Chapter 99 Techniques of liver replacement
Historical Overview
The steps by which liver replacement became the treatment of choice for numerous end-stage liver diseases (Starzl et al, 1989) were summarized in 2002 (Starzl). The basic operation was developed in dogs during the years 1958 through 1960 and was attempted clinically in 1963 under azathioprine-prednisone immunosuppression. The first humans to have liver replacement with prolonged survivals were reported by Starzl in 1969; however, not until the availability of cyclosporine in the 1980s did orthotopic liver transplantation became accepted worldwide as effective therapy. The results improved again with the advent of tacrolimus in the 1990s (see Chapter 96).
Elements other than immunosuppression have contributed to the success of liver replacement, including improved patient selection and pretransplantation management, noninvasive diagnostic techniques, new antibiotics, and advances in anesthetic and perioperative critical care (see Chapters 97A and 97B); however, perfection of the donor and recipient operations was the crucial factor on which all else ultimately depended. Surgical techniques used at the University of Colorado—and since January 1981, at the University of Pittsburgh—are presented in this chapter, with an emphasis on principles rather than details.
Donor Operation
The use of multiple organs from a single cadaveric donor became practical with the development of standard procurement methods in the early 1980s (see Chapter 98A). Subsequently, the University of Wisconsin (UW) and histidine-tryptophan-ketoglutarate (HTK) preservation solutions made storage of hepatic grafts relatively safe for 12 to 18 hours. The availability of this much time has allowed widespread sharing of livers while permitting an accurate assessment of the grafts by histologic and metabolic criteria.
Standard Liver Procurement
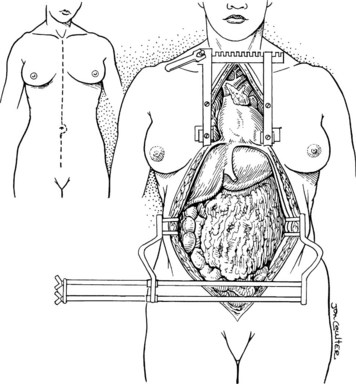
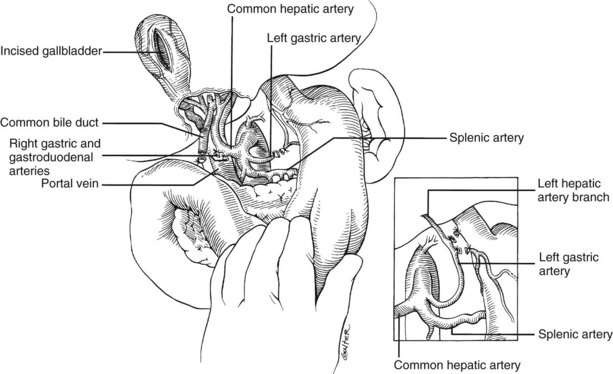
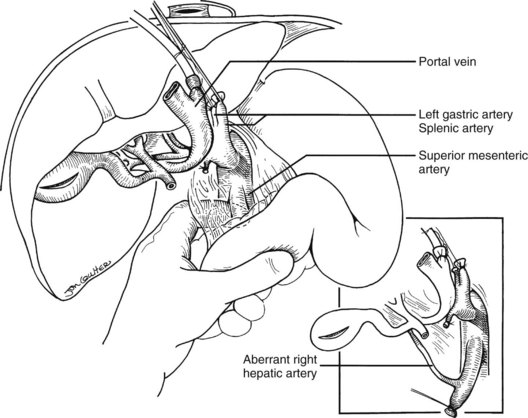
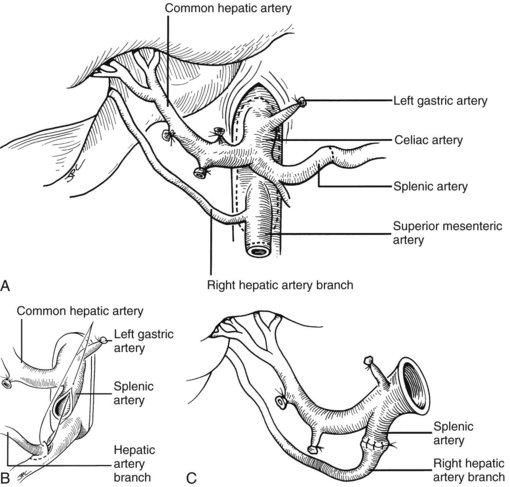
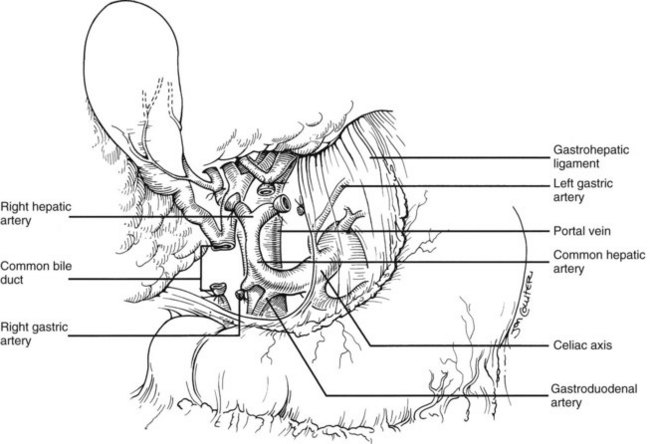
In the standard procurement technique, a midline incision is made from the suprasternal notch to the pubis to expose the abdominal and thoracic organs of potential interest (Fig. 99.1). After verification that the liver has a normal consistency and color, the left suspensory ligament is incised, allowing the left lobe to be retracted anteriorly and to the right. This retraction exposes the upper part of the gastrohepatic ligament, which contains the left gastric artery, the smallest branch of the celiac axis, and the arterial supply of the liver (Fig. 99.2). If an anomalous left hepatic arterial branch originates from the left gastric artery (Fig. 99.3), it must be preserved in continuity with the main left gastric artery (see Fig. 99.3, inset). When present, this anomalous left hepatic arterial branch is nearly always present just posterior to the vagus nerve branch, as it courses from the lesser curvature of the stomach through the gastrohepatic ligament to the liver.
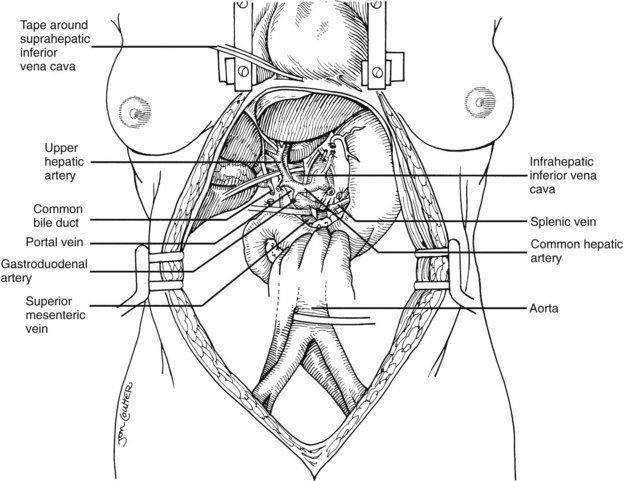
The largest branch of the celiac axis usually is the common hepatic artery. The right gastric and gastroduodenal arteries are ligated and divided (Fig. 99.4; see also 99.2). If the left gastric, right gastric, and gastroduodenal arteries are ligated in that order, the subsequent dissection of the common duct and the portal vein is rendered relatively bloodless. The common bile duct is transected near the duodenum, and the gallbladder is incised, permitting the bile to be irrigated out with saline (see Fig. 99.3); this avoids autolysis of the extrahepatic and intrahepatic bile duct epithelium during storage. The portal vein now is dissected inferiorly to the confluence of the splenic and superior mesenteric veins (see Fig. 99.4).
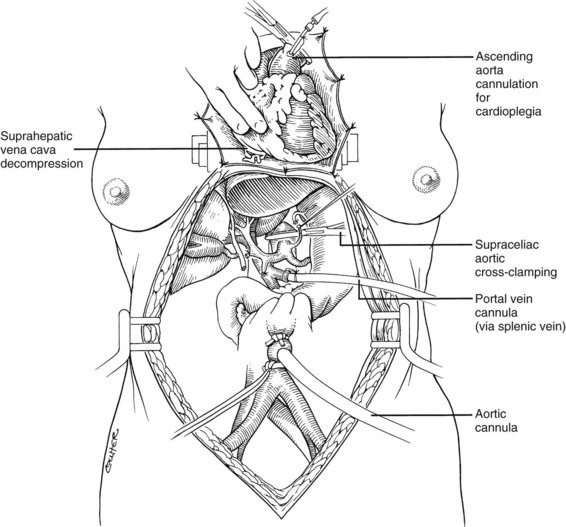
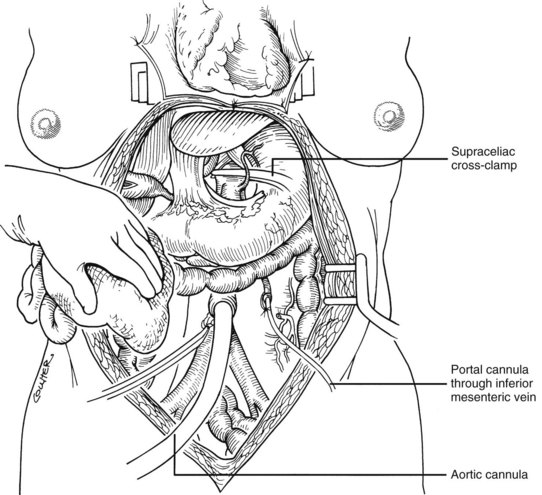
After completing the hilar dissection, the aorta is encircled superiorly, where it passes through the diaphragm, and inferiorly, just proximal to its distal bifurcation. Cannulae for infusion are placed into the inferior mesenteric vein (or splenic vein) and, after total body heparinization, into the distal aorta (see Fig. 99.4). When all procurement teams are ready, the aorta is cross-clamped at the diaphragm or in the chest by the abdominal surgeon (see Fig. 99.4), while the thoracic surgical team clamps the ascending aorta. Moderately rapid infusion of cold preservation solution is started into the portal circulation and aortic cannula. At the same time, a cardioplegia solution is infused into the midportion of the ascending aorta. Congestion of the various organs is prevented by an incision in the suprahepatic inferior vena cava at the level of the right atrium, which allows the blood and infusate to drain into the pericardium (see Fig. 99.4).
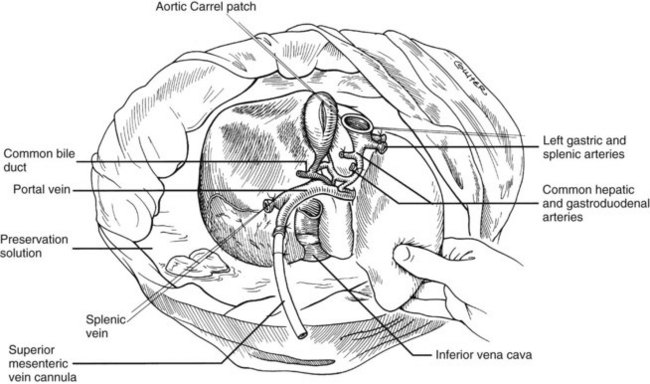
In adults, the liver is usually perfused with 2 L of HTK infused through the splenic vein or inferior mesenteric vein and 10 L infused through the aorta, although smaller volumes are used for children. When the liver becomes cold and blanched, and the heart has been removed, the total hepatectomy is completed. The remaining dissection must be performed expeditiously but methodically. If the celiac axis is retained with the graft, a proximal segment of its splenic arterial branch also should be conserved for potential reconstruction of an anomalous hepatic artery (see later). The most common hepatic artery anomaly is an aberrant right hepatic artery that originates from the superior mesenteric artery, commonly found posterior to the portal vein (Fig. 99.5; see Chapter 1B). If the pancreas is to be discarded, the anomalous retroportal artery can be kept in continuity with the superior mesenteric artery (see Fig. 99.5, inset), where its origin can be incorporated into a Carrel patch that is shared with the origin of the celiac axis.
The liver now remains attached primarily by the vena cava above and below the liver. The vena cava below the liver is transected above the entry of the left and right renal veins (Fig. 99.6). The vena cava above the liver is transected with a surrounding rim of diaphragm that is carefully excised on the back table. The retrohepatic vena cava is dissected free, including ligation of the right adrenal vein and posterior lumbar tributaries. The liberated liver is immediately placed in a solution-filled preservation bag packed in ice (Fig. 99.7); some surgeons flush the common bile duct with HTK before packaging the liver.
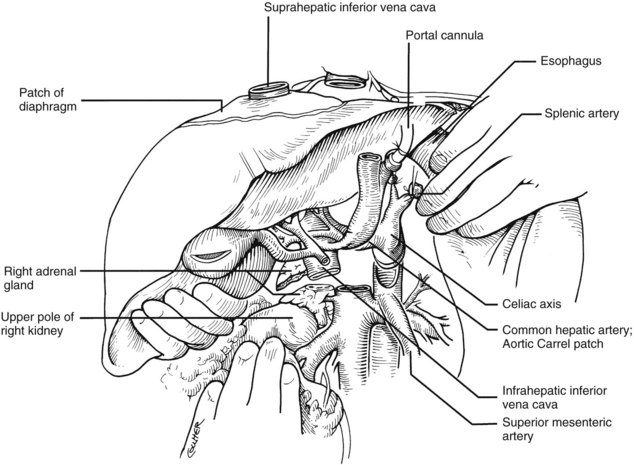
FIGURE 99.6 The suprahepatic vena cava has been transected with inclusion of a generous patch of diaphragm on the liver side. The infrahepatic vena cava is divided just above the origin of the renal veins, and the celiac axis is removed with a Carrel patch of anterior aorta. If an anomalous right hepatic artery originates from the superior mesenteric artery (SMA), the origin of the SMA may be included in the Carrel patch (see Fig. 99.10A).
Modified Donor Procedures
Rapid Procurement
Use of the standard technique in stable donors has allowed the training of relatively inexperienced surgeons in the performance of a donor hepatectomy. When the technique is mastered, faster methods can be applied electively or, if required, by urgent clinical circumstances. With the rapid techniques, little or no preliminary dissection is done except for encirclement of the supraceliac aorta and cannulation of the inferior mesenteric vein and terminal aorta (Fig. 99.8). If the heart is to be removed, the cardiac surgeon proceeds as if other organs are not to be harvested but gives warning before the circulation is stopped.
At the moment heart function ceases, the abdominal aorta is cross-clamped above or just below the diaphragm, and an infusion of cold HTK solution is started in the inferior mesenteric vein and distal aorta (see Fig. 99.8). The amount of preservation fluid with the rapid technique is approximately the same as that used for the standard method (i.e., 2 L into the inferior mesenteric vein (IMV)/splenic vein and 10 L through the aorta). When the liver becomes cold, the infusions are slowed. In the now bloodless field, the main vessels of the celiac axis can be quickly dissected, and the hilar dissection can be completed in a matter of minutes.
Super-Rapid Procurement
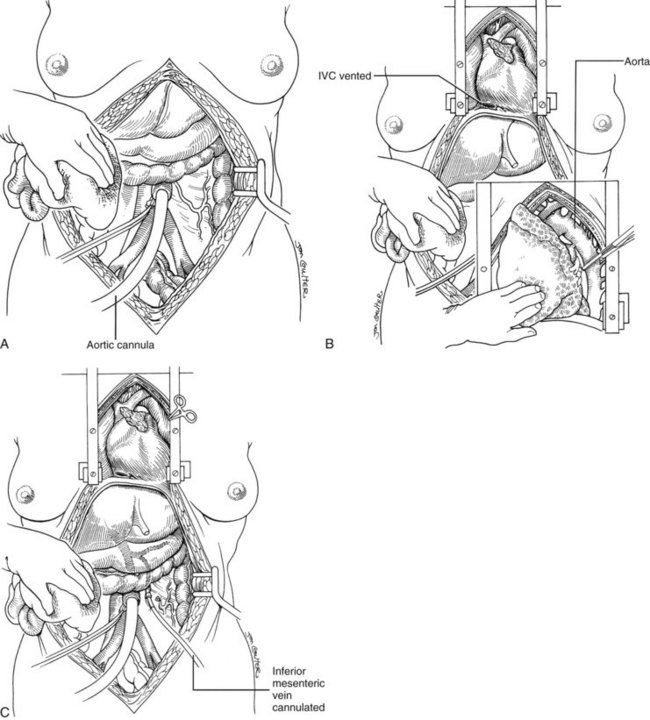
In arrested or non–heart-beating donors, an even quicker procedure can be used to procure satisfactory organs. This method also can be applied in countries that do not have “brain death” laws or under special legal or religious circumstances. Here, cooling requires urgent cannulation and cold fluid infusion into the distal aorta (Fig. 99.9A). Sternum splitting, thoracic aortic cross-clamping, and severance of the suprahepatic inferior vena cava for venous decompression are performed (Fig. 99.9B), deferring cannulation and perfusion of the portal venous system until after the various organs are at least partly cooled intraarterially (Fig. 99.9C). The various dissections are done in the same way as with the standard and rapid techniques. Effective application of this method requires an extremely high level of skill.
Back-Table Surgery
No matter which procurement method has been used, further preparation of the liver is performed on a separate back table before delivering the graft to the recipient surgeon. The liver should be kept cold by submerging it in a basin containing ice-cold preservation solution surrounded by a bag containing sterile ice slush (see Fig. 99.7). Back-table preparation includes the following:
1 Dissection and removal of extraneous tissue, such as diaphragm, adrenal gland, lymph node, pancreatic, peripancreatic, and ganglionic tissue
2 Preparation of cuffs of the suprahepatic and infrahepatic vena cava, cleaning of the portal vein and hepatic artery, and inspection of the bile duct
3 Verification of secure ligatures on small retrohepatic caval, portal vein, and hepatic arterial branches
4 Ensuring the continuity and integrity of all major structures that must be anastomosed to the companion recipient structures
Failure to have the graft completely ready for implantation when it is brought to the recipient operative field can result in irreversible damage to the graft or may make it impossible to complete the recipient operation. Several methods of back-table vascular reconstruction have been designed to repair technical accidents or to accommodate aberrant vessels or congenital anomalies. A common reason for back-table reconstruction is the presence of an anomalous right hepatic artery originating from the superior mesenteric artery (Fig. 99.10).
Recipient Operation
Abdominal Incision and Exposure
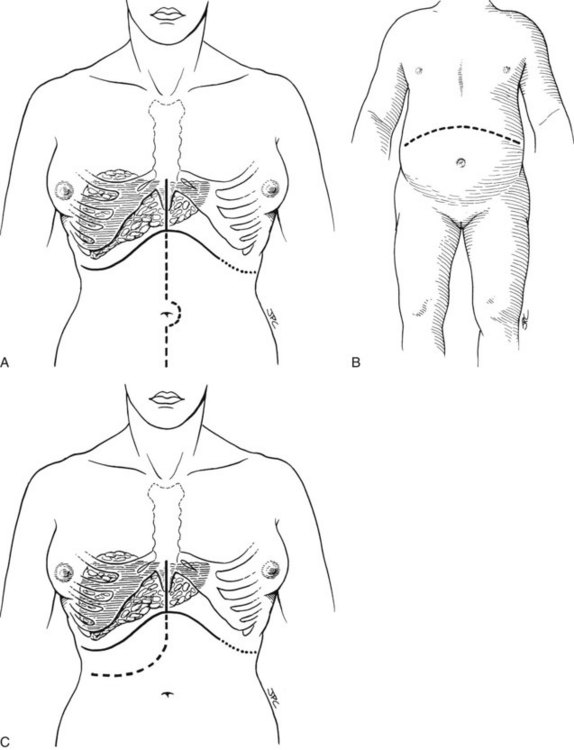
The exact location of the incision may be influenced by previous right upper quadrant surgery, the presence of an ileostomy, the size and configuration of the liver, or other factors. A bilateral subcostal incision is the most commonly used, extending on the right to just beyond the midaxillary line and on the left to the lateral edge of the rectus, with an upper midline extension and excision of the xiphoid process (Fig. 99.11A). In unusual circumstances, a lower midline extension may also be needed, especially if exposure of the distal aorta is required for reconstruction of the hepatic arterial supply. Thoracic extensions are rarely needed.
A bilateral subcostal incision (Fig. 99.11B) or a right subcostal incision with or without an upper midline extension (Fig. 99.11C) may also be used. The upper midline extension, shown in Figure 99.11C, is usually unnecessary in pediatric patients. Massive hepatomegaly, extensive prior abdominal surgery, or other factors may mandate the selection of a more extensive incision (see Fig. 99.11). In patients who require concomitant splenectomy or interruption of a prior splenorenal shunt, the incision may need to be extended to the left subcostal region.
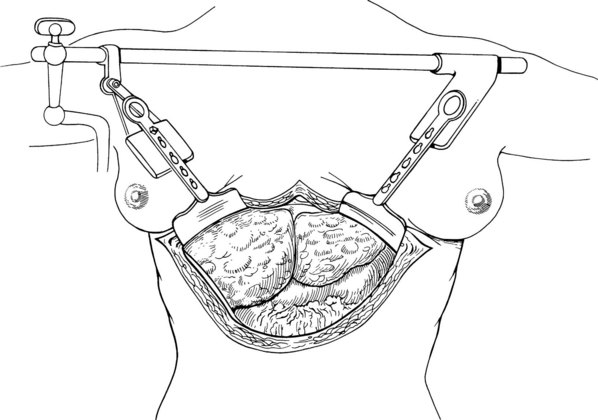
Improved exposure with any of these incisions can be obtained with use of the Bookwalter, Thompson, or another retractor that permits access to the hepatic veins and suprahepatic vena cava (Fig. 99.12). In making the incision, it may be necessary to abandon delicate celiotomy techniques of meticulous hemostasis and resort to continuous hemostatic suturing along the cut edges of the fascia and preperitoneum or to the use of hemostatic devices such as the Salient Surgical Technologies (Portsmouth, NH) Monopolar sealer. When the abdomen is entered, an effort must be made to find a plane of dissection just outside the liver capsule. Movement away from this plane risks an encounter with major venous collaterals, which can result in disastrous hemorrhage early in the operation.
Intraoperative Determination of Surgical Strategy
Venovenous Bypass
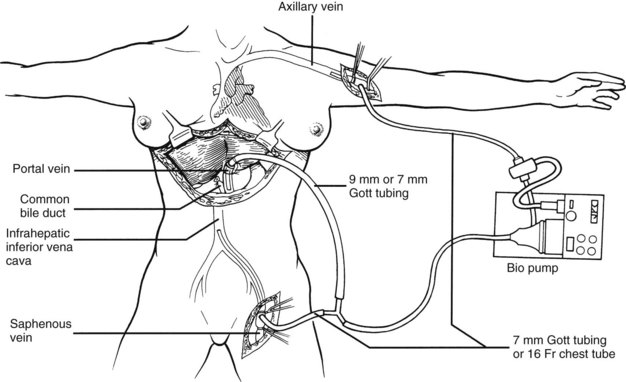
The most critical stage of the recipient operation is the anhepatic phase, during which the diseased liver is removed and replaced with the allograft. Obstruction of the portal vein and vena cava during this period results in splanchnic and lower body systemic venous hypertension. This situation can have devastating consequences in some patients. During the early 1980s, a pump-driven venovenous bypass, used without recipient heparinization, was developed in Pittsburgh to allow splanchnic and systemic blood to return to the heart by way of an inflow cannula placed in the axillary vein (Fig. 99.13). This technique permitted the hepatectomy and implantation to be done with significant reductions in blood loss, intestinal edema, and postoperative renal failure.
Hilar Dissection
In “easy” cases, the individual hilar structures can be readily skeletonized. The hepatic artery and common duct are ligated as close to the liver as possible, and the hepatic artery is dissected proximal to the origin of its gastroduodenal branch, facilitating exposure of the proximal portal vein (Fig. 99.14). If needed, venovenous bypass can be initiated with cannulation of the transected portal vein at this time or later.
Host Hepatectomy with or Without Vena Cava Removal
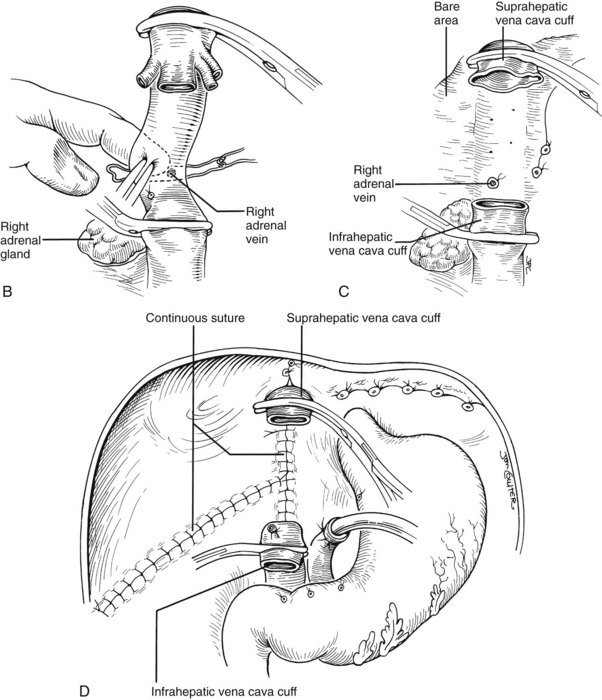
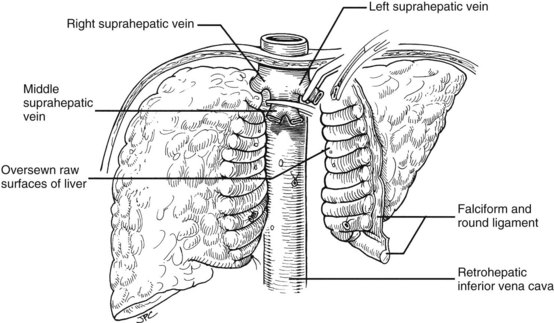
If the hilar dissection has been accomplished uneventfully, the liver is devascularized and now can be excluded from the circulation by cross-clamping the vena cava above and below the liver (Fig. 99.15A). The diseased organ with or without a segment of retrohepatic vena cava can be “peeled” out, working from the hilum up or from the diaphragm down. If the vena cava is part of the specimen, the obligatory ligation of the right adrenal vein (Fig. 99.15C) imposes a risk of adrenal infarction. If this occurs with venous hypertension and bleeding, the right adrenal gland should be removed immediately. Other systemic venous tributaries to the vena cava segment from the lumbar regions also must be scrupulously ligated. Because of regional venous hypertension in the right-sided bare area, it may be necessary then or later to obtain hemostasis by closing or oversewing the edges of the exposed bare areas with a continuous suture or by the use of such devices as the LigaSure (Covidien, Mansfield, MA) or the Monopolar or Bipolar sealer (Fig. 99.15D).
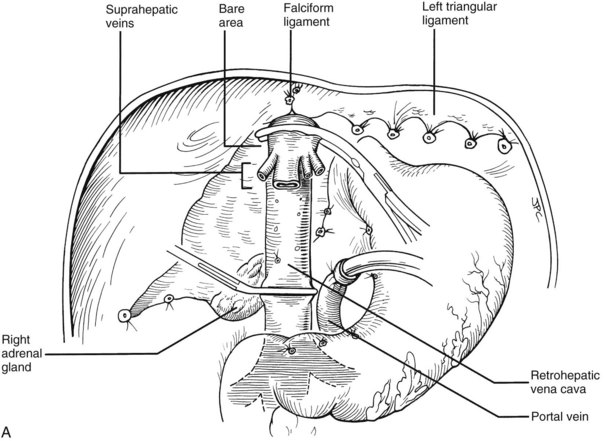
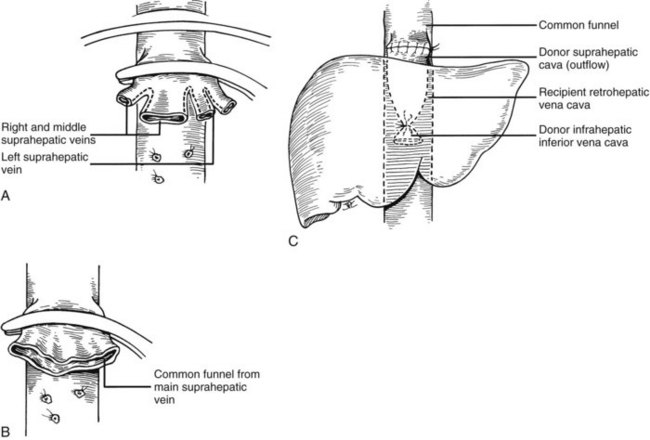
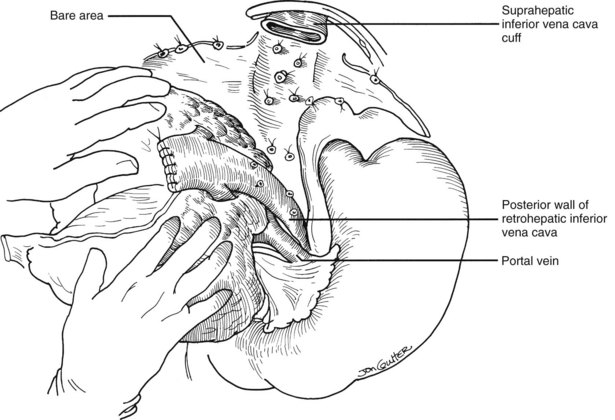
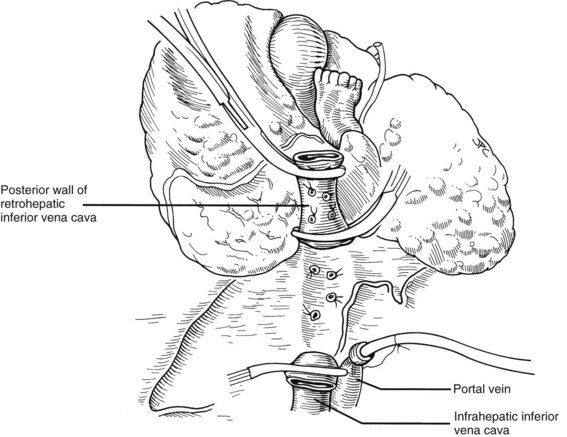
Many of these problems can be circumvented if the host retrohepatic vena cava can be conserved (see Fig. 99.15A). Separation of the diseased liver from the vena cava is done in the same way as in the classic experimental procedure of total canine hepatectomy. With this kind of hepatectomy, stumps of one or more of the main host hepatic veins are retained (Fig. 99.16A) for eventual receipt of the allograft’s venous outflow (Fig. 99.16B and C); this is known as the piggyback method of liver transplantation. To the extent vena cava flow can be maintained during the sometimes tedious and difficult denudation of the retrohepatic vena cava, the need for venovenous bypass is potentially eliminated, and the extent of retroperitoneal dissection is considerably reduced. Finally, the risk of right adrenal infarction is eliminated, unless the right adrenal vein is injured (see Fig. 99.15B).
Alternative Approaches to Hepatectomy
In many if not most cases, hepatectomy with or without inclusion of the retrohepatic vena cava is uncomplicated; however, dissection of the liver hilum sometimes is difficult or impossible because of scarring or the presence of varices. In these situations, the suprahepatic vena cava can be approached first. After transecting the suprahepatic vena cava, removal of the liver can be done from the top down, approaching the hilar structures from behind the liver (Fig. 99.17). If it is not possible to occlude the hepatic veins on the liver side, bleeding can be minimized by placing the fingers into the hepatic veins and retrohepatic vena cava or by squeezing shut the venous outflow of the liver. Alternatively, the inferior vena cava below the liver can be used as a “handle” to extract the liver from bottom to top, with cross-clamping or transection of the hilar structures at the earliest possible opportunity (Fig. 99.18). Finally, if adhesions are present that block access to the upper and lower vena cava and to the portal triad, the liver can be split in a superior-inferior direction, exposing the anterior surface of the retrohepatic vena cava from inside the liver (Fig. 99.19). Once bleeding from the raw surfaces is controlled, the two hepatic halves are stripped away from the surrounding structures. All these hepatectomy variations are made easier by venovenous bypass.
Vascular Anastomoses
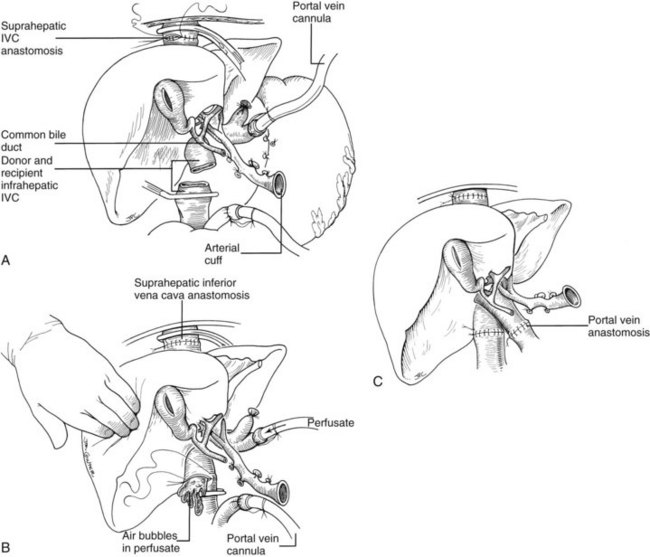
It is important to have the surgical field completely prepared for implantation before the new liver is brought from the back table. The first graft vessel to be anastomosed is always the segment of donor vena cava into which all the hepatic veins of the transplanted liver drain. If host hepatectomy has included removal of the retrohepatic vena cava, the anastomosis is an end-to-end, suprahepatic to suprahepatic vena cava connection at the diaphragm (Fig. 99.20A). With the piggyback operation, in which the host vena cava is conserved, the suprahepatic vena cava is emptied into a cuff of host hepatic veins (see Fig. 99.16) or by a side-to-side anastomosis between the two vena cava segments (not shown).
The order of the other vascular anastomoses may vary. With the caval-sparing piggyback operation, the infrahepatic vena cava of the graft is ligated or stapled (see Fig. 99.16). When the host caval segment is excised (standard technique), a common practice is to anastomose the infrahepatic vena cava (Fig. 99.20B), followed by removal of the recipient from the portal (but not systemic) bypass and subsequent portal vein anastomosis (Fig. 99.20C). These anastomoses may be done in reverse order (i.e., portal vein first). An experienced surgeon may prefer to perform the arterial anastomosis before the portal vein reconstruction or may complete all four anastomoses before unclamping. These decisions are influenced by the anatomic and physiologic circumstances in the individual case, including the efficiency with which the bypass system has functioned or the degree of venous hypertension when bypass is not used.
In all cases if the preservation solution used is UW solution, it is important before liver reperfusion to flush the allograft with Ringer’s lactate solution, although some surgeons prefer albumin or blood. Flushing is done through the cannula placed in the graft portal circulation at the time of procurement (see Fig. 99.20A), either on the back table before beginning implantation or in situ before beginning the portal vein anastomosis. After its passage through the microvasculature of the allograft, the infusate is vented from the vena cava (see Fig. 99.20B). The objectives are to remove air and to rid the graft of any high-potassium solutions (UW solution) used for organ preservation (see Fig. 99.20B). Failure to perform this flush can result in air embolism or hyperkalemic cardiac arrest or dysrhythmias, although this step is not necessary if HTK is used.
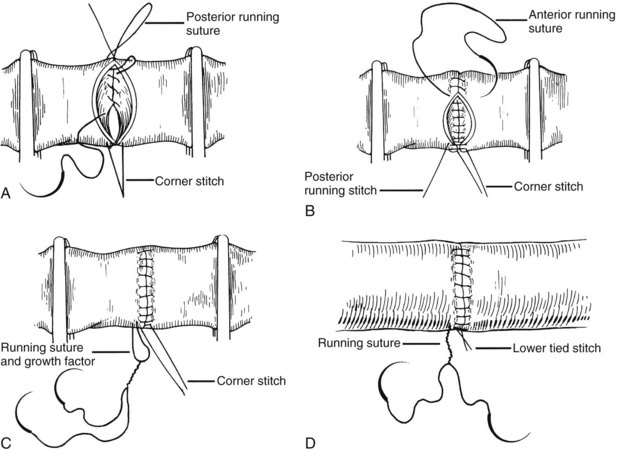
All of the venous vascular anastomoses are routinely performed with continuous suture. To avoid anastomotic strictures, particularly of the portal anastomosis, special techniques are developed that are made feasible by the ability of polypropylene (Prolene) suture to glide freely through tissue. A growth factor is left by tying the sutures at a considerable distance above the vessel wall. After flow is restored through the anastomosis, the excess polypropylene recedes back into the vessel and redistributes itself throughout the circumference of the suture line (Fig. 99.21). If leaks develop, these are readily controlled with additional single sutures.
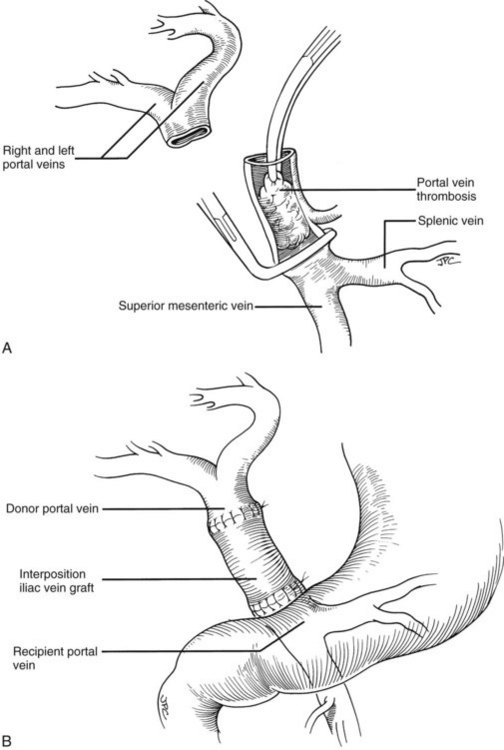
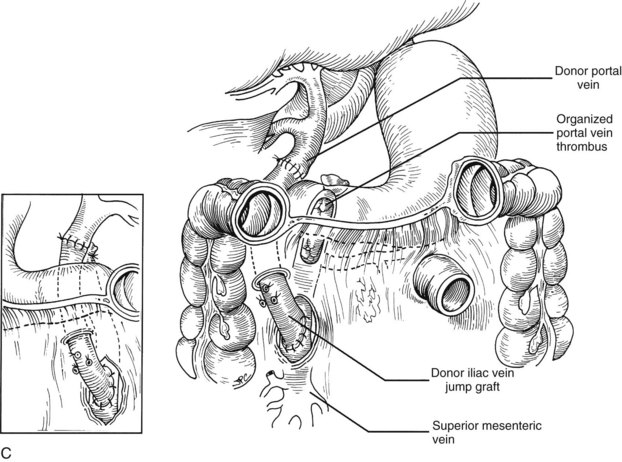
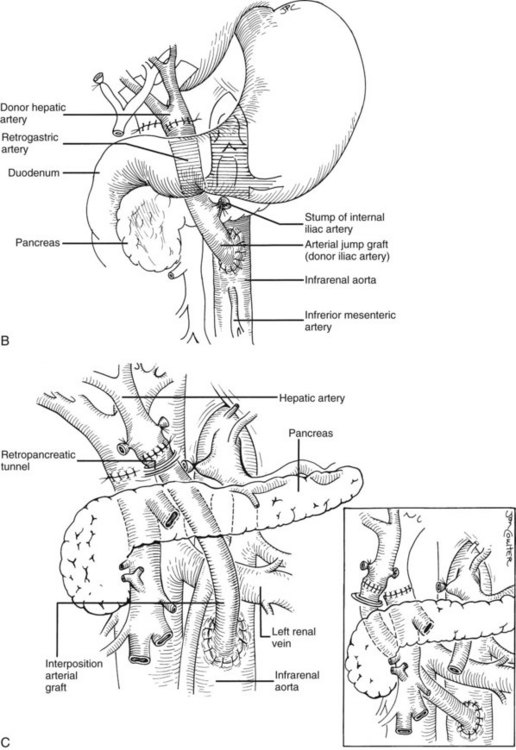
Rather than being whimsical, variations of the order and details of revascularization frequently are mandated by anatomic anomalies or by pathologic factors, including thrombosis of the portal vein that once contraindicated liver transplantation, until techniques were developed to deal with them. Declotting a thrombosed portal vein may be possible (Fig. 99.22A); if not, iliac or other veins from the donor may be used as interposition grafts (Fig. 99.22B) or as mesoportal jump grafts (Fig. 99.22C). A mesoportal graft may be anastomosed end-to-side to the superior mesenteric vein and tunneled through the transverse mesocolon in a relatively avascular plane anterior to the pancreas to reach the hepatic hilum for end-to-end anastomosis to the donor portal vein (see Fig. 99.22C).
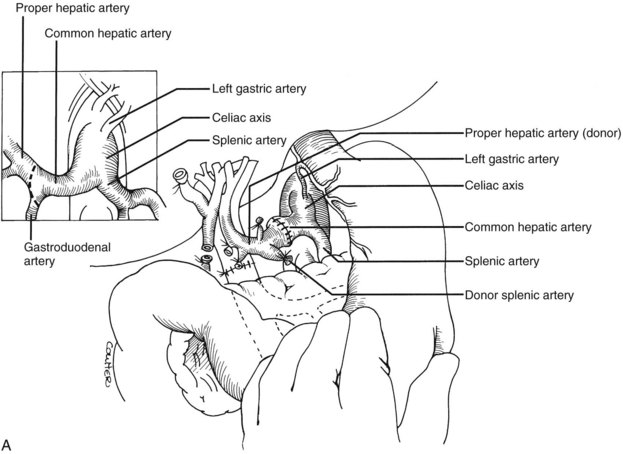
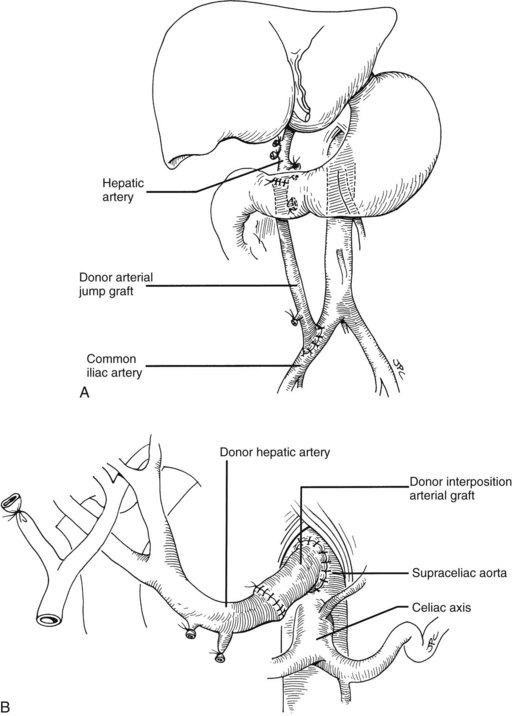
Numerous techniques also have been used to restore the hepatic arterial supply. The ideal reconstruction, when the allograft and recipient have normal arterial anatomy, is shown in Figure 99.23A. If anomalies, vascular injuries, or pathologic changes are present in the donor or recipient blood vessels, such as from radiation for cholangiocarcinoma, that preclude effective rearterialization, grafts obtained from the donor can be used (Fig. 99.24; see also Fig. 99.23B and C).
Biliary Tract Reconstruction
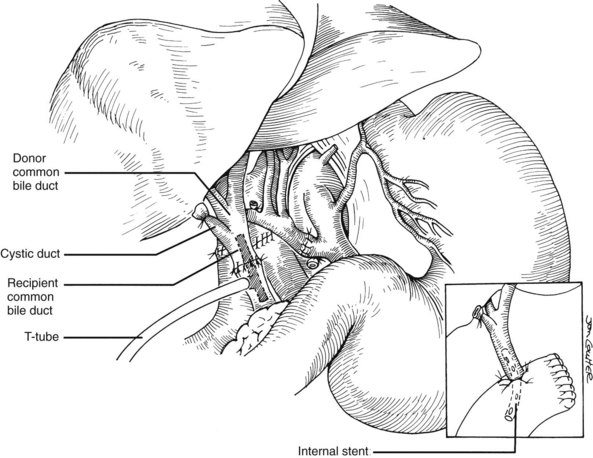
Good hemostasis must be achieved before the biliary reconstruction is performed. If the recipient duct is disease-free, and if there is a reasonable size match between the donor and recipient ducts, an end-to-end anastomosis is performed with or without a T-tube stent (Fig. 99.25). The anastomosis usually is performed with 8 to 10 interrupted absorbable sutures, such as 5-0 or 6-0 polyglycolic acid, or with a continuous suture, if the ducts are large enough. Because the integrity of the anastomosis depends primarily on an adequate blood supply of donor and recipient ducts, minimal dissection is performed in the periductal tissues. A small purse-string suture is usually placed around the T-tube exit site to prevent leakage, and the T-limb is brought out through a stab incision on the lateral side of the recipient duct.
If the recipient duct is diseased or otherwise inadequate for anastomosis, a choledochojejunostomy is performed. A 45-cm Roux-en-Y limb of proximal jejunum is brought, usually antecolic, to the hepatic hilum; the donor duct is then anastomosed end-to-side to the jejunal limb with a running or interrupted 5-0 or 6-0 absorbable polyglycolic acid suture with or without a stent (see Fig. 99.25, inset). When used, the stent is secured in place with a rapidly absorbed suture with the assumption that the stent will later pass spontaneously through the intestinal tract; however, occasionally the stent is retained and must be pushed into the bowel by an interventional radiologist, or it can be removed by push enteroscopy. Rarely does the stent require removal at laparotomy.
Reduced-Size Liver Transplantation
The scarcity of pediatric donors and the constraints of size matching often prohibit transplantation of a whole donor liver into a child or small adult (see Chapter 98C). Since 1980, the option of using a partial liver has been exercised at most large transplant centers with results equal to or approaching those achievable with whole liver transplantation. Partial liver transplantation, its application in live donors; and the use of divided cadaveric livers, which allow one organ to be used for two recipients, are described elsewhere in this text (see Chapters 98B and 98C).
Starzl TE. Experience in Hepatic Transplantation. Philadelphia: Saunders; 1969.
Starzl TE. The saga of liver replacement, with particular reference to the reciprocal influence of liver and kidney transplantation (1955-1967). J Am Coll Surg. 2002;195:587-610.
Starzl TE, et al. Medical progress: liver transplantation. N Engl J Med. 1989;321(part I):1014-1022. 321(part II):1092-1099