Chapter 115 Tarlov Cysts
Tarlov first described these cysts in 1938 during his autopsy studies of the filum terminale at the Montreal Neurological Institute.1 Since his seminal report, numerous cases of symptomatic Tarlov cysts have been published in the literature.2–7 With the advent of MRI, our ability to diagnose meningeal cysts, such as Tarlov cysts, has been enhanced.
Epidemiology and Histology
Tarlov, or perineurial, cysts are one of the most common forms of meningeal cyst. Estimates of the prevalence of meningeal cysts, including Tarlov cysts, in the general population vary, but generally are in the 5% range.8 In a study of 500 consecutive patients with back pain undergoing lumbosacral MRI, 5% were found to have one or more meningeal cysts. Among this latter group, the cyst was thought to be the source of the symptoms in 1% of the cases. Tarlov cysts, particularly those that are symptomatic, are more common among women. The reason for this is unclear, and we have postulated that there may be gender-related differences in the fundamental make-up of dura mater or spinal nerve roots that produce this epidemiologic disparity.
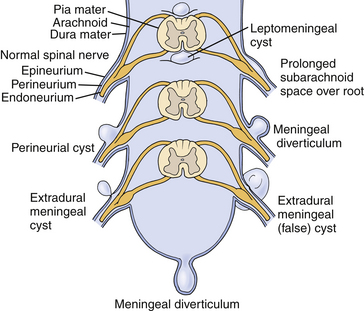
Tarlov distinguished perineurial cysts from other meningeal cysts based on several histologic criteria.1,9,10 He defined them as perineurial dilations that develop between the endoneurium and perineurium, typically of the S2 or 3 nerve roots, just proximal to the junction of the dorsal root ganglion and nerve root (Fig. 115-1). Simply stated, each cyst is a dilated spinal nerve root sheath, and the individual nerve fibers of that root are found running within the cyst cavity or its inner lining. Other meningeal cyst subtypes, such as meningeal diverticula and arachnoid cysts, typically are devoid of nerve root fiber elements.
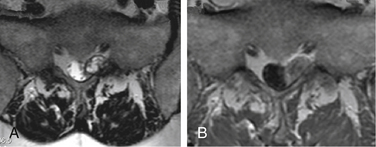
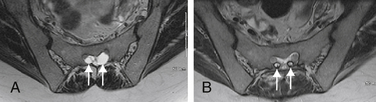
Tarlov cysts can be single or multiple, and can develop anywhere along the spine where nerve roots are present. Progressive cyst enlargement can cause significant bony erosion and impingement of adjacent spinal nerve roots, producing corresponding radiculopathies. For example, a Tarlov cyst in the sacral spinal canal arising from the S3 nerve root can cause symptomatic impingement of the ipsilateral S2 nerve root beside it, and of the S4 or S5 nerve root below (Fig. 115-2). A Tarlov cyst can also produce contralateral symptoms if it is large enough to extend across the midline and compress contralateral nerve roots. Additionally, the nerve root fibers running inside a Tarlov cyst often are attenuated and splayed out over the inner wall of the cyst. This neural fiber alteration and stretching also are suspected of causing symptoms.
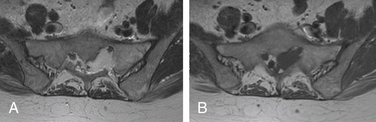
Tarlov cysts occasionally can be found in combination with other meningeal cysts. For example, patients with connective tissue disorders, such as Marfan syndrome, can have Tarlov cysts and large ectatic dural cysts so extensive that the distal spinal sac extends out into the pelvis (Fig. 115-3).
The pathogenesis of Tarlov cysts remains unclear. Tarlov proposed that cyst formation could be the result of trauma, ischemic degeneration, inflammation, or hemorrhagic infiltration from the subarachnoid space.1,9,10 Some patients with symptomatic Tarlov cysts report a history of sacral trauma, and evidence of old hemorrhage in the form of hemosiderin deposits and dystrophic calcification within Tarlov cyst walls supports prior trauma as an etiologic factor.7,11–13 Other reports have suggested that Tarlov cysts result from arachnoidal proliferation or blockage of perineurial fluid flow.14,15 Nabors et al. support a developmental origin, although an association between Tarlov cysts and spinal dysraphism is not as strong as that with other types of meningeal cysts.16 Only two patients with symptomatic Tarlov cysts and spina bifida have been reported, and the relationship could have been coincidental.7,17
Strully et al.18,19 and Smith20 proposed that Tarlov cysts form as a result of increased CSF hydrostatic pressure. They point out that spinal nerve roots are in communication with the thecal sac, and that there is myelographic evidence that spinal fluid flows within the nerve roots and could produce dilatation due to either higher hydrostatic pressure or inherent, traumatic, or iatrogenic weakness in the nerve root sheath. They also point out that the frequency and size of Tarlov cysts along the spine can be correlated with the rostral-caudal hydrostatic pressure gradient.17,18 Several reports on patients with Tarlov cysts have documented either a history of straining or coughing or an exacerbation of symptoms by these maneuvers.7,10,11,18 We also are aware of two cases of Tarlov cysts in patients with pseudotumor cerebri. However, no criteria have been established to determine who might benefit from CSF shunting for Tarlov cysts, and investigations are ongoing.
Diagnosis
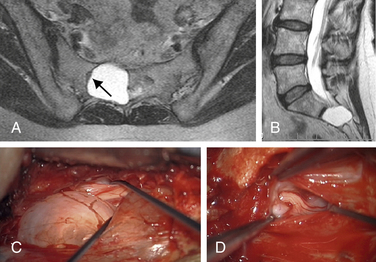
Even more unfortunately, we have encountered patients with symptomatic Tarlov cysts that were misdiagnosed with a variety of other ailments and treated unsuccessfully with a variety of procedures, such as hysterectomy, laparoscopic exploration, endometriosis surgery, oophorectomy, appendectomy, surgery for piriformis syndrome, sacroiliac joint fusion with implanted cages, fusion of degenerative discs in the adjacent spine, coccygectomy, and urinary bladder procedures (Fig. 115-4).
Differential Diagnosis
Other Masses
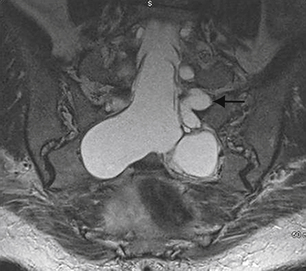
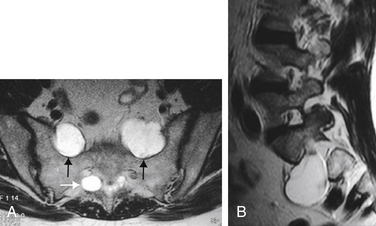
Schwannomas and neurofibromas can produce a radiculopathic pattern of symptoms similar to those of Tarlov cysts. They also can share imaging characteristics, such as a cystic appearance, lateral location in the spinal canal, and production of bone erosion and foraminal expansion (Fig. 115-5). However, Tarlov cysts do not enhance on MRI following the administration of gadolinium contrast, which is a characteristic typical of schwannomas and neurofibromas. Instead, they have signal characteristics similar to spinal fluid on all sequences, with the possible exception of differing signal when there has been hemorrhage or accumulation of stagnant, more proteinaceous, spinal fluid within a cyst.
We also have encountered other lesions with imaging characteristics similar to those of Tarlov cysts. For example, in one case, a large cystic lesion filling the left S1 foramen had the T2-weighted imaging appearance of a Tarlov cyst, but differed on other sequences (Fig. 115-6). Further workup revealed the lesion to be a large venous angioma within the spinal canal.
Radiography
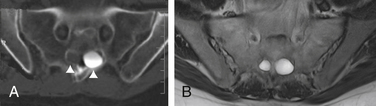
MRI currently is the gold standard for imaging meningeal cysts.21,22 Not only is it useful for differentiating them from other lesions, but it also can help distinguish among the different types of meningeal cysts, including Tarlov cysts. For example, Tarlov cysts typically are lateral in the spinal canal and arise from an individual spinal nerve root. Nerve root fibers often are identifiable inside the cyst on T2-weighted images (Fig. 115-7). In contrast, meningeal diverticula are found centrally in the spinal canal, and arise from the tip of the spinal sac, not from an individual nerve root. When they are large, Tarlov cysts can be seen to erode and expand the spinal canal or neural foramina and extend into the retroperitoneal pelvis (Fig. 115-8).
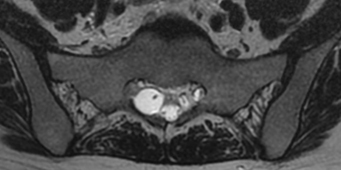
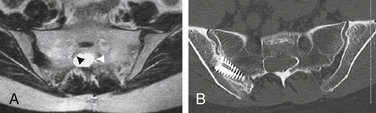
FIGURE 115-7 The nerve root fiber bundle inside this sacral Tarlov cyst is clearly seen on an axial T2-weighted MRI.
CT myelography previously was used preoperatively to distinguish Tarlov cysts from other forms of meningeal cysts based on the premise that Tarlov cysts filled poorly and in a delayed fashion.1,2,16,19 Other meningeal cysts, such as meningeal diverticula, were believed to fill more rapidly. However, these criteria are not reliable, because the extent of dye penetration into a cyst depends on the degree of its communication with the spinal sac, not on the cyst type. For example, we have encountered Tarlov cysts that communicate quite freely with the spinal sac, and filled readily on CT myelography (Fig. 115-9). Such cysts would have been erroneously categorized as non-Tarlov cysts using the more antiquated radiographic criteria.
Despite the fact that meningeal cysts, particularly Tarlov cysts, can be radiographically impressive, a careful search should be conducted to rule out other pathology that might explain the patient’s symptoms. For example, a complete workup of a sacral Tarlov cyst should include not only a sacral MRI but also an MRI and flexion-extension radiographs of the lumbar spine to evaluate for disc herniations, stenosis, spondylolisthesis, metastases, hemorrhages, and other possible pathologies.
Treatment
Needle Aspiration
Unfortunately, a significant number of patients with symptomatic Tarlov cysts undergo percutaneous needle aspiration procedures as an attempt at treatment, not for diagnosis. Such procedures are ineffective, because aspirated fluid within a Tarlov cyst typically is replaced rapidly with spinal fluid through the proximal nerve root in communication with the spinal sac.2,7,16 One study that assessed the effectiveness of percutaneous drainage of Tarlov cysts found that four of the five patients in that series suffered a recurrence of symptoms.8 A separate report in 2001 described patients who underwent percutaneous aspiration of their cysts preoperatively.7 None of those patients improved, and, in fact, some experienced marked worsening of their symptoms. This deterioration may have been the result of hemorrhage in the cyst wall, or nerve root injury.
Needle Aspiration and Fibrin Glue Injection
Treatment with percutaneous aspiration followed by fibrin glue injection also has been described. Authors of one report of four patients with symptomatic Tarlov cysts found that percutaneous fibrin glue therapy was effective in alleviating symptoms, although three patients developed postprocedural aseptic meningitis.23 Blind percutaneous introduction of multiple needles into a Tarlov cyst also increases the probability of causing nerve injury.
In our experience, fibrin glue treatment fails in a large number of patients, and those patients require subsequent surgery. Unfortunately, the introduction of fibrin glue or other substrates into a Tarlov cyst makes subsequent surgical treatment more difficult because it produces scarring or coating. The neural elements within the cyst are then much harder to identify and protect during surgery. We have encountered injured nerve roots intraoperatively in several patients following prior failed fibrin glue treatment (Fig. 115-10). The fibrin glue technique is falling out of favor, both in the United States and abroad, with only a few centers with a significant experience continuing its use.
Surgical Treatment
Patient Selection
As with most spinal pathology, the selection of patients for surgical treatment is based on the correlation among symptoms, physical examination, and radiographic findings. Additionally, the size of a Tarlov cyst is an important factor in determining the probability that it is symptomatic. In general, the larger the cyst the more likely it is to produce symptoms. One study found that patients with neurologic deficits that can be radiographically correlated with Tarlov cysts greater than 1.5 cm in diameter enjoyed substantial improvement following surgery.7 Furthermore, there was a very strong association between the presence of radicular symptoms and excellent outcome.
Surgery
The treatment of a symptomatic Tarlov cyst must take into account that the cyst is actually a dilated nerve root. Therefore, simple excision usually is not an option, because it can produce a critical deficit, particularly when in the lumbosacral region. This results in a treatment quandary: how does one eliminate a symptomatic Tarlov cyst without injuring the nerve root fibers inside and producing a neurologic deficit?
In the past, surgical treatments such as decompressive laminectomy, cyst fenestration, and complete cyst excision have fallen from favor due to a lack of success and unacceptable complication rates.2,3,6,24 More recent surgical techniques have targeted the underlying pathology causing nerve root dilation, that is, the process that allows spinal fluid to accumulate within an affected nerve root.
An initial step in this direction was the description of fenestration and imbrication techniques that involve opening a Tarlov cyst.6 The cyst is then reduced in size, either by imbrication or partial wall excision, thereby reconstituting a more normal caliber nerve root, which no longer compresses adjacent structures. However, such techniques do not prevent the continued flow of spinal fluid into the affected nerve root cyst, so they do not eliminate the risk of cyst reexpansion and spinal fluid leakage. Additionally, nerve fascicles in a Tarlov cyst often are found within the cyst wall itself, or so extensively splayed out over the internal surface of the cyst wall that they are not easily seen, even with the aid of an operating microscope. Cyst wall excision to decrease the overall size of a Tarlov cyst therefore increases the risk of producing deficits due to inadvertent nerve root fiber sectioning.
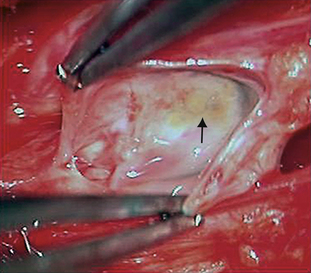
Current surgical techniques are focused on resolving the quandary of how to prevent spinal fluid flow into a symptomatic Tarlov cyst without injuring its nerve root fibers. To this end, we have focused on treatment of the ostium, where spinal fluid and nerve root fibers enter the cyst. We also have made efforts to confine cysts to prevent further compression of adjacent structures (Fig. 115-11).
Acosta F., Quinones-Hinojosa A., Schmidt M.H., et al. Diagnosis and management of sacral Tarlov cysts. AJNR Am J Neurosurg Focus. 2003;15:1-10.
Feigenbaum F., Henderson F. Surgical management of meningeal cysts, including perineural (Tarlov) cysts and meningeal diverticula. Semin Spine Surg. 2006;18:154-160.
Nabors N.W., Pait T.G., Byrd E.B., et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988;68:366-377.
North R.B., Kidd D.H., Wang H. Occult, bilateral anterior sacral and intrasacral meningeal and perineural cysts: case report and review of the literature. Neurosurgery. 1990;27:981-986.
Patel M.R., Louie W., Rachlin J. Percutaneous fibrin glue therapy of meningeal cysts of the sacral spine. AJR Am J Roentgenol. 1997;168:367-370.
Paulsen R.D., Call G.A., Murtagh F.R. Prevalencce and percutaneous drainage of cysts of the sacral nerve root sheath (Tarlov cysts). AJNR Am J Neuroradiol. 1994;15:293-297.
Strully K., Heiser S. Lumbar and sacral cysts of meningeal origin. Radiology. 1954;62:544-549.
Tarlov I.M. Perineurial cysts of the spinal nerve roots. Arch Neurol Psychiatry (Chic). 1938;40:1067-1074.
Voyadzis J.M., Bhargava P., Henderson F. Tarlov cysts: a study of 10 cases with review of the literature. J Neurosurg Spine. 2001;95:25-32.
1. Tarlov I.M. Perineurial cysts of the spinal nerve roots. Arch Neurol Psychiatry (Chic). 1938;40:1067-1074.
2. Acosta F., Quinones-Hinojosa A., Schmidt M.H., et al. Diagnosis and management of sacral Tarlov cysts. AJNR Am J Neurosurg Focus. 2003;15:1-10.
3. Landers J., Seex K. Sacral perineural cysts: imaging and treatment options. Br J Neurosurg. 2002;16:182-185.
4. Mummaneni P.V., Pitts L.H., McCormack B.M., et al. Microsurgical treatment of symptomatic Tarlov cysts. Neurosurgery. 2000;47:74-79.
5. North R.B., Kidd D.H., Wang H. Occult, bilateral anterior sacral and intrasacral meningeal and perineural cysts: case report and review of the literature. Neurosurgery. 1990;27:981-986.
6. Van de Kelft E., van Vyve M. Chronic perineal pain related to sacral meningeal cysts. Neurosurgery. 1991;29:223-226.
7. Voyadzis J.M., Bhargava P., Henderson F. Tarlov cysts: a study of 10 cases with review of the literature. J Neurosurg Spine. 2001;95:25-32.
8. Paulsen R.D., Call G.A., Mrutagh F.R. Prevalence and percutaneous drainage of cysts of the sacral nerve root sheath (Tarlov cysts). AJNR Am J Neuroradiol. 1994;15:293-297.
9. Tarlov I.M. Sacral nerve-root cysts: another cause of the sciatic or cauda equina syndrome. Springfield, IL: Charles C. Thomas; 1953. pp 56–116
10. Tarlov I.M. Spinal perineurial and meningeal cysts. J Neurol Neurosurg Psychiatry. 1970;33:833-843.
11. Basauri L., Hudson H., Bardales A. Case reports and technical notes: diverticuli of the nerve root sheaths. J Neurosurg. 1969;31:680-682.
12. Foreman S.M., Centeno R., Kerber C.W. Diagnosis of perineurial arachnoid cysts using computed tomography: technical and clinical considerations. J Manipulative Physiol Ther. 1986;9(1):23-26.
13. Kageyama Y., Machida A., Okada M., et al. Sacral perineurial cyst with ossification of the arachnoid membrane. Rev Rhum. 1998;65(2):153-156.
14. Dickenman R.C., Chason J.L. Cysts of dorsal root ganglia: report of 29 cysts and review of the literature. Arch Pathol. 1964;77:366-369.
15. Rexed B.A., Wennstrom K.G. Arachnoidal proliferation and cystic formation in the spinal nerve-root pouches of man. J Neurosurg. 1959;16:73-84.
16. Nabors N.W., Pait T.G., Byrd E.B., et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988;68:366-377.
17. Nishiura I., Koyama T., Handa J. Intrasacral perineurial cyst. Surg Neurol. 1985;23:265-269.
18. Strully K. Meningeal diverticula of sacral nerve roots (perineurial cysts). JAMA. 1956;161:1147-1152.
19. Strully K., Heiser S. Lumbar and sacral cysts of meningeal origin. Radiology. 1954;62:544-549.
20. Smith D.T. Cystic formations associated with human spinal nerve roots. J Neurosurg. 1961;18:654-660.
21. Boukobza M., Sichez J.P., Rolland E., et al. MRI evaluation of sacral cysts. J Neuroradiol. 1993;20:266-271.
22. Feigenbaum F., Henderson F. Surgical management of meningeal cysts, including perineural (Tarlov) cysts and meningeal diverticula. Semin Spine Surg. 2006;18:154-160.
23. Patel M.R., Louie W., Rachlin J. Percutaneous fibrin glue therapy of meningeal cysts of the sacral spine. AJR Am J Roentgenol. 1997;168:367-370.
24. Bartels R.H., van Overbeeke J.J. Lumbar cerebrospinal fluid drainage for symptomatic sacral nerve root cysts: an adjuvant diagnostic procedure and/or alternative treatment? Technical case report. Neurosurgery. 1997;40:861-864.