Chapter 203 Tetanus (Clostridium tetani)
Differential Diagnosis
Fully developed, generalized tetanus cannot be mistaken for any other disease. However, trismus may result from parapharyngeal, retropharyngeal, or dental abscesses or, rarely, from acute encephalitis involving the brainstem. Either rabies or tetanus may follow an animal bite, and rabies may manifest as trismus with seizures. Rabies may be distinguished from tetanus by hydrophobia, marked dysphagia, predominantly clonic seizures, and pleocytosis (Chapter 266). Although strychnine poisoning may result in tonic muscle spasms and generalized seizure activity, it seldom produces trismus, and unlike in tetanus, general relaxation usually occurs between spasms. Hypocalcemia may produce tetany that is characterized by laryngeal and carpopedal spasms, but trismus is absent. Occasionally, epileptic seizures, narcotic withdrawal, or other drug reactions may suggest tetanus.
Prevention
Wound Management
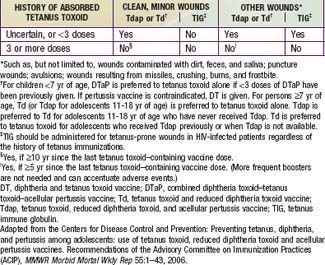
Tetanus prevention measures after trauma consist of inducing active immunity to tetanus toxin and of passively providing antitoxic antibody (Table 203-1). Tetanus prophylaxis is an essential part of all wound management, but specific measures depend on the nature of the injury and the immunization status of the patient. Regrettably, prevention of tetanus must now be included in planning for the consequences of bombings and other possible civilian mass-casualty events.
Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008;6:327-336.
Caleo M, Schaivo G. Central effects of tetanus and botulinum neurotoxins. Toxicon. 2009;54:593-599.
Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008. MMWR Recomm Rep. 2008;57:1-21.
Gaber TA, Mannemela S. Botulinum toxin for muscle spasm after tetanus. J R Soc Med. 2005;98:63.
Gibson K, Bonaventure-Uwineza J, Kiviri W, et al. Tetanus in developing countries: a case series and review. Can J Anaesth. 2009;56:307-315.
Groom AV, Washington ML, Smith PJ, et al. Underimmunization of American Indian and Alaska Native children. Pediatrics. 2008;121:938-944.
Lejtenyi D, Mazer B. Consistency of protective antibody levels across lots of intravenous immunoglobulin preparations. J Allergy Clin Immunol. 2008;121:254-255.
Moss WJ, Halsey NA. The effects of maternal malaria and HIV-1 infection on the effort to eliminate neonatal tetanus. J Infect Dis. 2007;196:502-504.
Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370:1947-1959.
Zarocostas J. UNICEF aims to eliminate tetanus in mothers and babies by 2012. BMJ. 2008;337:a1987.