Chapter 54 Tensioning Anterior Cruciate Ligament Grafts
Introduction
Graft selection, graft position, fixation, and postoperative rehabilitation are clearly implicated in the success and failure of anterior cruciate ligament (ACL) reconstruction. Tensioning of the ACL is another important factor in providing immediate and long-term stability in the reconstructed patient. Jones1 stated that tension applied to the ACL graft at the time of surgery should be enough to eliminate an anterior drawer sign but still allow a full range of motion. Excessive initial tension can lead to graft failure, fixation failure, loss of knee motion, excessively reduced anterior laxity, and cartilage degeneration.2–6 Lewis et al6 introduced the term overcorrected to describe the phenomenon in which the tibia was positioned posterior and externally rotated relative to the femur. Andersen and Jorgensen7 used a prosthetic ligament to study the consequence of overcorrected ACL reconstructions. These kinematic alterations result in increased graft forces at all flexion angles, increased forces in the posterior cruciate ligament (PCL), and alteration of the normal roll–glide mechanism during knee motion. Melby et al2 found that graft tension–related posterior tibial subluxation resulted in an increase in quadriceps force needed to achieve full knee extension and may lead to an extensor lag if quadriceps atrophy is present.
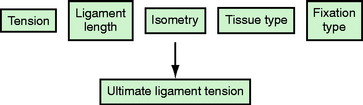
Initial tension affects the biology of the graft and is a time-dependent interplay of host incorporation and biomechanical forces. A number of factors affect graft tension, including initial tension (pretensioning and preconditioning), ligament length, graft isometry, tissue type, and fixation type (Fig. 54-1). For the purposes of this chapter, we will review the consequences of overtensioning the ACL graft both histologically and functionally, explore the factors in low-tension (bone–tendon–bone [BTB]) and high-tension (soft tissue) grafts, identify the ideal knee fixation angle, and provide suggestions for identifying tensioning problems and possible solutions. Historically, much debate has existed in the literature regarding these issues, with relatively few clinical studies focusing on graft tension; however, some consensus has been identified in recent years to lend clarity to this confusing topic.
Native Anterior Cruciate Ligament Tension
The goal of ACL reconstruction is to restore normal knee kinematics. Success requires a basic understanding of the forces affecting the normal ACL. Markolf et al8 and Wascher et al9 studied a series of loading experiments on cadaver specimens by isolating the bone plug that contained the ligament’s tibial insertion and attaching a load transducer. Passive extension of the knee generated forces in the ligament only during the last 10 degrees of extension, reaching 50 to 240N with hyperextension. Internal tibial torque generated greater forces in the ligament than did external tibial torque and increased as the knee was extended. The greatest forces (133–370N) were generated when 10 N/m of internal tibial torque was applied in hyperextension. A varus moment of 15 N/m generated forces of 94 to 177N at full extension, and a similar valgus moment generated a mean force of 56N independent of knee flexion.
Active knee motion near extension substantially increases forces across the ACL. Paulos et al10 reported that lengthening of the ACL was observed during active extension from 40 degrees of knee flexion. Active quadriceps contraction imparts significant strain on the ACL. Arms et al11 showed that over the first 45 degrees of flexion, the quadriceps increased strain, whereas it decreased strain at flexion angles greater than 60 degrees. Beynnon et al12 studied the forces on the anteromedial bundle of various exercises. Isometric quadriceps contraction at 15 degrees of knee flexion elicited the highest peak strain (4.4%), followed by open chain flexion-extension with a 45N weight (3.8%) and a Lachman test (3.7%). Isometric quadriceps contraction at 30 degrees contributed significantly less strain (2.7%) to the ACL.
More recent investigation has focused on the importance of both the anteromedial and posterolateral bundles in ACL function. Sakane et al13 examined in situ forces in nine human ACLs in response to applied anterior tibial loads at knee flexion angles of 0 to 90 degrees. Their results showed the magnitude of ACL force to be maximal with anterior tibial loads applied at 15 degrees of knee flexion, which differs slightly from Markolf’s earlier conclusions. The magnitude of force in the posterolateral bundle was larger than that in the anteromedial bundle at knee flexion angles between 0 and 45 degrees, reaching a maximum of 75N at 15 degrees of knee flexion under an anterior tibial load of 110N. The magnitude of force on the anteromedial bundle, in contrast, remained constant independently of flexion angle. They concluded that the magnitude of force in the posterolateral bundle was significantly affected by knee flexion angle and anterior tibial load and was the major restraint to anteroposterior (AP) instability in early flexion.
No graft currently in use matches the complex anatomy of the normal ACL. Woo et al14 compared anteromedial ACL reconstructions using hamstring and patellar tendon reconstructions versus the native ACL and found significant laxity to a combined rotational load involving internal rotation and valgus tibial torque at 15 and 30 degrees of flexion. This highlights the importance of the posterolateral bundle to knee stability in early knee flexion and has led some to question the anteromedial reconstructions and revisit the idea of anatomical double-bundle reconstructions.
Basic Science and Graft Histology
Evidence from animal experiments lends credence to a “window” of acceptable ACL graft tension. Graft tension that is too high can eventually lead to greater laxity and poorer results than knees fixed under low graft tension. Conversely, other animal studies demonstrate that deliberately de-tensioned grafts lose strength to a greater extent than the normally tensioned ACL. Although a stimulus is essential for the orientation of newly formed collagen during the remodeling phase,15 basic research studying the effect of graft tension cautions that high initial tension may be detrimental to the remodeling process. Yoshiya et al16 found that patellar tendon reconstructions in dogs exposed to high initial graft tension of 39N showed focal degeneration within the graft and replacement of the normal parallel arrangement of collagen fibrils by a myxoid, extracellular matrix. Microangiography demonstrated improved vascularity when the initial tension was 1N rather than 39N. Laxity measurements of the two different preloads showed increased stability of the highly tensioned graft at time zero. However, at 3 months, laxity between the two groups was similar.
The use of allograft tissue is gaining popularity in ligament reconstruction. The substantial decrease in graft strength during initial phases of healing in frozen allograft tissue relates to the inflammatory stages associated with revascularization, not the effects of freezing itself.17 Jackson et al18 studied the biomechanical outcomes of devitalized ACL at 0, 6, and 26 weeks after treatment with a freeze probe. At 6 weeks a significant reduction in maximum load to failure was observed. However, at 26 weeks, no differences were noted between frozen and contralateral controls relative to laxity, load to failure, stiffness, or modulus of elasticity.
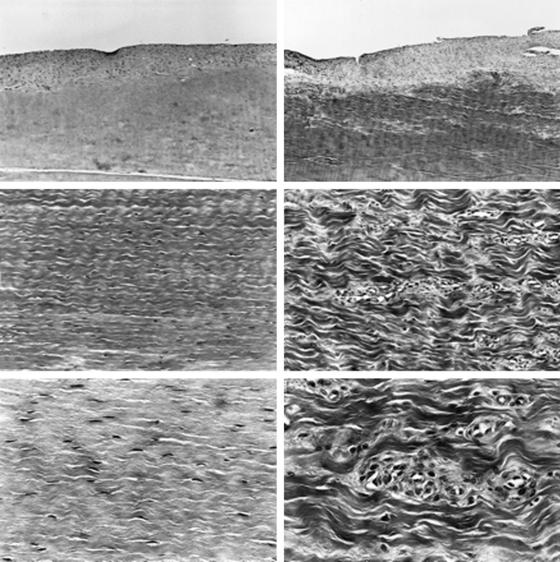
Katsuragi et al19 and Mikami et al20 studied nonphysiologically high initial tension after freezing ACL ligaments in dogs. After applying a freeze-thaw treatment to both ACLs, they applied initial tension of 20N to the test group and compared it with the physiologically tensioned contralateral extremity. The tensile strength in the highly tensioned knee decreased significantly at 6 and 12 weeks. Histologically, the collagen fibers in the highly tensioned knees were coarser and disoriented. In the midsubstance of the ACL, the physiological specimens had a spindle-shaped nucleus; however, the highly tensioned ACL had signs of degenerative changes (Fig. 54-2). Overtensioning causes significant degenerative changes in native, autologous BTB, and freeze-thaw–treated ACL. Over time, the mechanical properties of the overtensioned ACL graft deteriorate compared with physiological tension.
Graft-Specific Tensioning
Stiffness and elasticity vary among autograft tissues. Burks and Leland4 determined that the graft tension needed to restore normal anterior laxity is tissue specific. The material (stiffness) and geometrical (size and length) properties of the graft influence the amount of tension that needs to be applied. In cadaveric knees, they reconstructed the ACL using bone–patellar tendon–bone (BPTB), doubled semitendinosus, or iliotibial band grafts. They tensioned each graft to match translation of an applied 20-pound load (89N) using the KT-1000. The BPTB graft returned the knee to its preoperative condition with a mean of 3.6 pounds (16.2N); doubled semitendinosus graft, 8.5 pounds (38.3N), and iliotibial band graft, 13.6 pounds (61.2N). Due to the characteristics of BTB graft superstructure and fixation, they have been generalized as low-tension grafts, whereas the mechanical properties of soft tissue grafts have necessitated high tension for graft fixation. We will review some of the clinical evidence supporting this.
Low-Tension Grafts
Fleming et al21 studied the relationship between graft tensioning and AP laxity of the reconstructed goat knee. The AP laxity values of the intact knee were measured with the knee at various flexion angles. The ACL was then severed and the laxity measurements were repeated for nine different tensioning conditions: 30N, 60N, and 90N, each applied with the knee at 30, 60, and 90 degrees of flexion. They concluded that a 60N load applied at 30 degrees was the best combination for restoring normal AP laxity values. Pena et al22 developed a three-dimensional finite element model of the human knee joint. Under an anterior load of 134N, the closest anterior tibial translation to that of the intact knee was obtained with a pretension of 60N. However, they noted that because this load was likely to cause problems in revascularization and remodeling during postoperative healing, an initial tension of 40N was recommended.
There is no consensus for the ideal initial tension in BTB graft in clinical studies. Van Kampen et al23 prospectively randomized patients into 20N and 40N load groups and fixed them at 20 degrees of flexion. They found no significant difference in objective or subjective outcomes at 1 year after ACL reconstruction. However, they did note a slight tendency toward progressive instability in the 40N group. Yoshiya et al24 compared initial graft tension in two groups using 25N and 50N fixed at extension. In the immediate postoperative period, the reconstructed knee was similarly overconstrained in both groups. By 3 months, laxity of the surgically treated knee had increased significantly, was similar to the contralateral normal knee, and showed a slight increase between 3 and 6 months. No significant difference in the clinical outcome was observed between the groups at any time during follow-up.
Amis and Jakob25 surveyed a number of surgeons in an effort to establish a consensus on graft tensioning. Of those surveyed, the average graft tensioning protocols for BPTB reconstructions were 47N at 11 degrees of knee flexion. As previously mentioned, histological evidence of graft degeneration has been noted in animal models where the native ACL or BTB grafts were tensioned in a range of 20 to 40N. Although there were no direct consequences to knee stability, it reemphasizes the goal of “least amount of tension necessary to restore knee stability.” Applying a 20N load to a low-tension graft should provide adequate stability and reduce the risk of overconstraining the knee and degenerating the graft.
High-Tension Grafts
Multiple outcome studies have indicated that hamstring tendon autografts are associated with higher postoperative laxity measurements than patellar tendon autografts, with clinical instability rates ranging from 10% to 30%.26,27 Instability is defined as a 3-mm side-to-side difference in anterior tibial translation compared with the contralateral intact knee during manual maximum translation. Clinical instability rates ranging from 10% to 30% have been reported following reconstruction with hamstring tendon autografts. Soft tissue grafts present unique challenges. Namely, the length of the tendons are longer, more friction exists within the tunnels, and bone–bone healing is lacking. Considering the properties of soft tissue grafts, most surgeons reconstruct using higher tensions compared with BTB grafts.25 Burks and Leland4 suggested tension two to three times that required in BTB reconstructions to restore normal laxity patterns. Boylan et al28 applied three different loads in a cadaveric model: 68N, 45N, and 23N at 30 degrees of flexion. KT-1000 measurements of anterior translation demonstrated that the 68N load restored stability, whereas the knees loaded with 45N and 23N had significantly more laxity.
The clinical evidence offers some clarity to the issue. In a prospective randomized study, Yasuda et al29 followed the clinical results at 2 years in patients who received doubled semitendinosus/gracilis (2xST/Gr) grafts tensioned at 80N, 40N, or 20N. The average side-to-side difference in anterior laxity in the 80N group was significantly less than that in the 20N or 40N group. There were no complications, all patients regained maximum extension, and no difference in subjective outcomes were noted. Kim et al30 randomly allocated 48 patients to three groups in which three different tensions—8 kg (78N), 12 kg (117N), or 15 kg (147N)—were applied to a penta-semitendinosus graft. Postoperatively, the average side-to-side differences in anterior laxity were 1.3, 2.1, and 2.4 mm, respectively, at a minimum of 1 year. There were no significant differences among the groups in subjective clinical results, anterior laxity, or knee extensor strength.
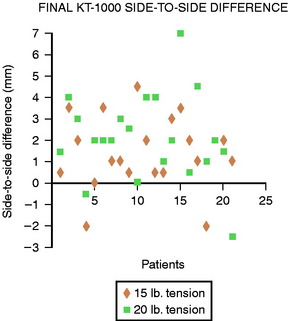
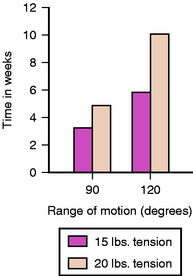
As outlined earlier, overcorrecting the joint can lead to deleterious effects on kinematics of the graft and loss of motion. Furthermore, the increase in cartilage compression that occurs with overconstraint and loss of knee motion may lead to a greater degree of degeneration over time. Vachtsevanos et al31 looked at the relationship of soft tissue tensioning and return of postoperative range of motion. They conducted a prospective randomized study using 61 patients reconstructed with autogenous 2xST/Gr. Patients were divided into two groups (15 pounds [68N] and 20 pounds [88N]) and followed in the postoperative period for an average of 15 months. The average side-to-side difference in anterior laxity was 1.7 ± 1.5 mm (68N) and 2.8 ± 2.0 mm (88N), which was significant (Fig. 54-3). Additionally, they found a significant delay in regaining extension in the 88N group (Fig. 54-4). Based on the evidence presented, it is reasonable to assume that an initial tension of 70N in soft tissue grafts provides consistent return of motion and clinical stability and that substantially greater tension does not improve results.
Stress Relaxation, Preconditioning, and Pretensioning
Due to the viscoelastic characteristic of graft tissue, the initial tension of ACL grafts is finite in nature. Beynnon et al32 reported that initial tension applied to the BTB graft at the time of fixation immediately decreased by creep elongation. Additionally, the percentage of stress relaxation is independent of the peak load applied to the graft.33 The data are similar for soft tissue grafts. Höher et al34 demonstrated a loss of 45% of graft tension in quadrupled hamstring grafts within 30 minutes due to stress relaxation. In vitro studies have been performed to determine the influence of cyclical loading on graft tension following ACL reconstruction. For studies performed with hamstring tendon grafts, bovine extensor tendon grafts, and patellar tendon grafts, cyclical loading reduced graft tension by 50% or more.28,35,36 Numazaki et al37 in a porcine ex vivo study fixed patellar tendon grafts with initial tensions of 20N, 80N, and 140N. A cyclical force–relaxation test was performed for 5000 cycles until the graft was stretched by 2 mm. The average peak load values after cycling were 105N, 157N, and 205N, respectively. Considering the in situ force of the ACL at full extension (16–87N),8 the authors concluded there was no benefit in tensioning the patellar tendon graft greater than 20N. For soft tissue grafts under the same initial loads, the average peak load values after cycling were 27N, 41N, and 39N. Increasing initial tension from 20 to 80N presented a significant increase in peak load after cycling; however, tensioning to 140N did not confer any benefit.
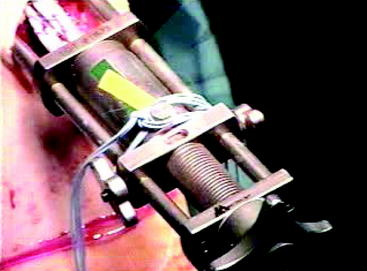
Attempts have been made to minimize graft viscoelasticity after reconstruction; pretensioning and preconditioning of the soft tissue grafts before final fixation have been recommended. To review, pretensioning refers to any loading of the graft that is performed before the graft is pulled into the knee (graft preparation board) (Fig. 54-5). Preconditioning refers to loading of the graft that is performed once the graft has been fixed in one of the tunnels (usually the femoral tunnel). Preconditioning may be further divided into cyclical and isometric preconditioning. The former denotes a load applied in multiple consecutive bouts (cycles), and the latter denotes a constant load applied on the graft before the final fixation of the graft.

Fig. 54-5 Example of pretensioning doubled semitendinosus/gracilis graft using Acufex Graftmaster board.
Despite the common use of pretensioning in graft preparation, there is little clinical evidence to support its effectiveness. Howard et al38 noted a 10% increase in length of patellar tendon grafts after applying an 89N load for 15 minutes. They concluded that without pretensioning, significant postimplantation graft creep will occur. Several pretensioning techniques have been developed for soft tissue grafts. Tension of 15 to 20 pounds (44–88N) for 10 to 20 minutes has been suggested for pretensioning hamstring tendon grafts. The initially set tension gradually decreases over time as the graft is stretched and the sutures are tightened around the tendon bundle. Accordingly, it would be misleading to claim that the graft was tensioned with a constant load during the entire time. However, it is reasonable to assume that pretensioning does eliminate some creep within the suture–graft interface and allow the surgeon to provide a more consistent initial tension, and it certainly improves the ease of graft preparation. There is no obvious utility in pretensioning BTB grafts.
More sophisticated studies looking at the effectiveness of preconditioning over time are inconclusive. Nurmi et al39 tested the effectiveness of preconditioning by assigning anterior tibialis grafts to three groups: control, cyclical preconditioned, and isometric preconditioned. The residual graft tension was then recorded immediately after the application of an initial graft tension of 80N and fixation into tibia with an interference screw. Immediately after screw insertion, the residual (AT) graft tensions were 79N ± 19N, 100N ± 17N, and 102N ± 15N, respectively. However, after 1 hour, a 60% decrease occurred in the initially set tension. Ejerhed et al40 prospectively randomized 53 patients undergoing patellar tendon autograft into two groups. One group of patients underwent isometric preconditioning by passive stretching at a constant load of 39N for 10 minutes immediately prior to implantation. The other group underwent no preconditioning before the implantation of the graft. At an average of 25 months, no significant objective or subjective differences were noted. To date, there is no clinical evidence proving that pretensioning or preconditioning of grafts will overcome the viscoelastic nature of graft tissue and improve clinical outcomes. However, cyclical preconditioning does allow the surgeon to identify the force–flexion curve of a specific graft before final fixation.
Knee Fixation Angle
When considering the ideal knee flexion angle at fixation, certain assumptions must be made. Namely, the femoral and tibial tunnels are placed near the native ACL origin and insertion. Excessively anterior placement of the femoral tunnel causes significant increases in ACL tension in flexion and laxity near extension. We know from kinematic studies that the forces generated in the graft as well as in the intact ACL are greatest at full extension and minimal between 30 and 60 degrees of knee flexion.41 Cadaveric studies by Bylski-Austrow et al5 concluded that a greater increase in force (50–115N) and greater posterior shifts in tibial position were produced by changing the flexion angle at tensioning from 0 to 30 degrees, rather than doubling the initial tension from 20 to 40N (15–35N). Gertel et al42 measured ACL graft force using BTB in cadaveric knees and concluded that graft forces are greater when the initial tension was applied at 30 degrees of flexion rather than at 0 degrees. This has led most surgeons to fix their grafts between 10 and 30 degrees of flexion.25
Asahina et al43 conducted one of the few clinical studies examining the importance of knee flexion angle at graft fixation. Using the modified Macintosh technique, 19 patients were fixed with the knees at full extension, whereas 25 patients were fixed at 30 degrees of flexion. A 70N force measured with a spring balance was used for both groups. At an average of 38 months, the range of motion in the extension group was significantly better. However, the stability of the knees and arthroscopic appearance of the grafts were significantly worse. This led the authors to suggest that graft fixation at 30 degrees of flexion is more effective in restoring stability and sustaining graft viability.
Tensioning Devices and Strategies
As mentioned previously, every graft produces its own individual force–flexion curve based primarily on the position of tunnel placement. After the graft is fixed in the femoral tunnel, the knee should be taken through a range of motion with tension on the tibial end of the graft (cyclical preconditioning). Based on graft length changes, which are assessed by motion of the graft within the tunnel, the point where the graft will be slack and where graft tension increases should be identified. Ideally, an isometric placed graft will show no significant motion and could be tensioned at any point in the flexion arc.44 Clinically, 2 to 3 mm of elongation is acceptable as long as the pattern of deviation throughout the range of motion reproduces that of the native ACL. Namely, in well-placed tunnels, this inflexion point occurs near extension as the graft begins to shorten in the tunnel.
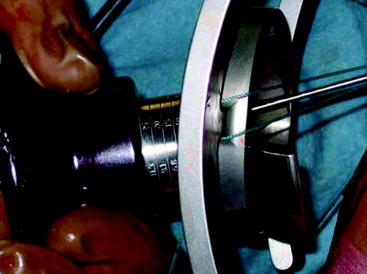
Cunningham et al45 studied the results of 13 orthopaedic sports medicine physicians who were asked to tension a soft tissue graft as they would in surgery and then maximally. They found that graft tensioning was highly variable and questioned whether tension should be more accurately measured and controlled intraoperatively. Commercially available ACL tensioning devices can be applied to the tibial end of the graft (Figs. 54-6 and 54-7). These allow the surgeon to dial in the set amount of tension, eliminating the need for an assistant. To date, no clinical studies have identified more consistent or improved outcomes using these devices.
1 Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg. 1963;45A:925-932.
2 Melby AIII, Noble JS, Askew MJ, et al. The effects of graft tensioning on the laxity and kinematics of the anterior cruciate ligament reconstructed knee. Arthroscopy. 1991;7:257-266.
3 Fleming B, Beynnon B, Howe J, et al. Effect of tension and placement of a prosthetic anterior cruciate ligament on the anteroposterior laxity of the knee. J Orthop Res. 1992;10:177-186.
4 Burks RT, Leland R. Determination of graft tension before fixation in anterior cruciate ligament reconstruction. Arthroscopy. 1988;4:260-266.
5 Bylski-Austrow DI, Grood ES, Hefzy MS, et al. Anterior cruciate ligament replacements: a mechanical study of femoral attachment location, flexion angle at tensioning, and initial tension. J Orthop Res. 1990;8:522-531.
6 Lewis JL, Lew WD, Hill JA, et al. Knee joint motion and ligament forces before and after ACL reconstruction. J Biomech Eng. 1989;111:97-106.
7 Andersen HN, Jorgensen U. The immediate postoperative kinematic state after anterior cruciate ligament reconstruction with increasing preoperative tension. Knee Surg Sports Traumatol Arthrosc. 1998;6:S62-S69.
8 Markolf KL, Gorek JF, Kabo JM, et al. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg. 1990;72A:557-567.
9 Wascher DC, Markolf KL, Shapiro MS, et al. Direct in vitro measurement of forces in the cruciate ligaments. Part I: the effect of multiplane loading in the intact knee. J Bone Joint Surg. 1993;75A:377-386.
10 Paulos L, Noyes FR, Grood E, et al. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. Am J Sports Med. 1981;9:140-149.
11 Arms SW, Pope MH, Johnson RJ, et al. The biomechanics of anterior cruciate ligament rehabilitation and reconstruction. Am J Sports Med. 1984;12:8-18.
12 Beynnon BD, Johnson RJ, Fleming BC, et al. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension. A comparison of an open and a closed kinetic chain exercise. Am J Sports Med. 1997;25:823-829.
13 Sakane M, Fox RJ, Woo SL, et al. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15:285-293.
14 Woo SL, Kanamori A, Zeminski J, et al. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon. A cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg. 2002;84A:907-914.
15 Frank C, Amiel D, Akeson WH. Healing of the medial collateral ligament of the knee. A morphological and biochemical assessment in rabbits. Acta Orthop Scand. 1983;54:917-923.
16 Yoshiya S, Andrish JT, Manley MT, et al. Graft tension in anterior cruciate ligament reconstruction. An in vivo study in dogs. Am J Sports Med. 1987;15:464-470.
17 Jackson DW, Grood ES, Wilcox P, et al. The effects of processing techniques on the mechanical properties of bone-anterior cruciate ligament-bone allografts. An experimental study in goats. Am J Sports Med. 1988;16:101-105.
18 Jackson DW, Grood ES, Cohn BT, et al. The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg. 1991;73A:201-213.
19 Katsuragi R, Yasuda K, Tsujino J, et al. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47-56.
20 Mikami S, Yasuda K, Katsuragi R, et al. Reduction of initial tension in the in situ frozen anterior cruciate ligament. Clin Orthop Relat Res. 2004;9:207-213.
21 Fleming BC, Abate JA, Peura GD, et al. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841-844.
22 Pena E, Martinez MA, Calvo B, et al. A finite element simulation of the effect of graft stiffness and graft tensioning in ACL reconstruction. Clin Biomech (Bristol, Avon). 2005;20:636-644.
23 van Kampen A, Wymenga AB, van der Heide HJ, et al. The effect of different graft tensioning in anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy. 1998;14:845-850.
24 Yoshiya S, Kurosaka M, Ouchi K, et al. Graft tension and knee stability after anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2002;394:154-160.
25 Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6:S2-S12.
26 Feller JA, Webster KE. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:564-573.
27 Freedman KB, D’Amato MJ, Nedeff DD, et al. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31:2-11.
28 Boylan D, Greis PE, West JR, et al. Effects of initial graft tension on knee stability after anterior cruciate ligament reconstruction using hamstring tendons: a cadaver study. Arthroscopy. 2003;19:700-705.
29 Yasuda K, Tsujino J, Tanabe Y, et al. Effects of initial graft tension on clinical outcome after anterior cruciate ligament reconstruction. Autogenous doubled hamstring tendons connected in series with polyester tapes. Am J Sports Med. 1997;25:99-106.
30 Kim SG, Kurosawa H, Sakuraba K, et al. The effect of initial graft tension on postoperative clinical outcome in anterior cruciate ligament reconstruction with semitendinosus tendon. Arch Orthop Trauma Surg. 2006;126:260-264.
31 Vachtsevanos JG, Lamberson KA, Paulos LE. Anterior cruciate graft tensioning. Tech Knee Surg. 2003;21:125-136.
32 Beynnon BD, Johnson RJ, Fleming BC, et al. The measurement of elongation of anterior cruciate-ligament grafts in vivo. J Bone Joint Surg. 1994;76A:520-531.
33 Johnson GA, Tramaglini DM, Levine RE, et al. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12:796-803.
34 Höher J, Scheffler SU, Withrow JD, et al. Mechanical behavior of two hamstring graft constructs for reconstruction of the anterior cruciate ligament. J Orthop Res. 2000;18:456-461.
35 Arnold MP, Lie DT, Verdonschot N, et al. The remains of anterior cruciate ligament graft tension after cyclic knee motion. Am J Sports Med. 2005;33:536-542.
36 Grover DM, Howell SM, Hull ML. Early tension loss in an anterior cruciate ligament graft. A cadaver study of four tibial fixation devices. J Bone Joint Surg. 2005;87A:381-390.
37 Numazaki H, Tohyama H, Nakano H, et al. The effect of initial graft tension in anterior cruciate ligament reconstruction on the mechanical behaviors of the femur-graft-tibia complex during cyclic loading. Am J Sports Med. 2002;30:800-805.
38 Howard ME, Cawley PW, Losse GM, et al. Bone-patellar tendon-bone grafts for anterior cruciate ligament reconstruction: the effects of graft pretensioning. Arthroscopy. 1996;12:287-292.
39 Nurmi JT, Kannus P, Sievanen H, et al. Interference screw fixation of soft tissue grafts in anterior cruciate ligament reconstruction: part 2: effect of preconditioning on graft tension during and after screw insertion. Am J Sports Med. 2004;32:418-424.
40 Ejerhed L, Kartus J, Kohler K, et al. Preconditioning patellar tendon autografts in arthroscopic anterior cruciate ligament reconstruction: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 2001;9:6-11.
41 Fleming B, Beynnon BD, Johnson RJ, et al. Isometric versus tension measurements. A comparison for the reconstruction of the anterior cruciate ligament. Am J Sports Med. 1993;21:82-88.
42 Gertel TH, Lew WD, Lewis JL, et al. Effect of anterior cruciate ligament graft tensioning direction, magnitude, and flexion angle on knee biomechanics. Am J Sports Med. 1993;21:572-581.
43 Asahina S, Muneta T, Ishibashi T, et al. Effects of knee flexion angle at graft fixation on the outcome of anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:70-75.
44 Arnold MP, Verdonschot N, van Kampen A. The normal anterior cruciate ligament as a model for tensioning strategies in anterior cruciate ligament grafts. Am J Sports Med. 2005;33:277-283.
45 Cunningham R, West JR, Greis PE, et al. A survey of the tension applied to a doubled hamstring tendon graft for reconstruction of the anterior cruciate ligament. Arthroscopy. 2002;18:983-988.