CHAPTER 196 Thalamic Tumors
Incidence
Although thalamic tumors constitute between 2% and 5% of all pediatric intracranial tumors1–3 versus 1% in adult series,4 the pediatric literature remains relatively sparse. Tumors do not necessarily respect the limits of the thalamus, and earlier studies have been difficult to interpret because they have included all age groups and tumors arising in adjacent regions.
In the St. Jude experience,5 12% of all childhood low-grade gliomas were found in the thalamus as compared with 20% in the cerebral hemispheres and 35% in the cerebellum. The mean age at diagnosis was 7 years, and 32% of the children were younger than 5 years. There does not appear to be a gender or lateralization bias.
Growth Pattern
On the basis of their growth pattern, tumors can be classified into one of three categories: focal, diffuse, or bilateral. Focal tumors have a discrete well-demarcated location within the thalamus. As growth progresses beyond the thalamic boundaries, the lesions expand into the surrounding white matter and particularly into the ventricular cavities. For low-grade gliomas, especially pilocytic astrocytomas, growth for the most part results in a mass effect that compresses the surrounding structures rather than infiltration. Superomedially, the ependymal surface is not usually breached. These lesions are most commonly accompanied by clinical signs of obstruction to flow of cerebrospinal fluid (CSF) and in many cases can be radically debulked and even cured surgically, with access facilitated by the enlarged ventricles. In contrast, fibrillary astrocytomas, both low grade and high grade, do not display such a well-demarcated brain-tumor interface and typically migrate beyond the confines of the thalamic boundaries; they also cause CSF obstruction or focal neurological deficits.6 These lesions present formidable challenges for treatment, although even with high-grade tumors, patients may obtain benefit from radical surgical resection.
Bilateral thalamic tumors are typically fibrillary astrocytomas and have traditionally been considered to be high-grade lesions with a poor prognosis. These lesions most often cause homogeneous symmetrical expansion of both thalami and focal deficits, as well as hydrocephalus. A recent review from the Necker Enfants Malades Hospital in Paris found that six of nine were surprisingly low-grade astrocytomas, although half of the patients were dead at 4 years.7 A so-called low-grade fibrillary astrocytoma therefore more commonly follows an aggressive course in this situation, and surgery has little role in treating these tumors other than providing a tissue diagnosis.
Imaging
The radiologic appearance of these lesions provides significant clues with regard to the histology and management options for these lesions. Colosimo and colleagues described four patterns that these lesions may demonstrate on computed tomography (CT) and MRI.8
The aforementioned classification offers a framework to assist in diagnosing such lesions, but interpretation by an experienced neuroradiologist and neurosurgeon is important. Interestingly, the authors of the Paris series reported that only half of their pilocytic lesions exhibited the typical appearance of a cystic tumor with an enhancing mural nodule and no surrounding edema.7
Histopathology
The overwhelming majority of pure thalamic lesions are glial, although there appears to be some discrepancy within the literature in terms of the exact proportion of low- and high-grade lesions. Including all sites, low-grade gliomas are the most common pediatric central nervous system tumors and account for approximately one third of all cases,9 whereas high-grade lesions are less frequent and account for 7% to 11% of all lesions.10 Earlier series reported that the vast majority of these lesions were juvenile pilocytic astrocytomas or fibrillary astrocytomas, but more recent studies looking specifically at thalamic tumors in children suggest that the proportion of high-grade lesions may be higher (Table 196-1). The largest series to date is from the Necker Enfants Malades Hospital in Paris,7 which reported on 69 children treated over a 14-year period: 54 lesions were unilateral, 32 low grade and 22 high grade. Thirty-three (61%) of the unilateral tumors were astrocytomas, divided almost in half into pilocytic and high-grade tumors. The remaining 21 unilateral tumors were composed of oligodendrogliomas, PNETs, neurocytomas, and gangliogliomas. Six tumors were thalamopeduncular, 4 of which were pilocytic astrocytomas, 1 was a malignant glioma, and 1 lesion was not sampled. Nine tumors were bilateral, and 6 of these were low grade or pilocytic. In the Marseille series, Fernandez and colleagues reported on 14 thalamic tumors, 5 of which were pilocytic astrocytomas, 7 were oligodendrogliomas, and 2 were glioblastomas.11 In the Pittsburgh series, Albright reported on 19 gliomas, 7 low grade and 12 high grade.12 Table 196-1 lists the histologic categories of thalamic tumors published in various series.
A point of note is the histologic uncertainty within the biopsied specimen, particularly those from deep locations. The Children’s Cancer Group CCC-945 trial reported that 18% of tumors thought to be high grade were changed to low grade after central review.13 Accordingly, the proportion of low- versus high-grade lesions reported in previous studies needs to be interpreted with caution, especially if the tissue diagnosis is based on small biopsy samples only (such as image-guided needle biopsies or endoscopic biopsies).
In their series, Puget and colleagues suggested that classification of tumors be based on location and recommended surgical removal for types 1 and 2.7 Type 1 tumors arise from one thalamus with possible extension into surrounding structures, type 2 consists of thalamopeduncular tumors arising at the junction of these two structures with relatively symmetrical supratentorial and infratentorial extension, and type 3, which is the rarest type, represents bilateral thalamic tumors.
Clinical Findings
Most studies report a mean age at diagnosis of between 8 and 10 years with a range spanning the entire pediatric population and a mean duration of symptoms of about 6 months.2,7,11,12,14 However, some children may have had symptoms for less than 2 months.7 This duration is less than that typically reported in adult series, and a shorter duration of symptoms before diagnosis may portend a worse prognosis, perhaps because of a higher pathologic grade.7 There is no sex predilection.
As a result of their deep locations, children harboring these lesions can exhibit a variety of symptoms, but they usually have increased intracranial pressure (ICP) secondary to obstruction of CSF pathways or an enlarging tumor mass, or focal neurological symptoms may be present. The most common symptoms, therefore, are headache, lethargy, nausea and vomiting, and decreased level of consciousness. Treatment of ICP by diversion of CSF flow is required in as many as 50% to 75% of these children when initially seen,2,3,7,14 approximately one third of whom were treated by third ventriculostomy in the Paris series.7 Lesions can cause obstruction of both foramina of Monro or posterior third ventricular/aqueduct occlusion.15
Motor deficits are common and sensory disturbances less frequent. In the Paris series, two thirds of all patients had a motor deficit at initial evaluation.7 Lesions in the ventrolateral thalamus are typically manifested in this manner given their close proximity to the internal capsule.15 These deficits are caused by either compression or frank tumor infiltration of the internal capsule. A classic thalamic syndrome, characterized by contralateral anesthesia (or hypoesthesia), contralateral weakness, ataxia, and frequently, persistent spontaneous pain, is rare.16
Visual symptoms or signs are present in as many as 50% of these patients7 and may consist of hemianopia, a myopic poorly reactive pupil, and ipsilateral oculomotor palsy. The visual field deficits are due to involvement of the optic tracts and the geniculate ganglia, and the oculomotor deficits are due to involvement of the retrolenticular segment of the internal capsule or the midbrain tegmentum.
In addition to the preceding, involvement of critical structures surrounding the thalamus and its role in a varied range of functions account for a number of other symptom complexes being reported in these patients. There is ample evidence of the role of the thalamus in speech generation, and these patients can initially be seen with speech problems. Involvement of the hypothalamus results in endocrinopathies, and seizures have been reported in a number of cases. Involvement of the mammillothalamic tracts or the fornices can lead to cognitive dysfunction and memory problems.16 Bilateral thalamic lesions have been associated with changes in personality, memory loss, confusion, hallucinations, hyperphagia, and bradyphrenia.17
Surgical Approach
With use of the latest imaging and neuronavigation systems, increasing numbers of surgical series for thalamic tumors in children are reporting good outcomes.2,7,12,18
A number of surgical approaches can be used to access these lesions:
In planning a surgical approach, it is essential for the surgeon to study the MRI scans in the three coaxial planes to identify the possible origin of the lesion and the direction of its growth. In general, only focal tumors are strongly considered for surgical removal. If resection is considered, it is to the surgeon’s advantage to approach a thalamic tumor from the ventricle. Tumors often project into the ventricles, which are also frequently enlarged and therefore facilitate access, and resection starts most easily at the tumor-CSF interface. Initial debulking of the tumor can then be performed with minimal disturbance of the surrounding parenchyma. The presence of hydrocephalus makes transcortical approaches easier. The surgeon should have a thorough knowledge of the surgical anatomy that will be encountered, including arterial and venous structures (Figs. 196-1 and 196-2).
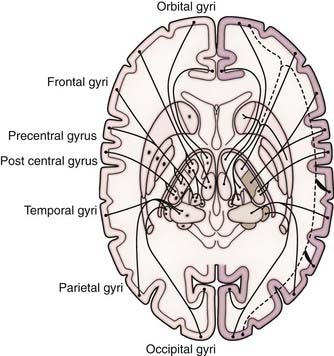
FIGURE 196-1 Thalamic connections.
(Modified from Nieuwenhuys R, ten Donkelaar HJ, Nicholson, eds. The Central Nervous System of Vertebrates. Heidelberg, Germany: Springer-Verlag; 1998.)
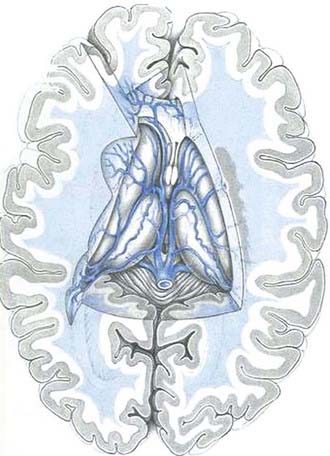
FIGURE 196-2 Deep venous anatomy of the thalamus.
(Modified from Yasargil MG. Microneurosurgery. Stuttgart, Germany: Thieme; 1984.)
The transcortical transventricular approach can be via the frontal lobe,2,14 the occipital lobe,19 or the temporal lobe20 or via a transparietal approach.12 These different trajectories are associated with their own sets of possible complications. Most commonly, a transcortical approach would be conducted transfrontally, from a coronal or precoronal trajectory. The transcortical approaches are reserved for very large masses that project toward the cortex or smaller masses associated with large ventricles.
With either of these approaches, once inside the lateral ventricle, landmarks must be identified to ensure correct orientation. Identification of the venous anatomy, the position of the choroid plexus and thalamostriate vein, the septum, and the foramen of Monro is key. Image guidance is extremely helpful because the anatomy can be very distorted by tumor. Generally, tumors can be radically debulked without sacrificing the intraventricular veins. The choroid plexus can be mobilized if necessary. For further access, some authors have described sectioning of the overlying thalamostriate vein, especially if the foramen of Monro needs to be enlarged.21 The incidence of venous infarction with this maneuver is reportedly low.22 However, we advise extreme caution in sacrificing any sizable deep or intraventricular vein.
The posterior interhemispheric parasplenial approach, popularized by Yasargil,23 offers access to more posterior lesions within the pulvinar. Occipital transventricular approaches can also be used for these tumors.
A transsylvian, transinsular approach, also described by Yasargil,23 can be used for thalamic tumors with a close relationship to the insula. In such cases, the working hypothesis is that the lesion is pushing the posterior limb of the internal capsule and the basal ganglia anteriorly and therefore an approach via the postcentral insular sulcus of the insular cortex should not damage these structures.
The aforementioned approaches can be used individually or in combination by staging the resections, a strategy that is recommended by certain authors7 as being preferential for larger tumors. In our view, however, most thalamic tumors, even large ones, can be treated with a single approach. It is obviously helpful for the surgeon to be familiar with a variety of different approaches to be able to achieve maximal safe resection. Intraoperative neuronavigation with MRI is essential, and we have also found that frequent use of ultrasound assessment at different stages during the resection is an excellent way of monitoring the progress of debulking. In general, the goal is to perform internal debulking, typically with the Cavitron ultrasonic aspirator (CUSA), while staying entirely within tumor and cautiously moving to the margins. If the tumor is discrete, the thinned-out tumor rim may “jump” into the CUSA with the aid of very gentle peripheral capsule dissection. Malignant tumors require a more cautious approach to the rim to avoid damaging the surrounding parenchyma. With these malignant tumors, as well as with many nonmalignant tumors, ultrasonic aspiration is used to shave the tumor down as close as possible to normal parenchyma, as opposed to developing a plane at the tumor-brain interface with microinstruments. One should recall that the genu of the internal capsule courses very close to the anterolateral aspect of the thalamus, and thus great care needs to be taken in this area to avoid hemiparesis. Tract stimulation will probably be used more widely in the future to ensure safe resection. We have found intraoperative somatosensory and motor evoked potential monitoring to be helpful; however, no monitoring can substitute for good anatomic knowledge and experience.
Complications
Diencephalic venous infarcts caused by obstruction of the internal cerebral venous system, although rare, can be catastrophic.24 Symptoms can range from hemiplegia to mutism or coma. The thalamostriate vein, which drains a large portion of the thalamus, is particularly at risk, even though some experienced authors advocate disrupting this structure electively, if necessary, for access.22 Perioperatively, the patient should be systemically optimized (such as adequate hydration) to reduce the chance of thrombosis of a deep cerebral vein.
Because the anterior thalamic nuclei are thought to play an important role in the neural pathways for memory processing and affect, diencephalic amnesia is a potential complication. Therapeutic creation of bilateral surgical lesions in the anterior thalamus has been used to treat a wide range of behavioral and pain problems with varying degrees of success. In terms of surgical approach, there have been no reported instances of these complications with unilateral manipulation of these nuclei.21 Of greater importance are the dorsomedial nuclei and the nearby mamillothalamic tracts. Although simultaneous bilateral damage to these structures is associated with a significant incidence of postprocedure amnesia, a unilateral dorsomedial thalamic lesion can in itself result in memory problems and frontal lobe signs. Korsakoff’s psychosis, typically seen with chronic alcohol consumption, is believed to be due to damage to the dorsomedial nuclei.
The fornices play an essential conduit role by connecting a number of basal structures, including the aforementioned dorsothalamic nuclei, the hippocampal formation, the mammillary bodies, the frontobasal areas, and others. Much has been written and debated about forniceal damage and its neuropsychological results.25 Sectioning or damage to a fornix is not entirely without risk, regardless of whether the contralateral fornix appears to be radiologically intact. In this context, forniceal damage in the anterior location appear to be less detrimental than posterior damage near the hippocampal commisure.8
Sectioning of the corpus callosum is expected to produce deficits in areas requiring interhemispheric interactions, such as problems with word-paired associate learning tasks in the Wechsler memory scale and left-sided motor apraxia in a right-handed person. These deficits are typically associated with large lesions, and short anterior surgical sectioning of the corpus callosum produces no clinically measurable deficits if no other lesions are present.26
Adjunctive Treatments
Surgery remains the mainstay of treatment of low-grade gliomas of the cerebral hemispheres,27 with radiotherapy and chemotherapy being reserved for recurrent or progressive inoperable disease. The extent of resection is of prognostic significance in these lesions both in the cerebral hemispheres27 and in the cerebellum.5 For thalamic tumors, resection alone of focal tumors, mainly pilocytic astrocytomas, can result in long-term disease control and even cure. If there is a small residual and the pathology is favorable (not high grade), observation with serial imaging is often favored and no further treatment is initiated. Repeat resection, if technically feasible, can be offered to patients who have tumors with favorable histology that regrow.
For incompletely resected or progressive lesions, most units use a radiation dose of 54 Gy administered in 30 fractions,28 although this has not been shown to provide an overall survival benefit in this cohort.27 Furthermore, the late effects of radiation therapy, such as endocrinopathies, vasculopathies, optic nerve damage, and loss of intellectual function, are particularly relevant given the anatomic location of these lesions in the thalamus. Chemotherapy is increasingly becoming the adjuvant therapy of choice, particularly in young children or in an attempt to delay radiation therapy.
The standard treatment of high-grade gliomas is surgery followed by radiotherapy. Traditionally, the role of surgery for these tumors has been underestimated, but there is good evidence that aggressive tumor debulking is associated with prolonged progression-free survival (PFS) and overall survival.13 Five-year PFS rates in the CCG-945 study were 44% versus 22% for anaplastic astrocytomas with or without radical resection and 26% versus 4% for glioblastoma. A number of chemotherapeutic agents have been used with mixed results. Bilateral thalamic tumors are typically treated with radiation therapy, regardless of whether they are histologically malignant.
Prognostic Factors and Outcomes
Although overall survival rates in children with low-grade gliomas are good, the thalamic location of these lesions portends a worse outcome. A St. Jude’s study quoted a 90% PFS rate for cerebellar low-grade gliomas, 85% for cerebral hemisphere tumors, but only 55% when these tumors were located in the thalamus5; perhaps these results will change with increasing aggressive use of surgical resection. In addition, children younger than 5 years had a worse prognosis than did the older age groups, and this difference was most pronounced in those with thalamic and hypothalamic lesions.5 Therefore, for low-grade gliomas, despite increasing roles for removal, significant challenges remain. For high-grade gliomas, radical surgical resection remains an important prognostic factor and should be considered unless the anticipated morbidity is considered severe.28
In their review of 69 cases, Puget and associates listed a symptom duration of longer than 2 months, lesions with low-grade histology, and tumor excision exceeding 90% as three favorable prognostic factors. Overall, of the 37 patients with long-term survival, 29 were functioning independently.7
For bilateral thalamic tumors, the prognosis remains relatively poor regardless of tumor histology. Surgical biopsy, typically stereotactic, is still considered to be standard to obtain a definitive histologic diagnosis. Many patients require shunting to control hydrocephalus. Radiation therapy and chemotherapy are typically offered, but adjuvant treatment has not been shown to provide any obvious survival benefit.3,7,29
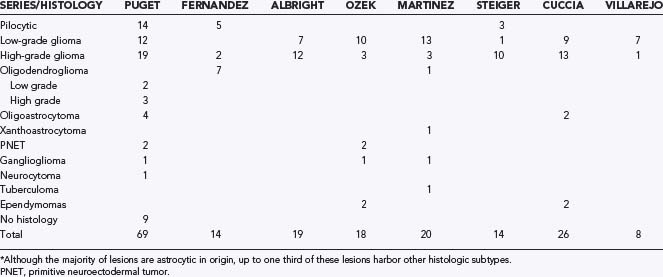
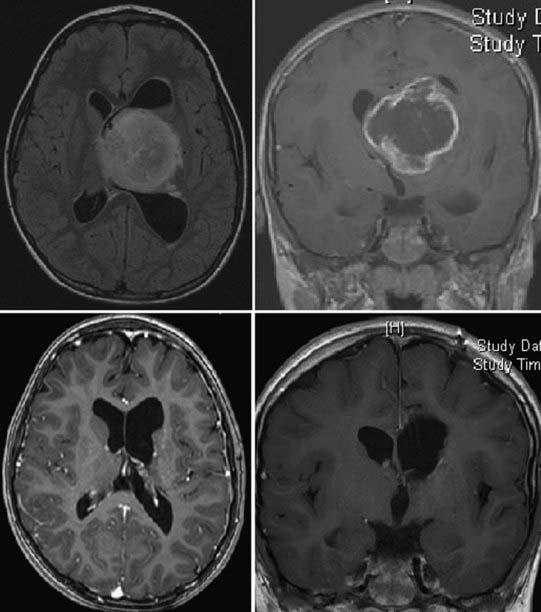
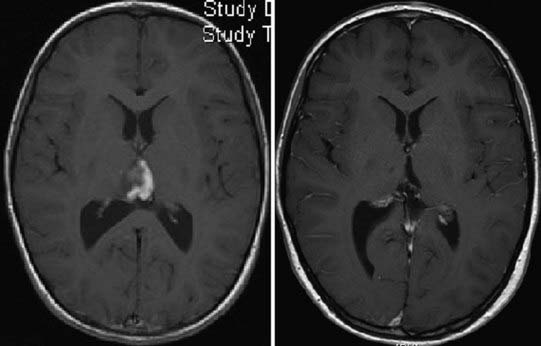
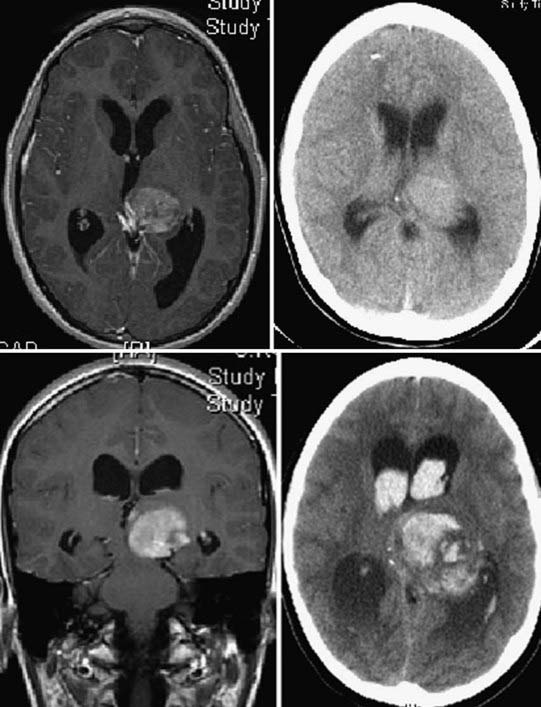
Figures 196-3 to 196-5 present examples of patients with thalamic tumors.
Albright AL. Feasibility and advisability of resections of thalamic tumors in pediatric patients. J Neurosurg. 2004;100:468-472.
Beks JW, Bouma GJ, Journee HL. Tumours of the thalamic region. A retrospective study of 27 cases. Acta Neurochir (Wien). 1987;85:125-127.
Bernstein M, Hoffman HJ, Halliday WC, et al. Thalamic tumors in children. Long-term follow-up and treatment guidelines. J Neurosurg. 1984;61:649-656.
Burger PC, Cohen KJ, Rosenblum MK, et al. Pathology of diencephalic astrocytomas. Pediatr Neurosurg. 2000;32:214-219.
Colosimo C, di Lella GM, Tartaglione T, et al. Neuroimaging of thalamic tumors in children. Childs Nerv Syst. 2002;18:426-439.
Cuccia V, Monges J. Thalamic tumors in children. Childs Nerv Syst. 1997;13:514-520.
Di Rocco C, Iannelli A. Bilateral thalamic tumors in children. Childs Nerv Syst. 2002;18:440-444.
Ehni G, Ehni BL. Considerations in transforaminal entry. In: Apuzzo MLJ, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:414-415.
Eick JJ, Miller KD, Bell KA, et al. Computed tomography of deep cerebral venous thrombosis in children. Radiology. 1981;140:399-402.
Estlin E, Lowis S. Central Nervous System Tumours of Childhood. London: Mac Keith Press; 2005.
Fernandez C, Maues de Paula A, Colin C, et al. Thalamic gliomas in children: an extensive clinical, neuroradiological and pathological study of 14 cases. Childs Nerv Syst. 2006;22:1603-1610.
Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15:2792-2799.
Heideman R, Packer RJ, Albright LA, et al. Tumours of the Central Nervous System. Philadelphia: Lippincott-Raven; 1997.
Hirsch JF, Zouaoui A, Renier D, et al. A new surgical approach to the third ventricle with interruption of the striothalamic vein. Acta Neurochir (Wien). 1979;47:135-147.
McKissock W, Paine KW. Primary tumours of the thalamus. Brain. 1958;81:41-63.
Ozek MM, Ture U. Surgical approach to thalamic tumors. Childs Nerv Syst. 2002;18:450-456.
Partlow GD, del Carpio-O’Donovan R, Melanson D, et al. Bilateral thalamic glioma: review of eight cases with personality change and mental deterioration. AJNR Am J Neuroradiol. 1992;13:1225-1230.
Pollack IF, Claassen D, al-Shboul Q, et al. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82:536-547.
Puget S, Crimmins DW, Garnett MR, et al. Thalamic tumors in children: a reappraisal. J Neurosurg. 2007;106:354-362.
Reardon DA, Gajjar A, Sanford RA, et al. Bithalamic involvement predicts poor outcome among children with thalamic glial tumors. Pediatr Neurosurg. 1998;29:29-35.
Reddy AT, Packer RJ. Chemotherapy for low-grade gliomas. Childs Nerv Syst. 1999;15:506-513.
Smith RR, Sanford RA, Schmidek HH. The deep veins. In: Apuzzo M, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:279-288.
Souweidane MM. Thalamic Gliomas. Philadelphia: Elsevier; 2005.
Souweidane MM, Hoffman HJ. Current treatment of thalamic gliomas in children. J Neurooncol. 1996;28:157-166.
Squire LR, Moore RY. Dorsal thalamic lesion in a noted case of human memory dysfunction. Ann Neurol. 1979;6:503-506.
Steiger HJ, Gotz C, Schmid-Elsaesser R, et al. Thalamic astrocytomas: surgical anatomy and results of a pilot series using maximum microsurgical removal. Acta Neurochir (Wien). 2000;142:1327-1336.
Villarejo F, Amaya C, Perez Diaz C, et al. Radical surgery of thalamic tumors in children. Childs Nerv Syst. 1994;10:111-114.
Wisoff JH, Boyett JM, Berger MS, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children’s Cancer Group trial No. CCG-945. J Neurosurg. 1998;89:52-59.
Yasargil M. Microneurosurgery, vol 4B. Stuttgart, Germany: Thieme; 1996.
1 Beks JW, Bouma GJ, Journee HL. Tumours of the thalamic region. A retrospective study of 27 cases. Acta Neurochir (Wien). 1987;85:125-127.
2 Cuccia V, Monges J. Thalamic tumors in children. Childs Nerv Syst. 1997;13:514-520.
3 Reardon DA, Gajjar A, Sanford RA, et al. Bithalamic involvement predicts poor outcome among children with thalamic glial tumors. Pediatr Neurosurg. 1998;29:29-35.
4 McKissock W, Paine KW. Primary tumours of the thalamus. Brain. 1958;81:41-63.
5 Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15:2792-2799.
6 Burger PC, Cohen KJ, Rosenblum MK, et al. Pathology of diencephalic astrocytomas. Pediatr Neurosurg. 2000;32:214-219.
7 Puget S, Crimmins DW, Garnett MR, et al. Thalamic tumors in children: a reappraisal. J Neurosurg. 2007;106:354-362.
8 Colosimo C, di Lella GM, Tartaglione T, et al. Neuroimaging of thalamic tumors in children. Childs Nerv Syst. 2002;18:426-439.
9 Reddy AT, Packer RJ. Chemotherapy for low-grade gliomas. Childs Nerv Syst. 1999;15:506-513.
10 Heideman R, Packer RJ, Albright LA, et al. Tumours of the Central Nervous System. Philadelphia: Lippincott-Raven; 1997.
11 Fernandez C, Maues de Paula A, Colin C, et al. Thalamic gliomas in children: an extensive clinical, neuroradiological and pathological study of 14 cases. Childs Nerv Syst. 2006;22:1603-1610.
12 Albright AL. Feasibility and advisability of resections of thalamic tumors in pediatric patients. J Neurosurg. 2004;100:468-472.
13 Wisoff JH, Boyett JM, Berger MS, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children’s Cancer Group trial No. CCG-945. J Neurosurg. 1998;89:52-59.
14 Bernstein M, Hoffman HJ, Halliday WC, et al. Thalamic tumors in children. Long-term follow-up and treatment guidelines. J Neurosurg. 1984;61:649-656.
15 Souweidane MM, Hoffman HJ. Current treatment of thalamic gliomas in children. J Neurooncol. 1996;28:157-166.
16 Souweidane MM. Thalamic Gliomas. Philadelphia: Elsevier; 2005.
17 Partlow GD, del Carpio-O’Donovan R, Melanson D, et al. Bilateral thalamic glioma: review of eight cases with personality change and mental deterioration. AJNR Am J Neuroradiol. 1992;13:1225-1230.
18 Ozek MM, Ture U. Surgical approach to thalamic tumors. Childs Nerv Syst. 2002;18:450-456.
19 Steiger HJ, Gotz C, Schmid-Elsaesser R, et al. Thalamic astrocytomas: surgical anatomy and results of a pilot series using maximum microsurgical removal. Acta Neurochir (Wien). 2000;142:1327-1336.
20 Villarejo F, Amaya C, Perez Diaz C, et al. Radical surgery of thalamic tumors in children. Childs Nerv Syst. 1994;10:111-114.
21 Smith RR, Sanford RA, Schmidek HH. The deep veins. In: Apuzzo M, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:279-288.
22 Hirsch JF, Zouaoui A, Renier D, et al. A new surgical approach to the third ventricle with interruption of the striothalamic vein. Acta Neurochir (Wien). 1979;47:135-147.
23 Yasargil M. Microneurosurgery. Stuttgart, Germany: Thieme, 1996;29-91. 252-312, 291-342
24 Eick JJ, Miller KD, Bell KA, et al. Computed tomography of deep cerebral venous thrombosis in children. Radiology. 1981;140:399-402.
25 Squire LR, Moore RY. Dorsal thalamic lesion in a noted case of human memory dysfunction. Ann Neurol. 1979;6:503-506.
26 Ehni G, Ehni BL. Considerations in Transforaminal entry. In: Apuzzo MLJ, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:414-415.
27 Pollack IF, Claassen D, al-Shboul Q, et al. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82:536-547.
28 Estlin E, Lowis S. Central Nervous System Tumours of Childhood. London: Mac Keith Press; 2005.
29 Di Rocco C, Iannelli A. Bilateral thalamic tumors in children. Childs Nerv Syst. 2002;18:440-444.