Chapter 48 The Assessment and Management of Septic Arthritis
Introduction
Septic arthritis is defined as a bacterial infection of the synovium and joint space. It is a relatively uncommon condition that may occur at any age being more common in children. In the pre-antibiotic era the mortality of all joint infections was 20% and morbidity in the region of 50%. Joint infection leads to rapid and irreversible damage to the articular cartilage. Any delay in the treatment of a septic joint will result in a poor outcome. While it is an infrequent presentation in the elbow, early recognition, diagnosis and treatment are essential to achieve a satisfactory outcome. This requires the clinician to have a high index of suspicion of sepsis in patients with predisposing conditions. In addition, infection can obviously follow any surgery to the elbow and is a particularly troublesome complication of elbow arthroplasty. This has been dealt with in Chapter 44 and will not be covered further in this chapter.
Epidemiology
In developed countries septic arthritis has shown no change in its incidence1 despite advances in antimicrobial therapy. Among children in Sweden the annual incidence is 10 per 100 000 children.2 In a separate study from the Mayo clinic over a 15-year period, the elbow accounted for 6% of all septic joints in the adult. To put this in context, however, the number of patients presenting to that unit with septic arthritis unrelated to previous surgery was only 10 new adult cases per year, thus emphasizing the uncommon nature of elbow sepsis. Most of these cases were over the age of 50 years with an equal sex ratio.3
The incidence in developing countries, particularly in Africa, is considerably higher.4,5 In contrast to the experience in Europe and North America, Goldschmidt and Hoffman5 reported a 100% increase in presentation of septic arthritis in Cape Town between 1983 and 1988, with a male to female ratio of 2:1. They also noted a seasonal variation with a 30% increase in incidence in the cooler months. This seasonal variation is the converse of their finding with other musculoskeletal infections which were 50% higher in the summer months. Other reports also suggest that musculoskeletal infections are commoner in warmer and more humid conditions.6
The presentation of septic arthritis may be monoarticular or polyarticular. The polyarticular form accounts for 10% of all presentations and, in adults, half of these are in rheumatoid arthritis patients.1
Aetiology and pathogenesis
There are four routes by which bacteria can spread to the elbow joint:
Haematogenous spread
Joint haemarthrosis may be due to direct trauma, a bleeding diathesis such as haemophilia or occur in patients on anticoagulant therapy. Neurological disorders resulting in a neuropathic (Charcot) joint may be more prone to haematogenous spread of infection, both due to the gross destructive change that may occur in the joint and due to any haemarthrosis.7 The presence of a haemarthrosis will create an inflammatory response within the joint with increased permeability of the basement membrane. In addition, the presence of blood within the joint will provide a rich culture medium for bacterial proliferation. These two factors make the joint susceptible to bacterial infection by a haematogenous route.
Systemic diseases may include those that affect joints directly and those that increase infection rates generally. The inflammatory arthropathies such as rheumatoid arthritis and the seronegative arthropathies have a significantly increased risk of developing septic joints, rheumatoid arthritis accounting for 50% of all adult polyarticular forms.1 This is likely to be multifactorial due to the joint damage, the nature of the condition and the disease-modifying drugs that these patients may be taking. Examples of such drugs used for treatment of rheumatoid arthritis include methotrexate, leflunomide and cytokine modulators such as the tumour necrosis factor (TNF) α inhibitors. These drugs are immunosuppressive and can significantly increase infection risk in their own right. Other medications that may predispose to infection include steroid therapy and the immunosuppressants used in the treatment of neoplasia and in transplant patients.
The rise in the incidence of human immunodeficiency virus (HIV) infection worldwide has been implicated in the increased incidence of septic arthritis, particular in Africa. Added to that, there is an increased incidence of haemophiliac people infected with the HIV virus.8 Other general conditions such as diabetes mellitus, malignancy, liver and renal disease have also been reported to increase the incidence of septic arthritis.9
Spread from a contiguous lesion
Spread from a contiguous lesion can occur from an overlying infected olecranon bursa, though any other local soft tissue infection such as cellulitis or an abscess can have the same effect. The olecranon bursa does not have a direct communication with the elbow joint thus bacterial spread in these local lesions must be by other routes. Contiguous needle aspiration of the bursa and aspiration of the elbow to exclude infection may be responsible for inoculation of the elbow joint with bacteria. Spread via the lymphatic system or local haematogenous spread accounts for the remainder. Olecranon bursitis is covered in Chapter 36 and will not be covered further in this chapter.
Spread from metaphyseal bone
Osseous infection principally affects the metaphysis due to the arrangement of the blood supply. The epiphyseal plate usually forms an effective barrier to the spread of bacteria from the metaphysis to the epiphysis. However, in children under the age of 18 months, blood vessels cross the epiphyseal plate allowing infection to spread from the metaphysis to the epiphysis, which is intracapsular. This allows spread of the infection into the joint.10
For transmission to occur from a metaphyseal infection directly to the joint after the age of 18 months, the metaphysis must be intracapsular. This arrangement of capsular attachment only occurs in few joints, one of which is the proximal radial metaphysis. As a consequence, the elbow is potentially susceptible to infection via this route. This again occurs more commonly in children. Accurate diagnosis in these cases may be difficult since the patient may have clear evidence of sepsis due to the metaphyseal infection masking the septic joint. Antibiotic treatment for the initial metaphyseal infection may also result in a negative culture from joint aspiration leading to the diagnosis of a reactive rather than a septic effusion.10,11
Pathology and microbiology
The commonest infective agent in joint sepsis in both the adult and child is Staphylococcus aureus (55%) with streptococcal infection accounting for 18% of infections, being commoner in patients over the age of 60.12,13 Meticillin-resistant S. aureus is increasing in frequency, causing a third of staphylococcal infections in one study. The affected patients were older and had more comorbidities and a higher 6-month mortality rate.14 N. gonorrhoeae infection is reducing in incidence but remains the commonest cause of septic arthritis in the young adult.15,16 A wide number of other infective agents have been described. There is, however, an association of different causative bacteria with age (Table 48.1).
| Age group | Organism | Comment |
|---|---|---|
| Neonates | Staphylococcus aureus | |
| Group B streptococci | ||
| Escherichia coli | ||
| Children and adolescents | Staphylococcus aureus | Less common with immunization |
| Haemophilus influenza | ||
| Streptococcus pyogenes | ||
| Adults | Staphylococcus aureus | Including meticillin-resistant |
| Neisseria gonorrhoeae | Young adults | |
| Staphylococcus epidermidis | Commonest with implants | |
| Proteus mirabilis | More common in debilitated patient | |
| Pseudomonas aeruginosa | More common in the immunocompromised | |
| Group B streptococci |
Natural history
The introduction of sulphonamides in the 1930s and the proliferation of antibiotic agents after 1940 led to a dramatic improvement in the outcomes of patients with septic arthritis. Before the antibiotic era there was 20% mortality and 50% morbidity from joint sepsis.17 However, even in the 1980s a 13% mortality rate was reported following disseminated joint infection in children.18 This therefore remains a serious condition particularly in the young, old and the immunocompromised.
The presence of active infection in the joint rapidly causes irreversible changes. In animal models, maximal arthritic symptoms occur 2 days following the inoculation of bacteria into a joint and at 7 days irreversible changes have taken place.19 The damage to the articular cartilage has historically been attributed solely to the effect of increased pressure within the joint resulting in the surgical drive to drain the joint as soon as possible. This has remained unquestioned until relatively recently. Other causes of high joint pressure are seen in effusions and haemarthrosis and yet the rapid destruction of the joint is not seen in these conditions. It is self-evident, therefore, that other factors which are not mechanical must have a part to play in the joint damage.
The effect of the inflammatory cascade releases proteolytic enzymes and cytokines into the joint. These have a rapid and devastating effect on the articular cartilage. Articular cartilage when exposed to fibronectin fragments undergoes proteoglycan depletion within 7 days.20 TNF-α, interleukin-1 and interleukin-6, which are released into the joint in sepsis, are inflammatory and cause rapid articular surface damage.21,22 These changes have been observed in the absence of mechanical factors. These studies demonstrating the rapid degradation of articular cartilage within a few days in animal models correlate with the clinical picture seen in humans and emphasize the need to diagnose and treat this condition early.
Clinical presentation
The patient will usually present with a painful and swollen elbow and a general feeling of malaise. In all patients presenting with these signs there should be a high index of suspicion for sepsis. Painful movements leading to a loss of function is also an early sign. As the effusion enlarges, the elbow is held in 80° of flexion, which is the position of comfort allowing the maximal joint volume.23
In the young, any pyrexia is usually marked and the child is plainly unwell. Under the age of 1 year local signs may be minimal, again making diagnosis difficult.24 Patients who have rheumatoid arthritis or who are immunosuppressed may present with rather vague symptoms of feeling unwell with few local clinical signs other than an effusion. These patients often have less redness and pain and more movement than one might anticipate with a septic joint. Again a high index of suspicion is required to make the diagnosis in these patients.
The differential diagnosis is that of a hot swollen joint. This will include other sepsis such as an olecranon bursitis or cellulitis, inflammatory arthritis, crystal arthritis, trauma and haemarthrosis. In children the presence of osteomyelitis can mask the diagnosis of a septic joint and lead to a delay in diagnosis.11 This may account for the significant number of poor results in children treated for osteomyelitis.10
Investigations
Plain radiographs are usually normal or show signs of an effusion. While radiographic signs of sepsis usually appear after 2–3 weeks, X-rays are useful by way of the differential diagnosis and to give a reference point for future radiological examination. Other imaging is possible but rarely contributes significantly in the acute situation. Ultrasound and magnetic resonance imaging are accurate in diagnosing an effusion, however, neither can distinguish between septic effusions and other causes.25 The technetium-99m phosphate three-stage bone scan is accurate in identifying increased uptake in septic joints in nearly all cases which may contribute to diagnosis but at the expense of delaying the onset of treatment. Likewise indium-111 labelled leucocytes have a sensitivity of 80% in the diagnosis of sepsis26 but this investigation takes 48 hours, which again leads to an unacceptable delay.
Joint aspiration is the most important confirmatory investigation and should be performed as soon as the diagnosis is in question.15 Aspiration should be performed using aseptic technique, both to reduce the risk of patient infection in the non-septic joint and reduce the risk of bacterial contamination of the specimen. Septic effusion is often viscous and a wide-bore (14 G) needle is recommended. Local anaesthetic infiltration of the skin is thus advisable, though the anaesthetic should not be injected intra-articularly prior to aspiration since local anaesthetics are bacteriostatic.
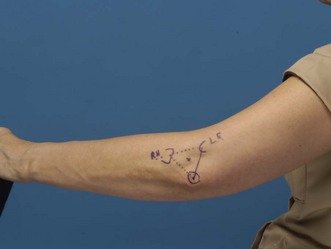
The recommended portal for aspiration is a lateral approach with the needle insertion being at the central point of the equilateral triangle formed by the lateral epicondyle, the olecranon process and the radial head. The radial head is often not palpable but its position may be surmised by drawing the base of the triangle between the other two easily palpable landmarks (Fig. 48.1).
Treatment
One area of contention currently among orthopaedic surgeons is whether or not drainage of the joint by needle aspiration, with or without needle washout, is a safe treatment technique or whether formal surgical drainage is required. As long as drainage to dryness can be achieved by needle aspiration it would appear that this method is acceptable.27 If the needle drainage is either not satisfactory or incomplete, or if deterioration occurs over 24 hours, formal washout by arthroscopic or arthrotomy methods should be undertaken.1,27
Antibiotic therapy should be started as soon as the possibility of a septic arthritis has become part of the differential diagnosis. The antibiotic choice before culture and sensitivities are available is based empirically on the most likely infective organism. This judgement can be aided by considering the patient’s age (Table 48.1), known foci of infection (such as urinary tract infection in the elderly) and the Gram stain of the aspirate. High-dose flucloxacillin or a cephalosporin is frequently used. The advice of a microbiologist is recommended (Table 48.2).
Table 48.2 Summary of recommendations for initial empirical antibiotic choice in suspected arthritis17
| Patient group | Antibiotic choice |
|---|---|
| No risk factors for atypical organisms | Flucloxacillin 2 g qds i.v. |
| ± gentamicin based on local policy | |
| Penicillin allergic: clindamycin 450–600 mg qds or third-generation cephalosporin | |
| High risk factor of Gram-negative sepsis (elderly, frail, recurrent urinary tract infection and recent abdominal surgery) | Second- or third-generation cephalosporin, e.g.: cefuroxime 1.5 g tds i.v. |
| ± i.v. Flucloxacillin with third-generation cephalosporin | |
| Gram staining may influence antibiotic choice | |
| Discuss allergic patient with microbiologist | |
| Meticillin-resistant Staphylococcus aureus (MRSA) risk | Vancomycin i.v. + second- or third-generation cephalosporin |
| Suspected gonococcus or meningococcus | Ceftriaxone i.v. or similar dependant on local policy or resistance |
| Intravenous drug users | Discuss with microbiologist |
| Intensive care unit patients with known colonization of organisms | Discuss with microbiologist |
Initial treatment should be given intravenously for 7–14 days following which oral antibiotics should be continued for a further 4–5 weeks.28 Monitoring the haematological indices such as the white cell count, ESR and CRP is recommended. The antibiotic regimen should be altered if necessary as soon as the sensitivities are available.
Direct intra-articular antibiotic delivery to a septic joint has also been suggested. There is historical evidence that sterilization of the joint is more rapid in cases where this is undertaken.29 However, there must also be some concern that such treatment can cause a chemical synovitis and thereby result in additional articular cartilage damage. In addition, intravenous administration of antibiotic gives a good delivery of antibiotic to the synovial cavity and this route rapidly achieves therapeutic antibiotic levels. Intra-articular antibiotic delivery is therefore not recommended.
Postoperatively the options are whether to splint or mobilize the elbow. A period of immobilization of 24–48 hours for pain relief has been suggested. However, early active mobilization has the advantage that further exudate can be expressed out of the joint by movement in cases that have had an arthrotomy and, to a lesser extent, an arthroscopic washout. This exudate may contain bacteria, harmful enzymes and cytokines. In addition, Salter et al found additional benefits of early mobilization in preventing adhesions and pannus, improving the nutrition of the articular cartilage and stimulating chondrocytes.30 Early active mobilization is thus recommended.
Since the main harmful effect of sepsis on the articular cartilage is chemical in origin and the result of this damage is catastrophic to the joint, there would be a benefit in directly neutralizing these harmful mediators. Much work has been done in this field in animal models. Neutralizing TNF-α and interleukin-1 with anti-TNF-α and anti-interleukin-1 has the potential to significantly reduce both inflammation and articular surface damage.21 Interleukin-4 and interleukin-10 block polymorphonuclear neutrophils in rat models of arthritis using mycobacterial antigen. The reduction in phagocytic activity in response to proinflammatory cytokines may make these agents useful.31 Interleukin-12 has been used in modifying the response in septic arthritis in mice infected with group B streptococci. A dose-dependent beneficial effect was found when the interleukin-12 was administered before the clinical signs become obvious.32
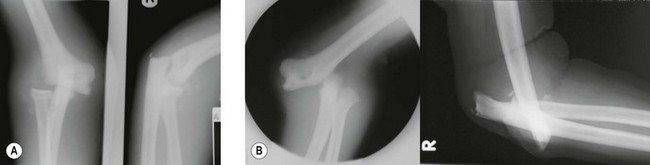
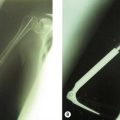
The potential for treatment by the reduction of the harmful effects of the inflammatory process in the septic joint is self-evident. The neuropathic elbow joint may become infected by haematogenous spread to the damaged joint, haemarthrosis, or by direct inoculation if skin breakdown occurs. These patients have an additional challenge of significant instability, which can become worse with the onset of sepsis (Fig. 48.2). Temporary stabilization by the application of an external fixator may aid treatment in these cases.7 Wound healing in the grossly unstable elbow is a problem and thus sepsis from haematological spread should be drained either by needle drainage or arthroscopy rather than arthrotomy.
Complications
Septic arthritis is a serious condition that still carries a high mortality. The high mortality rate with disseminated infection in children has already been alluded to.18 Gupta et al reported an 11% mortality rate in a prospective study of 75 patients with adult-onset septic arthritis. Many of these had concomitant disease, 46 patients had rheumatoid arthritis and 11 were intravenous drug users. Interestingly in this series those who had leg ulcers had 38% mortality.33 Mortality in these cases is due to septicaemia. In all patients and particularly those with an inflammatory arthropathy or those who are immunocompromised, the general effects of sepsis must not be overlooked. Patients can become rapidly severely ill with septic shock. Careful monitoring of the pulse, blood pressure, respiratory rate, temperature, urine output and oxygen saturation is required. An awareness of the local and national guidelines for the management of sepsis is essential.34
Tuberculous septic arthritis
Tuberculosis is an uncommon cause of sepsis in the elbow. However, the incidence worldwide is significant35 and the incidence in the West is increasing due in part to HIV infection.36,37 Martini et al38,39 in a large series of bone and joint tuberculosis identified 652 cases of which 297 were of the peripheral joints. Seventy-four cases affected the upper limb of which 42 involved the elbow. These patients present most commonly with a monoarthropathy. The onset is insidious with symptoms of progressive pain, swelling and stiffness.40 While tuberculous infection is often associated with the elderly and infirm, it may present at any age, including in children and young adults.41,42 Late presentation is, however, common and, in these cases, joint destruction occurs.42 A polyarticular presentation with swollen joints, which are sterile in association with tuberculous infection, is recognized (Poncet’s disease). This may be a reactive arthropathy though failure to culture acid-fast bacilli from an affected joint is recognized, leading to some controversy as to the nature of this condition.
The presence of pulmonary tuberculosis should alert one to the diagnosis.43 Aspiration resulting in the culture of acid-fast bacilli should be possible in 75% of cases.29 In cases where there is either a high index of suspicion or where the initial aspirate has been negative or not cultured for acid-fast bacilli, a synovial biopsy should be performed to make the definitive diagnosis.44 The characteristic findings on histological examination of the synovium include granulomatous inflammation, Langhans’ giant cells and epithelioid cells, lymphocytic infiltration and caseation.45 Later, radiographic examination may show a periosteal reaction and joint destruction.38,42
Patients who present with a possible tuberculous joint should undergo either an arthroscopic or open drainage and washout with a synovial biopsy. The washout may improve the range of movement.46 In late presentations and more advanced cases, therapeutic synovectomy and debridement may be required. Treatment with anti-tuberculous chemotherapy is the mainstay of management and is highly effective.42
Martini et al39 described the outcome of non-operatively managed tuberculous arthritis of the elbow. In 42 cases, 19 had a range of motion of more than 70°, 27 had what they described as a useful range of motion and the remainder had ankylosis. They also found rotation to be restricted.9 For patients with residual pain formal arthrodesis may be performed.47
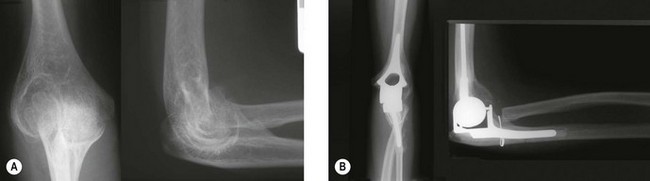
Functional results are related to the radiographic stage at presentation and not the duration of symptoms or the range of motion at presentation.42 Treatment for patients who have residual symptoms following successful treatment of the infection follows the same rationale as the treatment plan for the poor outcomes of acute infection. Initially debridement, synovectomy, radial head excision and arthrolysis may be considered. Arthrodesis is an option for patients who have residual pain and are young or high demand.47 Total elbow arthroplasty may be performed for either pain or in patients who require movement (Fig. 48.3).
1 Dubost JJ, Soubrier M, Sauvezie B. Pyogenic arthritis in adults. Revue du Rhumatisme. 2000;67(1):11-21.
2 Lidgren L, Lindberg L. Orthopaedic infections during a 5 year period. Acta Orthop Scand. 1972;43:325-334.
3 Kelly PJ, Martin WJ, Coventry MB. Bacterial (suppurative) arthritis in the adult. J Bone Joint Surg (Am). 1970;52:1595-1602.
4 Lavy CB. Septic arthritis in Western and sub-Saharan African children – a review. Int Orthop. 2007;31(2):137-144.
5 Goldschmidt RB, Hoffman EB. Osteomyelitis and septic arthritis in children. Curr Orthop. 1991;5:248-255.
6 Hedstrom SA, Lidgren L. Septic bone and joint infections. In: Klippel JH, Dieppe PA, editors. Rheumatology. London: Mosby, 1994.
7 Unnanuntyana A, Waikakul S. Neuropathic arthropathy of the elbow: a report of 2 cases. J Med Assoc Thailand. 2006;89(4):533-540.
8 Gilbert MS, Aledort LM, Seremetis S, et al. Long term evaluation of septic arthritis in haemophilic patients. Clin Orthop Relat Res. 1996;328:54-59.
9 Martini M, Gottesman H. Results of conservative treatment of tuberculosis in the elbow. Int Orthop. 1980;4(2):83-86.
10 Offiah AC. Acute osteomyelitis, septic arthritis and discitis: the differences between neonates and older children. Eur J Radiol. 2006;60(2):221-232.
11 Cole WG, Dalziel RE, Leith S. Treatment of acute osteomyelitis in childhood. J Bone Joint Surg (Br). 1982;64:218-223.
12 Dubost JJ, Soubrier M, De Champs C, et al. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Revue de Rheumatism. 2004;71(4):303-311.
13 Nolla JM, Gomez-Vaquero C, Corbella X, et al. Group B streptococcus pyogenic arthritis in non-pregnant adults. Medicine. 2003;82(2):119-228.
14 Al-Nammari SS, Bobak P, Venkatesh R. Methicillin resistant staphylococcus aureus versus methicillin sensitive staphylococcus aureus adult haematogenous septic arthritis. Arch Orthop Trauma Surg. 2007;127(7):537-542.
15 Vostrel P, Legout L, Hoffmeyeer P. Septic arthritis (non gonococcal) of the adult. Rev Med Suisse. 2006;2(92):2924-2930.
16 Dubost JJ, Soubrier M, De Champs C, et al. No changes in the distribution of organisms responsible for septic arthritis over a 20-year period. Ann Rheum Dis. 2002;61:267-269.
17 Trueta J. Studies in the development and decay of the human frame. London: Heineman; 1968.
18 Roberts JM, Drummond DS, Breed AL, et al. Subacute haematogenous osteomyelitis in children: a retrospective study. J Paediat Orthop. 1982;2:249-254.
19 Goldenberg DL, Reed JL. Bacterial arthritis. N Engl J Med. 1985;312:764-771.
20 Homandberg GA. Cartilage damage by matrix degredation products: Fibronectin fragments. Clin Orthop Relat Res. 2001;39(1):100-107.
21 Kuiper S, Joosten LA, Bendele AM, et al. Different roles of tumour necrosis factor alpha and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine. 1998;10(9):690-702.
22 Tissi L, Puliti M, Barluzzi R, et al. Role of tumor necrosis factor alpha, interleukin-1beta and interleukin-6 in a mouse model of group B streptococcal arthritis. Infect Immun. 1999;67(9):4545-4550.
23 O’Driscol SW, Morrey BF, An KN. Intra-articular pressure and capacity of the elbow. Arthroscopy. 1990;6:100-103.
24 Shetty AK, Gedalia A. Management of septic arthritis. Indian J Paediatr. 2004;71(9):819-824.
25 Jbara M, Patana M, Kazmi F, et al. MR imaging: arthropathies and infectious conditions of the elbow, wrist and hand. Magn Reson Imaging Clin N Am. 2004;12(2):361-379.
26  SÄ Lidgren L. Orthopaedic infections. Lund: Studentlitteratur; 1988.
SÄ Lidgren L. Orthopaedic infections. Lund: Studentlitteratur; 1988.
27 Coakley G, Mathews C, Field M, et al. on behalf of the British Society for Rheumatology Standards, Guidelines and Audit working Group. Guidelines for the management of the hot swollen joint in adults. Rheumatology. 2006;45:1039-1041.
28 Lavy CB, Thyoka M. For how long should antibiotics be given in acute paediatric septic arthritis? A prospective review of 96 cases. Tropical Doctor. 2007;37(4):195-197.
29 Argen RJ, Wilson CH, Wood P. Suppurative arthritis. Arch Intern Med. 1966;117:661-666.
30 Salter RB, Bell RS, Kelley FW. The protective effect of continous passive motion on living articular cartilage in acute septic arthritis. Clin Orthop. 1981;159:223.
31 Bober LA, Rogas-Triana A, Jackson JV, et al. Regulatoryeffects of interleukin-4 and interleukin-10 on human neutrophil function ex vivo and on neutrophil influx in a rat model of arthritis. Arthritis Rheum. 2000;43(12):2660-2667.
32 Puliti M, Orefici G, Tissi L. The beneficial effect of interleukin-12 on arthritis induced by group B streptococci is mediated by interferon-gamma and interleukin-10 production. Arthritis Rheum. 2002;46(3):806-817.
33 Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatologgy. 2001;40(1):24-30.
34 Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:1394-1396.
35 Alarcon GS. Arthritis due to tuberculosis, fungal infections and parasites. Curr Opin Rheumatol. 1992;4(4):516-519.
36 Domingo A, Nomdedeu M, Tomas X, et al. Elbow tuberculosis: an unusual location and diagnostic problem. Arch Orthop Trauma Surg. 2005;125(1):56-58.
37 Tuli SM. General principles of osteoarticular tuberculosis. Clin Orthop Relat Res. 2002;398:11-19.
38 Martini M, Ouahes M. Bone and joint tuberculosis: a review of 652 cases. Orthopaedics. 1988;11(6):861-866.
39 Martini M, Benkeddache Y, Gottesman H. Tuberculosis of the upper limbs. Int Orthop. 1986;10(1):17-23.
40 Ellis ME, el-Ramahi KM, al-Dalaan AN. Tuberculosis in peripheral joints: a dilemma in diagnosis. Tubercule Lung Dis. 1993;74(6):399-404.
41 Dix-Peek SI, Vrettos BC, Hoffman EB. Tuberculosis of the elbow in children. J Shoulder Elbow Surg. 2003;12(3):282-286.
42 Aggarwal A, Dhammi I. Clinical and radiological presentation of tuberculosis of the elbow. Acta Orthop Belg. 2006;72(3):282-287.
43 Hortas C, Ferreiro JL, Galdo B, et al. Tuberculous arthritis of peripheral joints with previous rheumatic disease. Brit J Rheum. 1988;27(1):65-67.
44 Chen WS, Wang CJ, Eng HL. Tuberculous arthritis of the elbow. Int Orthop. 1997;21(6):367-370.
45 Wang CT, Sun JS, Hou SM. Mycobacterial infection of the upper extremities. J Formosan Med Assoc. 2000;99(9):710-715.
46 Titov AG, Nakonechniy GD, Santavirta S, et al. Arthroscopic operations in tuberculosis. Knee. 2004;11(1):57-62.
47 Arafiles RP. A new technique of fusion for tuberculosis of the elbow. J Bone Joint Surg (Am). 1981;63(9):1396-1400.