CHAPTER 239 Techniques and Options in Nerve Reconstruction and Repair
Anatomic Principles
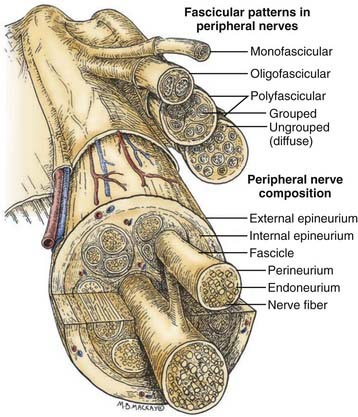
Before one can even consider the repair of a peripheral nerve, an understanding of the connective tissue layers, as well as of the fascicular anatomy of a nerve, is important. As seen in Figure 239-1, which contains a diagram of the peripheral nerve architecture and its components, an external epineurial sheath, composed of connective tissue and longitudinal blood vessels, surrounds each peripheral nerve. There is an external epineurium and an internal epineurium. The internal epineurium demarcates fascicles and groups of fascicles within the nerve. Each individual fascicle is surrounded by perineurium. The axons themselves are contained within fascicles, in close association with Schwann cells and the basement membrane that surrounds Schwann cells, the endoneurial basal lamina (also referred to as endoneurial tubes).1
Nerves may be generally divided into four basic patterns of intraneural architecture, based on their fascicular structure (see Fig. 239-1).2 Nerves containing one large fascicle are termed monofascicular, whereas those containing a few or discrete number of fascicles are oligofascicular. Most nerves contain many fascicles of varying sizes and are termed polyfascicular. The polyfascicular pattern may exist with grouping of fascicles or with a more diffuse (ungrouped) arrangement throughout the cross section of the nerve. The fascicular nature of a nerve changes as it extends from proximal to distal in the extremity. For example, the ulnar nerve is polyfascicular as it comes off the brachial plexus and then generally becomes organized into usually four fascicles at the level of the elbow. These fascicles are further segregated into motor and sensory groupings at the level of the wrist, and finally, the terminal digital branches are monofascicular in the fingers.
The proportion of connective tissue within the nerve varies considerably, from 25% to 85%, across the cross section of the nerve.2 In general, there is more connective tissue in the nerve where it crosses the joint. The connective tissue, particularly the perineurium, is the source of the main tensile strength to the nerve. It is also the layer that can take and hold a suture. From a practical viewpoint, the smallest component of nerve that can therefore be repaired using current microsurgical technique is the fascicle.
It must be stressed at the outset that a peripheral nerve repair is not a type of cellular repair but is actually a repair done at the level of the connective tissue to coapt a healthy proximal nerve to a healthy distal nerve stump. This then provides the appropriate anatomic environment so that axons from the proximal stump can regenerate into endoneurial tubes within the distal nerve stump and, hence, be led to end organs to restore function. Note that a nerve graft functions as a conduit, whose axons are destined to undergo wallerian degeneration as soon as it is removed from its harvest site. Thus, the graft provides an endoneurial tube network available to be exploited by regenerating axons from the proximal host nerve stump.3 It also provides viable Schwann cells, as long as the caliber of the nerve graft is not too large. For this reason, small caliber cutaneous nerves are most commonly used as graft material (see later section on donor graft harvesting techniques). The small caliber nerves, when sutured in a series of parallel segments, are in close proximity to tissue fluid and are therefore nourished. They also undergo rapid revascularization and thus remain viable.
Physiologic Principles and Patient Selection for Surgery
Peripheral nerves, once injured, have the potential to regenerate axons and reinnervate end organs, with resulting good functional recovery.4 Indeed, this is the case with all minor nerve injuries, such as neurapraxia, in which the axon remains intact. After a nerve injury resulting in axotomy (Sunderland grade II injury or greater), the distal axon undergoes wallerian degeneration. In purely axonotmetic injuries, in which axons are interrupted but the degree of connective tissue damage is minimal, regenerating axons use their existing endoneurial pathways to specifically reinnervate their own precise target end organs, as confirmed in recent experiments using bioengineered fluorescent mice.5 Outcome after more severe peripheral nerve injury, however, remains variable and often very poor. Most of these injuries exhibit both a loss of axon continuity and a significant disruption in the internal connective tissue structures. The resulting scarring within the nerve or a frank gap (with lacerating injuries) presents a formidable barrier to regenerating axons, preventing them from effectively innervating the distal nerve stump. These are currently managed with a repair of the divided nerve or, for the usual scenario of longer gaps or scar segments that need to be resected, placement of interposed nerve grafts.
Simplistically, exploration and repair of the peripheral nerve is indicated in clinical situations in which there is either the absence or the lack of expectation that there will be effective spontaneous regeneration. This will be the case in all patients with lacerating nerve injuries and in many of the patients who harbor the more severe injuries in continuity. As a practical rule, nerves known or expected to be sharply lacerated should be explored and repaired primarily and without delay, whereas bluntly lacerated nerves should be repaired after a period of 2 to 4 weeks. Patients with nerve injuries in continuity should be followed for about 3 months with repeat clinical and electrophysiologic evaluation. After 1 to 2 months have elapsed from the time of the trauma, the initial effects of any tissue damage will have resolved, and magnetic resonance neurography may then provide an early view of neuroma formation or of complete discontinuity. In patients failing to demonstrate clinical or electrical evidence of regeneration, the nerve should be explored within 4 to 6 months.6 The findings at surgery, including intraoperative electrophysiologic tests (briefly discussed later but in detail elsewhere in this text), determine the fate of the nerve injury (neuroma)–in-continuity.7
Operative Principles and Fundamental Techniques
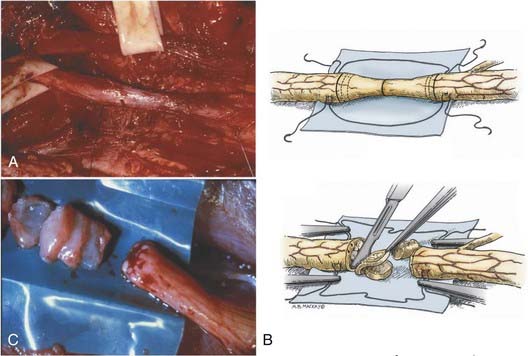
Isolation of the nerve itself should be performed using sharp dissection. The surgeon identifies normal nerve proximal and distal to the zone of injury and then works toward the area of injury. In clean lacerating injuries, the area of exposure may be relatively small. However, most injuries leave the nerve in continuity; because these injuries are also explored weeks to months following trauma, there is considerable scar formation and distortion of tissue, necessitating a wide and extensile exposure. Using sharp dissection techniques, the area of injured nerve is circumferentially exposed; that is, an external neurolysis is performed. With this type of circumferential mobilization, the gross anatomic details of the injury are identified. With the aid of an operating microscope, finer anatomic details can be appreciated. If the nerve is in clear discontinuity, nerve repair is necessary. However, most lesions are in-continuity. As demonstrated by Kline and Happel, recording of intraoperative nerve action potentials is useful in assessing these lesions.8 Specifically, the presence of a nerve action potential across the lesion argues for the lesion not to be resected (Fig. 239-2A). However, the lack of evidence of spontaneous regeneration (the absence of a nerve action potential) dictates resection of the neuroma and appropriate reconstruction of the resulting nerve injury gap.
The placement of lateral stay sutures using 6-0 monofilament (as illustrated in Fig. 239-2A and B) helps maintain the topographic alignment of the nerve. Under the operating microscope, the surgeon then cuts across the center of the neuroma. Small segments of the nerve are sliced in perfect cross section, using a fresh blade, until a healthy fascicular pattern is identified both at the proximal and at the distal stump9 (Fig. 239-2B and C). This step is critical because attempting to appose or graft scarred proximal and distal stumps is a major cause of nerve repair failure. Healthy fascicular tissue is recognized when the epineurium retracts slightly and the endoneurium appears to “pout” or mushroom out of the fascicles (because of positive endoneurial pressure). Fine bleeding from endoneurial microvessels may also be appreciated. This type of adequate débridement invariably leaves some degree of gap between the proximal and the distal stump. If the gap is short and the two ends can be brought together without undue tension, a direct repair is appropriate. One good way to determine the degree of tension present at the suture line is to bring the ends together using the stay epineurial sutures. If this can be performed without suture distraction, a direct repair is appropriate. However, if the ends are under considerable tension and the suture line appears to tear out, a graft repair must be performed.
Nerve Repair Techniques
Methods of peripheral nerve repair fall under two basic categories: direct repair (neurorrhaphy) and bridge procedures, in which most commonly, autologous nerve grafts are used. The suture repair may be performed using an epineurial, group fascicular, or fascicular technique or various combinations of these methods.10
Direct Repair
Epineurial Repair
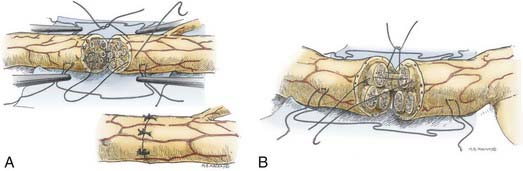
Epineurial suture repair has been a traditional method of nerve coaptation. These repairs are most appropriate for monofascicular (e.g., digital) nerves and diffusely grouped polyfascicular (most proximal limb and plexus element) nerves. Simplistically, this method achieves continuity of the connective tissue from the proximal to the distal stumps, without tension and with appropriate rotational alignment of both stumps. The goal is to obtain good coaptation of proximal and distal fascicular anatomy. Freshening of the two nerve ends to débride the nerve and remove scar tissue is therefore critical. Achieving appropriate nerve alignment can be aided by inspecting for longitudinal blood vessels in the epineurium as well as attending to fascicular alignment. The use of lateral stay sutures (see Fig. 239-2) also aids this process. Neurorrhaphy is performed using 8-0 to 10-0 nonabsorbable nylon sutures. A small bite of the internal and the external epineurium (being careful to avoid perineurium) is taken from both stumps, and the suture is tied using only mild to moderate tension (Fig. 239-3A). It is critical to avoid tying the knot under too great a tension because this will cause overriding or an accordion effect on the fascicles or, in fact, pouting out of a fascicle from the epineurial repair site, thus defeating the purpose of suturing. Two initial sutures are placed 180 degrees apart. If needed, this distance is then divided in half, and two further sutures are positioned. The number of epineurial sutures required varies; in most cases, four to eight sutures suffice for approximating the proximal and the distal stumps in a tension-free manner. Excess sutures may result in additional scarring and are to be avoided.
Grouped Fascicular Repair
As in the epineurial repair method, the nerve ends are matched by resecting damaged tissue. Débridement is followed by careful analysis, under the operating microscope, of the anatomic cross-sectional appearance of the nerve stump. Using the cross-sectional appearance, the longitudinal blood supply and other spatial landmarks (e.g., branching of nerve just proximal and distal to the injury site), the proximal and distal stumps can be matched. Interfascicular dissection is then performed within the internal epineurium to draw out groups of fascicles (Fig. 239-3B). Groups of fascicles may vary from two to several, each surrounded by a variable amount of internal epineurium, with the external epineurium dissected away. After groups of fascicles are adequately matched, 8-0 or 9-0 microsutures are placed through the interfascicular epineurial tissue and perineurium, allowing coaptation of fascicular groups from the proximal to the distal stump (see Fig. 239-3B).
Nerve Autograft Repair
The surgical technique of nerve grafting is similar in many ways to that of direct nerve repair.11 From a practical viewpoint, when direct repair cannot be performed without undue tension, nerve grafting must be undertaken. A corollary to this is that when nerve grafting is performed, there must be no tension at the proximal and distal repair sites to prevent postoperative distraction.12 Therefore, nerve grafting is generally performed with the extremity in full extension. In general, a nerve graft should be about 10% longer than the existing nerve gap. Frequently, the cross-sectional area of the host nerve stump will be several-fold larger than the diameter of the nerve graft. Several segments of nerve graft will therefore be needed to allow coverage of the entire cross-sectional surface of the host nerve stumps. This must be taken into consideration when harvesting the donor nerve. In general, it is imperative to harvest the maximal amount of nerve graft material available.
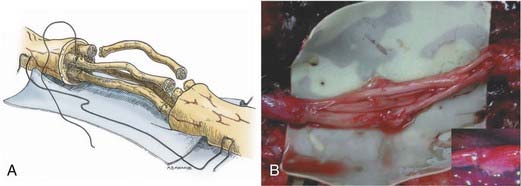
The graft repair technique is similar to the various repair methods already outlined, with the exception that there are two suture lines. When preparing the proximal and the distal stumps, the interfascicular and external epineurial tissue must be dissected away to allow groups of fascicles (or fingers) to be created at each stump (Fig. 239-4A). The diameter of the group of fascicles should approximate that of the cutaneous nerve graft obtained. The nerve graft is then sewn in place to the proximal group of fascicles, using epineurial and interfascicular epineurial techniques (Fig. 239-4B). Sutures, about 180 degrees apart, are positioned and passed through the epineurium of the nerve graft and then through the interfascicular epineurium of the host stump, spreading the cross section of the graft in a fish-mouth configuration (see Fig. 239-4A). The end of the graft segments then covers as much of the fascicular area of the host nerve stump as possible, allowing capture of the maximal number of regenerating axons. Typically, one or two sutures are required for each nerve graft at each coaptation site. The nerve graft segments are sutured to the distal group of fascicles in a similar manner. It is important to reinspect all proximal and distal coaptation sites at the completion of repair to ensure that no graft ends were disconnected while others were being sutured in place. Fibrin glue is then applied to reinforce each of the repair sites (Fig. 239-4B, inset).
As much as possible, the grafts are aligned in orientation so that groups of proximal fascicles will be directed to appropriate groups of distal fascicles. This allows for the most precise repair possible. For each nerve treated, an understanding of the gross anatomy and branching pattern of the nerve, as well as knowledge of interfascicular anatomy, is helpful. As Sunderland has shown, the plexiform nature of the more proximal nerve elements, whereby groups of fibers are exchanged among fascicles in a longitudinal manner, defeats exact matching.2 In general, when dealing with proximal nerves and nerve elements such as the brachial plexus, spatial alignment of fascicles allows the best possible matching. In the most distal nerves (e.g., the median and the ulnar nerve at the wrist), more precise anatomic matching with regard to groups of fascicular and, occasionally, specific fascicular anatomy is possible.13
Nerve Graft Harvesting Techniques
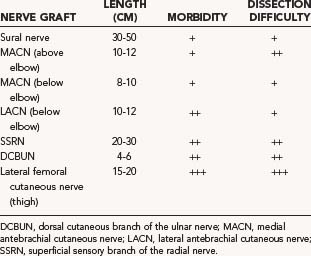
The donor nerves sought for nerve grafting are typically cutaneous nerves of the upper and lower extremities (Table 239-1). In the upper extremity, these include the medial antebrachial cutaneous nerve (MACN), the lateral antebrachial cutaneous nerve (LACN), the superficial sensory branch of the radial nerve (SSRN), and the dorsal cutaneous branch of the ulnar nerve (DCBUN). Lower extremity nerves harvested for graft purposes include the frequently used sural nerve and the less frequently used lateral femoral cutaneous nerve. Table 239-1 indicates the approximate length of graft that can be obtained for each donor nerve as well as the potential donor site morbidity. By far, the maximal amount of nerve graft available is provided by the sural nerve. Along with relative ease of dissection and limited donor site morbidity, this accounts for the popularity in the use of the sural nerve as graft material in most peripheral nerve reconstructive procedures (see Table 239-1).
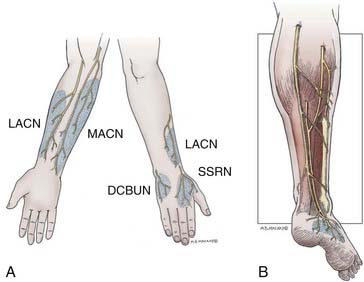
Upper extremity donor nerves provide a limited amount of graft material (see Table 239-1). The typical sensory deficits following harvesting of upper extremity donor nerves are outlined in Figure 239-5A. The LACN is the terminal sensory portion of the musculocutaneous nerve. The nerve can be found just lateral to the biceps tendon lying superficial to the surface of the brachialis muscle in the subcutaneous tissue. There is an anterior and a posterior division. Either or both divisions may be taken with impunity, except in situations in which a median nerve injury preexists because there may be overlap in sensation to the dorsal lateral thumb and significant loss of sensation in this area (see Fig. 239-5A). About 12 cm of nerve can be obtained at and below the elbow. More favored than the LACN for grafting is the MACN. This nerve is derived from the medial cord of the brachial plexus. The MACN closely follows the brachial vein entering into the subcutaneous space, about midway in the upper arm, where it also divides into an anterior and a posterior division (see Fig. 239–5A). About 20 cm of nerve can be obtained through a longitudinal medial forearm incision. Because the more proximal nerve comes off the medial cord, it is larger in caliber and has been used as a single trunk graft for repair of axillary and other nerve injuries. The more distal nerve becomes smaller in caliber and is of value for use in a cable fashion for grouped interfascicular and distal nerve injury repairs. This nerve should not be used in situations of ulnar nerve injury because of potential sensory overlap (see Fig. 239–5A). The SSRN is the terminal sensory component of the radial nerve. It continues along the course of the main nerve trunk, just deep to brachioradialis muscle in the proximal forearm, after the takeoff of the posterior interosseous nerve. The sensory distribution is to the dorsum of the hand, with a fairly small area of autonomous innervation to the anatomic snuffbox (see Fig. 239-5A). This area is not clinically important and can be easily sacrificed. The SSRN is an especially good nerve for reconstruction of proximal radial nerve injuries because sensation has already been lost. Moreover, the surgeon can direct the distal portion of the graft to the posterior interosseous nerve, thereby preventing wasting of regenerating axons into the unimportant sensory nerve stump.
The sural nerve is the most commonly used donor nerve. From its origin in the popliteal fossa, as a branch of the tibial nerve or peroneal nerve, to the level of the ankle where it starts to divide into many small branches, about 40 cm of nerve can be obtained from each leg.14 The nerve is deep to the fascia from the popliteal fossa to the level of the midcalf (Fig. 239-5B). In most cases, there is a contribution at the level of its emergence from the fascia by the lateral sural nerve (a branch of the peroneal nerve). In about 20% of cases, the contribution is quite large, and in some cases, the entire nerve originates from the peroneal nerve. In the latter two instances, dissection of the peroneal communicating branch is especially important to obtain the maximal amount of graft material.
There are two alternative open techniques for the incision to expose the sural nerve. The classic approach is to use a single lengthy longitudinal incision, extending from just behind the lateral malleolus to the level of the popliteal fossa. Other surgeons prefer a small longitudinal incision along with a series of small transverse “step” incisions along the course of the nerve. Some surgeons now prefer to harvest the sural nerve using endoscopic techniques.15 In either case, one starts with a longitudinal incision several centimeters in length, fashioned about 1 cm posterior and superior to the lateral malleolus and extending cephalad from there. The sural nerve is easily identified in the subcutaneous plane, in association with and usually just posterior to the lesser saphenous vein. The nerve is circumferentially exposed, surrounded with a vasoloop, and raised from the subcutaneous tissue. Gentle traction on the nerve and palpation confirm the course of the nerve, and the incision is appropriately extended obliquely up the leg toward the posterior midline of the calf. The surgeon continues to mobilize the nerve and free it from the subcutaneous tissue. At about the midcalf level, the nerve dives deep under fascia overlying the gastrocnemius muscle. At this point, there can be a substantial contribution from the peroneal communicating branch of the lateral sural nerve. If this is the case, the peroneal communicating branch can be dissected out to obtain extra graft material, as required. The peroneal communicating branch remains subcutaneous in its course toward the main peroneal nerve. However, the main sural nerve from midcalf level to the popliteal fossa remains deep to the fascia, requiring incision of the fascia up and along the posterior midline of the leg. The superior extent of the incision to harvest the sural nerve stops just below the knee crease. The knee crease should not be crossed because this may result in a painful scar. It should also be noted that the incision at the ankle level is designed to avoid contact with conventional footwear and should not become too troublesome for the patient. Additionally, if the entire sural nerve is excised and the proximal stump is left in the high calf or popliteal region, the chance for painful neuroma formation is minimized. The sensory deficit sustained is quite predictable, usually involving the lateral surface of the foot (see Fig. 239-5B). Over the course of time, this diminishes to a relatively small patch of skin anterior and inferior to the lateral malleolus along the lateral surface of the foot. Most patients tolerate the sensory deficit without complaint.
Nerve Transfers
Nerve transfers, although technically a type of direct (end-to-end suture) nerve repair, are a special category of procedure that merits discussion as an important option to consider when deciding on what type of nerve repair to perform on a patient. A nerve transfer essentially involves the coaptation of a proximal foreign (donor) nerve to the distal denervated (recipient) nerve so that the latter’s end organs will be reinnervated by the donor neuronal population. The concept is to sacrifice the function of a less valued donor nerve to revive function in the recipient nerve and muscle that will undergo reinnervation, for example, a hypoglossal-facial nerve transfer. Nerve transfers are becoming more liberally used for repair of devastating brachial plexus injuries.16 A common indication for a nerve transfer procedure is the brachial plexus nerve root avulsion or very proximal intraforaminal injury close to the spinal cord where there is no or poor nerve stump available to lead nerve graft. A C5 and C6 avulsion may thus be managed with distal targeted transfers, namely accessory nerve to suprascapular nerve, triceps branch nerve to axillary nerve, and ulnar nerve fascicle to biceps branch of musculocutaneous nerve (see ![]() Video 239-1 in the supplementary online version of the text).17
Video 239-1 in the supplementary online version of the text).17
Another, more controversial, indication for pursuing a transfer over attempted direct (or graft) repair at the injury site includes the presence of significant vascular or bony injuries in the region of the brachial plexus to avoid the difficult dissection through the injured, scarred area and also to potentially avoid unnecessary damage to vital structures. Delayed presentation and long interval from injury to surgery (the ideal time for direct nerve repair is up to 6 months after the injury), as well as previously failed brachial plexus or proximal nerve repair, have also been used as criteria for considering nerve transfers. In general, a reasonable indication is in patients with very proximal injury with a long distance to the target muscle. For instance, a high (axillary or arm) ulnar nerve lesion, in which it would be unlikely to reestablish any hand intrinsic motor function with a very proximal repair, may be palliated with a distal anterior interosseous nerve transfer to the motor branch of the ulnar nerve in the distal forearm.18
Nerve Tube Repairs
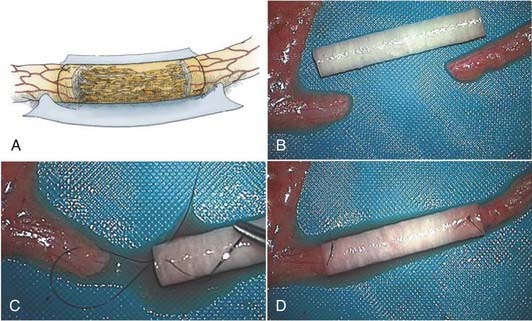
Although nerve autografts remain the gold standard for peripheral nerve repair, nerve autografting has inherent flaws. Procuring the donor nerve incurs a new neurologic deficit, in addition to donor site morbidity, such as scar and occasionally neuroma pain. Additionally, there may be insufficient length and diameter of autogenous nerve to optimize reconstruction. Moreover, an artificial (not nerve) conduit interposed between the proximal and distal nerve stumps may provide a more suitable environment for regenerating nerve fibers to sample and respond to appropriate directional and trophic cues derived from migrating Schwann cells and soluble growth factors emanating from both nerve stumps21,22 (Fig. 239-6A). With the basis of a large experimental experience, several clinical trials with nerve conduits to repair nerve injuries have been reported. For example, in a randomized prospective study, biodegradeable polyglycolic acid tubes achieved comparable outcomes to nerve grafts in the clinical repair of digital nerves with defects up to 3 cm.23 Nerve tubes are therefore appropriate for use in the repair of the smaller diameter nerve injuries where gap lengths are less than 3 cm.
To undertake the repair with a nerve tube, the nerve ends are trimmed until a good fascicular pattern is visible at each nerve stump (see Fig. 239-6B and C). An appropriate diameter nerve tube is chosen so that the inner luminal diameter is about 20% larger than the cross-sectional diameter of the nerve to be repaired (Fig. 239-6B). The nerve ends are then inserted into each end of the tube. This requires the placement of a single microsuture in a U fashion from outside to inside of the tube then through the epineurium of the nerve 1 to 2 mm back from the nerve stump, then again from the inside of the nerve tube to the outside where the knot is tied (Fig. 239-6C). This type of stitch at each end keeps the nerve stumps constrained within the tube lumen (Fig. 239-6D). The interior of the lumen is then filled with saline, with a small-gauge needle and syringe, to flush out any air bubbles. The ends of the nerve tube and nerve junction can be further reinforced, if needed, with fibrin glue.
Nerve Repair in the Future
A major shortcoming with the nerve graft technique is the biologic constraint, which cannot be overcome by further progress in microsurgical techniques. Even with the most meticulous repair, regenerating axons at the site of suture or within the graft become misdirected or lost, leading to inappropriate (nonspecific) and incomplete reinnervation, respectively.13 Innovative recent investigations with nanoscale engineered devices suggest that some day surgery at the cellular level to splice and repair individual axons may at least be feasible.24
Recent insights into the nuances of axonal regeneration, however, provide potential for some new therapies that may be readily accessible soon. We now understand that axonal outgrowth is not synchronous, but rather staggered, so that some pioneering axons grow out early, whereas others lag far behind. In fact, many of the lagging axons are delayed at suture repair sites and—in the case of a nerve graft—are compounded, involving two separate repair locations. Exciting recent work by Gordon and colleagues suggests that epochs of electrical stimulation as short as 1 hour have a significant influence not only on synchronizing the initial regrowth of motor axons but also possibly on enhancing specificity of motor reinnervation in rodent models.25 Clinical trials to assess the utility of short-duration electrical stimulation on improving the outcome of nerve repair are therefore warranted.
In an attempt to provide a more suitable environment for regenerating axons to sample and respond to appropriate endogenous directional cues, many investigators have proposed using bioengineered graft that may allow the introduction of exogenous therapies that build on our rapidly expanding knowledge of axonal guidance.26 The clinical utility of such a strategy is apparent when considering the example of repair of nerves to the hand, in which the median nerve is paramount for sensation and the ulnar nerve is critical for discrete motor function of the digits. In these instances, the nerve tube repair could be endowed with specific growth factors or other molecules that will bias the regeneration toward a population of axons (motor versus sensory) to achieve improved specificity of reinnervation. Advances in bioengineering, coupled with our understanding of how to effectively deliver growth factors, cell adhesion molecules, and other therapies within the artificial nerve graft, should lead to major advances in improving both the quantity and specificity of axonal regeneration through the nerve tube.
Brushart TME. The mechanical and humoral control of specificity in nerve repair. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:215-230.
Chang DW. Minimal incision technique for sural nerve graft harvest: experience with 61 patients. J Reconstr Microsurg. 2002;18:671-676.
Chang WC, Hawkes EA, Kliot M, Sretavan DW. In vivo use of a nanoknife for axon microsurgery. Neurosurgery. 2007;61:683-691.
Dahlin LB, Lundborg G. Use of tubes in peripheral nerve repair. Neurosurg Clin N Am. 2001;12:341-352.
Dellon AL. Resection: nerve repair’s most neglected technique. Plast Surg Tech. 1995;1:191-199.
de Moura W, Gilbert A. Surgical anatomy of the sural nerve. J Reconstr Microsurg. 1984;1:31-39.
Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67-116.
Gordon T, Brushart TM, Amirjani N, Chan KM. The potential of electrical stimulation to promote functional recovery after peripheral nerve injury—comparisons between rats and humans. Acta Neurochir Suppl. 2007;100:3-11.
Harris ME, Tindall SC. Techniques of peripheral nerve repair. Neurosurg Clin N Am. 1991;2:93-104.
Hudson AR, Hunter DA, Kline DG, Bratton BR. Histological studies of experimental interfascicular graft repairs. J Neurosurg. 1979;51:333-340.
Kline DG. Physiological and clinical factors contributing to the timing of nerve repair. Clin Neurosurg. 1977;24:425-455.
Kline DG, Happel LT. A quarter century’s experience with intraoperative nerve action potential recording. Can J Neurol Sci. 1993;20:3-10.
Malessy MJ, Bakker D, Dekker AJ, et al. Functional magnetic resonance imaging and control over the biceps muscle after intercostal-musculocutaneous nerve transfer. J Neurosurg. 2003;98:261-268.
Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
Midha R, Kline DG. Evaluation of the neuroma in continuity. In: Omer GE, Spinner M, Van Beek AL, editors. Management of Peripheral Nerve Problems. 2nd ed. Philadelphia: Saunders; 1998:319-327.
Midroni G, Bilbao JM. Normal anatomy of peripheral (sural) nerve. In: Midroni G, Bilbao JM, editors. Biopsy Diagnosis of Peripheral Neuropathy. Boston: Butterworth-Heinemann; 1995:13-33.
Millesi H. Indications and techniques of nerve grafting. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction, Vol 1. Philadelphia: JB Lippincott; 1991:525-543.
Millesi H, Meissl G, Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972;54:727-750.
Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5:861-867.
Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg. 2002;18:459-464.
Oberlin C, Beal D, Leechavengvongs S, Salon A, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232-237.
Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65-82.
Seddon HJ. Three types of nerve injury. Brain. 1943;66:238-288.
Sunderland S. Nerve and Nerve Injuries. Baltimore: Williams & Wilkins; 1968.
Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036-1045.
1 Midroni G, Bilbao JM. Normal anatomy of peripheral (sural) nerve. In: Midroni G, Bilbao JM, editors. Biopsy Diagnosis of Peripheral Neuropathy. Boston: Butterworth-Heinemann; 1995:13-33.
2 Sunderland S. Nerve and Nerve Injuries. Baltimore: Williams & Wilkins; 1968.
3 Hudson AR, Hunter DA, Kline DG, Bratton BR. Histological studies of experimental interfascicular graft repairs. J Neurosurg. 1979;51:333-340.
4 Seddon HJ. Three types of nerve injury. Brain. 1943;66:238-288.
5 Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5:861-867.
6 Kline DG. Physiological and clinical factors contributing to the timing of nerve repair. Clin Neurosurg. 1977;24:425-455.
7 Midha R, Kline DG. Evaluation of the neuroma in continuity. In: Omer GE, Spinner M, Van Beek AL, editors. Management of Peripheral Nerve Problems. 2nd ed. Philadelphia: Saunders; 1998:319-327.
8 Kline DG, Happel LT. A quarter century’s experience with intraoperative nerve action potential recording. Can J Neurol Sci. 1993;20:3-10.
9 Dellon AL. Resection: nerve repair’s most neglected technique. Plast Surg Tech. 1995;1:191-199.
10 Harris ME, Tindall SC. Techniques of peripheral nerve repair. Neurosurg Clin N Am. 1991;2:93-104.
11 Millesi H, Meissl G, Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972;54:727-750.
12 Millesi H. Indications and techniques of nerve grafting. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction, Vol 1. Philadelphia: JB Lippincott; 1991:525-543.
13 Brushart TME. The mechanical and humoral control of specificity in nerve repair. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:215-230.
14 de Moura W, Gilbert A. Surgical anatomy of the sural nerve. J Reconstr Microsurg. 1984;1:31-39.
15 Chang DW. Minimal incision technique for sural nerve graft harvest: experience with 61 patients. J Reconstr Microsurg. 2002;18:671-676.
16 Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
17 Leechavengvongs S, Witoonchart K, Uerpairojkit C, et al. Combined nerve transfers for C5 and C6 brachial plexus avulsion injury. J Hand Surg Am. 2006;31:183-189.
18 Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg. 2002;18:459-464.
19 Oberlin C, Beal D, Leechavengvongs S, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232-237.
20 Malessy MJ, Bakker D, Dekker AJ, et al. Functional magnetic resonance imaging and control over the biceps muscle after intercostal-musculocutaneous nerve transfer. J Neurosurg. 2003;98:261-268.
21 Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67-116.
22 Dahlin LB, Lundborg G. Use of tubes in peripheral nerve repair. Neurosurg Clin N Am. 2001;12:341-352.
23 Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036-1045.
24 Chang WC, Hawkes EA, Kliot M, Sretavan DW. In vivo use of a nanoknife for axon microsurgery. Neurosurgery. 2007;61:683-691.
25 Gordon T, Brushart TM, Amirjani N, Chan KM. The potential of electrical stimulation to promote functional recovery after peripheral nerve injury—comparisons between rats and humans. Acta Neurochir Suppl. 2007;100:3-11.
26 Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65-82.