of implant | Implant resorption and replacement with soft tissue or bone |
Metals
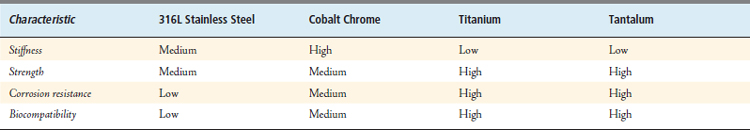
Current implantable metal alloys with wide use in orthopedics are 316L stainless steel, cobalt-chromium alloys, titanium alloys, and tantalum (Table 70-2). In general, metals are used routinely for weight-bearing or load-bearing implants such as plates, nails, stems, and screws. Though biocompatibility is good with metals, there are issues of concern. Corrosion, metallic toxicity, hypersensitivity, genotoxicity, and carcinogenesis all have been described in the literature with the use of metallic implants.
Metal Types
Tantalum
Tantalum is a gray, heavy, and very hard metal. When pure, it is ductile and can be drawn into fine wire, which is used as a filament for evaporating metals such as aluminum. Tantalum is almost completely immune to chemical attack at temperatures below 150° C, and is attacked only by hydrofluoric acid, acidic solutions containing the fluoride ion, and free sulfur trioxide. At high temperatures, tantalum becomes much more reactive. Tantalum is used to make a variety of alloys with desirable properties such as high melting point, high strength, and good ductility. Tantalum readily forms oxides and is most stable as +5 tantalum pentoxide. Elemental tantalum unites strength and corrosion resistance with excellent biocompatibility. Tantalum is the metal used in the construction of Trabecular Metal (Zimmer). The cellular structure of Trabecular Metal resembles bone and approximates its physical and mechanical properties more closely than any other prosthetic material. Its unique, highly porous, trabecular configuration is conducive to bone formation, enabling rapid and extensive tissue infiltration and strong attachment.
Corrosion
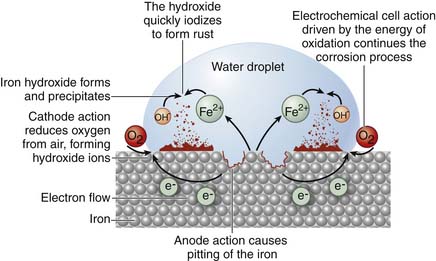
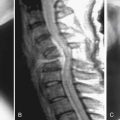
Most fluids in the human body are of similar chloride content and pH to sea water (20 g/L and 7.4); therefore many metals used in orthopedic implants have been those most resistant to corrosion in sea water. Corrosion is, simply, the dissolution of metallic ions in aqueous solution. Electrochemical cells are produced in the body when these metallic implants are used and equilibria of metallic ions in solution are achieved within body fluids over time (Figure 70-2).
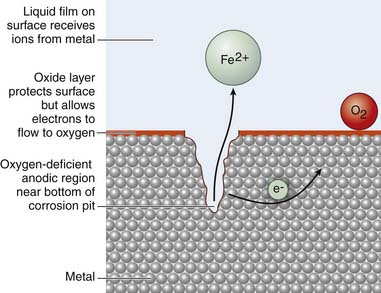
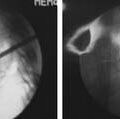
Generally three types of corrosion exist with the use of metallic implants and include (1) galvanic, (2) crevice or pitting, and (3) fretting corrosion. Galvanic corrosion is corrosion due to the use of dissimilar metals in contact with one another or electrochemical dissolution. Pitting corrosion is a form of localized corrosion that leads to the creation of small holes or defects in the metal (Figure 70-3). The driving power for pitting corrosion is the lack of oxygen around a small area. This area becomes anodic while the area with excess of oxygen becomes cathodic, leading to very localized galvanic corrosion. The corrosion penetrates the mass of the metal, with limited diffusion of ions, further increasing the localized lack of oxygen. The mechanism of pitting corrosion is probably the same as crevice corrosion. Finally, fretting corrosion, as defined by the ASM Handbook on Fatigue and Fracture, is: “A special wear process that occurs at the contact area between two materials under load and subject to minute relative motion by vibration or some other force.” The relative small motion causes mechanical wear and material transfer at the surface of the metals, followed by oxidation of that debris and the freshly exposed surface. This debris then acts as an additional abrasive product that is often harder than the original metal and perpetuates the process.
Distribution of Metal in Body Fluids
A prosthetic device constitutes a pool of trace elements or alloy in the body, which, when mobilized by corrosion, dissolution, and wear, are distributed in local tissue or potentially at sites distant to the original site of implantation. Metallic particles can be found in local tissues such as articular joint capsules, muscle, and regional lymph nodes, or at distant tissues such as abdominal paraaortic lymph nodes, liver, spleen, and pancreas. Studies have looked at distribution of metal ions in body fluids following prosthetic joint implantation. Only slight increased levels of Co and Cr in serum and urine have been noted in patients 2.5 years after implantation of the prosthesis.1 Another paper reported increased deposition of metallic particles in the liver, spleen, and abdominal paraaortic lymph nodes, in a postmortem study. Larger metallic burdens were seen in patients with failed total joint replacements. In most of the patients evaluated in the postmortem study, the concentration of metallic particles in the liver and spleen was low, and no toxic effects were apparent on histological exam of the surrounding tissue.2
Mutagenesis
Metallic particles disseminated throughout the body are feared to have potentially deleterious effects. Some early studies were published raising the question of biomaterials being responsible for mutagenesis at distant sites in the body. Mutagenesis, or genotoxicity, is the disruption of DNA resulting in the production of aberrant proteins that lead to cellular or tissue dysfunction. Genotoxicity or mutagenesis can serve as an indicator for the potential carcinogenicity of a material. A paper in 2003 looked at potential mutagenesis of cobalt-chrome and titanium implants. The conclusion was that neither material showed evidence of mutagenesis in bacterial assay and mammalian cell assays. Although, by itself, this is not enough evidence to be able to state that these implants are not mutagenic or genotoxic, in combination with the reports of cancer (next section) in patients with Co-Cr or titanium implants, it appears to be supported clinically.
Polymers
Introduction
Synthetic polymers are occupying a growing role in implant construction. They accord numerous advantages including radiolucency and elasticity. While the vast majority are biologically inert, their wear and degradation processes and properties reflect on their suitability as implants. The following sections will outline the principal polymers used in disc arthroplasty, in fusion, and as bioabsorbable interbody spacers.
Hydrogels
Synthetic Hydrogels
Synthetic polymers exhibit low toxicity, and have been used in medical applications for a period of more than 60 years. Many polymer systems have been employed, including polyacrylonitrile, polyamides, polyethylene, polymethylmethacrylate, polytetrafluoroethylene, polyurethanes, and silicones. Products made from these polymers have been used as bone and tissue replacements, as drug delivery devices, and have been variously employed in nearly all medical disciplines, including heart surgery, orthopedic surgery, ophthalmology, gynecology, and plastic surgery with remarkable success. Synthetic polymers are also utilized in topical applications and as coatings for stents and other implants.
Hydrolyzed Pan Hydrogels – Development and History
Additional HPAN advantages as compared to other hydrogels are:
Biologics
Bone Graft
The bone grafts and their substitutes are divided according to their properties of osteoconduction, osteoinduction, osteogenesis, or a combination of these. Osteogenic refers to a material that produces bone-forming cells that directly lay down new bone in an area. Osteoinductive refers to a material that can stimulate the differentiation of stem cells into osteogenic cells. Osteoconductive materials are those that provide a porous scaffold to support the formation of new bone. In addition, there are materials that provide more than one of the above characteristics and are considered combination materials (Table 70-3).
| Bone Graft Family | Description | Classes |
|---|---|---|
| Osteoconductive | Provides structure or scaffold to support bone formation | Ca-Phosphate, ceramics, synthetic polymers, bioactive glass, allograft |
| Osteoinductive | Induces differentiation of cells and new bone growth | BMP, demineralized bone matrix |
| Osteogenesis | Provides stem cells with osteogenic potential that directly form new bone | Bone marrow aspirate |
| Combined | Combinations of above | Autograft |
Osteoconduction refers to the process in which the three-dimensional structure of a substance is conducive to the ongrowth and ingrowth of new bone. Osteoconductive bone graft substitutes are commercially available and vary in chemical composition, structure, and resorption rates. Understanding the basics of each type and the reason to use a specific one of them will assist with surgical success. The Table 70-3 groups these materials into classes and describes some of their basic properties.
1. M. Rang, The story of orthopaedics, Saunders Publishing Philadelphia, 2000
2. Anderson J.M., et al. Foreign body reaction to biomaterials. Sem Immunology. 2008;20:86-100.
3. Lewis C.G., et al. Metal carcinogenesis in total arthoplasty. Clin. Orthop. 1996;329S:S264-S268.
4. Disegi J.A., et al. Stainless steel in bone surgery. Injury. 2000;31:S-D2-6.
5. American Society for Testing and Materials, Handbook.
6. Sunderman F.W., et al. Cobalt, chromium, and nickel concentrations in body fluids of patients with porous-coated knee or hip prostheses. J. Ortho. Research. 1989;7:307-315.
7. Urban R.M., et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Joint Surg. 2000;82-A(4):457-477.
8. Katzer A., et al. In vitro toxicity and mutagenicity of CoCrMo and Ti6Al wear particles. Toxicology. 2003;190:145-154.
9. International Agency for Research on Cancer. Surgical implants and other foreign bodies,. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 74, 1999.
10. Hallab N., et al. Metal sensitivity in patients with orthopaedic implants. J. Bone Joint. 2001;Surg 83-A(3):428-436.
11. Kurtz S.M., et al. The clinical performance of UHMWPE in the spine. In: Kurtz S.M., editor. Ultra-high molecular weight polyethylene in total joint replacement and medical devices. Academic Press, 2009.
12. Ingham E., Fisher J. Biological reactions to wear debris in total joint replacement. Inst. Mech. Eng. 2000;214(1):21-37.
13. Kurtz S.M., Devin J.N. PEEK Biomaterials in trauma, orthopaedic, and surgical implants. Biomaterials. 2007;28(32):4845-4869.
14. Ciccone W.J., et al. Bioabsorbable implants in orthopaedics: new developments and clinical applications. JAAOS. 2001;9(5):280-288.
15. Hak D.J. The use of osteoconductive bone graft substitutes in orthopaedic trauma. JAAOS. 2007;Vol. 15(9):525-536.
16. Electrochemical Corrosion. http://www.chem1.com/acad/webtext/elchem/ec7.html. Accessed February 18, 2010.