Chapter 76B Technique of portocaval shunting
Overview
Surgical shunts for portal hypertension and variceal hemorrhage are rarely undertaken today, in large part as a result of the recent popularity gained by transjugular intrahepatic portosystemic shunting (TIPS) (see Chapter 76E), a general lack of interest by surgeons, and the rising number of liver transplant centers across the United States. As a result, the number of hepatobiliary surgeons who have expertise in surgical shunting is declining, which is not to imply that this is appropriate, but it is true. Although current data document that surgical shunting is superior to TIPS in regard to patency, survival, risk of rebleeding, and maintaining effective hepatic blood flow, TIPS now predominates current therapy for variceal bleeding, likely because of its less invasive nature. The scope of this chapter is to describe the technical aspects of performing a native side-to-side portacaval shunt and a small-diameter prosthetic H-graft portocaval shunt. Unlike an end-to-side portacaval shunt, these shunts potentially preserve effective hepatic blood flow and are not ascitogenic.
Variceal hemorrhage secondary to portal hypertension presents an enormous challenge and responsibility to surgeons. Varices that are actively bleeding should be treated pharmacologically and endoscopically, if possible, prior to surgical management (see Chapters 75A and 75B). In the opinion of most providers, it should be a priority to stabilize the patient with nonoperative measures rather than attempt an emergency surgical shunt. The primary goal of surgery is to control ongoing hemorrhage and to prevent future life-threatening hemorrhage without inducing hepatic dysfunction. Patients should not be candidates for imminent liver transplantation (i.e., within 6 months), and an optimal candidate should have a Model for End-Stage Liver Disease (MELD) score less than 14 or have a Child-Turcotte-Pugh (CTP) class of A or B. However, for patients with advanced liver disease without prospects for transplantation, shunting is advocated for resource conservation and control of hemorrhage, albeit with increased risk of hepatic decompensation. With a recent episode of hemorrhage, the total bilirubin may be elevated, which increases the MELD score at the time of hospitalization. In these circumstances, it is helpful to extrapolate the patient’s MELD score or CTP class back to a time prior to the most recent hospitalization and/or hemorrhage.
Surgical Shunts in the Age of Tips
TIPS is a “definitive” nonsurgical treatment option for portal hypertension, often coined a “bridge to transplantation,” but with many critical issues. The most common causes of TIPS failure are shunt thrombosis and stenosis, both of which lead to variceal rehemorrhage. Numerous reports have shown that hepatic decompensation is unacceptably common after TIPS. In addition, the survival data after TIPS are not comparable to surgical shunts, most notably small-diameter prosthetic HGPCSs (Clark et al, 2010). Of concern is that TIPS excessively diverts nutrient effective hepatic blood flow (EHBF) from liver parenchyma that is already compromised by increased sinusoidal resistance to portal blood flow (Rosemurgy et al, 1995, 2003), thus potentially accelerating hepatic decompensation. Although TIPS does successfully partially decompress the PV and corrects the abnormal physiologic pressure gradient between the PV and IVC, its failures and shortcomings should be recognized, as this treatment option is undertaken frequently, without thorough consideration of its consequences. Although TIPS is viable for portal pressure decompression and temporary relief from variceal bleeding, the “bridge” to transplantation built by this procedure is seldom crossed; less than 10% of patients who undergo TIPS undergo liver transplantation.
Both TIPS and surgical shunts decompress the portal system, but surgical shunts—notably small, calibrated, side-to-side portacaval shunts and HGPCSs—relatively preserve effective hepatic blood flow. With TIPS, a decompression of portal venous pressure, along with the ensuing decrease in sinusoidal pressure, results in a concomitant increase in arterialization of the liver. However, after TIPS, increases in arterial blood flow, or “arterialization of the liver,” are not sufficient to compensate for relative excessive diversion of portal blood flow from the hepatocytes. Excessive decreases in total nutrient blood flow (EHBF) to hepatocytes result after TIPS relative to small-diameter calibrated and reinforced side-to-side portacaval shunts, such as an HGPCS (Rosemurgy et al, 2003). The critical, life-threatening issues of decreased EHBF and variceal rehemorrhage loom over an otherwise successful TIPS decompression.
Variceal hemorrhage after TIPS is relatively common and often results from shunt stenosis or thrombosis. Despite strict follow-up and assessment, TIPS patients experience more shunt occlusion and stenosis, although occlusion can usually be corrected by interventional radiology. The issue is that patients require multiple interventions to maintain shunt patency, whereas with an HGPCS, occlusion is less common. Vigilance with follow-up is necessary after TIPS; often the first sign of TIPS malfunction is variceal rehemorrhage (Rosemurgy et al, 2005).
The long-term complications of nonpatency, shunt stenosis, and thrombosis associated with TIPS are well documented. In 2005, a randomized controlled trial of 132 patients undergoing TIPS versus HGPCS was reported (Rosemurgy et al, 2005). The data demonstrated that stenosis and thrombosis after TIPS placement occurred in significantly more patients, and with greater frequency, compared with HGPCS. In the 32 patients who received TIPS, 66 interventions and/or revisions were required to maintain shunt patency during a 1-year follow-up period. In this study, only seven patients required intervention for patency after HGPCS, and five patients had irreversible TIPS occlusion. Most importantly, irreversible shunt occlusion often presented as major variceal hemorrhage. Of the 32 patients who underwent revisions for TIPS stenosis or thrombosis, 20 of these patients had major variceal rehemorrhage within 30 days, two patients rehemorrhaged after 30 days, and none of the patients had stenotic or thrombosed shunts following HGPCS (Rosemurgy et al, 2005). Also, TIPS has proven to be more expensive than pharmacologic, endoscopic, or surgical shunt treatments, owing to the number of interventions necessary to maintain patency (Kravetz, 2007). Indeed, the “Achilles heel” of TIPS is its lack of patency, and the success of TIPS as a portal decompression procedure is masked by continuing stenosis and thrombosis, requiring more cost for intervention and follow-up. Proponents of TIPS believe this has changed with the advent of covered stents.
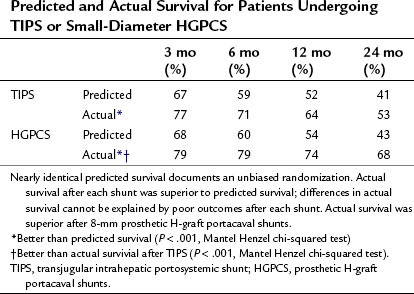
A critical issue of TIPS is subsequent mortality. Median time to death after shunting was 29 months (41 ± 39.4 months) after TIPS compared with 56 months (53 ± 40.1 months) after H-graft shunts. Predicted survival was identical in both groups, and actual survival was significantly better for each shunt than predicted, using survival data at 3, 6, 12, and 24 months after shunting (Table 76B.1). However, actual survival with H-graft shunts was superior to survival after TIPS. Furthermore, the time to failure for patients undergoing TIPS or HGPCS was significantly different. For all patients of all CTP classes, TIPS had a median time to failure of 14 months (29 ± 33.2) compared with a median time to failure of HGPCS of 43 months (48 ± 41.9). In a patient with a severely impaired liver, a difference of nearly 30 months is critical, as that time could be utilized to undergo transplantation, if such were indicated and possible.
For patients with poor hepatic reserve, the implications of reduced EHBF to an already diseased liver parenchyma can be devastating. EHBF is nutrient-rich portal blood flow—carrying venous return from the spleen, small intestine, and colon—and oxygen- and nutrient-rich arterial blood flow. This venous inflow to the liver also carries protein products and toxins that need to be neutralized, reduced, detoxified, or broken down to some degree by the liver. Given the changes in portal blood flow associated with TIPS, hepatic “arterialization” after TIPS is inadequate to maintain EHBF (Rosemurgy et al, 2003), therefore hepatic decompensation is promoted after TIPS.
Technique of Side-To-Side Portacaval Shunts
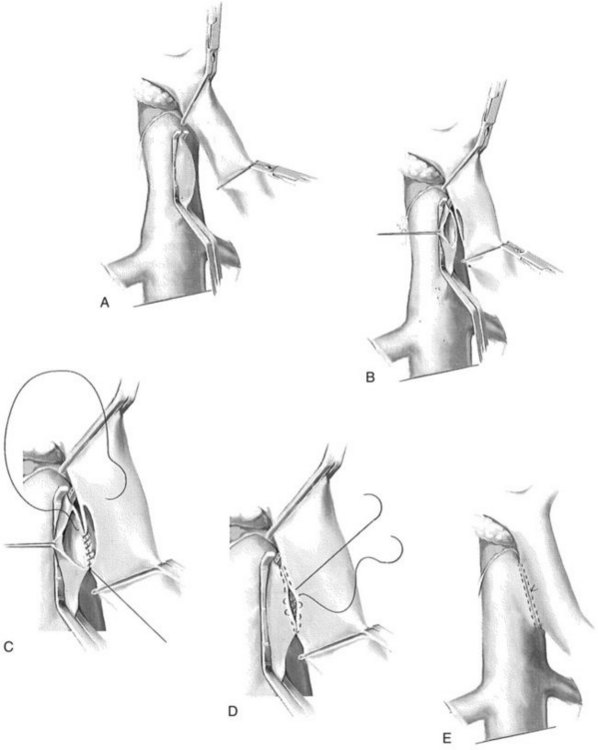
A side-biting Satinsky clamp is placed over the ventral aspect of the vena cava, and two right-angled vascular clamps are placed approximately 5 cm apart on an overlying segment of PV. A 2.5-cm longitudinal strip of vena cava is excised following venotomy. Excision of this “window” is to ensure adequate outflow for the shunt. Similarly, a 2.5-cm strip of PV is excised along the posterolateral aspect of the vein. Heparinized saline is injected within the lumina of both vessels. Beginning in the middle of the left/back wall of the anastomosis, a 5-0 double-armed polypropylene is used in a running manner at the cephalad and caudal extents (Fig. 76B.1). The sutures are tied at each corner of the anastomosis, at the cephalad and caudad extents. The right side or front wall is completed with two 5-0 polypropylene sutures, beginning at each corner; these are tied to the free ends of the posterior layer, and the right side is run from each end toward the midportion of the anastomosis. Prior to tying the sutures together, the vascular clamps are partially released, beginning with the vena cava, to release thrombus that may have formed. Upon completion of the anastomosis, the clamps are sequentially released, beginning with the vena cava, followed by the distal PV clamp and finally the proximal vein clamp. Portal and caval pressures should be similar—that is, equal or near equal—following completion of the shunt. An elevated gradient above 4 to 5 mm Hg should alert the surgeon to a technical problem that must be addressed expediently to decrease the risk of PV or shunt thrombosis.
Technique of Small-Diameter (8 MM) H-Graft Portacaval Shunts
For a small-diameter HGPCS, the patient is placed supine on the operating table and rolled into a 30-degree left lateral decubitus position by placing a rolled sheet beneath the patient’s right side. A transverse incision is utilized from the midline, extending just beyond the lateral border of the rectus sheath. The incision is placed over the liver edge, if palpable. Because of collaterals that may be present, the falciform ligament is carefully divided if necessary. Our preference is to use the table-mounted Omni retractor in conjunction with wound protectors; once the wound is saturated with Clorpactin solution and well padded, a sternal blade is placed cranially (Rosemurgy, 2007).
A Kocher maneuver is initiated at the foramen of Winslow and carried caudad, using electrocautery liberally. The goal is to expose approximately 5 cm of IVC, including only about one half of the ventral circumference of the vessel, thus facilitating placement of a side-biting Satinsky vascular clamp (Fig. 76B.2). Because the patient is rotated to the left, a common error is to carry the dissection lateral to the vena cava into the renal hilum; this should be avoided.
FIGURE 76B.2 Kocherization and preparation of the vena cava.
(From Rosemurgy A, et al, 2007: 8-mm Interposition Portacaval Shunt. In Clavian P, Sarr M, Fong Y [eds]: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 675-685.)
Once the vena cava is adequately exposed, attention is then turned to the hepatoduodenal ligament. A splanchnic blade is placed over a well-padded gallbladder. This retraction should be toward the patient’s left shoulder, effectively rotating the common bile duct and any replaced/accessory right hepatic artery ventrally and medially. The dissection is then carried parallel and dorsal to the common bile duct. Lymphatics are predominantly found in this area, overlying the PV, and these should be ligated to avoid postoperative ascites formation. Care should be taken to identify an accessory or replaced right hepatic artery and to avoid injuring it. A vein retractor is placed over the common bile duct, and it is retracted medially to facilitate exposure of the PV. As the PV becomes visible, a generous portion of the vein should be grasped with Russian forceps. The enveloping tissue of the posterior hepatoduodenal ligament may be similarly grasped, and the plane is developed with a Yankauer sucker. Gentle, small strokes will separate the enveloping tissue from the PV without injuring any small branches. The ventral and medial surface of the PV is exposed in a similar fashion, grasping the vein with Russian forceps, retracting laterally, and passing the Yankauer sucker along the ventral surface of the vein. A right-angle clamp is placed around the PV, followed by a vessel loop (Fig. 76B.3). The vessel loop may then be used to retract the vein laterally and expose a generous circumferential portion of the PV if necessary, although circumferential dissection of the PV is generally superfluous.
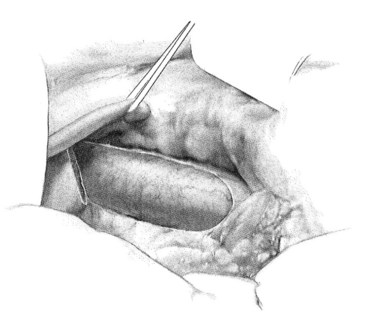
FIGURE 76B.3 Exposure of portal vein.
(From Rosemurgy A, et al, 2007: 8-mm Interposition Portacaval Shunt. In Clavian P, Sarr M, Fong Y [eds]: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 675-685.)
A ringed forceps is used to grasp the anterior wall of the vena cava, retracting ventrally as a side-biting Satinsky clamp is placed upon the vein. The vascular clamp must be fully closed to prevent the wall of the IVC slipping from the clamp. A portion of the vena cava wall is then excised that should measure approximately 4 mm long and 2 mm wide ex vivo. The anastomosis to the vena cava is sewn with running 5-0 polypropylene suture, beginning at the cephalad apex of the anastomosis and progressing along the back, or left, medial wall (Fig. 76B.4). Begin by placing a horizontal mattress suture at the heel/cephalad apex of the anastomosis, and run the suture in-to-out on the vein and out-to-in on the graft until beyond the toe of the graft. At that point, the other limb of the 5-0 polypropylene is utilized to complete the lateral front right wall of the anastomosis. The IVC-graft anastomosis can be assessed by occluding the graft with a right-angle clamp and releasing the occlusion of the Satinsky clamp (Fig. 76B.5). The Satinsky clamp is then replaced on the vena cava, and the graft is irrigated with heparinized saline to remove blood and clot.
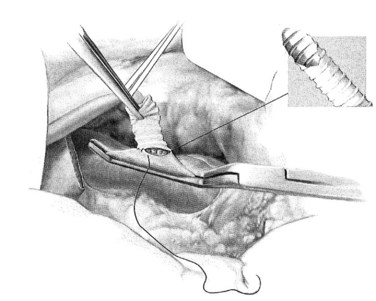
FIGURE 76B.4 Graft-caval anastomosis.
(From Rosemurgy A, et al, 2007: 8-mm Interposition Portacaval Shunt. In Clavian P, Sarr M, Fong Y [eds]: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 675-685.)
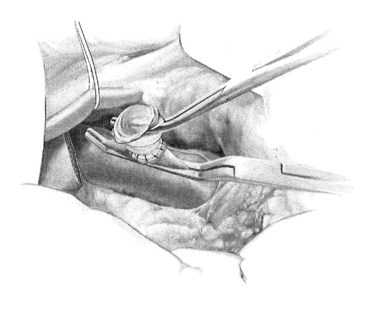
FIGURE 76B.5 Testing the graft-cava anastomosis.
(From Rosemurgy A, et al, 2007: 8-mm Interposition Portacaval Shunt. In Clavian P, Sarr M, Fong Y [eds]: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 675-685.)
In constructing the PV-graft anastomosis, the posterolateral wall of the PV is grasped with a Russian forceps and retracted laterally. A right-angled side-biting clamp is then placed on the PV, although the PV need not be completely occluded to perform the anastomosis. An 11-blade scalpel is used to achieve a venotomy, which is then extended with Potts scissors. A retraction suture is placed in the mid-ventral venotomy to facilitate exposure (Fig. 76B.6). The anastomosis begins at the mid-dorsal/posterior aspect of the venotomy using a double-armed 5-0 polypropylene suture. Again, a horizontal mattress suture is placed, followed by a running suture (Fig. 76B.7), sewing in-to-out on the vein and out-to-in on the graft in a cephalad then caudal direction. Once the back row is complete, the suture is drawn tight with a nerve hook. The ventral layer is then sewn with each free end of the double-armed suture, converging upon the midportion of the venotomy. Prior to closure, the PV clamp is partially released to blow clot and debris. Again, once the clamp is reapplied, vigorous irrigation of the graft and PV with heparinized saline is carried out.
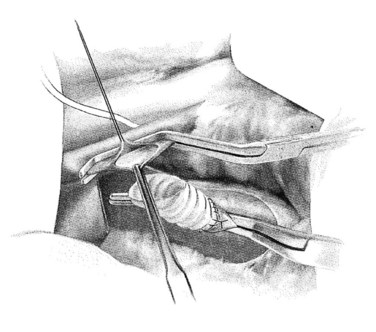
FIGURE 76B.6 Preparation of the portal vein–graft anastomosis.
(From Rosemurgy A, et al, 2007: 8-mm Interposition Portacaval Shunt. In Clavian P, Sarr M, Fong Y [eds]: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 675-685.)
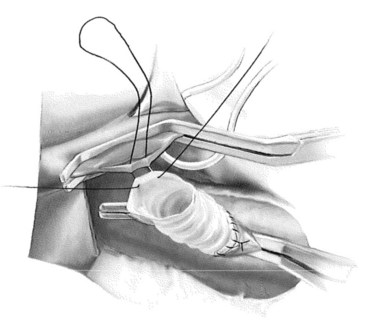
FIGURE 76B.7 Portal vein–graft anastomosis.
(From Rosemurgy A, et al, 2007: 8-mm Interposition Portacaval Shunt. In Clavian P, Sarr M, Fong Y [eds]: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 675-685.)
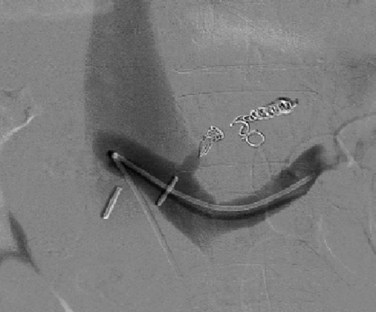
Next, the sutures are tied along the anterior aspect of the anastomosis; this requires that arm of the suture be reversed to tie the sutures from opposing sides of the anastomosis (Fig. 76B.8). The clamp on the vena cava is removed prior to removing the clamp on the PV; a palpable thrill should be present in the vena cava cephalad to the anastomosis. Two metallic clips are preferentially placed cephalad and caudal to the caval anastomosis to allow radiologists to identify and cannulate the anastomosis (Fig. 76B.9).
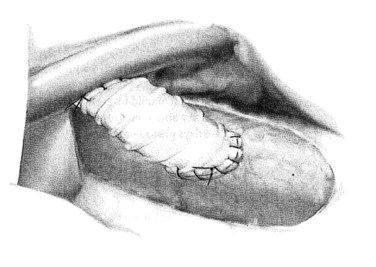
FIGURE 76B.8 The completed small-diameter prosthetic H-graft portacaval shunt.
(From Rosemurgy A, et al, 2007: 8-mm Interposition Portacaval Shunt. In Clavian P, Sarr M, Fong Y [eds]: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. New York, Springer, pp 675-685.)
Clark WC, et al. Surgical shunting versus transjugular intrahepatic portasystemic shunting for bleeding varices resulting from portal hypertension and cirrhosis: a meta-analysis. Am Surg. 2010;76(8):857-864.
Kravetz D. Prevention of recurrent esophageal variceal hemorrhage: review and current recommendations. J Clin Gastroenterol. 2007;41:S318-S322.
Rosemurgy AS. Small-diameter interposition shunt. In: Fischer, JE, Bland, KI. Mastery of Surgery. 5th ed. Philadelphia, Lippincott: Williams & Wilkins; 2007:1346-1351.
Rosemurgy AS, Thometz DP, Zervos EE. Portal blood flow, effective hepatic blood flow, and outcome after partial portal decompression. J Surg Res. 2003;117:64-70.
Rosemurgy AS, et al. The effect of partial portal decompression on portal blood flow and effective hepatic blood flow in man: a prospective study. J Surg Res. 1995;59:627-630.
Rosemurgy AS, et al. H-graft portacaval shunts versus TIPS: ten-year follow up of a randomized trial with comparison to predicted survivals. Ann Surg. 2005;241(2):238-246.