178 Tetanus
• Puncture wounds pose the highest risk for tetanus. Tetanus also occurs following clean, minor wounds, abscesses, and cellulitis.
• Keep current the tetanus immunization status of patients with any injury, even minor, clean wounds.
• Give tetanus immune globulin to patients with wounds, other than clean, simple wounds, who have never completed a primary tetanus immunization series.
• Tetanus is a clinical diagnosis. Begin treatment when the diagnosis is suspected in any patient with unexplained rigidity.
• Tetanus treatment consists of tetanus immune globulin, antibiotics, and local wound care.
Epidemiology
Tetanus is a rare disease in the United States, with only 20 cases reported in 2003. In 1990 to 2000, the average number of cases in the United States was 50 per year. The average annual incidence from 1995 to 2000 was approximately 0.16 cases per million population.1,2 Tetanus is more common among people 60 years old or older (0.35 cases per million population), patients 60 years old or older who have diabetes (0.70 cases per million population), and Hispanic persons (0.37 cases per million population). Injecting drug users are at unique risk for tetanus and accounted for 15% of cases of tetanus from 1998 to 2000.1 Most (74%) injecting drug users who developed tetanus reported injecting heroin, and 100% reported “skin popping” rather than intravenous injection.3
Tetanus morbidity and mortality remain high, even with appropriate treatment. Current vaccination status decreases the severity of the disease and the likelihood of death from tetanus. In 1998 to 2000, 18% of patients with tetanus died. Of fatal cases, 75% occurred in patients who were 60 years old or older. No patients with an up-to-date vaccination status died of tetanus.1
Perspective
In addition to recognizing the clinical presentation of tetanus, EPs play a vital role in the prevention of the disease. Primary pediatric vaccination and regular decennial booster vaccination are the mainstays of disease prevention and severity modulation.4 Herd immunity does not occur with tetanus. Therefore, only people who receive the vaccination benefit from immunization. In the United States, the prevalence of tetanus immunity decreases by age, after 40 years of age. At 40 years of age, 80% of the population is immune to tetanus. By the age of 80 years, only 30% of the population remains immune. This decrease is most striking in women and Mexican Americans.5 Only 36% of persons age 65 years old or older report receiving tetanus vaccination in the past 10 years.6,7 Most cases of tetanus and fatalities resulting from tetanus are in patients who either have never been vaccinated or have not had a booster in the past 10 years.8 EPs have the opportunity to provide booster vaccination at times of minor to severe injury and skin infection. In light of pertussis epidemics, providers should also consider a patient’s pertussis immunization status when choosing the tetanus vaccine.
From Centers for Disease Control, September 2005. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/00041645.htm
![]() Facts and Formulas
Facts and Formulas
Tetanus Booster Based on Age
| Infant to 7 years | DTap (diphtheria, tetanus, acellular pertussis) |
| DT (pediatric diphtheria, tetanus preparation), if pertussis is contraindicated | |
| Age 7 to 10 years | dT |
| Tdap, if not previously given once in primary series | |
| Age 11 to 18 years | Tdap (tetanus, diphtheria, acellular pertussis) preferred |
| Td (tetanus, diphtheria) acceptable | |
| Adult | Td |
| In people aged 11 to 64 years, if primary series not completed, can substitute Tdap for one Td; substitution should be done in people aged 11 to 18 years who have not completed a primary series |
Anatomy
Most cases of tetanus are associated with acute trauma (Table 178.1), but many cases are associated with abscesses, cellulitis, chronic ulcers, dental infections, frostbite, and gangrene. In one study of injecting drug users with tetanus, 69% had an abscess at the injection site.3 Tetanus affects postpartum women, with an increased risk after unsanitary birth or abortion practices.1
| MOST THREATENING | MOST COMMON | |
|---|---|---|
| Location of injury | Face | Lower extremity Upper extremity Head and trunk |
| Type of injury | Puncture wound Crush injury Burn Chronic ulcer |
Puncture wound Laceration Chronic wound Abrasion |
| Patients | Diabetic patients Age > 60 yr Neonates No prior tetanus immunization |
Intravenous drug users Age > 60 yr Hispanic ethnicity Diabetic patients No prior tetanus immunization Last immunization >10 yr ago |
Puncture wounds are the most frequent type of acute trauma associated with tetanus. Puncture wounds include nail injuries to the foot, splinters, barbed wire injuries, tattoos, drug injection, penetrating eye injuries, and spider bites. Crush injuries, burns, and eye injuries are also portals of tetanus infection. In patients with tetanus, approximately 50% of injuries are located on the lower extremity, 36% on the upper extremity, 10% on the head or trunk, and 5% on other areas.1
The occurrence of tetanus following minor or trivial wounds is well documented in the literature. Tetanus results from minor wounds and abrasions when proper wound care is not administered.2,9–12 Nearly half of the wounds that resulted in tetanus in 1998 to 2000 occurred indoors.1
C. tetani enters the body through a wound and produces two exotoxins: tetanolysin and tetanospasmin. Tetanolysin causes local cell death and creates an anaerobic environment in the wound site.13 Tetanospasmin interferes with the transmission of inhibitory impulses in the central nervous system. It creates a presynaptic blockage of the inhibitory Renshaw cells and Ia fibers of alpha motoneurons that transmit gamma-aminobutyric acid (GABA) and glycine. Renshaw cells that transmit acetylcholine are not affected as strongly. Tetanospasmin binding prevents inhibitory signals in the central nervous system.
Tetanus becomes a systemic disease as the toxin spreads through the body. Tetanospasmin binds to nerve terminals, is internalized, and travels in retrograde fashion to the cell synapse. The toxin travels at 75 to 250 mm/day, and it affects synapses of shorter nerves before synapses of longer nerves.14 The toxin also travels by lymphatic and blood flow to remote nerves. The toxin exhibits local effects first and then spinal motor effects. The autonomic system is the last to be affected because of the length of the nerves. Tetanospasmin also inhibits acetylcholine release, a process that leads to flaccid paralysis between episodes of spasticity.15
The result of the general loss of inhibitory signals is rigidity with periods of spasticity. The reflex inhibition of antagonizing muscles is lost, thus allowing agonist and antagonist muscle groups to contract simultaneously. Autonomic disinhibition occurs late in the disease. Toxin binding appears to be irreversible; the growth of new nerve terminals is required to overcome the effects.16
Clinical Presentation
The average incubation period from time of injury to the onset of symptoms is 7 to 10 days, with a range of 1 to 60 days. Shorter incubation times are associated with more severe clinical presentation and a poor prognosis.8,16 Tetanus is usually an afebrile disease until autonomic instability occurs late in the disease. Fever suggests coinfection of the wound or other infectious causes. Generalized tetanus, or tetanus affecting the whole body, is the most common form of tetanus.
Before modern mechanical ventilation, death resulted from respiratory failure or aspiration.17 With modern mechanical ventilation, death is more commonly caused by autonomic events.18,19
The second week of illness involves autonomic instability in addition to the muscle spasms. The sympathetic nervous system is more strongly affected. Sudden increased autonomic tone, with elevated circulating catecholamine levels, increased vascular tone, hypertension, and tachycardia alternate with profound hypotension, bradycardia, and even cardiac arrest. Cardiac dysrhythmias occur, and the patient may develop hyperpyrexia at this point.20–23
Recovery begins in the third or fourth week. Muscle spasms decrease, but rigidity may persist. The duration of recovery, in those who survive, ranges from 2 to 4 months, as new axon terminals grow. Most patients return to their baseline with no residual deficit.8
Variations
Cephalic tetanus is another rare form of disease. Cephalic tetanus follows a head or facial wound or, rarely, otitis media. The cranial nerves are initially affected. Spasticity or flaccid paralysis may be the presentation. Cephalic tetanus generally progresses rapidly to generalized tetanus and is associated with a severe course.24
Neonatal tetanus is generalized tetanus of the neonate. It occurs in infants born to mothers who are inadequately immunized. The port of entry is usually the umbilicus, with increased risk if the umbilicus is cut with a nonsterile instrument or is packed with contaminated material, such as soil, dung, or clay. Symptoms manifest on day 3 to 9 of life.25
The initial presentation is failure to feed in a child who previously fed normally. Neonatal tetanus progresses to generalized tetanus as described previously. The mortality rate of neonatal tetanus, when treated, is 25% to 90%.26 Thirteen cases of neonatal tetanus were diagnosed in the United States from 1992 to 2000. In these cases, 85% of the neonates had not been vaccinated because of parental religious or philosophic objections.27
Because tetanus is a rare disease in the United States, it is difficult to suspect and diagnose (Box 178.1). Tetanus should be considered in the differential diagnosis of patients with rigidity or spasticity. The disease can be found in the setting of a minor or trivial injury that may not be remembered by the patient or family.2,9–11 Tetanus is more common in patients without vaccination or without booster in the last 10 years. Unusually, from 1998 to 2000, 6% of tetanus cases occurred in patients who reported being up to date on tetanus vaccination.1
Older patients and patients with chronic illness may have a decreased immune response to vaccination and therefore may lack protective antibody levels to tetanus in spite of appropriate vaccination. This situation is increasingly true in patients aged 65 years or older.28,29
Diagnostic Testing
The diagnosis of tetanus is clinical. The Vaccine-Preventable Diseases Surveillance Manual defines tetanus as “the acute onset of hypertonia or by painful muscular contraction (usually, initially of the jaw and neck) and generalized muscle spasms without other apparent medical cause.”27 No laboratory tests confirm or refute the diagnosis of tetanus. The organism is rarely recovered from wounds and can be cultured from patients without clinical tetanus. Serologic testing to detect anti-etanus antibody levels plays a small role in the diagnosis of the disease. Patients can develop tetanus with “protective” levels of antibody.27 The aim of testing is to rule out other causes of rigidity and spasticity. If tetanus is suspected, begin treatment immediately. Do not delay treatment, because no confirmatory test exists.
Check the patient’s electrolytes, primarily to evaluate for hypocalcemia. Order a strychnine level determination if concern exists about exposure to strychnine, with the understanding that illegally imported pesticides contain strychnine.30–32 Ask patients about pesticide exposure, and consider accidental ingestion in children.33,34
The spatula test has been used to distinguish tetanus from other forms of spasticity. In this test, a blunt instrument such as a tongue blade is used to touch the oropharynx. A patient without tetanus gags and attempts to expel the instrument. In a patient with tetanus, the stimulus triggers masseter spasm, resulting in a reflex bite of the blade.35 Although this test is reported to have a sensitivity of 94% and a specificity of 100%, the results may not be applicable to the United States, where tetanus is rare.
Management
Prevention
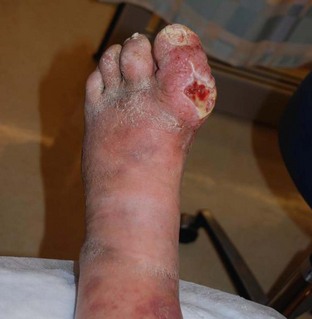
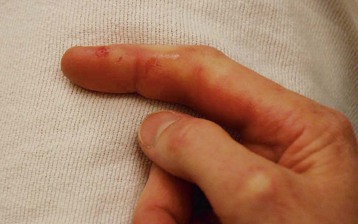
Prevention through vaccination and proper wound care remains the mainstay of therapy for tetanus. Update the patient’s tetanus immunization, and administer tetanus immune globulin according to Centers for Disease Control and Prevention guidelines (Fig. 178.1). Maintain the patient’s current tetanus immunization status in cases of cellulitis, abscesses, eye injuries, chronic ulcers, burns, injecting drugs, minor abrasions, acute lacerations, punctures, and crush injuries (Figs. 178.2 to 178.4). Clean wounds to remove any contaminants.
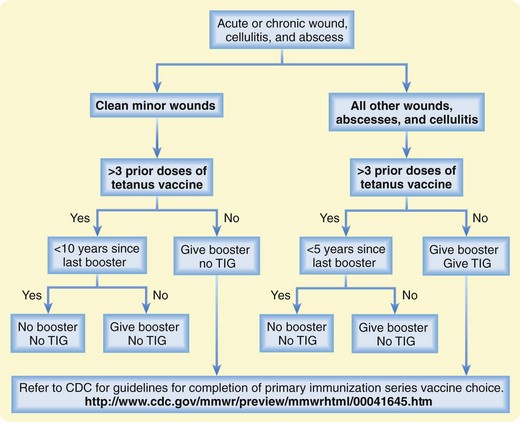
Fig. 178.1 Approach to tetanus prevention.
CDC, Centers for Disease Control and Prevention; TIG, tetanus immune globulin.
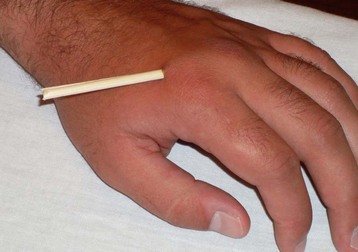
Fig. 178.4 A puncture wound to the hand from this organic material introduces a significant tetanus inoculum.
Some patients have decrease in tetanus titers earlier than 10 years. For this reason, patients with wounds that are not clean or are more than minor should receive a tetanus booster at 5 years after the last booster.27 Clinical tetanus does not confer immunity. Therefore, survivors of tetanus require immunization when they are clinically stable. Pregnant women can receive tetanus prophylaxis, if indicated.36–38 No confirmed risk to the fetus has been determined from tetanus-diphtheria or tetanus immune globulin.36
Treatment
Antitoxin Therapy
Administer tetanus immune globulin at a dose of 3000 to 5000 units intramuscularly to the pediatric or adult patient. Some sources recommend infiltration of some immune globulin around the wound site, if identified.27 Intravenous immune globulin has tetanus antitoxin and may be administered if tetanus immune globulin is not available in a reasonable amount of time.
Antibiotic Therapy
Metronidazole is the drug of choice for tetanus. Administer intravenously, 1 g every 12 hours or 500 mg every 6 hours, to adult patients. Administer intravenous metronidazole at 30 mg/kg/day, divided every 8 or 12 hours, to pediatric patients. Penicillin G is the second choice of antibiotics. Penicillin antagonizes GABA with unknown clinical significance. The dose of penicillin is 24 million units intravenously, divided every 4 to 6 hours, for adults. The pediatric dose of penicillin G is 100,000 to 250,000 units/kg/day, divided every 6 hours.39–43 Erythromycin, doxycycline, tetracycline, chloramphenicol, and clindamycin are alternatives if metronidazole and penicillin are contraindicated.43,44
Supportive Therapy
Muscle spasms are controlled with large doses of benzodiazepines to augment GABA activity. Continuous infusions improve effectiveness. Control pain with generous doses of morphine or another opiate, but avoid meperidine. If respiratory depression results from sedation, intubate the patient. Magnesium in a continuous intravenous infusion has been used as an adjunct to benzodiazepines in the treatment of muscle spasms. Magnesium contributes to respiratory depression.45–48 Sedation with propofol at levels equivalent to those used during general anesthesia decreases muscle rigidity and spasm. Intubate the patient before using propofol.49,50
If deep sedation with benzodiazepines and opioids fails to control muscle spasm, intubate the patient. Intrathecal baclofen has been used with success in a few patients. The large doses of baclofen required result in respiratory depression and coma that necessitate intubation in many patients.49,50 If intrathecal baclofen is not available on an emergency basis or if it fails to produce an improvement, administer a neuromuscular blocking muscle agent.
Tips and Tricks
| Type of Wounds | Wound Care Tips |
|---|---|
| Puncture | Copiously irrigate. Remove foreign bodies as indicated. |
| Simple laceration | Copiously irrigate. |
| Complex laceration | Copiously irrigate. Débride nonviable tissue. |
| Abscess | Incise and drain. Débride avascular tissue. |
| Cellulitis | Débride any necrotic tissue. |
| Crush | Copiously irrigate. Débride avascular tissue. |
| Abrasion | Copiously irrigate. |
| Avulsion | Copiously irrigate. Débride nonviable tissue. |
Patients with tetanus are at increased risk of aspiration because of the loss of laryngeal reflexes, atony of the stomach, and forceful contraction of the abdominal wall. Empty the patient’s stomach to decrease the chances of aspiration. Autonomic instability is a late finding and is not likely to be treated in the ED. Sedation with morphine and maintenance of a quiet, low-stimulus environment are critical in decreasing autonomic instability.48,51,52 Esmolol has been used to control hyperadrenergic states. Propranolol and labetalol are both linked to increased mortality.52,53
1 Pascual FB, McGinley EL, Zanardi LR, et al. Tetanus surveillance: United States, 1998-2000. MMWR CDC Surveill Summ. 2003;52:1–8.
2 Bardenheier B, Prevots DR, Khetsuriani N, Wharton M. Tetanus surveillance: United States, 1995-1997. MMWR CDC Surveill Summ. 1998;47:1–13.
3 Centers for Disease Control and Prevention. Tetanus among injecting-drug users: California, 1997. JAMA. 1998;279:987.
4 Centers for Disease Control and Prevention. Update on adult immunization: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40:1–94.
5 Gergen PJ, McQuillan GM, Kiely M, et al. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761–766.
6 National Center for Health Statistics. National health interview survey, 1995. Hyattsville, Md: National Center for Health Statistics; 1995.
7 McQuillan GM, Kruszon-Moran D, Deforest A, et al. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med. 2002;136:660–666.
8 Atkinson W, Hamborsky J, McIntyre L, Wolfe S. Centers for Disease Control and Prevention: epidemiology and prevention of vaccine-preventable diseases, 9th ed, Washington, DC: Public Health Foundation, 2006.
9 Centers for Disease Control and Prevention. Tetanus: United States, 1982-1984. MMWR Morb Mortal Wkly Rep. 1985;34:602. 607-11
10 Centers for Disease Control and Prevention. Tetanus: United States, 1987 and 1988. MMWR Morb Mortal Wkly Rep. 1990;39:37–41.
11 Centers for Disease Control and Prevention. Tetanus: Puerto Rico, 2002. MMWR Morb Mortal Whly Rep. 2002;51:613–615.
12 Rhee P, Nunley MK, Demetriades D, et al. Tetanus and trauma: a review and recommendations. J Trauma. 2005;58:1082–1088.
13 Rottem S, Cole RM, Habig WH, et al. Structural characteristics of tetanolysin and its binding to lipid vesicles. J Bacteriol. 1982;152:888–892.
14 Davies J, Tongroach P. Tetanus toxin and synaptic inhibition in the substantia nigra and striatum of the rat. J Physiol. 1979;290:23–36.
15 Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87:477–487.
16 Duchen LW, Tonge DA. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973;228:157–172.
17 Trujillo MH, Castillo A, Espana J, et al. Impact of intensive care management on the prognosis of tetanus: analysis of 641 cases. Chest. 1987;92:63–65.
18 Tsueda K, Oliver PB, Richter RW. Cardiovascular manifestations of tetanus. Anesthesiology. 1974;40:588–592.
19 Udwadia FE. Haemodynamics in severe in tetanus. In: Udwadia FE, ed. Tetanus. New York: Oxford University Press, 1994.
20 Kerr JH, Corbett JL, Prys-Roberts C, et al. Involvement of the sympathetic nervous system in tetanus: studies on 82 patients. Lancet. 1968;2:236–241.
21 Kelty SR, Gray RC, Dundee JW, McCulloch H. Catecholamine levels in severe tetanus. Lancet. 1968;2:195.
22 Kanarek DJ, Kaufman B, Zwi S. Severe sympathetic hyperactivity associated with tetanus. Arch Intern Med. 1973;132:602–604.
23 Udwadia FE, Lall A, Udwadia ZF, et al. Tetanus and its complications: intensive care and management experience in 150 Indian patients. Epidemiol Infect. 1987;99:675–684.
24 Jagoda A, Riggio S, Burguieres T. Cephalic tetanus: a case report and review of the literature. Am J Emerg Med. 1988;6:128–130.
25 World Health Organization. Neonatal tetanus. In: Immunizations, vaccines, and biologicals. http://www.who.int/vaccines/en/neotetanus.shtml.
26 Stanfield JP, Galazka A. Neonatal tetanus in the world today. Bull World Health Organ. 1984;62:647–669.
27 Nagachinta T, Cortese MM, Roper MH, et al. Tetanus. In: Wharton M, Hughes H, Reilly M. Vaccine-preventable diseases surveillance manual. 3rd ed. Atlanta: Centers for Disease Control and Prevention; 2002:1–8.
28 Carson PJ, Nichol KL, O’Brien J, et al. Immune function and vaccine responses in healthy advanced elderly patients. Arch Intern Med. 2001;160:2017–2024.
29 Solomonova K, Vizez S. Secondary response to boostering by purified aluminum-hydroxide–adsorbed tetanus in aging and aged adults. Immunobiology. 1981;158:312–319.
30 Cone J. 2005 NYC DOHMH health alert no.16: investigation of possible tainted heroin [letter]. New York: New York City Department of Health and Mental Hygiene; 2005.
31 Centers for Disease Control and Prevention. Scopolamine poisoning among heroin users: New York City, Newark, Philadelphia, and Baltimore, 1995 and 1996. MMWR Morb Mortal Wkly Rep. 1996;45:457–460.
32 O’Callaghan WG, Joyce N, Counihan HE, et al. Unusual strychnine poisoning and its treatment: report of eight cases. BMJ. 1982;285:478.
33 Belson M, Kieszak S, Watson W, et al. Childhood pesticide exposures on the Texas-Mexico border: clinical manifestations and poison center use. Am J Public Health. 2003;93:310–315.
34 Centers for Disease Control and Prevention. Poisoning by an illegally imported Chinese rodenticide containing tetramethylenedisulfotetramine: New York City, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:199–201.
35 Apte NM, Karnad DR. Short report. The spatula test: a simple beside test to diagnose tetanus. Am J Trop Med Hyg. 1995;53:386–387.
36 American College of Obstetricians and Gynecologists. ACOG committee opinion no. 282, January 2003: immunization during pregnancy. Obstet Gynecol. 2003;101:207–212.
37 Demicheli V, Barale A, Rivetti A. Vaccines for women to prevent neonatal tetanus. Cochrane Database Syst Rev. 4, 2005. CD002959
38 Centers for Disease Control and Prevention. General recommendations on immunization. MMWR Recomm Rep. 2002;51:1–35.
39 Ahmadsyah I, Salim A. Treatment of tetanus: an open study to compare the efficacy of procaine penicillin and metronidazole. BMJ. 1985;291:648–650.
40 Yen LM, Dao LM, Day NPJ, et al. Management of tetanus: a comparison of penicillin and metronidazole. Prestented at Symposium of Antimicrobial Resistance in Southern Viet Nam; 1997; Ho Chi Minh City, Vietnam.
41 Bleck TP. Clostridium tetani. 4th ed. Mandell GL, Bennett JE, Dollin R, eds. Principles and practice of infectious diseases. New York: Churchill Livingstone; 1995;vol 2:2173–2178.
42 Bhatia R, Prabhakar S, Grover VK. Tetanus. Neurol India. 2002;50:398–407.
43 Gilbert DV, Moillering RC, Eliopoulo GM, Sande MA. The Sanford guide to antimicrobial therapy 2005. 35th ed. Hyde Park, Vt: Antimicrobial Therapy; 2005:30.
44 Edmondson RS, Flowers MW. Intensive care in tetanus: management, complications and mortality in 100 cases. BMJ. 1979;1:1401–1404.
45 Ceneviva GD, Thomas NJ, Kees-Folts D. Magnesium sulfate for control of muscle rigidity and spasms and avoidance of mechanical ventilation in pediatric tetanus. Pediatr Crit Care Med. 2003;4:480–484.
46 Attygalle D, Rodrigo N. New trends in the management of tetanus. Expert Rev Anti Infect Ther. 2004;2:73–84.
47 Thwaites CL, Farrar JJ. Magnesium sulphate as a first line therapy in the management of tetanus. Anaesthesia. 2003;58:286.
48 Attagylle D, Rodrigo N. Magnesium sulphate for the control of spasms in severe tetanus. Anaesthesia. 1999;54:302–303.
49 Borgeat A, Dessibourg C, Rochani M, Suter PM. Sedation by propofol in tetanus: is it a muscular relaxant? Intensive Care Med. 1991;17:427–429.
50 Borgeat A, Popovic V, Schwander D. Efficiency of a continuous infusion of propofol in a patient with tetanus. Crit Care Med. 1991;19:295–297.
51 Rie MA, Wilson RS. Morphine therapy controls autonomic hyperactivity in tetanus. Ann Intern Med. 1978;88:653–654.
52 King WW, Cave DR. Use of esmolol to control autonomic instability of tetanus. Am J Med. 1991;91:425–428.
53 Rocke DA, Wesley AG, Pather M, et al. Morphine in tetanus: the management of sympathetic nervous system overactivity. S Afr Med J. 1986;70:666–668.