Chapter 9 Complications of Supracondylar Fractures and Their Management
Background/aetiology
The incidence of vascular injuries following supracondylar fractures varies between 5% and 35%.1,2 In addition, patients may also suffer from vascular compromise, which can range from an absent or weak pulse to a frankly ischaemic limb.
Peripheral nerve injuries occur in 5–15% of supracondylar fractures.3,4 All nerves crossing the elbow may be injured, with the commonest being the median and its anterior interosseous branch. The median nerve is most at risk with posterolaterally displaced fractures, while the radial nerve is more likely to be damaged if displacement is posteromedial. The greater the bony displacement, the greater is the risk of nerve injury. Although most nerves are damaged at the time of the fracture, it is important to appreciate that they are also at risk during surgical treatment. In particular, the ulnar nerve may inadvertently be injured during pin insertion for fracture fixation.
Presentation, investigation and treatment options
Vascular injuries
If the hand is obviously ischaemic, the arm should be immediately manipulated and splinted in an extended position. This will often restore the circulation to the hand but if this fails the child should be taken immediately to the operating room for closed reduction and pinning. The role of arteriography is controversial.5,6 While plainly any information about the vascular status is helpful, undertaking the technique should not lead to undue delay with treatment. The rationale is that this is an emergency situation and, if the circulation does not return following reduction and pinning of the fracture, exposure of the brachial vessels should be performed. If the artery is trapped between bony fragments then the fixation should be taken down and the artery liberated. If the artery is crushed, transacted or has an intimal tear, reconstruction will be required, usually with help from a vascular or micro trained surgeon. In this situation it is also important to perform a fasciotomy of the deep fascia of the forearm, particularly if there has been significant ischaemia time.
Malunion
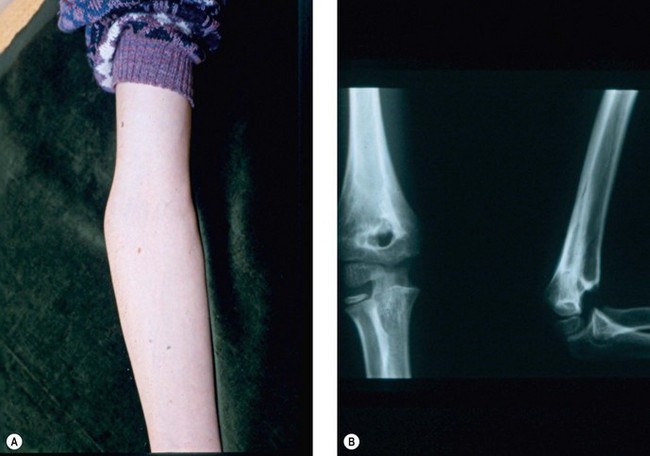
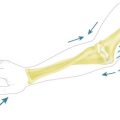
Cubitus varus or valgus is assessed by measuring the carrying angle of the arm. This is the angle created by the medial border of the fully supinated forearm and the medial border of the humerus when the elbow is extended (Fig. 9.1). The angle typically varies between the sexes, with the normal range lying between 6° and 12°. It is generally greater in female than male patients and is best assessed by comparison with the contralateral side. In addition, as the normal elbow extends, the carrying angle increases, i.e. becomes more valgus. With malunions, however, hyperextension tends to accentuate a cubitus varus deformity, while a flexion contracture can create the appearance of cubitus valgus. Measurement of rotational deformity is more difficult but can be assessed by asking the child to bend forward such that the thoracolumbar spine is parallel to the ground. Each arm is then placed in turn behind the patient’s back and rotation measured as the angle between the forearm and the patient’s thoracolumbar spine when the arm is maximally rotated.
Gurkan et al7 described posterior instability of the shoulder in three patients with cubitus varus deformity.
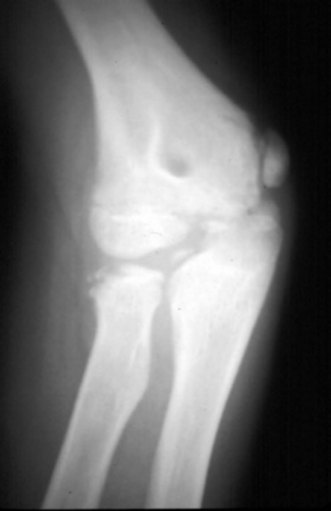
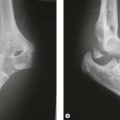
A less commonly reported complication of supracondylar fractures of the humerus in children is the dissolution of a portion of the trochlea at a variable time after fracture (Fig. 9.2). If this defect to the trochlea is severe it will allow migration of the ulnar proximally.8 A similar complication on the lateral side of the elbow was reported by Vocke-Hell et al.9 They noted that this could occur at any time between 1 and 4 years after the fracture and may result in secondary radial head dislocation.
Surgical techniques and rehabilitation
Vascular injury
Technique 1 – exposure of the neurovascular structures at the front of the elbow
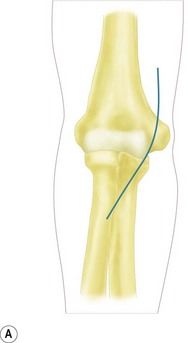
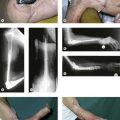
With the patient supine, the arm abducted, the elbow extended and the forearm supinated, an incision is made over the anteromedial aspect of the elbow. This should be angled or stepped as it crosses the anterior elbow crease (Fig. 9.3A). The subcutaneous fat is dissected carefully and the medial cutaneous nerve of the forearm isolated and retracted as appropriate.
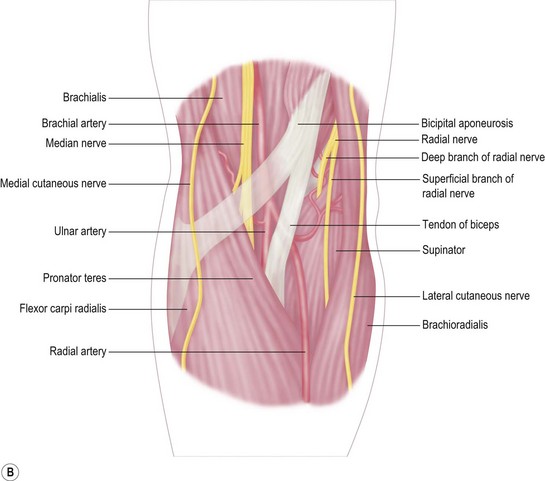
Distally the radial artery crosses over the insertion of the pronator teres and the origin of the flexor pollicis longus, ultimately disappearing between the tendons of the abductor and the extensor pollicis brevis. The ulnar artery disappears from the cubital fossa, passing deep to the deep head of the pronator teres and beneath the fibrous arch of the flexor digitorum superficialis. Finally the median nerve exits the cubital fossa, between the two heads of the pronator teres (Fig. 9.3B).
Peripheral nerve injury
The exploration of peripheral nerves around the elbow in children is no different from that in adults. Generally the author would recommend the use of loupe magnification during these procedures. Routine exposures of the ulna, median and radial nerves are described in Chapter 31. Following a supracondylar fracture the approaches are the same.
Malunion
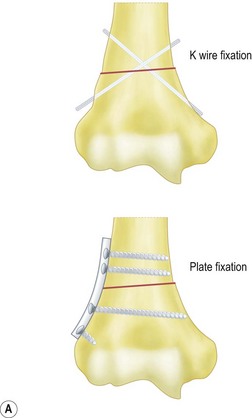
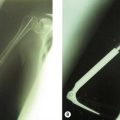
Fixation of the osteotomy can be undertaken with pins as well as plates and screws. At the author’s institution the latter is more commonly used with the application of a small fragment AO semi-tubular plate (Fig. 9.4). This fixation is supplemented by plaster cast splintage for 3–4 weeks.
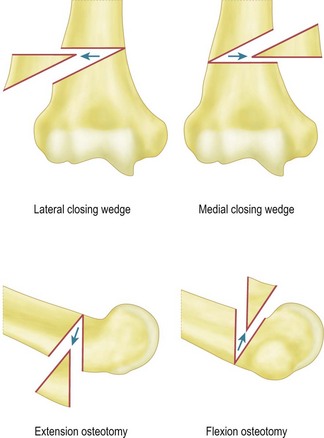
Surgical correction of cubitus varus and cubitus valgus deformities
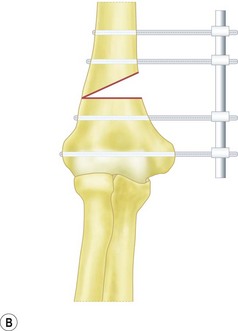
For flexion and extension osteotomies a lateral approach can again be used. In this instance, however, the osteotomy is angulated in the anteroposterior rather than the lateromedial plane (Fig. 9.5). Fixation is once more achieved using a plate and screws. As an alternative in very young children the osteotomy can be held with crossed K-wires and external plaster splintage.
Stiffness
It is unusual for any child who has suffered a supracondylar fracture to require a secondary procedure to treat stiffness. Usually a course of physiotherapy and ‘skilful neglect’ is all that is required. Certainly it is well accepted that soft tissue tightness will improve with time. If, however, there is a persistent soft tissue flexion or extension contracture in an older child, surgical correction may be required. The technique of anterior and posterior capsular release is described in Chapter 29.
Outcomes and complications of treatment
Outcomes of treatment for vascular complications
A review of the literature reveals areas of agreement and controversy in the management of a pulseless arm associated with a supracondylar fracture. All clinicians agree that in such a scenario the patient should undergo an immediate reduction and fixation of the fracture, usually with K-wires. Thereafter, the arm should be splinted in extension, further reducing compression of the artery and vein.10 Elevation of the arm is also useful to prevent oedema. In the majority of patients treated this way the radial pulse is usually restored. Plainly, this situation will require regular monitoring in the early postoperative/injury phase. It is also important to identify any signs of a compartment syndrome.
If the pulse does not return, the management options vary. Some clinicians, predominantly from the United States, advocate emergency exploration of the brachial artery. Authors report finding arterial entrapment and in some cases intimal tears often needing a vascular graft.11–13 In most patients repairs remain patent, although a progressive postoperative deterioration in circulatory status has been seen 24–36 h after surgery in a small number of cases. This group required further investigation and surgery.
In contrast, the majority of orthopaedic surgeons in the United Kingdom faced with the same scenario would manage the arm conservatively, provided that following reduction of the fracture the hand remains pink and well perfused, and passive movement of the fingers is pain free. The patient would undergo regular review with the expectation that as the swelling subsides the circulation will be restored.14 In this situation further investigation, specifically pressure monitoring, duplex scanning and magnetic resonance angiography, will enable the clinician to confirm that there is no surgically treatable cause for the absent pulse.
There is no doubt, however, that recovery following severe upper limb ischaemia is limited. Motor recovery does not take place, leaving the patient with significant permanent contractures. Although some degree of sensory recovery may occur, it can take between 6 and 12 months to maximize.15
Compartment syndrome was noted by Blakemore et al16 in three out of a series of 33 children, all of whom had displaced extension type supracondylar fractures. This gives an incidence for this complication of 7%. Fasciectomy resulted in a satisfactory outcome in all cases. Ramachandran et al17 identified 11 patients with this condition, 10 of whom presented with severe swelling around the elbow. In addition, for one reason or another all had surgery delayed by an average of 24 h. Interestingly, all patients presented with low-energy injuries and an intact radial pulse. The authors concluded that significant swelling at presentation and delay to fracture reduction were warning signs for the development of this dangerous complication.
Outcomes of treatment following nerve injury
The incidence of nerve injuries following supracondylar fractures varies from 5% to 15%. Although all of the nerves crossing the elbow may be injured, the most commonly reported are the median and anterior interosseous nerves. Injury can occur with all types of supracondylar fracture but are more common in cases with posterolateral displacement.18
The next most commonly involved nerves are the radial and posterior interosseous, which are injured in fractures with posteromedial displacement. Injuries to the ulnar nerve may also occur but usually follow percutaneous K-wire fracture fixation rather than as a direct result of the fracture. They occur in 5% of cases.19
For those who do not recover within that time period, exploration is required. Generally this is undertaken between 3 and 6 months following the injury. The findings at exploration may include bony entrapment, scarring, neuroma formation and complete nerve transection. In the majority of reported cases neurolysis is the commonest procedure undertaken, generally with a good outcome. Some patients do, however, ultimately require exploration and neurolysis and occasionally excision of neuroma and nerve grafting.20 If these techniques are unsuccessful at restoring function, tendon transfers may be required.
Ulnar nerve injuries that become apparent after supracondylar fracture fixation require urgent treatment. This initially involves removal or repositioning of fixation wires after, which recovery has been reported to occur relatively quickly.21 If this is not the case, however, exploration and decompression or rarely reconstruction of the ulna nerve may be required.
Outcome of procedures to correct bony deformities
Several reports clearly show that fractures treated by immediate fixation, particularly K-wires, result in fewer and milder cases of cubitus varus deformity when compared to other treatment techniques.22,23 This minimizes the cosmetic deformity that many patients find unacceptable and reduces the need for corrective surgery. In addition to tardy ulnar nerve palsy seen in cubitus valgus deformity, instability of the ulnar nerve has also been reported.24,25 The latter is often associated with an unstable or snapping medial triceps.
Posterolateral rotatory instability has been described as a complication of long-standing varus deformity. Following an initial case report by Abe et al,26 O’Driscoll et al27 described 24 cases (25 limbs) of this condition, which occurred two to three decades after the original injury. All patients presented with lateral elbow pain and recurrent instability. The average varus deformity was 15°. The explanation for this complication was stated to be repetitive external rotation torque on the ulna, resulting in stretching of the lateral collateral ligament complex. Surgical treatment included a supracondylar valgus osteotomy and transposition of a portion of the medial head of the triceps to the lateral aspect of the olecranon. Although this was generally successful, two patients in this series continued to have snapping of the medial head of the triceps.
A biomechanical analysis of the effect of distal humeral varus deformity on strain to the lateral ulna collateral ligament was undertaken by Beuerlein et al.28 Using fresh frozen cadaveric elbow joints they measured strain in the ligament while stressing the ulnar humeral joint in several positions of varus. They confirmed that increasing deformity increased strain on the lateral ulnar collateral ligament.
Correction of the deformity is achieved by performing a closing valgus wedge osteotomy. Bellemore et al29 from Sydney reported their results in 32 patients over a 10-year period. Half of these underwent the technique described by French30 to fix the osteotomy. This involved the insertion of a screw either side of the osteotomy with a figure-of-eight wire around the screws to provide compression at the osteotomy site. Overall, apart from an unsightly scar in four cases, they reported a significant improvement in cosmesis, within 5° of the contralateral side. There were, however, some failures due to inadequate osteotomy fixation. These occurred principally in the group fixed by K-wires alone. Range of motion was generally preserved and, overall, they recommended this technique. The problems with K-wire fixation have also been reported by Rang,31 who in a series of 20 patients experienced loss of fixation, infection, nerve palsy and an aneurysm of the brachial artery. Oppenheim et al32 from the United States reported their results of 45 osteotomies performed in 43 children with an average follow-up of 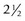 years. While good to excellent results were obtained in most patients, unsatisfactory results were seen in 12. There was also a significant complication rate of 24%, which included neuropraxia, sepsis and cosmetically unacceptable scarring. It should be noted again that in this series fixation was undertaken by threaded Steinmann pins alone.
years. While good to excellent results were obtained in most patients, unsatisfactory results were seen in 12. There was also a significant complication rate of 24%, which included neuropraxia, sepsis and cosmetically unacceptable scarring. It should be noted again that in this series fixation was undertaken by threaded Steinmann pins alone.
A variation on this technique was described by DeRosa and Graziano in 1987,33 when they reported a step-cut osteotomy that allowed fixation by a single screw. In their series of 11 patients, they were able to obtain an average correction of 28.4°, leaving a carrying angle of 9.3° in most patients. There were no complications.
New techniques of osteotomy fixing include the use of plates and external fixation.34 Devnani in 199735 reported his results in nine children undergoing osteotomies that were internally fixed with a two-hole plate. Generally the results were good, with no loss of correction due to implant failure. The implant was usually removed 12 months after surgery. Many clinicians would now use a four-hole plate.
In 1997 Matsushita and Nagano36 from Japan reported the technique of an arc or dome osteotomy. This technique allowed simultaneous correction of both the angulatory deformity and any lateral shift. In 12 patients followed for 28 months the average carrying angle was corrected from 22° of varus to 6° of valgus with no complications. Movements were also well maintained. Finally, Usui et al37 from Japan described a three-dimensional corrective osteotomy for the treatment of cubital varus. This osteotomy, which is again principally lateral based, also allows correction of any hyperextension or internal rotation deformity.
1 Pirone AM, Graham HK, Krajbich JI. Management of displaced extension-type supracondylar fractures of the humerus in children. J Bone Joint Surg (Am). 1988;70:641-650.
2 Louahem DM, Nebunescu A, Canavese F, et al. Neurovascular complications and severe displacement in supracondylar humeral fractures in children: defensive or offensive strategy? J Paediatr Orthop B. 2006;15(1):51-57.
3 Mehlman C, Crawford A, McMillion T, et al. Operative treatment of supracondylar fractures of the humerus in children: the Cincinnati experience (Review). Acta Orthop Belg. 1996;62(Suppl.):41-50.
4 Sairyo K, Henmi T, Kanematsu Y, et al. Radial nerve palsy associated with slightly angulated pediatric supracondylar humerus fracture. J Orthop Trauma. 1997;11:227-229.
5 Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
6 Sabharwal S, Tredwell SJ, Beauchamp RD, et al. Management of pulseless pink hand in pediatric supracondylar fractures of humerus. J Pediatr Orthop. 1997;17:303-310.
7 Gurkan I, Bayrakci K, Tasbas B, et al. Posterior instability of the shoulder after supracondylar fractures recovered with cubitus varus deformity. J Pediatr Orthop. 2002;22:198-202.
8 Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg (Am). 1984;66:557-562.
9 Vocke-Hell AK, von Laer L, Slongo T, et al. Secondary radial head dislocation and dysplasia of the lateral condyle after elbow trauma in children. J Pediatr Orthop. 2001;21:319-323.
10 Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop. 2002;22:431-439.
11 Shaw BA, Kasser JR, Emans JB, et al. Management of vascular injuries in displaced supracondylar humerus fractures without arteriography. J Orthop Trauma. 1990;4:25-29.
12 Schoenecker PL, Delgado E, Rotman M, et al. Pulseless arm in association with totally displaced supracondylar fracture. J Orthop Trauma. 1996;10:410-415.
13 Copley LA, Dormans JP, Davidson RS. Vascular injuries and their sequelae in pediatric supracondylar humeral fractures: toward a goal of prevention. J Pediatr Orthop. 1996;16:99-103.
14 Malviya A, Simmons D, Vallamshetla R, et al. Pink pulseless hand following supra-condylar fractures: an audit of British practice. J Pediatr Orthop. 2006;15:62-64.
15 Sundararaj GD, Mani K. Pattern of contracture and recovery following ischaemia of the upper limb. J. Hand Surg. 1985;10:155-161.
16 Blakemore LC, Cooperman DR, Thompson GH, et al. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Rel Res. 2000;376:32-38.
17 Ramachandran M, Birch R, Eastwood DM. Clinical outcome of nerve injuries associated with supracondylar fractures of the humerus in children. J Bone Joint Surg (Br). 2006;88:90-94.
18 Kiyoshige Y. Critical displacement of neural injuries in supracondylar humeral fractures in children. J Pediatr Orthop. 1999;19:816-817.
19 Ikram M. Ulnar nerve palsy: a complication following percutaneous fixation of supracondylar fractures of the humerus in children. Injury. 1996;27:303-305.
20 Culp RW, Osterman AL, Davidson RS, et al. Neurological complications associated with supracondylar fractures of the humerus in children. J Bone Joint Surg (Am). 1990;72:1211-1214.
21 Lyons JP, Ashley E, Hoffer MM. Ulnar nerve palsies after percutaneous cross-pinning of supracondylar fractures in children’s elbows. J Pediatr Orthop. 1998;18:43-45.
22 Weiland AJ, Meyer S, Tolo VT, et al. Surgical treatment of displaced supracondylar fractures of the humerus in children. J Bone Joint Surg (Am). 1978;60:657-661.
23 Prietto CA. Supracondylar fractures of the humerus. J Bone Joint Surg (Am). 1979;61:425-428.
24 Abe M, Ishizu T, Shirai H, et al. Tardy ulnar nerve palsy caused by cubitus varus deformity. J Hand Surg (Am). 1995;20:5-9.
25 Spinner RJ, O’Driscoll, Dabids JR, et al. Cubitus varus associated with dislocation of both the medial portion of the triceps and the ulnar nerve. J Hand Surg (Am). 1999;24:718-726.
26 Abe M, Ishizu T, Morikawa J. Posterolateral rotatory instability of the elbow after posttraumatic cubitus varus. J Shoulder Elbow Surg. 1997;6:405-409.
27 O’Driscoll SW, Spinner RJ, McKee MD, et al. Tardy posterolateral rotatory instability of the elbow due to cubitus varus. J Bone Joint Surg (Am). 2001;83:1358-1369.
28 Beuerlein MJ, Reid JT, Schemitsch EH, et al. Effect of distal humeral varus deformity on strain in the lateral ulnar collateral ligament and ulnohumeral joint stability. J Bone Joint Surg (Am). 2004;86:2235-2242.
29 Bellemore MC, Barrett IR, Middleton RWD, et al. Supracondylar osteotomy of the humerus for correction of cubitus varus. J Bone Joint Surg (Br). 1984;66:566-572.
30 French PR. Varus deformity of the elbow following supracondylar fractures of the humerus in children. Lancet. 1959;1:439-441.
31 Rang M. Children’s fractures. Philadelphia, PA: JB Lippincott; 1974.
32 Oppenheim WL, Clader TJ, Smith C, et al. Supracondylar humeral osteotomy for traumatic childhood cubitus varus deformity. Clin Orthop Rel Res. 1984;188:34-39.
33 DeRosa GP, Graziano GP. A new osteotomy for cubitus varus. Clin Orthop Rel Res. 1988;236:160-165.
34 Levine MJ, Horn BD, Pizzutillo PD. Treatment of posttaumatic cubitus varus in the pediatric population with humeral osteotomy and external fixation. J Pediatr Orthop. 1996;16:597-601.
35 Devnani AS. Lateral closing wedge supracondylar osteotomy of humerus for post-traumatic cubitus varus in children. Injury. 1997;28:643-647.
36 Matsushita T, Nagano A. Arc osteotomy of the humerus to correct cubitus varus. Clin Orthop Rel Res. 1997;336:111-115.
37 Usui M, Ishii S, Miyano S, et al. Three-dimensional corrective osteotomy for treatment of cubitus varus after supracondylar fracture of the humerus in children. J Shoulder Elbow Surg. 1995;4:17-22.