CHAPTER 74 TECHNIQUES IN THE MANAGEMENT OF COMPLEX MUSCULOSKELETAL INJURY: ROLES OF MUSCLE, MUSCULOCUTANEOUS, AND FASCIOCUTANEOUS FLAPS
Injuries involving skin and subcutaneous tissue loss require reconstructive solutions. In many cases, skin grafting alone may be sufficient. However, when skeletal fractures, tendons, viscera, or hardware is exposed, vascularized soft tissue coverage of the wound using muscle, musculocutaneous, and fasciocutaneous flaps is the preferred technique. The objective is to provide wound healing, optimal function, and the best possible aesthetic result.
DIAGNOSIS
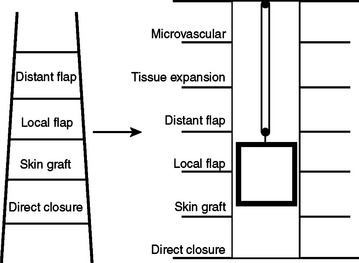
The concept of the “reconstructive ladder” recommends the use of the least invasive repair that will satisfactorily close a wound. The methods begin with secondary healing and progress through skin grafting to microvascular free-tissue transfer. This paradigm has been largely supplanted by the “reconstructive elevator” concept.1 With this approach, the surgeon evaluates the wound and decides on the best option, not necessarily the least complicated one (Figure 1).
ANATOMY
The most important anatomical aspect of muscle flaps is their vascularity. Because blood supply is usually the limiting factor in flap success, flaps are most often categorized by the vascular system on which they are based. McGregor proposed the concept of “random” and “axial” pattern flaps based on the importance of the presence or absence of a major vessel running along the axis of the flaps.2 Random pattern flaps do not incorporate a dominant vascular supply, relying on the networks of small-diameter vessels to sustain the transferred tissue. They are limited in size and may require delay for successful transfer. Axial pattern flaps incorporate an anatomically recognized arteriovenous system running along the long axis of the tissue which permits successful transfer of vascularized flaps with high length-to-breadth ratios. They obtain their vascular supply from the musculocutaneous and fasciocutaneous systems, both of which rely on multiple “perforator” arteries. Knowledge of muscle vascular anatomy is helpful in predicting the viability of overlying skin territories based on such perforating vessels.
The now classic schema of Nahai and Mathes has divided muscles into groups according to their principal means of blood supply.2 A type I muscle, such as the gastrocnemius or tensor fascia lata (TFL), is supplied by a single pedicle. A type II muscle, such as the trapezius or gracilis, has a dominant pedicle, with one or more minor pedicles. A type III, the serratus anterior (SA) or gluteus maximus (GM), for example, has dual dominant pedicles. A type IV, such as the tibialis anterior (TA) or sartorius, has segmental pedicles. The type V, such as the internal oblique muscle or latissimus dorsi (LD), has a dominant pedicle, with secondary segmental pedicles. Most muscles fall into the type II group. Types I, III, and V are the most reliable because complete muscle viability can be sustained by a single vessel. Sometimes the muscle territory of a minor pedicle is poorly captured by the dominant pedicle in a type II muscle. Owing to their segmental means of perfusion, type IV muscles would potentially only allow small flaps that have limited application.
SURGICAL MANAGEMENT: PRIMARY FLAPS
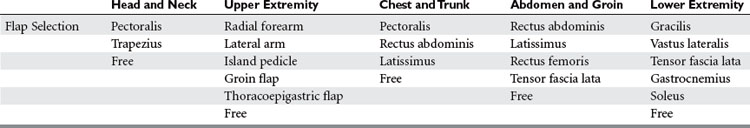
Musculoskeletal trauma will be grouped into five soft tissue coverage regions: head and neck, upper extremity, chest and trunk, abdomen, and the lower extremity (Table 1).
Head and Neck
The pectoralis myocutaneous flap can be designed with a skin paddle centered over the lower portion of the muscle. It can be used to resurface the neck, cheek, oral cavity, palate, tonsillar area, and nasopharynx, tongue, floor of mouth, mandible, and cervical area. The flap has an upward arc of rotation of 180 degrees and may be raised as high as the orbits; however, in practicality, it is difficult to secure the closure without a significant downward pull on the muscle. The flap can be modified with an extended random skin component or with two separate skin paddles that can be divided. A rib may be harvested with the flap for bony reconstruction. Higher elevation of the flap can be performed with the division of the clavicle. The pectoralis was one of the early workhorse flaps, but it has largely been supplanted by free flaps.
Upper Extremity
The groin flap (GF), reported by McGregor and Jackson in 1972, revolutionized the reconstruction of upper extremity wounds.2 It is a versatile flap with a reliable vascular supply that is capable of covering large defects of the hand and wrist and provides an excellent tissue bed for subsequent procedures such as tendon reconstruction.
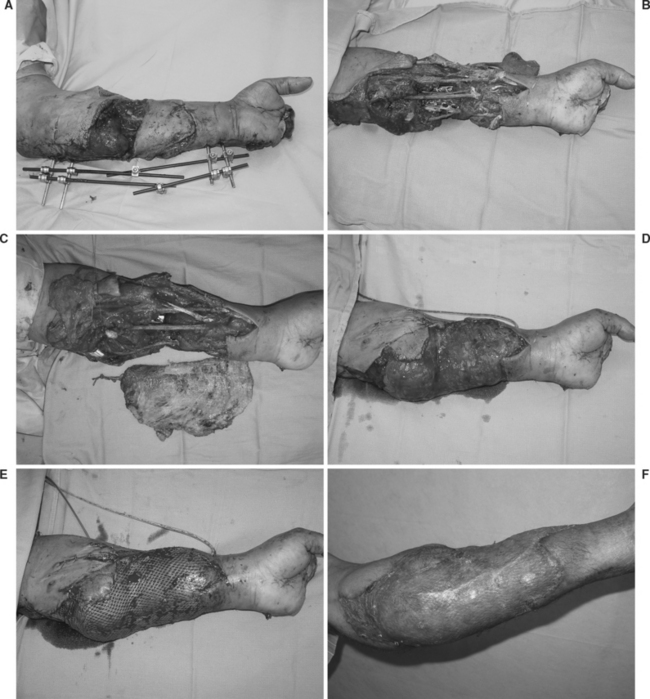
Various situations preclude the use of local, regional, or distant pedicle flaps. Involvement of the hand and forearm in an injury may eliminate all potential local or regional flap options. Although distant pedicle flaps, such as the GF, are useful for coverage of large defects, defects resulting from circumferential injuries are extremely difficult to cover with such a flap. Occasionally, defects involving the volar and dorsal aspects of the hand or more proximal circumferential defects can be covered by a combined groin and epigastric flap. However, free flaps offer far greater versatility and availability of donor sites for coverage of extensive defects. Additionally, tailoring of the free flap to suit the reconstructive needs of the wound is more easily accomplished because of the diversity of available flaps. Limb elevation, early mobilization, and the ability to cover even extensive circumferential defects are additional advantages to the use of free-tissue transfer (Figure 2).
Chest Wall and Trunk
The principles of chest wall reconstruction follow: (1) adequate debridement and resection of all tumors, osteoradionecrotic tissue, infection, or crushed soft tissue or bone; (2) obliteration of intrathoracic dead space; (3) skeletal stabilization if more than four ribs or greater than 5 cm of chest wall are resected en bloc; (4) adequate soft tissue coverage; and (5) aesthetic consideration. Muscle and musculocutaneous flaps are the tissues of choice for chest wall reconstruction. Local skin flaps, regional pedicle flaps, and thoracoabdominal tube flaps were used until the popularization of muscle and musculocutaneous flaps in the mid 1970s. The pectoralis major, LD, SA, and RA muscles are most frequently used.
In reconstruction of the posterior trunk, muscle coverage can be divided into thirds based on the location of the wound: the trapezius muscle for upper-third wounds, the latissimus dorsi for middle-third wounds, and the gluteus maximus for lower-third wounds. The scapular or parascapular fasciocutaneous flaps also may be used for small upper-third defects. Paraspinous muscle flaps may be advanced for relatively small vertical defects of the spine and paraspinal regions.2
Abdominal Wall and Groin
Like the TFL, the rectus femoris (RFe) is an excellent flap choice for reconstruction of the ipsilateral or lower abdominal wall. For extensive defects, a larger cutaneous paddle may be incorporated with the adjacent fascia lata in the musculocutaneous flap. The tip of the flap reaches a point midway between the umbilicus and xiphoid. The flap is supplied by the lateral femoral circumflex vessels. It also can cover the entire suprapubic region and extend to the contralateral anterosuperior iliac spine. After transposition, the vastus lateralis and vastus medialis are approximated to prevent a functional deficit resulting in loss of the final 15 degrees of knee extension. Sacrifice of this muscle in ambulating patients causes minimal functional debility. The “mutton chop” or extended rectus femoris myocutaneous flap, described by Dibbell and colleagues, allows reconstruction of large full-thickness abdominal wall defects, including the epigastrium, without prosthetic material.2
Lower Extremity
Knee
The alternatives for soft tissue coverage of the knee are muscle flaps, fasciocutaneous flaps, and free-tissue transfer. The gastrocnemius muscle flap is readily available for knee coverage; however, it does not reliably cross the midline to the contralateral aspect of the knee or to the superior aspect of the knee. The distally based muscle flaps are unreliable. The saphenous fasciocutaneous flap, as described by Walton and Bunkis, is useful if available.2 For extensive defects covering the entire surface of the knee, free-tissue transfer of a large fasciocutaneous flap or muscle flap is the most reliable.
Proximal Third
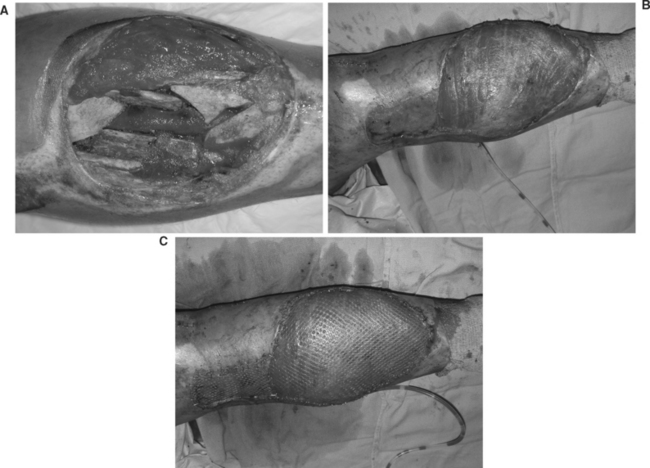
For soft tissue coverage of the proximal tibia, local muscle flap options include the medial and lateral gastrocnemius. The lateral is usually the shorter muscle and is compromised by the position of the peroneal nerve. Fasciocutaneous flaps include the saphenous flap, or a distally based medial or lateral fasciocutaneous flap. Free flaps are indicated when the local soft tissue injury contraindicates the use of local muscle or fasciocutaneous flaps or when the deficit is extensive (Figure 3).
Foot
Indications for flap coverage of the foot are exposed bypass grafts, chronic osteomyelitis, open fractures, and tendon and nerve exposure. Indications for free-tissue transfer include large or circumferential defects exposing fractures, open joints, or the Achilles tendon; incisions or soft tissue trauma that compromise the lateral or medial fasciocutaneous areas; and compromise of the distal arterial flow, which may prevent the use of lateral supramalleolar or dorsalis pedis flaps.
COMPLICATIONS MANAGEMENT
In general, microvascular tissue transfers are a large metabolic challenge for patients; all of the complications common to major surgery are possible. Specifically, flap necrosis is the only serious complication. Loss of a few millimeters, or even 1–2 cm, at a margin of the flap usually has no deleterious effect. Loss of the major portion of the flap occurs in about 4% of the cases and is almost always explained by an obvious precipitating cause. A major flap loss may require the performance of a second flap, though most compromised free flaps can be salvaged. Venous congestion owing to insufficient venous outflow is the most common complication which, with early recognition, may be rectified by flap revision or the use of medicinal leech therapy. Godina demonstrated the benefits of achieving healthy, soft tissue wound coverage over open lower extremity fracture within 5 days after injury.2 This approach minimizes the risk of acute and chronic infection and maximizes flap survival.
1 Bennet N, Choudary S. Why climb a ladder when you can take the elevator? Plast Reconstr Surg. 2000;105(6):2266.
2 Mathes SJ, Nahai F. Reconstructive Surgery: Principles, Anatomy, and Technique. New York: Churchill Livingstone, 1997.
3 Marsh JL. Decision Making in Plastic Surgery. St. Louis, MO: Mosby, 1993.
4 Strauch B, Vasconez LO, Hall-Findlay EJ. Encyclopedia of Flaps. Boston: Little, Brown, 1990.
5 Conley J, Patow C. Flaps in Head and Neck Surgery. New York: Thieme, 1989.
6 Aston SJ, Beasley RW, Thorne CH M. Grahb and Smith’s Plastic Surgery. New York: Lippincott-Raven, 1997.