Chapter 22 Targeted Radionuclide Therapy—Expanded Content
Selective delivery of radionuclides to cancer cells using an antibody or other conjugate has been under investigation for more than 20 years. In 2002, the Food and Drug Administration (FDA) granted its first approval for a radiolabeled antibody. Initially, this form of radionuclide therapy mainly involved the use of antibodies or antibody-derived constructs as carriers of radionuclides and is therefore called radioimmunotherapy (RIT). However, because the concept also includes binding to nonantigen receptors, targeted radionuclide therapy (TaRT) is a more comprehensive term. Paul Wallner coined the acronym STaRT for systemic targeted radionuclide therapy. The development of TaRT has required the cooperation of basic scientists in the areas of radiation biology, chemistry, physics, and immunology with multiple clinical specialists. With the exception of some gene therapy approaches, TaRT differs from external beam radiation therapy in that selective targeting can be at the cellular level rather than the target volume level. Among its potential applications, TaRT provides a means of irradiating multiple tumor sites throughout the body with relative sparing of normal tissues. A number of challenges hampering the use of TaRT have been overcome, and others are areas of active investigation. Many of these are covered in more detail in other reviews.1–15
Tumor Targeting
Antibody Targeting
The efficacy of RIT is dependent on a number of factors, including properties of the targeted antigen or receptor, tumor, and targeting agent. Antigen/receptor variables include affinity, avidity, density, availability, shedding, and heterogeneity of expression.16 Tumor factors include vascularity, blood flow, and permeability. Antibody features to consider are specificity of the binding site, which affects selective tumor uptake; immunoreactivity, which can affect localization; stability in vivo; and both avidity and affinity.16–18 Affinity can be described by an intrinsic association constant K that characterizes binding of a univalent ligand (formation of a stable antibody-antigen complex) and can be calculated from the ratio of the rate constants for association and dissociation. Because intact antibodies and most antigens are multivalent, the tendency to bind depends upon the affinity, number of binding sites, and other nonspecific factors involved in aggregation. Therefore the term avidity is used to describe the overall tendency of antibody to bind to antigen.
The optimal level of binding affinity for maximal in vivo effectiveness is controversial, and binding affinity does not always correlate with activity in vivo.17,19 One might expect that high-affinity antibodies compared with low-affinity antibodies would result in increased tumor uptake, increased retention, and improved efficacy, as has been shown in some studies.20–22 However, high-affinity antibodies may preferentially bind to perivascular regions in the periphery of tumors, whereas antibodies of lower affinity are able to penetrate deeper into tumors.23 The optimal amount of unlabeled antibody remains undetermined for most agents, but relatively high doses have been as good as or better than lower doses in most imaging studies.24,25 Efforts by the Seattle transplant team to optimize the amount of unlabeled antibody for individual patients by tracer studies with varying amounts often showed the highest concentration studied to be as good as or better than direct RIT with iodine-131 (131I) antibody conjugates.26 Recent modeling with alpha-emitting conjugates attempts to maximize tumor cell kill while remaining within renal tolerance by individualization based on antigen ratio, adjuvant chemotherapy cytoreduction, and dose fractionation.13,27 A wide variety of antibodies have been made against a number of tumor-specific and tumor-associated antigens, which, although present on some normal cells, are usually expressed at lower levels on normal cells than on the targeted tumor cells. Most RIT trials have used monoclonal antibodies, and many have used intact murine immunoglobulin G (IgG) antibodies. Early on, the immunogenicity of the nonhuman antibodies was recognized as a serious limitation of RIT.28 With the exception of patients with lymphoma, who are less prone to develop an immune response to murine antibodies (human antimouse antibody [HAMA] response), more than 80% of patients usually develop an immune response against a therapeutically administered murine or other species antibody after a single injection of antibody.28 Such an immune response can occur even after doses as small as 1 mg used for imaging studies or following administration of antibody fragments or smaller constructs (but with less frequency than is found following administration of intact IgG). Administration of antibody in a patient with a HAMA response can result in a severe immune reaction and rapid blood clearance, limiting tumor uptake of radiolabeled antibody.29 Several approaches have been employed in an effort to ameliorate the problem of antibody immunogenicity, such as (1) the development of less immunogenic (chimeric and humanized) antibodies, (2) the use of immunosuppression by cyclosporine or deoxyspergualin to prevent an immune response, (3) the removal of HAMA directed to the therapeutic antibody by administration of an excess of unlabeled antibody before the administration of the radioimmunoconjugate, or (4) post-treatment removal of immune complexes by passing the patient’s blood through an extracorporeal immunoadsorption column.30–33
Genetic engineering has been successful in providing numerous targeting agents with reduced immunogenicity and has allowed for optimization of other aspects of the therapy.34–36 Genetic constructs have been produced that vary in specificity and can be altered by conjugating them to other agents (e.g., cytokines, toxins, or radiosensitizers) to form fusion proteins.37,38
Technologic advances have provided a wide variety of antibodies and constructs varying in specificity, size, and number of antigen combining sites, rapidity of distribution, immunogenicity, and immunologic function.39 Use of small antibody constructs such as single-chain antigen-binding proteins may be most useful for diagnostic studies or for multistep targeting strategies.40 The decreased immunogenicity of fragments/constructs and human and humanized antibodies now allows for administration of repeat courses or fractionated RIT dosing.41 As immunogenicity of antibodies has been ameliorated, some investigators have had concern about the potential immunogenicity of other components of therapy. For example, as more macrocyclic chelators have been developed to improve stability of conjugates, some data suggest that these molecules may be immunogenic as has been the streptavidin used in some pretargeting schemes.42–44 Therefore work continues to diminish immunogenicity problems from these other components.
Nonantibody Targeting
Several of the factors affecting antibody development/efficacy for TaRT are also pertinent to nonantigen targeting, such as receptor density, availability, affinity, shedding, and heterogeneity of receptor expression, as well as features of the tumor, including vascularity, blood flow, and permeability. The discovery made more than a decade ago that many tumors overexpress receptors for peptide hormones opened the field for TaRT and other forms of targeted therapy. Radiolabeled peptide analogs such as those for somatostatin, bombesin, calcitonin, oxytocin, and epidermal growth factor have been studied. Analogs of somatostatin are the most studied to date, with radiolabeled peptides being used for detection and treatment of a variety of neuroendocrine tumors.10,45,46 Initial treatment of tumors expressing somatostatin receptor subtype 2 was with higher doses of the diagnostic agent (111In-DTPA)-octreotide.47 Subsequently, the higher-affinity (DOTA, Tyr3) octreotide and (DOTA, Tyr3) octreotate were introduced and used with yttrium-90 (90Y) and lutetium-177 (177Lu) labeling as the therapeutic beta emitter for peptide receptor radionuclide therapy (PRRT). (Tyr3) octreotate differs from octreotide by substitution of tyrosine as the third residue and the replacement of threoninol by threonine. Early studies show impressive response rates and also show that fractionation of the administered dose as well as infusion of amino acids has ameliorated the dose-limiting renal toxicity.3,48,49 Various multicenter studies have been conducted for optimization of 90Y-(DOTA,Tyr3) octreotide, 177Lu-(DOTA,Tyr3) octreotate, and 90Y-(DOTA) lanreotide, another somatostatin analog.50 In addition to objective clinical response rates that are often higher than 25% and improved survival rates, clinical benefit in terms of decreased symptoms has usually been observed in the majority of patients with progressive neuroendocrine tumors.14,46,49,51 Radiolabeled peptides targeting tumor neovasculature are also under investigation and may provide therapy for nonendocrine cancers.52 Another targeting entity under investigation is the use of liposomes or nanoparticles as carriers of radionuclides.53–55 There continues to be progress on strategies that are not aimed at one tumor type, such as the targeting of tumor blood vessels and the evolving applications of nanoparticles.56–58
Selection of Radionuclides
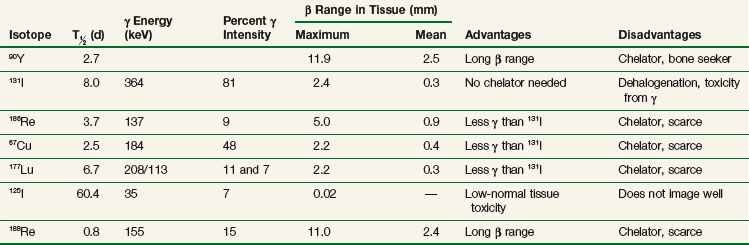
Early reviews provide detail on radionuclide selection, primarily with regard to the radiolabeling of whole immunoglobulins with beta emitters.59–61 Some later publications have focused on the selection of radionuclides for use with smaller engineered, antibody-derived constructs,62–64 and small nonantibody targeting delivery entities.65 Alpha emitters are also of increasing use.53,66–70 The emission profile of radionuclides determines their suitability for therapy and imaging. Particle energy and mean path length in tissue are important determinants of therapeutic efficacy, whereas the presence of gamma emissions at an appropriate energy is best for imaging. Some radionuclides such as (131I) have beta and gamma emissions that allow for both therapy and imaging. The therapeutic effect from such radionuclides is primarily because of the beta radiation, whereas the gamma component allows for imaging but contributes substantially more to normal tissue toxicity than efficacy. Important considerations for selection of radionuclides for TaRT include the size and biologic features of the tumor and antibody (or other targeting agent). In a one-step RIT administration using directly labeled antibodies, matching of the physical half-life of the radionuclide with the initial peak of tumor to nontumor antibody concentration was reported to be the most important factor for optimal radionuclide selection.59 For example, a good therapeutic ratio would not be obtained if one used a radionuclide that decays within several hours linked to an antibody that has a circulation time of several days, because most of the radiation exposure would have occurred before the radiolabeled antibody localized in the tumor. In this case, the resulting radiation dose to normal tissues would be nearly as great as that to the targeted tumor. Pretargeting strategies, discussed in more detail in the optimization section, allow for use of radionuclides with shorter half-lives because unlabeled methods of tumor targeting precede administration of the radionuclide.
The development of labeling and chelation chemistry for the production of stable radioconjugates has greatly facilitated TaRT. 131I can be directly attached to antibody without a chelator. However, if a targeted antigen/receptor undergoes modulation (internalization), the tumor cells can dehalogenate the conjugate and release free iodine. 131I can also be attached by a secondary molecule conjugated through an iodinatable linker rather than directly linked to antibodies. These radioimmunoconjugates appear to be more resistant to dehalogenation.71 A variety of increasingly stable chelators have been developed for 90Y and other metal radionuclides, but some have been found to be immunogenic (probably due in part to aggregation).42,44 The chemistry, stability, and in vivo processing of radioimmunoconjugates also have implications for toxicity. The filtration of small radioconjugates or their metabolites through the kidneys may result in considerable amounts of radiation being delivered to the kidneys. Renal toxicity has been reported to be the dose-limiting toxicity for some small-molecular-weight peptide conjugates and alpha emitter conjugates.68,72 Hepatic uptake, metabolism of radioimmunoconjugates, and retention of 90Y-complexes in the liver are potentially dose limiting in myeloablative TaRT.73 With some antibodies, comparison of radionuclides for optimization purposes has been studied using human tumors in xenograft models or theoretical modeling based on prior patient pharmacokinetics data.74–76 In summary, there are a variety of radionuclides that are suitable for TaRT. These include alpha emitters, low-, medium-, and high-energy beta emitters, and those that work through electron capture and/or internal conversion (Auger electrons). Beta emitters (e.g., 131I and 90Y) have been the most popular radionuclides for clinical TaRT trials to date. These radionuclides have the advantage of not needing to target every individual tumor cell, because the mean radiation range is approximately 0.4 mm and 2.5 mm, respectively. Features of some of these radionuclides are compared in Table 22-1. Alpha emitters offer promise for the future, especially when they are used to treat microscopic disease or are administered intratumorally. Their suitability includes the relatively short half-lives for most alpha emitters (e.g., 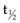 = 45.6 minutes for bismuth-213 (213Bi) and 7 hours for astatine-211 (211At) and a short range for the high-LET alpha particles (e.g., limited to 40 to 85 microns). These radionuclides may prove to be advantageous for the treatment of small-volume disease.77 Use of some alpha emitters with a longer half-life will facilitate more widespread application as it will be feasible to ship products.68,78,79
= 45.6 minutes for bismuth-213 (213Bi) and 7 hours for astatine-211 (211At) and a short range for the high-LET alpha particles (e.g., limited to 40 to 85 microns). These radionuclides may prove to be advantageous for the treatment of small-volume disease.77 Use of some alpha emitters with a longer half-life will facilitate more widespread application as it will be feasible to ship products.68,78,79
Designing chelators with special characteristics can also affect radionuclide “matching” with the targeting agent. For example, selective degradation of a cathepsin-sensitive chelate reduced liver uptake of radiometal.80 The use of such chelators may allow for dose escalation of radiometal conjugates such as curium-67 (67Cu) that otherwise would be limited by hepatic toxicity. Other conditionally cleavable chelators are also under study.81 The synthesis of the 2-(4-isothiocyanotobenzyl)-1,4,7,10-tetraaza-1,4,7,10-tetra-(2-carbamonyl methyl)-cyclododecane (TCMC) chelator has allowed progress in the use of lead-212 (212Pb).82 Macrocyclic chelators such as DOTA, which have been developed for stable chelation to 90Y, and other beta-emitting radiometals have shown acid lability with 212Pb and likely with other alpha emitters.83 To overcome this, the bifunctional chelating agent TCMC was synthesized, characterized, and subsequently used in several animal studies.82
Radiobiology
Biologic effects of TaRT may be associated with both the radionuclides and their carriers, either alone or in combination.84,85 This is particularly true for certain antibody carriers that may have antitumor efficacy alone (e.g., anti-CD20 antibodies). Antibody-induced responses can include apoptosis, complement-dependent cytotoxicity, and antibody-dependent cell-mediated cytotoxicity.86 Radiation effects can result in cell death during mitosis or by apoptosis, necrosis, and/or a metabolic cell death. The relative importance of these types of cell death depends on factors such as the inherent genetic makeup of the cells. For lymphomas, apoptosis is an important mechanism of cell death, whereas apoptosis usually plays less of a role in killing solid tumor cells. Lymphomas are especially sensitive to apoptosis induced not only by radiation but by a variety of cytotoxic agents, including antibody therapy alone.87–90 Several signal transduction pathways have been found to be associated with radiation-induced apoptosis. These have been reviewed by Hernandez and Knox.84 Antibody-induced intracellular signaling may also function to enhance the effects of RIT, and dose rate effects may be very important to the efficacy of RIT as well.91,92
Several studies have shown that cells exposed to either continuous or exponentially decreasing low-dose-rate (LDR) radiation are arrested in the radiosensitive G2/M phase of the cell cycle and undergo apoptosis.93–96 This G2/M block from LDR radiation as delivered via TaRT may also sensitize tumor cells to killing by other therapies such as external beam irradiation.97 Data from some experiments showing this effect suggest that therapy may be optimized by using TaRT before external beam irradiation.97,98 External beam irradiation has also been given before TaRT to increase tumor uptake of radionuclide conjugates, as will be discussed later. In addition, regulating cell cycle distribution or altering the underlying apoptotic potential by drugs or biologic response modifiers in tumor cells can affect the susceptibility of such cells to radiation-induced apoptosis.99 Recent studies include exploration of the use of gene transfer technology to induce expression of high-affinity membrane receptors to enhance the specificity of radioligand localization or the use of genetically engineered antibody molecules (e.g., diabodies: noncovalent single-chain Fv dimers) as carriers for TaRT that may be able to enhance the efficacy of this therapy.100–103
Although there is usually a positive correlation between the level of radiation-induced cell killing and the dose rate, an inverse dose rate effect has been reported with low-dose-rate irradiation in the range of about 0.3 Gy/hour, with some cell types being more sensitive to this inverse dose rate effect than others.104 Knox and colleagues105 showed lymphoma cells to be very sensitive to 131I-labeled antibody low-dose-rate radiation in a direct comparison to high-dose-rate external beam irradiation. At least one mechanism for this observation appears to be greater arrest in the G2/M phase of the cell cycle. Another mechanism of lymphoma radiation sensitivity may be less efficient DNA repair among lymphomas compared with some other cell types.84,85 Ongoing studies will help elucidate the radiobiology of TaRT, which will facilitate optimization of TaRT therapy in the future and provide the prerequisite understanding of underlying biologic mechanisms to determine how best to use TaRT in combination with other treatment modalities.
Clinical Results
Several routes of administration have been employed, including intravenous, intra-arterial, intrathecal, intraperitoneal, intratumoral, and, rarely, intrapleural administration. The most prevalent route of administration has been the intravenous route for systemic therapy. However, for localized disease, regional or intracavitary administration may be advantageous. Intraperitoneal RIT has generally been more efficacious than intravenous RIT for the treatment of small-volume disease confined to the peritoneal cavity, but there are conflicting reports concerning the relative merits of arterial and venous routes of administration of radiolabeled antibodies for other sites.106–110
Radioimmunotherapy of Nonhematologic Malignant Disease
Radioimmunotherapy (RIT) for solid tumors continues to be actively investigated as a form of systemic targeted radiation therapy in a variety of tumor types and clinical settings. Initial clinical efforts were with polyclonal antibodies produced in immunized rabbits and other species.111 Beginning in the 1970s with the development of hybridoma technology, large-scale production of clinical grade monoclonal antibodies (Mabs) selected against specific tumor-associated antigens became possible and greatly expanded the number of agents evaluated in clinical trials. Subsequent molecular engineering efforts allowed for the customized design of antibody constructs with properties better optimized for therapy.
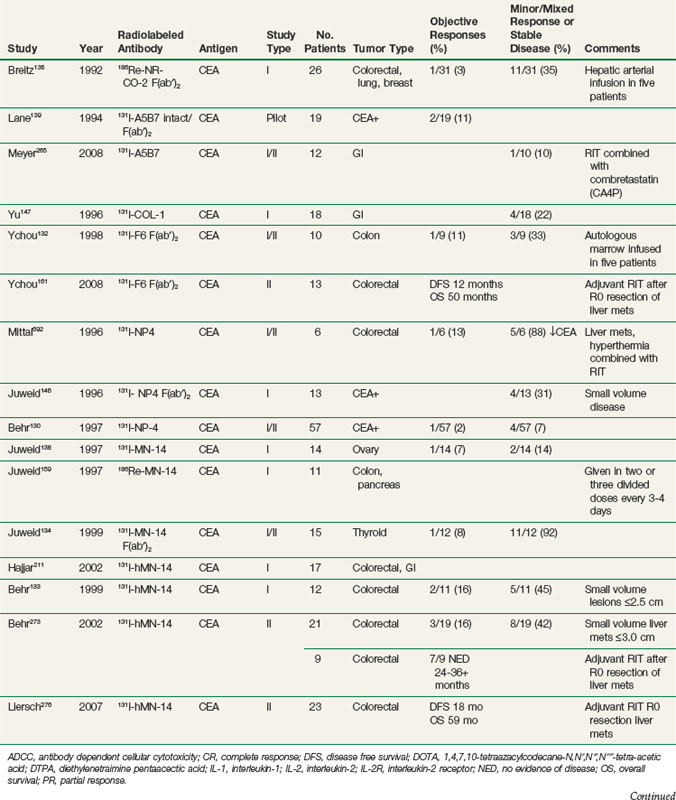
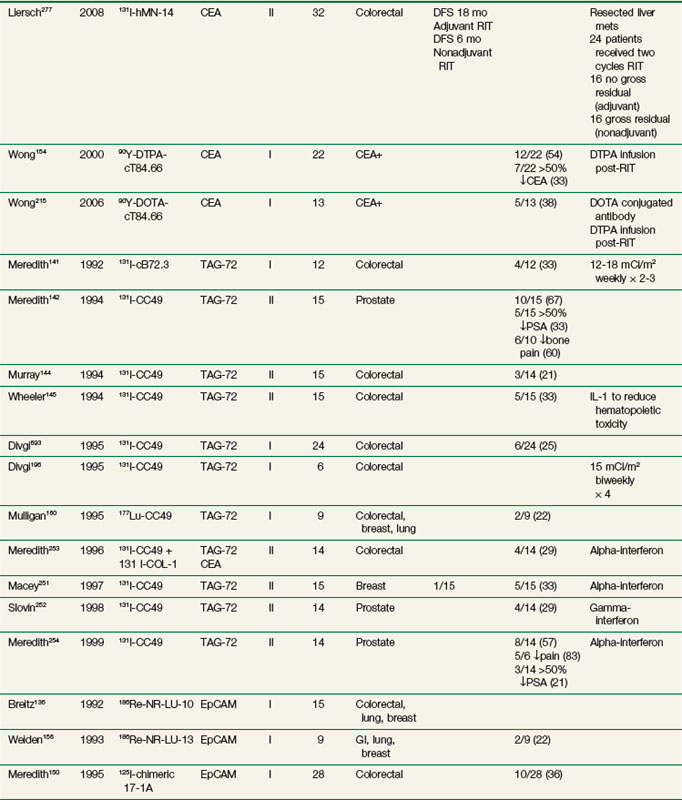
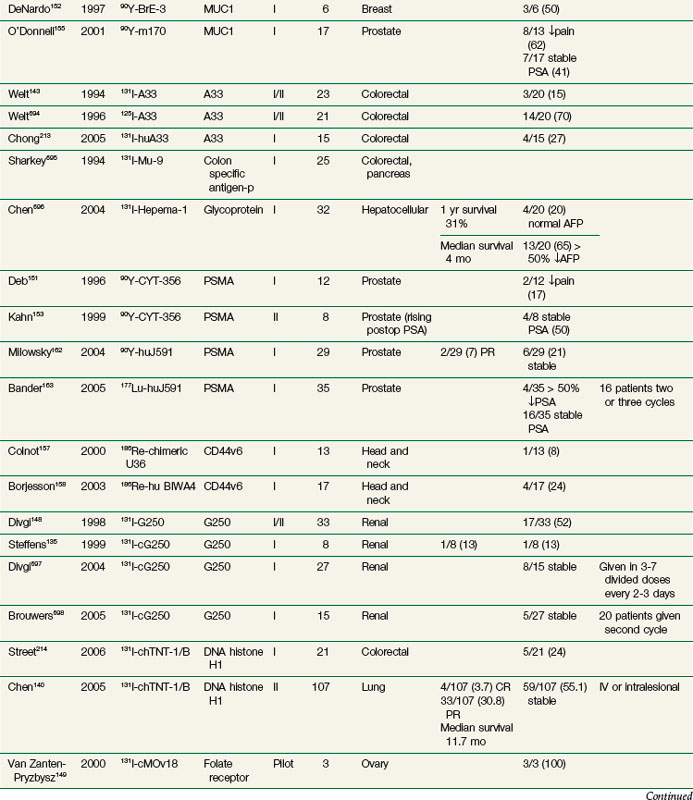
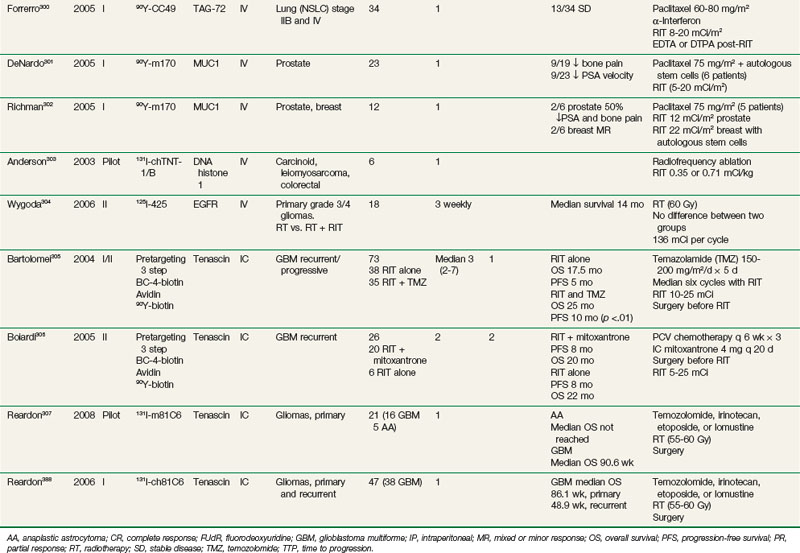
RIT demonstrated initial promise in animal models, with dozens of studies in tumor-bearing animal models documenting significant tumor growth delay and cures in a wide variety of solid tumor types.112–115 Tumor doses often exceeded 10 to 15 Gy after a single infusion.116,117 In some preclinical studies, RIT achieved comparable tumor growth delays as equivalent doses of single-fraction or conventionally fractionated external beam radiation therapy116–122 or equitoxic doses of chemotherapy.123 Initial clinical trials evaluating biodistribution and pharmacokinetics confirmed tumor targeting and demonstrated that therapeutic doses of radiation to tumor were potentially achievable.124–129 However, RIT in solid tumors has not realized the same results in the clinic as had been predicted from preclinical studies. Table 22-2 provides an overview of Mabs that have been evaluated in clinical trials. RIT has been evaluated in most major disease sites, including gastrointestinal, breast, brain, ovarian, head and neck, medullary thyroid, melanoma, renal, lung, and prostate cancers. Results from selected solid tumor trials evaluating systemically administered nonmyeloablative doses of RIT as a single modality are summarized in Table 22-3. Early efforts used Mabs labeled with 131I because of availability and ease of labeling, with subsequent trials using radiometals such as 90Y, rhenium-186 (186Re), and 177Lu. Almost all of the antibodies studied have been administered at low protein doses (~5 to 50 mg), have had no significant clinical immunomodulatory or antitumor properties, and have therefore served primarily as a delivery vehicle for radiation. Dose-limiting toxicities have primarily been thrombocytopenia and leukopenia. Results have been comparable regardless of disease site or the particular radionuclide-antibody combination.
TABLE 22-2 Examples of Monoclonal Antibodies Evaluated for RIT
| Malignancy | Antigen | Antibody |
|---|---|---|
| Colorectal cancer | CEA | cT84.66, hMN-14, A5B7, F6 |
| TAG-72 | B72.3, CC49 | |
| A33 | anti-A33 | |
| EpCAM | NR-LU-10, NR-LU-13, 17-1A | |
| DNA histone H1 | chTNT-1/B | |
| Breast cancer | MUC1 | huBrE-3, m170 |
| L6 | chL6 | |
| TAG-72 | CC49 | |
| CEA | cT84.66 | |
| Ovarian cancer | MUC1 | HMFG1 |
| Folate receptor | cMov18 | |
| TAG-72 | B72.3, CC49 | |
| Prostate cancer | PSMA | huJ591, CYT-356 |
| TAG-72 | CC49 | |
| Lung cancer | DNA histone H1 | chTNT-1/B |
| TAG72 | CC49 | |
| Head and neck cancer | CD44v6 | U36, BIWA4 |
| Gliomas | EGFR | 425 |
| Tenascin | 816C, BC4 | |
| Melanoma | p97 | 96.5 |
| Renal cancer | G250 glycoprotein | cG250 |
| Medullary thyroid | CEA | cT84.66, hMN-14, NP-4 |
| Neuroblastoma | Ganglioside GD2 | 3F8 |
| NCAM | UJ13A, ERIC-1 |
Until recently, most of the clinical experience involved phase I trials, evaluating RIT as monotherapy in patients with advanced, bulky, chemotherapy-refractory disease progressing into therapy. As expected in this patient population, partial and complete responses have been infrequent regardless of disease type,130–140 with most antitumor effects reported as stable disease and serologic, mixed, or minor responses.137,141–163 This is in contrast to clinical results observed with RIT in lymphomas with objective responses ranging from 30% to 85%.164–172
A number of factors have been put forth to explain the limited responses seen. With hematologic malignant disease, at least some of the observed response rates can be attributed to the immunologic effects of the antibody itself,173 which is not the case for most radiolabeled antibodies directed against solid tumors. Other factors involve those that restrict antibody targeting and uptake to tumor more so for solid tumors than for lymphomas. These include differences in tumor physiology, tumor vascularity, antigen accessibility, and heterogeneity of antigen expression.174,175 However, these factors probably play a minor role because the clinical literature demonstrates no significant differences in estimated RIT dose to tumor between solid tumors and hematologic cancers. This suggests that the difference in radiosensitivity between solid tumors and hematologic cancers is the major factor in explaining the difference in RIT response rates.
Table 22-4 compares tumor doses achieved after a single administration of nonmyeloablative activities of RIT for solid tumors and lymphomas and demonstrates that tumor doses are comparable for most antibodies evaluated in the clinic. Median and mean doses are approximately 10 to 20 Gy, with select tumors occasionally achieving doses of 20 to 70 Gy or greater. In our experience with 90Y-cT84.66 anti-CEA RIT, approximately 25% of tumors achieve tumor doses of more than 15 to 20 Gy after a single infusion. These data support the hypothesis that the differences in response rates between solid tumors and hematologic cancers are largely because of differences in radiosensitivity, not the result of differences in tumor antibody uptake. This is even after accounting for effects of unlabeled antilymphoma antibodies, because clinical trials have demonstrated no significant response rates with unlabeled antibodies such as Lym-1.176,177 In the case of 90Y-ibritumomab, a 24% increase in response rate was observed with the addition of radiolabeled antibody compared with unlabeled antibody alone in a recent randomized trial.173 Similarly for tositumomab, there was significant improvement in all therapeutic outcomes by additional 131I-labeled antibody.178
TABLE 22-4 Tumor Doses and Objective Response Rates from Selected Solid Tumor and Lymphoma RIT Trials
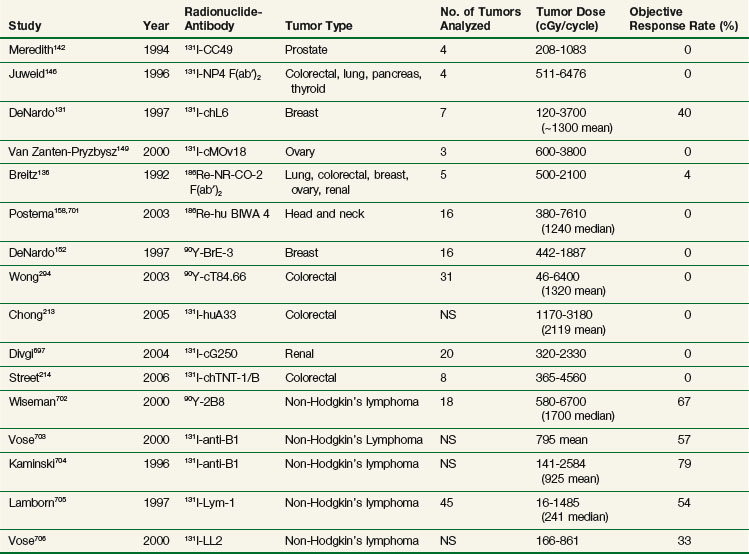
Although tumor doses with RIT are a fraction of what can be delivered by standard teletherapy or brachytherapy, doses currently achievable are at levels that can potentially result in clinically important antitumor effects in solid tumors if applied in the appropriate clinical setting, particularly in patients with subclinical or microscopic disease. With conventionally fractionated external beam radiotherapy, Withers and associates179 reviewed the clinical literature evaluating adjuvant radiation for solid tumors and noted an inverse linear relationship between radiation dose and tumor recurrence rate with doses ranging from 2000 to 5000 cGy. More importantly, there was no threshold effect and doses in the range of 2000 cGy had measurable effects in the adjuvant setting. Clinical trials have also demonstrated important antitumor effects in rectal cancer with doses in the range of 3000 cGy.180,181 Finally, doses as low as 3000 cGy conventionally fractionated have been combined with chemotherapy in esophageal and anal cancer, resulting in pathologic complete responses.182–184 Therefore centiGray doses to tumor achievable through RIT may have more clinical importance in low-tumor burden situations and/or as an additional agent added to a multiagent systemic therapy approach.
Autologous Stem Cell Support (see Table 22-3)
Because hematologic toxicity is dose limiting, groups have explored dose intensification strategies using stem cell reinfusion after myeloablative doses of radiolabeled Mabs. Most have been phase I studies treating a limited number of patients. Stem cell–supported RIT studies primarily in breast cancer have reported objective responses.185,186 Other studies have reported fewer or no objective responses.132,187–190 Stem cell reinfusion has permitted higher myeloablative doses to be administered, but it appears that dose intensification alone will not result in the level of objective response rates initially hoped for and will need to be combined with other optimization strategies.
Fractionation of Administered Activity (see Table 22-3)
Fractionated RIT usually involves administration of the total activity through divided infusions spaced days to a week apart instead of as a single infusion and takes advantage of the differences in uptake and clearance kinetics of activity between tumor and normal tissues, such that with the appropriate fractionation schedule the ratio between tumor dose and marrow dose can be amplified. The optimum schedule therefore may be dependent on a complex interplay of multiple factors, including uptake and clearance kinetics of the antibody construct, decay kinetics of the radionuclide, tumor size, radiosensitivity of tumor and organs, and clearance kinetics of the individual patient. A number of groups have reported in animal models reduced hematopoietic toxicity and greater antitumor effects with fractionation.191–194 Meredith and colleagues141 used a fractionated RIT schedule with 131I-chimeric B72.3 in patients with colorectal cancer who received 28 or 36 mCi/m2 in 2 to 3 weekly fractions of 12 to 18 mCi/m2. There was a modest but statistically significant (p = .04) decrease in hematopoietic toxicity adjusted for whole body dose when compared with a similar population treated in an earlier trial195 who received similar doses of 131I-chimeric B72.3 as a single infusion. Divgi and colleagues196 also evaluated a fractionated schedule with the anti-TAG-72 antibody CC49 radiolabeled with 131I. Six patients with colorectal cancer received 15 mCi/m2 biweekly up to a maximum of four infusions. This schedule was well tolerated with no dose-limiting toxicities observed.
Engineered Immunoconstructs
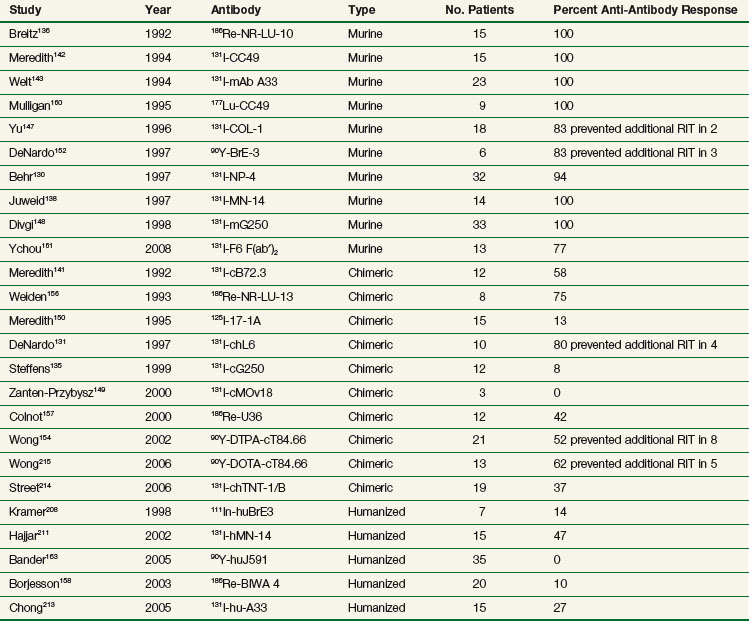
Molecular engineering of antibodies with properties optimized for therapy continues to be pursued. Reducing antibody immunogenicity to permit multiple administrations has been successful in the clinic. Murine-derived Mabs have the disadvantage of being recognized as foreign by the patient’s immune system, which can lead to the formation of human antimouse antibodies (HAMA) in 50% to 100% of patients after a single administration,197–200 resulting in faster blood clearance of subsequently administered antibody and potentially compromised efficacy.199,201 Reduction in antibody immunogenicity has been observed with the use of immunosuppressive agents such as cyclosporine A.186,202,203 Others have reduced antibody immunogenicity through modification of the antibody molecule using recombinant DNA techniques to replace murine with human antibody domains (e.g., chimeric antibodies, where the murine variable domains are retained, and humanized antibodies, where only the murine complementarily determining region (CDR) antigen recognition sites are retained152,157,158,204–215 (Table 22-5).
Recombinant antibodies can be designed with improved biodistribution and pharmacokinetic properties to optimize their use for therapy. Antibody fragments and other smaller-molecular-weight antibody constructs may ultimately prove superior for therapy because of their faster clearance, greater tumor penetration,216 more uniform distribution in tumor,217 higher initial dose rate in tumor,218 decreased immunogenicity,219 higher tumor to background ratios,115 and potential for higher administered activities and tumor doses for the same level of toxicity. Initial efforts evaluated radiolabeled Fab and F(ab′)2 fragments generated from whole immunoglobulin through enzymatic digestion132,136,139,146,220 (see Table 22-3). Unfortunately, antitumor effects appeared to be no better than with radiolabeled intact antibody. Although blood clearance may be more rapid with fragments, retention time in tumor is also reduced, resulting in no significant improvement in radiation dose to tumor over the intact antibody for equitoxic administered activities.
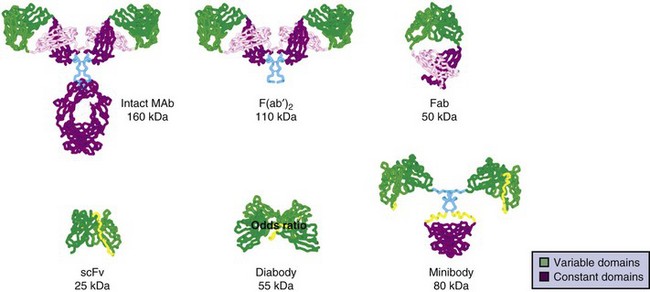
More recent efforts have used recombinant DNA technology to genetically engineer antibody constructs with properties to improve tumor to normal organ biodistribution, enhance in vivo stability, reduce immunogenicity, aid in conjugation and radiolabeling, and increase clearance kinetics. Constructs have ranged from approximately 25 to 160 kDa in size. The genes encoding the antibody’s variable light (VL) and variable heavy (VH) domains can be cloned and linked by a contiguously encoded peptide into a single-chain (sc) Fv construct. This small monovalent scFv, 28 kDa, clears rapidly from the circulation, increasing the tumor/blood ratio, making it attractive as a potential imaging agent.221,222 However, the absolute peak uptake and retention time in tumors are often reduced, limiting its use as a therapy agent.221–224 In addition, low- molecular-weight constructs can filter through the renal glomeruli, resulting in increased kidney uptake and radiation dose compared with higher-molecular-weight constructs. A more suitable construct for therapy might be bivalent, of intermediate molecular weight, and exhibit uptake and retention in tumor comparable to intact antibodies, but with faster clearance times than an intact Mab, resulting in an improved therapeutic ratio. Multivalent intermediate-molecular-weight constructs have been produced through covalent linking of monovalent fragments.193,223,225–233 Alternatively, single-chain monomers can be engineered with properties that promote spontaneous formation of dimeric and trimeric species. For example, Figure 22-1 shows an engineered series of recombinant fragments derived from the intact anti-CEA T84.66 Mab.234 This cognate family of anti-CEA constructs was radioiodinated and evaluated in animal biodistribution studies. The minibody (80 kDa), formed by the self-association of two scFv-CH3 single-chain monomers, was potentially suitable for therapy and resulted in peak tumor uptakes of over 20% to 30% injected dose per gram, which exceeded that of the diabody and F(ab′)2 and approached that of the intact antibody.235
A pilot imaging trial236 evaluating the biodistribution and tumor targeting properties of the 123I-cT84.66 minibody in 10 patients with colorectal cancer demonstrated faster blood clearance than intact cT84.66.237 To further evaluate its potential as a therapy agent, a follow-up pilot study evaluated 111In-DOTA-cT84.66 minibody, with four of five patients demonstrating tumor imaging. Predicted mean radiation dose to liver was 19.6 cGy, kidney 33.3 cGy, lung 1.5 cGy, and marrow 2.2 cGy per mCi of 90Y. Mean tumor dose was 12.4 cGy (6.8 to 25.1 cGy) per mCi of 90Y. Tumor dose to marrow dose ratio was 5.6, comparable to that seen in murine models, but less than the ratio of 10.5 seen for intact 90Y-DTPA-cT84.66154 and 11.6 for 90Y-DOTA-cT84.66215 seen in previous phase I trials.154,215
Other groups have investigated similar strategies.224,238–242 Forero and colleagues243 recently reported on a 153-kDa 131I-labeled humanized CH2 domain deleted construct (HuCC49ΔCH2) derived from CC49 anti-TAG72. Blood clearance 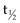 was 20 hours, comparable to cT84.66 minibody. A phase I dose escalation therapy trial in metastatic colorectal cancer was initiated, and preliminary results indicate reduced hematologic toxicity compared with intact murine 131I-CC49. At the initial dose level of 75 mCi/m2, only 25% of patients (one of four) developed hematopoietic toxicity higher than grade 3, compared with more than 60% of patients with 131I-murine CC49 in a previous trial.244
was 20 hours, comparable to cT84.66 minibody. A phase I dose escalation therapy trial in metastatic colorectal cancer was initiated, and preliminary results indicate reduced hematologic toxicity compared with intact murine 131I-CC49. At the initial dose level of 75 mCi/m2, only 25% of patients (one of four) developed hematopoietic toxicity higher than grade 3, compared with more than 60% of patients with 131I-murine CC49 in a previous trial.244
Modifying the Tumor Environment (see Table 22-3)
Up-regulation of tumor antigen expression as a means of increasing antibody targeting and radiation dose to tumor has been explored. Alpha and gamma interferon have been shown to increase CEA and TAG-72 expression,245–248 resulting in increased tumor antibody uptake and RIT efficacy in animal models.249,250 In the clinic, interferon has also increased tumor antigen expression and antibody uptake251–254 in breast, colorectal, and prostate cancer. However, antitumor effects remain modest with this strategy alone, leading investigators to now combine this approach with other optimization strategies.
Strategies to modify the tumor vasculature to increase blood flow or vascular leakage, using vasoactive agents, vasoactive immunoconjugates, radiation therapy, or hyperthermia, have been explored primarily in experimental models.255 Interest has focused on an emerging class of agents directed against the tumor neovasculature. Jain and colleagues256,257 and others have proposed using anti-angiogenic agents, such as anti-VEGF (vascular endothelial growth factor) antibodies, to “prune” or normalize the tumor vasculature, decrease tumor interstitial pressure, and increase tumor perfusion, which should increase macromolecule delivery. In some experimental models, tumor oxygenation is also increased,257 which can further enhance the antitumor effects of RIT. In a similar strategy, Burke and associates258 have looked at using cyclic RGD peptide, which recognizes the ανβ3 integrin receptor on tumor neovasculature. In a tumor-bearing breast cancer mouse model, the combination of RGD peptide and 90Y-chimeric L6 RIT resulted in higher tumor uptake of antibody and a greater percentage of mice cured than either therapy alone.258,259 In colorectal cancer tumor–bearing mice, Kinuya and colleagues260 combined the anti-angiogenic agent 2-methoxyestradiol with 131I-A7 RIT and demonstrated prolonged survival with the combination compared with either agent alone. Recently, Bodet-Milin and colleagues261 combined 131I-F6 anti-CEA RIT and bevacizumab (anti-VEGF) in medullary thyroid cancer–bearing mice and reported an increase in tumor growth delay with the combination compared with either agent alone.
Agents that increase vascular permeability also have been evaluated. A response-selective peptide agonist to human C5a resulted in an increase in 131I-B72.3 anti-TAG72 Mab and a twofold increase in colon cancer xenograft growth delay in vivo.151,262 The vascular disrupting agent combretastatin A-4-phosphate (CA4P) has been shown to increase tumor retention of 131I-AB7 anti-CEA antibody, resulting in an increased radiation dose to tumor263 and a significant increase in tumor growth suppression compared with 131I-AB7 RIT alone.264 A phase I/II trial was initiated and preliminary results recently reported.265 Twelve patients received 1311-A5B7 anti-CEA Mab given on day 1 and CA4P on days 2 and 3. Dynamic contrast-enhancing magnetic resonance imaging (MRI) demonstrated reduction in tumor vascular kinetics in 9 of 12 patients. Dose-limiting hematopoietic toxicity was observed at 131I-A5B7-administered activities of 43.2 mCi/m2 and CA4P of 54 mg/m2. Of 10 evaluable patients, 1 had stable disease.
Minimal Tumor Burden Setting (see Table 22-3)
Tumor size plays a dominant role in influencing antibody uptake, and clinically important outcomes are predicted if systemic RIT is applied in the subclinical or microscopic disease setting. Jain and colleagues216,266 have identified key physiologic factors that limit uptake of macromolecules. These factors include spatial heterogeneity of tumor vascularity, increased interstitial pressure in poorly vascularized areas, and limited diffusion distances of macromolecules, which work to significantly limit the ability of the antibody to reach all sites within the tumor. These factors are amplified as the tumor grows, resulting in an exponential decrease in tumor antibody uptake that has been demonstrated in vivo267–270 and in clinical trials.271,272 Given this inverse exponential relationship, a very modest reduction in tumor size will result in a substantial increase in antibody uptake.
RIT should therefore have its greatest impact in the adjuvant setting and in the treatment of minimal or microscopic disease. For example, Behr and colleagues133 and Vogel and associates194 successfully demonstrated improved survival rates and prevention of liver metastases in animal models with RIT delivered with adjuvant intent. Subsequent clinical trials evaluated this strategy in patients with small-volume disease. Behr and colleagues133 reported initial phase I trial results in 12 colorectal cancer patients with metastatic lesions of 2.5 cm or less. Patients received a single administration of 50 to 70 mCi/m2 131I-hMN14 anti-CEA RIT. Of 11 evaluable patients, 2 partial responses and 5 minor/mixed responses of up to 12 months’ duration were observed (see Table 22-3). This was followed by a phase II study of 30 patients with colorectal cancer and liver metastases who received a single administration of 131I-hMN14 anti-CEA RIT (60 mCi/m2).273 Of 19 evaluable patients who had small-volume disease of 3 cm or less, 3 patients experienced a partial response of 3 to 15 months’ duration and 8 patients had a minor response of 3 to 14 months’ duration. Five patients received a second cycle at time of progression at 8 to 16 months, which resulted in partial responses in two patients. In addition, nine patients received RIT for microscopic disease similar to the results in an adjuvant setting after a complete resection of hepatic metastases. Seven of nine remained disease free at more than 24 to 36 months, which compared favorably with historical controls at the same institution receiving 5-FU-based regimens following hepatic resection (see Table 22-3). These initial encouraging observations led to a phase II adjuvant RIT trial of 131I-hMN14 administered after resection of colorectal cancer liver metastases274,275 (see Table 22-3). In the most recent report, 23 patients who underwent R0 resection of liver metastases from colorectal cancer received a single administration of 40 to 60 mCi/m2 of 131I-hMN14 anti-CEA.276 Results were compared with those of a contemporaneous control group of 19 patients from the same institution treated following resection with 5-FU-based chemotherapy regimens. Median follow-up for the RIT group was 91 months and for the control group was 51 months. A statistically significant improvement in median overall survival rates in the RIT group (58 months vs. 31 months) was reported. Median disease-free survival time was greater for the RIT group (18 months vs. 12 months), but the difference did not reach statistical significance. The investigators concluded that a prospective randomized trial to evaluate 131I-hMN14 as adjuvant therapy in this patient population was warranted given the suggestion of a survival advantage. This same group recently reported preclinical results of an ongoing trial evaluating the feasibility of administering two courses of RIT after resection of liver metastases in patients with colorectal cancer.277 Thirty-two patients received a dose of 40 to 50 mCi/m2 131I-hMN14 anti-CEA, with 24 receiving a second RIT administration. Sixteen patients had no residual disease detectable after surgery and comprised the group that received adjuvant RIT. With follow-up of 21 months, disease-free survival time was 18 months for the adjuvant group and 6 months for the nonadjuvant group. The authors concluded that a second cycle of RIT is feasible with acceptable toxicity. A future randomized trial is planned in the same population that will compare two cycles of RIT versus RIT and chemotherapy as adjuvant therapy.
An adjuvant RIT trial in colorectal cancer was recently carried out by Ychou and colleagues,161 who administered a preoperative “diagnostic” dose of 131I-F6 F(ab′)2 anti-CEA (8 to 10 mCi) before planned surgery to 22 patients with one to four liver metastases. After complete resection (R0), 13 patients, including 10 who had tumor to liver uptake ratios of more than 5, received 180 to 200 mCi of 131I-F6 F(ab′)2 anti-CEA RIT. With a median follow-up of 127 months, median disease-free survival time was 12 months and median overall survival time was 50 months. One patient remained disease free at 93 months. The investigators concluded that RIT is feasible after surgery in this poor prognostic group and that RIT combined with chemotherapy should be further evaluated in this clinical setting.
At City of Hope, a recently completed phase I trial evaluated the feasibility and toxicities of 90Y-cT84.66 anti-CEA RIT combined with hepatic arterial fluorodeoxyuridine (FUdR) and systemic gemcitabine in colorectal cancer patients after resection and or radiofrequency ablation of liver metastases. No dose-limiting toxicities were observed in 13 patients with FUdR of 0.1 to 0.2 µg/kg/day for 14 days, gemcitabine 105 mg/kg, and RIT 16 .6 mCi/m2. Four patients remain progression free at over 4 to 37 months (see Table 22-3).
Combined-Modality Approaches (Table 22-6)
RIT and Chemotherapy
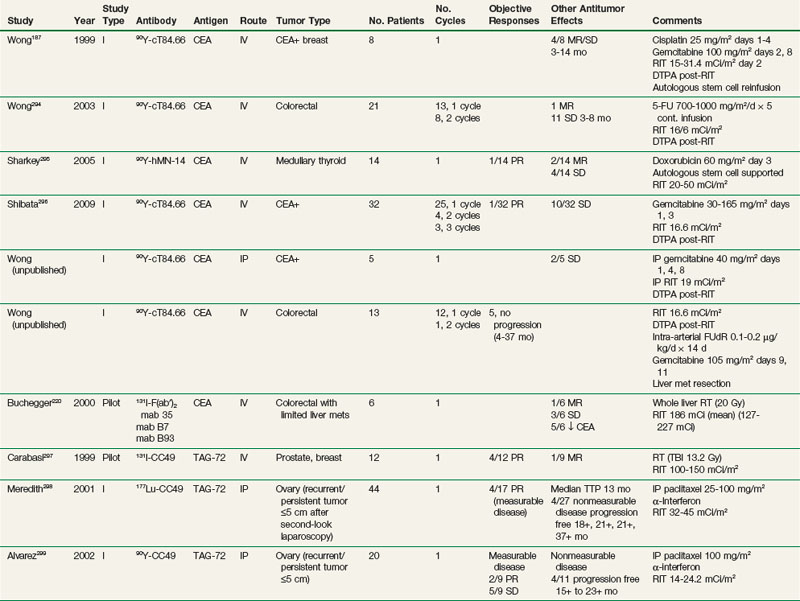
Multiple preclinical studies have documented additive or supra-additive antitumor effects when RIT is combined with radiation-enhancing chemotherapy or other systemic agents, including gemcitabine,278,279 taxanes,280,281 cisplatin,282 5-fluoruracil (5-FU),283–285 doxorubicin,286 halogenated pyrimidines,287 topoisomerase inhibitors,283,288,289 tirapazamine,290,291 epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors,292 and anti-EGFR Mabs.293 A growing number of clinical trials are evaluating the feasibility and potential improved efficacy of concomitant chemotherapy and RIT, either by adding chemotherapy to maximum tolerated doses of RIT or adding RIT as additional therapy to established chemotherapy regimens. Table 22-6 summarizes RIT-chemotherapy trials performed in a number of different solid tumor types, including ovarian, breast, prostate, medullary thyroid, and colorectal cancer.187,220,294–307 In some studies, the strategy of chemotherapy and RIT has often been combined with other strategies, including regional delivery, interferon, and hematopoietic stem cell support.
Radiolabeled anti-CEA Mabs have been evaluated in phase I trials in combination with single-agent chemotherapy. One of the first reported phase I trials combined 90Y-cT84.66 at maximum tolerated doses of 16.6 mCi/m2 (defined from an earlier trial154) with 5-day continuous-infusion 5-FU at doses escalating from 700 to 1000 mg/m2/day.294 One patient with a mixed response and 11 patients with stable disease of up to 8 months’ duration were reported in 21 patients treated with up to two courses of combination therapy. 5-FU did not appear to alter the pharmacokinetics and biodistribution of cT84.66. Human antichimeric antibody (HACA) responses were over 50% less frequent compared with those seen with 90Y-cT84.66 alone,154 suggesting that the 5-FU may have played a role in reducing immune response to the chimeric antibody. This was followed by a phase I trial of similar design combining 90Y-cT84.66 at doses of 16.6 mCi/m2 on day 1 with gemcitabine 30 to 165 mg/m2 on days 1 and 3. The maximum tolerated dose of gemcitabine was 150 mg/m2. Of 32 patients treated with one to three courses of therapy, 1 patient with a partial response and 10 patients with stable disease were observed.296 The HACA response was less frequent than with 90Y-cT84.66 alone,154 suggesting that, as with 5-FU, the addition of gemcitabine may have reduced the incidence of HACA. Phase I trials using autologous stem cell support with full-intensity chemotherapy have also been reported (Table 22-6). The established chemotherapy regimen of gemcitabine and cisplatin was combined with a single infusion of 90Y-cT84.66 (15 to 31.4 mCi/m2) in eight patients with metastatic breast cancer. Four patients demonstrated mixed responses or stable disease of 3 to 14 months’ duration.187 In medullary thyroid cancer, escalating doses (20 to 50 mCi/m2) of 90Y-hMN-14 anti-CEA were combined with single-cycle doxorubicin at 60 mg/m2 in 14 patients, with one partial response, two mixed responses, and four with stable disease observed.295 Taxanes have shown promise in combination with systemic and intraperitoneal RIT. 90Y-m170 at 5 to 20 mCi/m2 with paclitaxel 75 mg/m2 has been evaluated in prostate and breast cancer.301,302 Recently, 90Y-CC49, paclitaxel 60 to 80 mg/m2, and interferon, to increase tumor antigen expression, was administered to 34 patients with stage IIB and IV non–small cell lung cancer, with 13 demonstrating stable disease. Regional combined-modality approaches remain promising. In patients with ovarian cancer with recurrent or refractory microscopic disease or macroscopic disease smaller than 5 cm, combination therapy was evaluated, consisting of a single cycle of intraperitoneal 177Lu-CC49 RIT, intraperitoneal paclitaxel, and subcutaneous alpha-interferon.298 Four of 17 patients with cutaneous measurable disease had a partial response, and 4 of 27 patients with nonmeasurable disease remained progression free at 18+ to 37+ months. A subsequent phase I study of the same design evaluated intraperitoneal 90Y-CC49 in combination with intraperitoneal taxol and alpha-interferon.299 Two of 9 patients with measurable disease demonstrated a partial response and 4 of 11 with nonmeasurable disease remained progression free at 15+ to 23+ months. A recently completed pilot study of intraperitoneal 90Y-cT4.66 anti-CEA RIT and intraperitoneal gemcitabine in patients with CEA-expressing cancers with disease primarily confined to the peritoneal cavity demonstrated that the combination was well tolerated with no dose-limiting toxicities observed (see Table 22-6, unpublished data). Results were encouraging with four of seven patients demonstrating antitumor effects (one with a mixed response and three with stable disease of 2 to over 15 months’ duration). One had complete resolution of malignant ascites, and another had a reduction of ascites of more than 50%, no longer requiring frequent abdominal paracenteses. Regional RIT is also actively being evaluated in combination with chemotherapy in patients with high-grade gliomas. These studies are described in more detail below.
RIT and External Beam Radiotherapy
RIT in combination with external beam radiation therapy is an attractive concept for a number of reasons.308 The two modalities combined may deliver a higher cumulative radiation dose to the tumor than either modality alone. If external beam radiotherapy precedes RIT, radiolabeled macromolecule uptake can potentially be increased through reduction in tumor size,309 increase in vascular permeability,310,311 or increase in antigen expression.312–314 If RIT precedes external beam radiation therapy, low-dose-rate RIT irradiation has been shown to potentiate tumoricidal effects of subsequent external beam radiotherapy in vitro through mechanisms that involve a G2 block315 or through a phenomenon termed “protracted exposure radiosensitization” as described by Williams and colleagues.316
A limited number of external beam radiotherapy and RIT combined-therapy efforts have been reported (see Table 22-6). Markoe and associates317 treated 53 patients with 125I-17-1A anti–Ep-Cam antibody in a phase I study. Forty-six patients with liver metastases also received 1500-cGy whole liver radiotherapy in 10 daily fractions to increase RIT uptake to hepatic lesions. No significant hepatotoxicity was reported. One partial response, one mixed response of 12 months’ duration, and 10 patients with stable disease of 3 to 8 months’ duration were observed. Buchegger and colleagues220 combined 131I-anti-CEA F(ab′)2 RIT with liver radiotherapy (20 Gy in 2- to 2.5-Gy daily fractions) in six patients with metastatic colorectal cancer. Hematopoietic toxicity was the primary toxicity, and grade 1 to 3 transient elevations in liver transaminases and alkaline phosphatase were observed. One minor response, three patients with stable disease, and five patients with a decrease in CEA were reported. Clinical trials combining locoregional external beam radiotherapy with RIT have been completed with acceptable toxicity in patients with hepatobiliary cancers317–320 and high-grade gliomas.321–323
Combining total body irradiation as a form of systemic external beam radiotherapy with RIT in a myeloablative transplant setting has also been performed by one group in patients with breast and prostate cancer.297 Twelve patients received 1320-cGy total body irradiation in 8 fractions over 4 days combined with a single infusion of 100 to 150 mCi/m2 of 131I-CC49 anti-TAG72 RIT, followed by autologous hematopoietic cell reinfusion, with four partial responses and one mixed response observed.
RIT and Hyperthermia
Hyperthermia has demonstrated promise in experimental models as a means of enhancing the efficacy of RIT through a number of possible mechanisms, which include increasing tumor antigen expression,324,325 increasing tumor blood flow and/or vascular permeability, decreasing tumor interstitial pressure, radiosensitization by inhibition of sublethal damage and potentially lethal damage repair,326–328 and direct cytotoxic effects on tumor. Mittal and colleagues329 treated LS174T colon cancer–bearing mice with 131I-anti-CEA C110, followed 2 days later with hyperthermia of 42.5° C for 45 minutes. Greater tumor growth delay was seen when hyperthermia was added to RIT. The same group then performed a phase I/II combined-therapy trial of 13II-NP4 anti-CEA RIT combined with hyperthermia in six patients with liver metastases. RIT (30 or 60 mCi/m2) was administered intravenously followed 24 hours later by 42° C interstitial hyperthermia to the liver lesions. One partial response was observed, and two of three patients had resolution of abdominal pain after therapy.
RIT Using Antibodies with Immunomodulatory or Biologic Activity
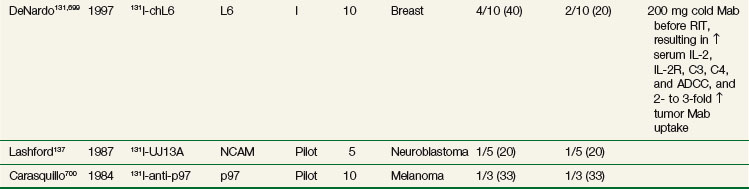
Although most Mabs evaluated to date have had no significant biologic activity, there have been some exceptions. In a pilot trial of 10 patients with breast cancer, unlabeled chL6 (200 mg) was administered before the 131I-chL6 therapy dose. Giving chL6 resulted in an increase in serum interleukin-2 and interleukin-2–receptor levels, a decrease in serum C3 and C4 complement levels, and an increase in antibody-dependent cellular cytotoxicity (ADCC) activity. In addition, an approximately twofold increase in radiolabeled antibody uptake to tumor was seen, which the authors hypothesized was secondary to changes in tumor vascular permeability as a result of chL6 bioactivity.131 This may explain, in part, the higher than usual response rate in this trial, with four partial and two mixed responses reported (see Table 22-3). HuJ591, which recognizes an external domain of prostate-specific membrane antigen (PSMA), may have similar bioactivity. HuJ591 has been radiolabeled with 90Y and 177Lu and evaluated in phase I RIT trials with encouraging results162,163 (see Table 22-3). Unlabeled huJ591 has also been evaluated in several trials in patients with hormone-refractory metastatic prostate cancer. The initial phase I trial treated 14 patients with four weekly doses of huJ591, with dose levels of 25, 50, 100, or 200 mg/m2.330 Prostate-specific antigen (PSA) stabilization was observed in three patients. This was followed by a pilot trial of 14 patients, with each patient receiving four escalating doses of unlabeled Mab of 10, 25, 50, and 100 mg.331 Increasing ADCC activity was seen with increasing antibody dose. One patient demonstrated a more than 50% reduction in PSA. Given the ability of huJ591 to mediate ADCC, a phase II trial of huJ591 and low-dose interleukin-2 therapy has been initiated, with stabilization of PSA observed in some patients.128
Using radiotherapy and RIT as a means to enhance immunotherapy through other mechanisms has also received recent attention.332,333 Tumor cell death after irradiation can release antigen, which can then activate or potentiate an immune response against tumor.333 Radiation therapy can alter the tumor cell phenotype by increasing expression of tumor-associated antigens, such as CEA, up-regulate tumor chemokines and adhesion molecules,334 and up-regulate Fas and major histocompatibility complex (MHC) molecule335 expression, which can work in concert to enhance immune recognition. Low-dose total body irradiation in murine models can enhance effects of subsequent adoptive transfer of tumor-specific T cells by selectively depleting cell populations that compete for needed cytokines.336 Recently, Charkraborty and colleagues337 evaluated the combination of anti-CEA RIT (90Y-COL-1) and vaccine therapy in a colon cancer–bearing transgenic mouse model for human CEA. The combination resulted in a significant increase in survival compared with either therapy alone. RIT resulted in an up-regulation of Fas by tumor cells and an increase in infiltrating CEA-specific CD8+ T cells in tumor.
Antibodies now used as part of standard regimens in nonhematologic cancers are also being evaluated as potential radioimmunotherapeutics. This concept is attractive because these antibodies have known antitumor effects that can add to the cytotoxic effects of RIT. Trastuzumab (Herceptin) is one the first monoclonal antibodies to be approved for use in the treatment of nonhematologic cancers and recognizes EGFR 2 (HER2/neu), which is overexpressed by 20% to 30% of breast cancers. Trastuzumab radiolabeled with the beta emitters 90Y, 177Lu, and 188Re, the Auger electron emitter 111In, and the alpha-particle emitters 212Pb, 211At, and actinium-225 (225Ac) has been evaluated in in vitro and in vivo preclinical therapy studies with encouraging results.338–345 Recently, imaging doses of 111In-trastuzumab were administered to 17 patients with metastatic HER2 overexpressing breast cancers.343 The tumor detection rate was 45%, with new tumor lesions detected in 13 of 15 evaluable patients.
Cetuximab, a monoclonal antibody (Mab) directed against epidermal growth factor receptor (EGFR), is used in the treatment of head and neck cancer with radiotherapy and in colorectal cancer in combination with standard chemotherapy regimens. The antitumor effects of the antibody may also enhance the efficacy of radiotherapy,346–348 although the exact mechanism is unclear. Preclinical in vivo studies have also reported increased RIT efficacy when combined with EGFR-inhibiting agents.292,349 Radiolabeled anti-EGFR Mabs have been evaluated in animal models and in a limited number of clinical trials to assess biodistribution and tumor targeting, as well as their potential as radioimmunotherapy agents. Goldenberg and colleagues350 evaluated biodistribution and tumor localization of 111In-labeled murine 225 anti-EGFR in mice bearing a high EGFR-expressing vulvar squamous cell cancer tumor. This was followed by an imaging and biodistribution trial with 111In-Mab 225 in 19 patients with squamous cell lung cancer.351 At the 300-mg level, the mean percentage of injected dose (%ID) per tumor tissue was 3.1%, liver 21.6%, heart 4.3%, and bowel 24.5%. The low tumor uptake and low tumor to organ uptake ratios appear to limit the potential of Mab 225 as an RIT agent. In contrast to this, Milenic and colleagues352 evaluated 111In-cetuximab in mice bearing a variety of tumor xenografts. For the human colon cancer line LS174T, tumor uptake at 72 hours was high at 52 %ID/g, with blood uptake of 10.6 %ID/g and liver uptake of 7.9 %ID/g. The authors concluded that 111In-cetuximab may be a useful radioimmunoconjugate for therapy.
Bevacizumab, an anti-VEGF (vascular endothelial growth factor) Mab, has recently been introduced as part of standard systemic therapy regimens for metastatic colorectal, lung, and breast cancers.353,354 Preclinical models also report that bevacizumab combined with RIT can potentially increase antitumor effects compared with RIT alone.261 Evaluation of bevacizumab as a possible radioimmunoconjugate for imaging and therapy are in the preclinical stages. Stollman and associates355 recently performed imaging and biodistribution studies in LS174T colon cancer–bearing mice administered 111In-labeled bevacizumab. At 3 days following administration, mean tumor uptake of 111In-bevacizumab was 19.4 %ID/g, which is comparable to the levels observed for 111In-anti-CEA antibodies evaluated in the same tumor-bearing mouse model. Bevacizumab labeled with zirconium-89 (89Zr) or alpha particle-emitting radionuclides356,357 has also been evaluated in vivo and has demonstrated targeting to tumor and successful tumor imaging.
Pretargeting Delivery Systems for RIT (see Table 22-7)
Pioneering work utilized the high-binding affinity streptavidin- or avidin-biotin system, in which an antibody-streptavidin conjugate or a biotinylated antibody is coupled to a reciprocal ligand bearing the therapeutic radionuclide.358–360 Results using pretargeted approaches have been promising in animal models361,362 and are now being actively studied in the clinic.363–370 However, immunogenicity to the bacterial-derived reagents, such as streptavadin, has limited the ability to deliver multiple administrations.
Bispecific antibodies provide an attractive alternative for pretargeting by fusing antitumor targeting to the capture of the small-molecular-weight hapten.371 Bispecific antibodies have one arm of the antibody recognizing the tumor antigen and the other recognizing the radiolabeled ligand. Although initial studies used chemically conjugated372 or quadroma fusions,373 recombinant DNA technology has enabled the production of human or humanized molecules to lower the potential for immunogenicity. The antihapten antibody can be highly specific for the radiometal-chelate complex, exhibiting vastly reduced binding affinity if a different metal is used to coordinate the complex.374 To circumvent this metal specificity, antibodies against the hapten peptide histamine-succinyl-glycine (HSG) have been generated, which is independent of the radionuclide or chelate.375 This provides a platform technology that enables multivalency and multifunctionality, where, for example, two antitumor Fabs can self-assemble with an anti-HSG Fab.376
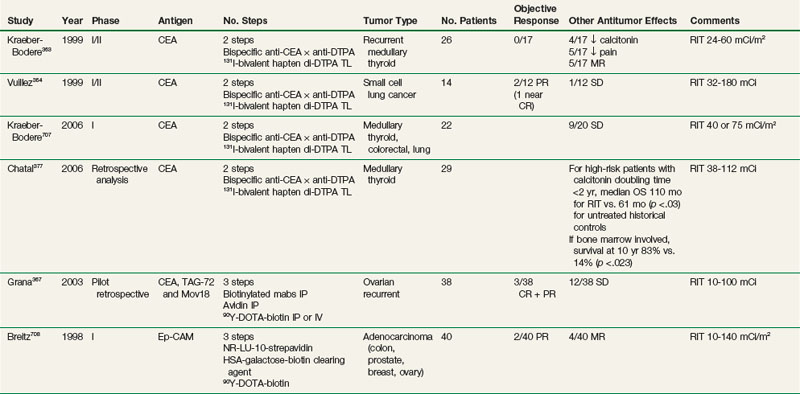
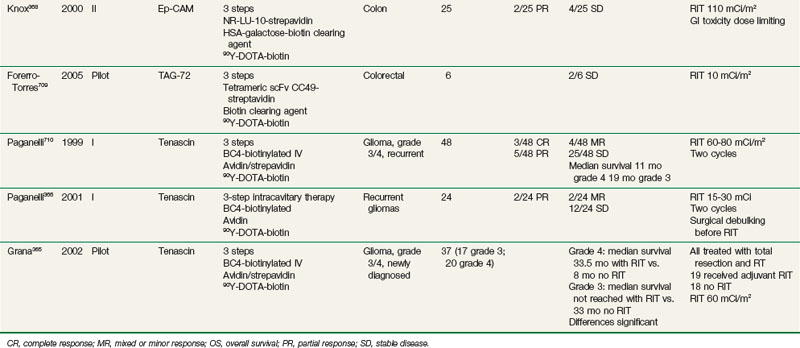
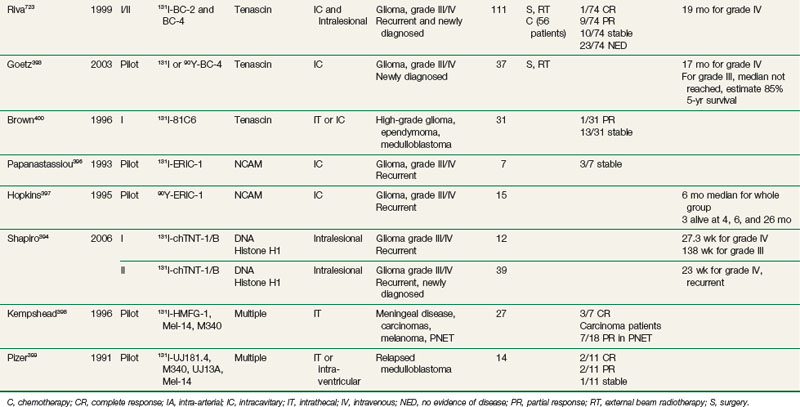
Table 22-7 summarizes some of the clinical therapy trials performed to date using pretargeting approaches. Trials in colorectal, ovarian, lung, medullary thyroid, and brain cancers have been carried out, with most trials reporting objective responses. Chatal and colleagues377 recently reported on 29 patients with metastatic medullary thyroid cancer treated using a bispecifc anti-CEA Mab. The median overall survival time for high-risk patients with a calcitonin doubling time of less than 2 years was 110 months, which compared favorably to the 61-month time for historical controls. Grana and colleagues,365 using the antitenascin Mab BC-4, reported on 37 patients with newly diagnosed high-grade gliomas. After standard therapy of surgical resection and external beam radiotherapy, 19 patients received adjuvant intracavitary RIT. There was a significant improvement in the median overall survival time for grade 4 gliomas of 33.5 months with RIT versus 8 months without RIT. In summary, results from pilot phase I/II trials using pretargeting approaches are encouraging, and this approach warrants further investigation.
Regional Administration of RIT (Tables 22-8 and 22-9)
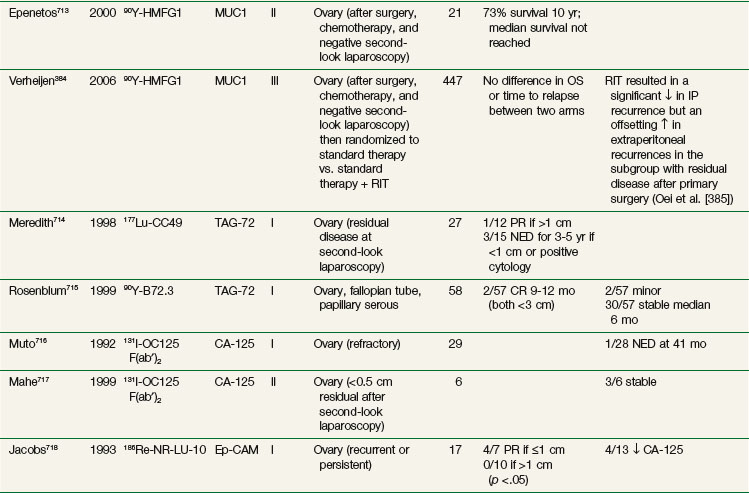
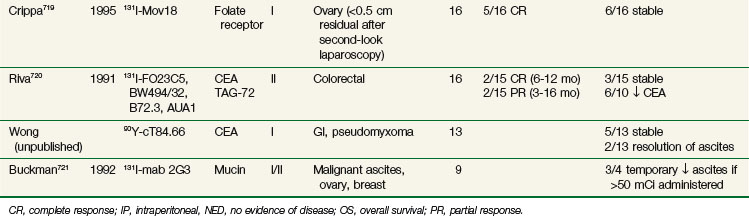
Regional administration (intraperitoneal, intracavitary, intralesional, and intra-arterial systems) to optimize uptake at the tumor site relative to dose-limiting normal organs has been extensively explored, with the most encouraging results in the minimal tumor burden and adjuvant settings. Table 22-8 summarizes RIT trials in which therapy has been administered through the intraperitoneal route. Preclinical378 and clinical379 studies indicate that intraperitoneal administration has pharmacologic advantages over intravenous administration for cancers confined to the peritoneal cavity, because of reduced circulating activity and lower hematologic toxicity secondary to increased antibody uptake in tumor, particularly for small-volume disease.380,381
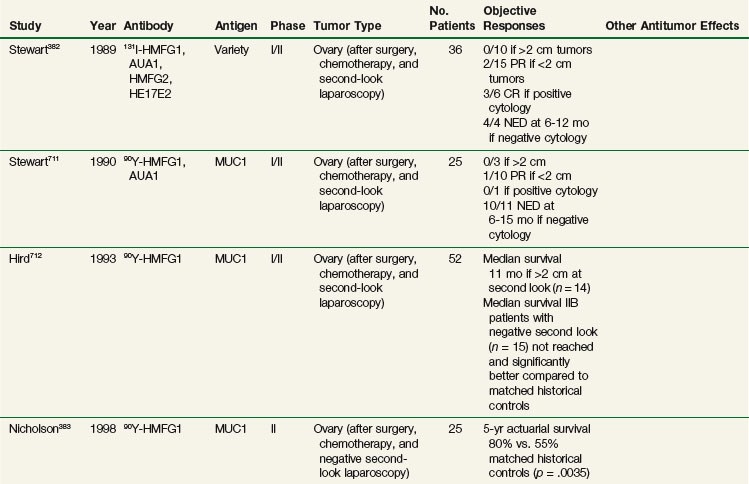
The agent most extensively studied is 90Y-HMFG1. Epenetos and colleagues382 first evaluated HMFG1, along with a panel of other antiovarian cancer antibodies radiolabeled with 131I in patients with ovarian cancer after debulking surgery, first-line chemotherapy, and second-look laparoscopy. Objective responses were more frequent in patients with smaller tumors or lower tumor burden. This led to a phase II trial for evaluating adjuvant intraperitoneal 90Y-HMFG1 at 18 mCi/m2 in patients with initial-stage Ic to IV tumors after surgery, first-line chemotherapy, and negative second-look laparoscopy.383 With a median follow-up of 59 months, a 5-year actuarial survival rate of 80% was reported, which was significantly better than the 55% rate observed for a matched historical control group (p = .0035). This was followed by a multinational phase III trial entering 447 stage Ic to IV ovarian cancer patients with negative second-look laparoscopy after surgery and platinum-based chemotherapy. Patients were randomized to standard treatment versus standard treatment plus a single intraperitoneal infusion of RIT (18 mCi/m2).384 With a median follow-up of 3.5 years, there was no significant difference in time to relapse, relapse-free survival rates, or overall survival rates between the two arms. The overall survival rate at 5 years was 55% to 60%, lower than that reported in the phase II study. The authors put forth a number of factors to explain the lack of benefit with RIT, including the facts that the type of standard treatment varied with each center and that more patients in the control arm received consolidative chemotherapy than patients in the RIT arm (19.7% vs. 12.5%). A subsequent analysis of the data reported a significant decrease in intraabdominal recurrence rates for those who received intraperitoneal therapy. Patients who received extraperitoneal therapy had a significant increase in time to relapse and recurrence rates.385 Strategies to improve on intraperitoneal RIT in this population may involve more effective systemic therapy delivered concomitantly with intraperitoneal RIT, multiple administrations of intraperitoneal RIT, and/or combined intraperitoneal RIT-chemotherapy approaches, which are currently being evaluated by a number of groups (see Table 22-8).
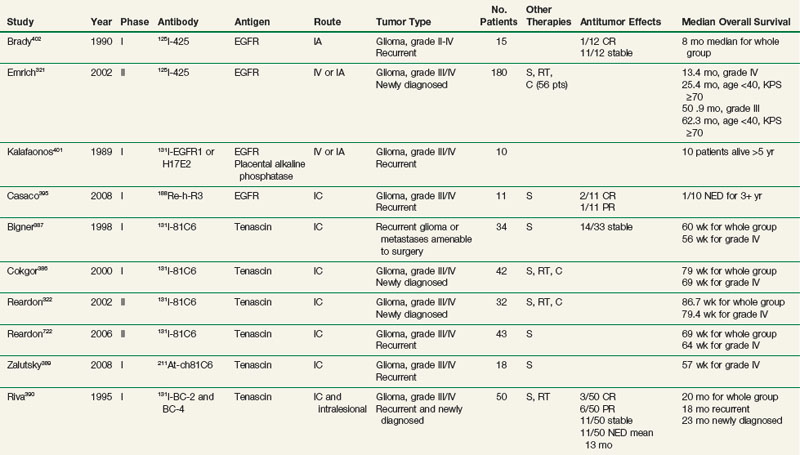
Regional delivery of RIT has also been evaluated in central nervous system cancers, primarily high-grade gliomas (see Table 22-9). Studies have investigated intracavitary administration after surgical resection, as well as intralesional, intra-arterial, and intrathecal administration. Early efforts in patients with recurrent disease were encouraging. The findings led to trials evaluating this approach as adjuvant therapy in combination with standard surgical resection, external beam radiotherapy, and chemotherapy in patients with newly diagnosed high-grade gliomas. Reported toxicities have been acceptable, primarily consisting of hematologic and neurologic complications, including temporary headache, seizures, and worsening of neurologic symptoms. A few studies reported irreversible neurologic symptoms in a small subset of patients at maximum tolerated doses.322,386 Intracavitary and intralesional delivery of radioiodinated antibodies against tenascin, an extracellular matrix protein expressed by gliomas but not by normal brain tissue, has been evaluated as singlecycle therapy by investigators at Duke University and as multicycle therapy in Italy. Bigner and colleagues have evaluated a single administration of intracavitary 131I-81C6386,387 in recurrent and newly diagnosed high-grade gliomas (see Table 22-9). This led to a phase II adjuvant RIT trial.322 Thirty-three patients with previously untreated high-grade gliomas received a single administration of 120 mCi 131I-81C6 directly into the surgical resection cavity, followed by conventional radiation therapy and 1 year of alkylator-based chemotherapy. The average radiation dose from RIT to the 2-cm rim around the resection cavity was 48 Gy. A median survival time of 86.7 weeks for all patients and 79.4 weeks for glioblastoma patients was observed.
A pilot study with 131I-murine 81C6 was recently completed307 (see Table 22-6) in which RIT was combined with standard therapy in 21 patients with newly diagnosed high-grade gliomas. After surgical resection, RIT was administered into the surgical cavity to deliver 44 Gy to the cavity rim, followed by external beam radiotherapy with 55 to 60 Gy and chemotherapy. Toxicities were acceptable, demonstrating the feasibility of this combined-modality first-line therapy approach. With a median follow-up time of 151 weeks, the median overall survival time was 90.6 weeks for patients with glioblastomas. Recently, a phase I study evaluated a 131I-labeled chimeric version of 81C6 in a similar combined-modality approach also demonstrating encouraging results388 (see Table 22-6). Finally, chimeric 81C6 radiolabeled with the alpha emitter 211At is being evaluated in a phase I setting.389 Riva and colleagues in Italy have also investigated intracavitary and intralesional radiolabeled antitenascin RIT in resected high-grade glioma patients390–393 (see Table 22-9). Recent efforts have focused on pretargeting approaches combined with chemotherapy305,306 (see Table 22-6). In a phase I/II trial of 73 patients with recurrent glioblastomas, 38 received RIT alone versus 35 who received RIT and temozolamide. A median number of three cycles of therapy were administered. With RIT and temozolamide, the overall survival time was 25 months and the progression-free survival time was 10 months; these times compared favorably to those of the group who received RIT only, for whom the overall survival time was 17.5 months and the progression-free survival time was 5 months.
Other antibodies have been evaluated in a similar approach,394,395 with mean doses to the cavity rim of 5120 cGy396 and 5500 cGy.397 In addition, regional intrathecal administration has been evaluated in meningeal carcinomatosis and medulloblastoma, with objective responses observed.398–400 Central nervous system cancers have been treated through intra-arterial, intravenous, and intracavitary approaches using antibodies directed against EGFR.395,401,402 Brady and colleagues321,402–404 have administered 125I-Mab 425, an internalizing murine IgG2a directed against EGFR, in patients with high-grade gliomas. Emrich and associates321 reported results for 180 previously untreated patients who were given 125I-Mab 425 as adjuvant therapy (see Table 22-9). Most patients underwent prior surgical debulking and conventional radiotherapy, with approximately one third also receiving chemotherapy. For patients with glioblastoma, the median survival time was 13.4 months, and it was 25.4 months for patients who were under 40 years of age and had a Karnofsky performance status score of 70 or higher, with 10 patients still alive more than 5 years after receiving therapy.
In summary, regional delivery of RIT continues to show promise, particularly in brain tumors in the adjuvant setting. Randomized phase III trials evaluating the addition of adjuvant RIT to conventional multimodality therapy are needed to confirm the encouraging results in high-grade gliomas. Finally, hepatic arterial delivery of RIT in hepatocellular carcinoma has been investigated. Zeng and colleagues reported their single-institution experience and observed a significantly higher rate of conversion to resectability for the group receiving hepatic arterial 131I-Hepama-1 RIT compared with the group who received hepatic arterial 5-FU, cisplatin, and doxorubicin405 or chemoembolization and 46-Gy external beam radiotherapy.406 A 5-year survival rate of 28.1% for the RIT group was reported, which compared favorably with 9.1% for the chemotherapy group. In a recent multicenter phase I/II trial, 134 patients with unresectable hepatoma received one to two cycles of 131I-metiximab via the hepatic artery, with a reported 8.2% partial response rate and a median survival time of 19 months.407
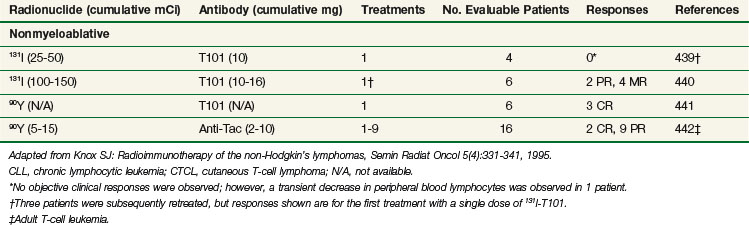
Rit for Lymphoma and Leukemia
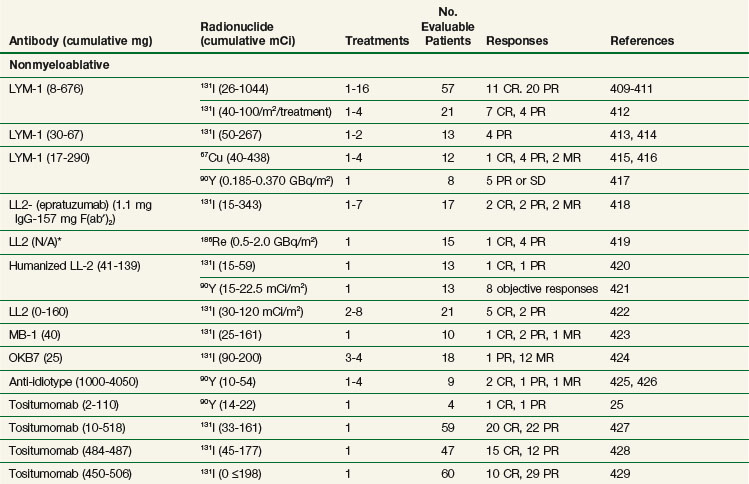
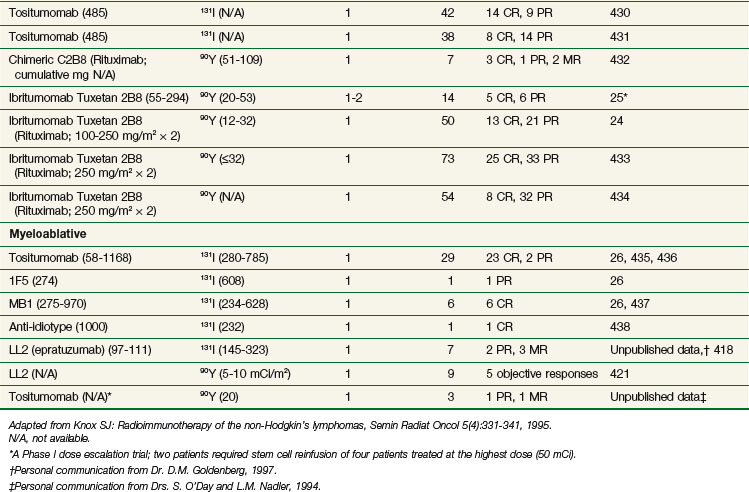
RIT has been most successful for the treatment of lymphoma and leukemia.408 Tables 22-10 and 22-11 summarize representative clinical trials using RIT for B-cell non-Hodgkin’s lymphoma,24–26,409–438 and T-cell lymphoma, and leukemia439–442 in terms of general study design (nonmyeloablative vs. myeloablative), radionuclide, and antibody used, number of treatments, number of treated evaluable patients, and responses. Myeloablative studies have included bone marrow and/or peripheral stem cell collection and reinfusion. These trials differ in terms of eligibility criteria, antibody and radionuclides used, dose, number of treatments, doses of unlabeled antibody preinfused or coinfused, and biodistribution or dosimetry estimations required for administration of a therapeutic dose of radiolabeled antibody. The results summarized in Table 22-10 are particularly promising and show a relatively high response rate with a number of durable PRs (50% or more decrease from baseline in overall tumor size for 1 month or longer) and CRs (complete response with no evidence of disease for more than 1 month), with a subset of these responses ongoing at 5 years or longer and many patients experiencing remissions of longer duration than had been achieved with previous chemotherapeutic regimens. The highest overall response rates and CR rates, and the longest remission durations, have been reported in patients treated with very high doses of radiolabeled antibody in single doses in conjunction with autologous bone marrow transplant or stem cell transplant.26,435–438 Although higher doses of RIT have tended to be more efficacious, there has not been a direct correlation between dose and response in most reported studies. These results are particularly encouraging because all of the patients treated in these trials have had recurrent disease following at least one form of conventional therapy. Many of the patients had failed multiple courses of therapy, and some were unable to tolerate additional chemotherapy for a variety of reasons.
Two anti-CD20 Mabs conjugated to either 131I (131I-tositumomab or Bexxar) or 90Y (90Y-ibritumomab tiuxetan or Zevalin) have been approved by the FDA for patients with relapsed or refractory low-grade or transformed B-cell non-Hodgkin’s lymphoma,428,429,433 and have demonstrated efficacy in patients even after the use of rituximab (Rituxan).431,434 90Y-ibritumomab was also approved for initial treatment of previously untreated patients with low-grade B-cell non-Hodgkin’s lymphoma in 2009. The Bexxar (tositumomab and 131I-tositumomab) regimen consists of the sequential administration of a dose of an unlabeled murine Mab, tositumomab, to optimize biodistribution and increase tumor localization443,444 of the 131I-tositumomab that is administered thereafter. An initial biodistribution study uses a dosimetric dose in calculating a patient-specific therapeutic dose, so that an absorbed whole body dose of 75 cGy can be delivered to patients with a platelet count of 150,000/µL or more. FDA approval was based on a study in 40 patients with relapsed or refractory disease after rituximab therapy and was further supported by the demonstration of durable responses in four other studies enrolling 190 patients with relapsed or refractory disease following chemotherapy. Objective tumor responses occurred in approximately 60% of patients, with CRs in approximately 30%. Median response durations have been in excess of 12 months, with occasional durable complete responses428,429,445,446 lasting for years.
The 90Y-ibritumomab tiuxetan or Zevalin regimen uses the chimeric anti-CD20 antibody rituximab for predosing. The pivotal study that supported FDA approval of Zevalin randomized 143 patients to receive either rituximab alone (four weekly doses of 375 mg/m2, as it is used as a therapeutic agent) or RIT with a lower dose (250 mg/m2) of rituximab for purposes of optimizing the biodistribution of 111In-labeled ibritumomab tiuxetan for an imaging/dosimetry study. Seven days later patients received 250 mg/m2 of rituximab followed by 0.4 mCi/kg of 90Y-labeled ibritumomab tiuxetan. Based on the International Workshop Non-Hodgkin’s Lymphoma Response Criteria, the overall response rate was 80% for Zevalin versus 56% for rituximab (p = .002). CRs were achieved in 30% of patients with Zevalin versus 16% for rituximab (p = .04).433 Of patients who have since progressed, the time to the next therapy was 11.5 months after Zevalin and 7.8 months after rituximab.
Two randomized studies of either 90Y-anti-CD20 Mab433 or 131I-anti-CD20 Mab430 versus unlabeled anti-CD20 Mab have demonstrated the importance of the radiation associated with RIT as a determinant of both the efficacy and the toxicity of the RIT treatment. The radiobiology of RIT targeting the CD20 antigen is critical to tumor and normal tissue effects associated with this therapy and has been reviewed.84 Retreatment with radiolabeled anti-CD20 Mab has also been reported to be well tolerated and efficacious in a subset of patients.25,422,447
The optimal time to use RIT in the course of non-Hodgkin’s lymphoma has not been defined.12,408 A trial using 131I-tositumomab as primary therapy for low-grade disease in 76 previously untreated follicular lymphoma patients has shown impressive results, with a response rate of 95% and evidence of conversion to molecular negativity by polymerase chain reaction (PCR) testing for Bcl-2 rearrangements in 80% of patients with a complete response. The actuarial 5-year survival rate was 59%, with a median progression-free survival rate of 6.1 years.448 Patients who have failed bone marrow transplant have experienced excellent tumor responses to RIT as well.421,449 In addition, RIT is being studied in other histologic types such as diffuse large cell lymphoma (DLCL), mantle cell lymphoma,450 and multiple myeloma451 with encouraging results.452–454 For example, in a study of 104 patients with relapsed B-cell DLCL following treatment with either chemotherapy alone or with chemotherapy and rituximab, the overall response rate was 44%, with a median overall survival time of approximately 22 months for patients previously treated with chemotherapy and 4.6 months for patients who had failed chemotherapy plus rituximab.452
RIT has also been useful as a preparatory regimen for transplantation.26,435–438 In a clinical trial comparing high-dose RIT with conventional high-dose chemotherapy with hematopoietic stem cell transplant, the RIT group had higher overall survival and progression-free survival rates than the group who received the conventional preparatory regimen for transplantation.455 Other trials of 131I-tositumomab combined with etoposide, cyclophosphamide, and autologous stem cell transplant (ASCT) compared with total body irradiation combined with etoposide, cyclophosphamide, and autologous stem cell transplant also demonstrated improved rates of overall survival and progression-free survival in the group receiving RIT.47,456 The same regimen has yielded very encouraging results in patients with mantle cell lymphoma, with a 3-year progression-free survival rate of 61%.457 131I-tositumomab has also been studied in combination with BEAM (BCNU, etoposide, Ara-C, and melphalan) followed by autologous stem cell transplant. Toxicity was similar to BEAM alone. The CR rate was 59%, and the overall response rate was 68%, with a 3-year event-free survival rate of 39% and an overall survival rate of 55%.458 A similar approach using 90Y-ibritumomab with BEAM has also yielded promising results.459 A variety of extended phase II trials using RIT combined with high-dose chemotherapy are ongoing to better define the safety and efficacy of this therapeutic approach. 90Y-ibritumomab therapy with stem cell support is also being studied, with encouraging early results,460 including studies of RIT as part of a reduced-intensity conditioning regimen for allogeneic stem cell transplant.461,462
Other approaches to increase the efficacy of RIT in B-cell non-Hodgkin’s lymphoma include the use of a pretargeting regimen with an anti-CD20-fusion peptide,463 which is discussed later in this chapter. In this approach, antibody is delivered separately from the radionuclide and a clearing agent is used between the two deliveries to eliminate unbound circulating antibody that if radiolabeled, would be a source of nonspecific radiation and, therefore, toxicity. Proof of principle for this platform technology has been demonstrated in a clinical trial463 with significantly increased ratios of tumor to whole body, tumor to blood, and tumor to normal organ than have been achievable with directly labeled Mab.
Another area of active investigation is the study of RIT as part of a combined-modality approach with either chemotherapy or conventional radiation therapy.464,465 Recently, the combination of RIT, CHOP, and involved field radiation therapy in aggressive DLCL (Southwest Oncology Group [SWOG] 0313) resulted in outcomes that compared favorably with historical experience at relatively short follow-up times.466 Numerous trials are currently studying the combination of RIT with a variety of chemotherapy drugs. For example, the combination of fludarabine and CHOP chemotherapy with RIT as up-front therapy in phase II trials has resulted in high overall response rates and excellent progression-free survival rates.467,464 In a recent phase II trial (SWOG 9911) of CHOP chemotherapy combined with 131I-tositumomab as consolidation in 90 newly diagnosed follicular non-Hodgkin’s lymphoma patients, the CR rate was 39% with CHOP alone and 69% with CHOP plus RIT, respectively. Five-year progression-free survival and overall survival rates were 67% and 87% for patients receiving the combination therapy.464 These results provided the rationale for an ongoing phase III trial (SWOG 0016) that is comparing CHOP plus rituximab with CHOP followed by 131I-tositumomab. Results from this trial will help to clarify the utility of up-front cytoreduction followed by RIT in previously untreated patients with follicular non-Hodgkin’s lymphoma, because clinical outcomes with RIT alone in this patient population were similar to those observed with chemotherapy followed by RIT. Chemotherapy combined with RIT has also been studied in 20 previously untreated elderly patients with DLBCL, using CHOP and 90Y-ibritumomab.468 In this phase II trial, the overall response rate was 100%, with 95% CR rates. Importantly, four of five patients with an initial PR to CHOP alone converted to CR following consolidative RIT. With a median follow-up of 15 months, the 2-year progression-free survival rate was 75% and the 2-year overall survival rate was 95%. In a study of 414 patients with advanced-stage follicular non-Hodgkin’s lymphoma, patients were randomized to receive induction chemotherapy followed by observation or 90Y-ibritumomab. The progression-free survival rate was 13.5 months in the observation group versus 37 months for the group receiving RIT consolidation. The progression-free survival rate for patients obtaining a PR or CR was 6.3 months versus 29.7 months for PRs, and 29.9 months versus 54.6 months for CRs, respectively. Furthermore, RIT converted 77% of PRs to CRs.469 Ongoing SWOG and Eastern Cooperative Oncology Group (ECOG) studies are investigating the role of 90Y-ibritumomab therapy as consolidative therapy following CHOP and CHOP/rituximab therapy, respectively, in early-stage disease. Encouraging results have been reported in patients with untreated mantle cell lymphoma using 90Y-ibritumomab following four cycles of rituximab plus CHOP chemotherapy regimen with a 75% RR (43% CR) and 93% overall survival rate at 18 months.450 It is noteworthy that patients with non-Hodgkin’s lymphoma who undergo RIT therapy can well tolerate subsequent chemotherapy regimens or autologous stem cell transplantation,470 with toxicity from subsequent therapy being similar to that observed in patients not previously treated with RIT. More recently, RIT is being studied in combination with biologic response modifiers such as bortezomib, in order to try to further increase the therapeutic index of RIT.471 Results from clinical studies of RIT for the treatment of patients with recurrent Hodgkin’s disease have been encouraging. These studies have used 131I- or 90Y-labeled antiferritin antibody.472–476 Overall, 134 patients with recurrent Hodgkin’s disease have been treated in five different studies with radiolabeled antiferritin antibody.477 Results obtained with 90Y-antiferritin antibody were better than those obtained with 131I-antiferritin antibody. 90Y-antiferritin antibody therapy resulted in a response rate of 60%, with a CR rate of 30%. The extent of response was associated with increased survival times, with 50% of patients with CR, PR, and progressive disease alive at 2 years, 1 year, and 4 months, respectively.477 Responses in these studies were more common in patients with a disease history longer than 3 years, those with tumor volumes less than 30 cm3, and those who received at least 0.4 mCi/kg of 90Y-labeled antiferritin antibody per kilogram.477 More recently, 131I-tositumomab has been studied in recurrent lymphocytic–predominant Hodgkin’s lymphoma.478
A variety of radiolabeled antibodies have also been safely administered to patients with advanced acute leukemias and have been shown to have significant antileukemic activity and to enhance the antileukemic effects of stem cell transplantation conditioning regimens (e.g., beta-emitting 131I-anti-CD33, 90Y-anti-CD33, 131I-anti-CD45, and 188Re-anti-CD66c). Radioimmunoconjugates that emit alpha particles with a very short range (e.g., 213Bi) may be most useful for low-volume and/or microscopic residual leukemic disease.68,479 Studies using RIT targeting the CD33 antigen, which is expressed on early myeloid progenitor cells and myeloid leukemia cells, have yielded promising results that may improve the efficacy of marrow transplantation. In a dose escalation trial of 131I-murine anti-CD33 (M195) in patients with relapsed or refractory myeloid leukemias,480 8 of 24 patients treated with 50 to 210 mCi/m2 in divided doses had sufficient marrow cytoreduction to proceed to bone marrow transplant, and 3 of these patients achieved marrow remission, but 37% of assessable patients developed HAMA. 131I-M195 (120 to 230 mCi/m2) was combined with busulfan and cyclophosphamide in an effort to intensify therapy before a first or second bone marrow transplant.481 All patients engrafted, and 18 achieved CRs, with 3 patients following first bone marrow transplant remaining in unmaintained remission for 18+ and 29+ months. Six patients relapsed, and 10 patients died in CR of transplant-related complications.481 HAMA occurred in 37.5% of evaluable patients. Nevertheless, these studies provided data that suggest that leukemic cytoreduction could be safely achieved with 131I-anti-CD33, even in multiply relapsed or chemotherapy-refractory leukemia, and that RIT may be useful as part of a preparatory regimen for bone marrow transplant in myeloid leukemia.
Next, a study was performed with 131I-M195 (50 or 70 mCi/m2) in seven patients with relapsed acute promyelocytic leukemia (APL) in second remission following treatment with all–trans retinoic acid.482 Two of six patients with detectable promyelocytic leukemia gene/retinoic acid receptor-alpha (PML/RAR-α) messenger RNA after all-trans retinoic acid therapy had negative reverse transcriptase-polymerase chain reaction determinations following 131I-MI95. The median disease-free survival rate was 8 months and the median overall survival rate was longer than 21+ months, demonstrating that this regimen compared favorably with other approaches for the treatment of relapsed acute promyelocytic leukemia,482 but HAMA developed in five of the seven patients. Because of the immunogenicity of the murine M195 antibody, a humanized M195 antibody was developed that had avidity 4 to 8.6 times higher than the murine antibody.483 A homodimeric humanized IgG1 antibody was also developed that had improved effector function as well as the ability to internalize and retain radioisotope in targeted leukemia cells.483 This humanized M195 (HuM195) was subsequently used in a phase 1B trial,484 in which patients with relapsed or refractory myeloid leukemia were treated at dose levels of 0.5 to 10 mg/m2 in six doses over 8 days. Optimal biodistribution occurred at 3 mg/m2, and HAMA responses were not observed.484 131I-M195 and HuM195 have also been studied in combination with chemotherapy as a conditioning regimen for allogeneic transplant. Absorbed doses to the bone marrow ranged between 272 and 1470 cGy. The median survival time was 4.9 months (range, 0.3 to 90+ months), and three patients with relapsed acute myelogenous leukemia (AML) have ongoing CRs at 59+ to 90+ months following bone marrow transplant. These results demonstrate the feasibility of using radiolabeled anti-CD33 in combination with a standard bone marrow transplant preparatory regimen, but randomized trials will be needed to determine whether or not there is a statistically significant benefit to intensified conditioning with RIT.485
Other very encouraging results have been achieved using RIT targeting the CD45 antigen with 131I-anti-CD45 (BC8 antibody) combined with cyclophosphamide and total body irradiation as a marrow transplant regimen for acute leukemia and myelodysplastic syndrome (MDS).486 Forty-four patients received a biodistribution dose of 0.5 mg/kg of 131I-anti-CD45, which delivered 6.5 ± 0.5 cGy/mCi to the marrow, with a mean dose to the spleen of 13.5 ± 1.3 cGy/mCi. Doses to the liver, lung, kidney, and total body were significantly less, and 84% of patients had a favorable biodistribution of antibody, with higher estimated radiation absorbed doses to the marrow and spleen than to normal organs. Thirty-four patients received a therapeutic dose of 131I-anti-CD45 labeled with 76 to 612 mCi 131I in addition to cyclophosphamide and total body irradiation. The maximum tolerated dose delivered 10.5 Gy to the liver. Seven out of 25 treated patients with acute myelogenous leukemia/myelodysplastic syndrome survived disease-free for a median time of 26 to 100 months following transplantation, and 3 of 9 patients with acute lymphoblastic leukemia (ALL) were disease-free 34 to 82 months following transplantation. This study demonstrates that RIT with 131I-anti-CD45 can be used safely to deliver additional targeted radiation to bone marrow (approximately 24 Gy) and spleen (approximately 50 Gy) when combined with cyclophosphamide plus total body irradiation.486
A subsequent study with this preparative regimen in patients with relapsed acute myelogenous leukemia following first remission or advanced myelodysplastic syndrome suggested that marrow doses higher than 7 cGy/mCi may lead to lower relapse rates following transplantation.487 This was followed by a trial of 131I-anti-CD45 combined with busulfan and cyclophosphamide in 49 patients with acute myelogenous leukemia in first remission.488 In this study, the estimated mean dose to marrow was 11 Gy, with almost 30 Gy to the spleen. The results were superior to that expected for treatment with busulfan and cyclophosphamide alone (≥30% relapse rate), with a relapse rate of only 20% and an estimated disease-free survival time of 3 years after transplantation.489 131I-anti-CD45 is also being studied in combination with fludaribine and 200-cGy total body irradiation in an allogeneic transplant trial in poor-risk, advanced, older acute myelogenous leukemia patients and high-risk myelodysplastic syndrome patients.490 In this study, mean doses of at least 27 Gy to the marrow and 81 Gy to the spleen were delivered without an increase in day 100 mortality rates. 90Y- and 188Re-labelled anti-CD66 have also been studied as part of a dose-reduced conditioning regimen for patients 55 years of age or older with acute leukemia and myelodysplastic syndrome, with a cumulative risk of relapse of 55% at 30 months following transplantation.491,492
In an effort to overcome the limitations of using beta-emitting radionuclides for the treatment of leukemia, alpha-emitting radioimmunoconjugates have been studied with promising results. Alpha emitters have the potential to increase the efficacy of RIT, without the nonspecific effects of beta-emitting radionuclides, and also have promise for treatment of patients in a minimal disease state. In an early study using an alpha emitter, 213Bi was conjugated to HuM195. 213Bi has a t1/2 of 45.6 minutes and emits high-LET alpha particles (8 MeV) with a path length of approximately 60 to 90 µm in tissue. Therefore 213Bi-labeled Mab can theoretically kill leukemia cells with one or two alpha particles with a minimal bystander effect. A phase I dose escalation study at Memorial Sloan-Kettering Cancer Center (MSKCC) using 213Bi-HuM195 in patients with refractory and relapsed myeloid leukemias demonstrated rapid targeting of disease sites within minutes, with evidence of antileukemic effects even at low dose levels.493 In a study of 18 patients with relapsed and refractory acute myelogenous leukemia or chronic myelomonocytic leukemia treated with 37 MBq/kg of 213Bi-HuM195, there was no significant extramedullary toxicity. The median time to hematologic recovery was 22 days. Target to background and target to whole body ratios were greatly enhanced compared with those seen with immunoconjugates using beta emitters in this patient population. With this therapy, 78% of patients had a decrease in the percentage of bone marrow blasts.494 With the further development of chelator chemistry for linking alpha-emitting radionuclides to antibodies, it has become possible to use other alpha emitters, with longer half-lives and potentially longer marrow residence times than those achievable with 213Bi. 225Ac has a 10-day half-life and is currently being studied in a phase I trial of 225Ac-HuM195 for advanced myeloid leukemias at MSKCC.495 Another advantage of 225Ac is its intracellular decay to short-lived therapeutic daughter alpha particles (francium-221 [221Fr], 217At, 213Bi), which significantly contribute to the increased effectiveness of 225Ac as compared with 213Bi constructs.496 211At-labeled anti-CD25 and anti-CD30 Mabs are also now being studied as potential new antileukemia therapeutics, which have the additional advantage of being expressed by a high proportion of targeted neoplastic cells but not normal resting cells.497,498
Toxicities Associated with Tart and Methods to Ameliorate These Effects
There are a number of well-established factors that influence radionuclide toxicity. Among these are the patient’s genetic makeup, immune factors, physiologic conditions, age, and gender; radionuclide characteristics; dose rate and heterogeneous dose distribution; receipt of other prior and concomitant therapies; recovery from prior therapy; and radioprotector/sensitizer use. Many of these have been discussed in more detail in prior reviews.499–501
Bone marrow suppression has been the primary dose-limiting toxicity of most conventional systemic and intraperitoneal TaRT regimens. That is changing, however, with the increasing use of small-molecule radionuclide delivery. Among multiple factors that may influence the extent and duration of myelosuppression, marrow reserve, recovery from prior therapy, and radioconjugate stability appear to be predominant determinants of this effect.502,503 Hematologic support with transfusions and growth factors has been helpful, and bone marrow or peripheral stem cell reinfusion has allowed dose escalation of radioconjugates to at least three times the dose that resulted in dose-limiting hematopoietic toxicity.455 Interleukin-1 and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been reported to have radioprotective effects on the hematopoietic system.504,505 The adjuvant use of these cytokines with TaRT has resulted in modest protection. Additionally, the radioprotector amifostine has been helpful in preclinical radionuclide studies for the therapy of thyroid disease with 131I506,507 and for reduction of renal toxicity from small-molecule conjugates.508 Similar effects with radionuclide conjugates other than 131I and 90Y are expected, but this potential effect has not yet been well studied. Adjuvant use of chelating agents in combination with unstable metal radioconjugates can help to minimize toxicity, but some have had only a minor protective effect on myelosuppression when used with stable chelators.509,510 In addition to chelators that remove unbound radioactivity from the blood, “chasing” the radiolabeled antibody with a second unlabeled antibody directed against the radiolabeled antibody has been reported to modestly decrease toxicity.511 Pretargeting approaches, which dissociate and delay the delivery of the radionuclide from that of the targeting agent, can decrease toxicity and are discussed in more detail in the optimization section. Although the second dose-limiting organ toxicity for myeloablative TaRT may vary with radionuclide, cardiopulmonary and liver toxicities have been reported for 131I and anticipated for 90Y immunoconjugates, respectively.73,436,512
The importance of stable chelators also affects other potential toxicities as demonstrated with alpha emitters. Renal accumulation leading to nephrotoxicity has been noted from a 212Bi conjugate with an unstable chelator and may be dose limiting for some applications. This was reported by Machlis and colleagues after intraperitoneal administration of 212Bi-anti-Thy 1.2 IgM conjugate to mice bearing EL-4 tumor cells. In the Machlis study, 8% of the injected radioactivity was in the cardiac blood pool by 2 hours and accumulation to kidneys was similar to that of free 206Bi, indicating instability of the 212Bi conjugate with the DTPA chelator.83 Subsequently, nephrotoxicity has been reduced with stable chelate-conjugates, including in clinical experience in acute myelogenous leukemia patients receiving 1 mCi/kg 213Bi, which was mitigated by the use of adjuvant agents.513,514
Acute symptoms from systemic RIT are usually related to administration of antibody products, not the radioactivity. The symptoms have usually been mild and have included rash, fever, chills, myalgia, diaphoresis, pruritus, nausea, vomiting, diarrhea, nasal congestion, and hypotension.515 These side effects are very transient and usually respond to antihistamines, acetaminophen, and nonsteroidal anti-inflammatory agents. Rarely, more severe symptoms, including rigors, bronchospasm, or laryngeal edema, have been noted. These often respond quickly to steroids, antihistamines, oxygen, and meperidine. With central nervous system administration, various transient symptoms have been described, which are usually mild but can include cerebral edema, nuchal rigidity, aseptic meningitis, increased intracranial pressure, and seizure. Steroids have usually ameliorated these symptoms. A serum sickness-type of phenomenon has been commonly observed with intraperitoneal administration of murine antibody conjugates (affecting about one-third of patients), but it is rare among patients receiving intravenous RIT, even with the same radioimmunoconjugate. Serum sickness-like symptoms occur about 2 weeks after treatment and may persist for more than a week. Variation in the manifestation of symptoms may depend, in part, on the particular radioimmunoconjugate used because skin rash has been noted by the Hammersmith group, whereas fever with joint and/or muscle aches but no rash has been common among patients treated at the University of Alabama at Birmingham with a different radiolabeled Mab.516,517
Other acute effects can include a temporary increase in bone pain for radionuclide targeting of bone metastases, as well as gastrointestinal disturbance, which usually manifests as nausea. Nausea has been more commonly associated with PRRT than conventional RIT, as discussed below. However, nausea from PRRT may be more related to the amino acid infusion given to reduce renal toxicity than to the radionuclide therapy itself. The targeting of receptors along the gastrointestinal tract may also contribute to nausea in PRRT. Nausea and vomiting can occur after oral 131I is used in thyroid ablation or treatment of metastatic disease sites. 131I therapy can also lead to sialadenitis, taste changes, transient swelling, and pain at sites of metastasis, ocular dryness, nasolacrimal duct obstruction, and bone marrow suppression.518 Radiation sickness has been reported with doses in excess of 300 mCi.519 There are various reports concerning effects on fertility from 131I thyroid therapy. Short-term gonadal function may be impaired and the incidence of miscarriages increased, but there appears to be no increase in malformed offspring.520,521 Also, the risk for development of certain cancers shifts after 131I thyroid therapy, with increases for cancers of some organs with excess exposure, such as the stomach and salivary glands, but decreases for cancers of the lung and cervix.518,522
In TaRT using small molecules such as peptides or radionuclide conjugates in pretargeting approaches, the kidney and bladder usually receive much higher doses than other normal organs. For these therapies, renal rather than bone marrow toxicity can be dose limiting.10,44,48,523 Fractionated radionuclide delivery may reduce toxicity and increase efficacy.10,14,524 Limited clinical studies as well as radiobiologic considerations of fractionated TaRT have been reviewed elsewhere.525 Fractionation of dosing has been helpful for decreasing toxicity associated with peptide-radionuclide therapy and has been investigated in other TaRT applications.525 Acute symptoms from PRRT are often related to adjuvant agents (amino acids plus amifostine) used to decrease renal toxicity, rather than to a “foreign substance reaction” that may occur with larger immunoglobulin products. The nausea and vomiting commonly associated with use of the adjuvant agents may be ameliorated with antiemetics and other measures.
The importance of dose rate in urinary tract (renal and bladder) toxicity is suggested by early experience with PRRT and in myeloma patients receiving holmium-166 (166Ho). Although the calculated renal dose from high-dose 166Ho-DOTMP was only 710 cGy when 40 Gy was delivered to the marrow, 30% of patients experienced grade 2 to 4 renal toxicity, with severe thrombotic microangiopathy. The risk of hemorrhagic cystitis was decreased by continuous bladder irrigation during the 166Ho infusion.526
Unexpected rates of renal toxicity were also noted in early use of PRRT. Although the mean renal doses were well below tolerance limits for fractionated external beam radiation, the rapid accumulation of radioactivity in the renal cortex resulted in higher doses in that area, which were delivered at a relatively high dose rate. Further study has shown that renal toxicity of PRRT is associated with risk factors such as prior renal insufficiency and diabetes, whereas adjuvant infusion of amino acids and amifostine can help to prevent toxicity.63,523 Incorporation of “protective” measures in PRRT regimens has allowed escalation of the fractionated radioactivity administered by as much as 50%.508 The impact of the administration of lysine is illustrated in Figure 22-2 from an animal study directly comparing autoradiographs of the same radionuclide conjugate with and without amino acid infusion.527 A 40% reduction of 111In-octreotide accumulation in rat kidney was obtained in the lysine-treated animal compared with controls. This was achieved with either intravenous or oral administration of lysine and did not affect distribution in other organs. A similar illustration of the impact of lysine administration is demonstrated in single-photon emission computed tomography (SPECT) images of a patient by Valkema and colleagues.508,527 Other efforts to understand and ameliorate the problem of renal toxicity by more accurate prediction of dose parameters includes publication of six age-dependent models of the Medical Internal Radiation Dose (MIRD) Committee of the Society of Nuclear Medicine that allow subregion radionuclide dose calculation,528 a continuing education session at the 2003 Society of Nuclear Medicine Meeting from which contributions have been published, and MIRD suborgan modeling.529,530
The latest publication, MIRD #20, examines renal toxicity caused by radionuclide therapy by modeling the effects of (1) the dose rate (biologic effective dose [BED]), (2) the heterogeneity of activity distribution over time, and (3) The correlation of the absorbed radiation dose and the biologic effective dose with observed clinical toxicity. The impact of application of patient-specific dosimetry on renal toxicity is illustrated by Barone and associates.531
Nonsystemic administration of radionuclides has been associated with other types of toxicity, dependent on the site of administration and other factors. Late bowel toxicity after intraperitoneal RIT has been infrequent, which is an advantage compared with the effects of nonspecific radionuclide therapy (e.g., phosphorus-32 [32P] chromic phosphate solution). However, the most severe toxicities of 32P therapy include fibrosis and necrosis of the bowel or body wall and are the result of misplacement of the therapeutic agent into the bowel lumen, loculation sac, or body wall rather than the intraperitoneal space (FDA Medwatch). Even with proper administration, there has been an increased risk of bowel complications compared with chemotherapy, especially among patients who also received external beam irradiation to the pelvis. Although nearly three-quarters of postsurgical ovarian patients receiving 15 mCi 32P intraperitoneally in an early Gynecologic Oncology Group (GOG) trial (#7602) reported no side effects, with some of the remaining patients having mild to moderate gastrointestinal symptoms, one developed bacterial peritonitis and four developed bowel obstruction.532 A later GOG study compared cyclophosphamide/cisplatin with intraperitoneal 32P after surgical debulking. More patients treated with 32P than with chemotherapy had bowel problems. Some had suboptimal distribution of the radionuclide within the peritoneal cavity with areas of loculation and likely would not currently have been considered candidates for intraperitoneal therapy.533 With targeted therapy using antibody carriers for radionuclides and pretreatment scans to rule out loculation, severe bowel toxicity has been infrequent.534–536 In more than 90 patients treated at the University of Alabama at Birmingham with intraperitoneal 90Y- or 177Lu-CC49 antibody therapy for relapsed ovarian cancer, no incidences of bowel toxicity severe enough to require surgery were observed, although some patients who underwent surgery for recurrent disease were found to have areas of palpable fibrosis.537
32P has also been injected into malignant lesions and other cavities such as the pleural space for treatment of malignant effusions and the intra-articular spaces for hemarthoses.519 Relatively high concentrations of 32P injected directly into malignant lesions have been well tolerated locally and have occasionally resulted in dose-limiting toxicity in other organs such as the stomach and lungs from shunting. To overcome this potential hazard, pretreatment scans have been obtained to assess trace radionuclide distribution before administration of a therapeutic dose.538 32P injected directly into subcutaneous nodules and pretargeted interstitial administration of 90Y radionuclide into more diffuse areas of chest wall recurrence of breast cancer have been well tolerated.539 In contrast, accidental delivery of radionuclide to subcutaneous tissues from intravenous infiltration has resulted in local necrosis.540 32P applied as a skin patch for treatment of basal cell carcinoma has been well tolerated and effective.541
Higher concentrations of beta and alpha emitters have been injected into glioma cavities than could be tolerated systemically, with infrequent reports of severe toxicity.541–543 Edema and seizures are the most common adverse events reported. Intrathecal radionuclide therapy has resulted in aseptic meningitis and occasional seizure activity.544
Other organs that are at risk for toxicity have not been extensively addressed. For instance, cataracts would be anticipated as a risk after high-dose total body irradiation based on external beam data, but they have not been reported in long-term follow-up studies.545 Lung fibrosis is of concern for 131I therapy of lung metastases and is under study in animals after plutonium inhalation.546,547 Animal model radium inhalation studies have shown an increased risk of lung cancer, which is enhanced by smoking.548
Thus far, late organ dysfunction following RIT with 131I-labeled antibodies has been uncommon, except for abnormal thyroid function.435 Despite measures to block thyroid uptake, elevated thyroid-stimulating hormone levels occurred in 59% of patients treated with myeloablative doses of 131I-anti-CD20 antibodies, at a median time of 6 months after treatment.435 Some patients who received nonmyeloablative doses of 131I have also had elevated thyroid-stimulating hormone levels at later times. Salivary gland inflammation and damage can be an acute and/or a late toxicity. Parotiditis, which may result in xerostomia, has been more common with treatment of thyroid cancer than high-dose radioconjugate therapy. However, this remains a potential toxicity of 131I-antibody therapy.549
Risk of a second malignancy is of concern even if it has not been noted for any particular radionuclide therapy. A small percentage of patients treated with high-dose RIT for lymphoma have developed second cancers. Myelodysplasia is the potential TaRT treatment-related toxicity of most concern. It has been difficult to quantify any increased risk for myelodysplasia resulting from various TaRT applications because patients have often received alkylating agents with or without conventional external beam radiation (also a risk factor in combination with alkylating agents) and some patients have shown evidence of dysplasia before radionuclide therapy.429,550,551 Leukemia risk for adults treated with 32P for polycythemia appears dose related, whereas leukemia has been noted after lower doses (0.6 to 1.5 mCi ) of intra-articular 32P administration in children.552 Second cancers will be especially of concern as more radionuclide therapies are developed for children (e.g., meta-iodobenzylguanidine [MIBG], 90Y-ibritumomab) because age appears to be a factor in induction of thyroid cancer and other cancers after 131I exposure and of myelodysplastic syndrome after MIBG therapy.553,554
Dosimetry
Dosimetry is the process of relating the administered amount of radioactivity to the absorbed radiation dose in tumors, organs, or the whole body. Dosimetry is important for dose correlation with clinical results and, in some instances, for treatment planning to avoid excess toxicity. In general, the doses calculated for TaRT are less accurate than those for external beam radiation therapy for a variety of reasons. These include limited radiation dose input data (e.g., few sample points for a therapy using continuous exponentially decreasing irradiation), inhomogeneous dose distributions, and the assumptions/calculation methodology used to estimate TaRT absorbed doses.555 Dose calculation is also more complicated for internally distributed radionuclides than for external beam irradiation. Alpha particle dosimetry adds the complication of decay and daughter products that may have a different distribution than the parent radionuclide.69,556,557
Data required for TaRT dose estimate calculations include the mass of tumors and normal organs, the cumulative radioactivity taken up by organs and tumors, and the pharmacokinetics of the administered radioactivity.558 Data are usually acquired by serial gamma camera imaging. Bone marrow dose estimates are based upon imaging studies of bony regions of active marrow such as the spine and/or blood pharmacokinetics.559,560 Although there have been attempts to provide dosimetry based on Bremsstrahlung images from radionuclides that do not have gamma emissions, this is not commonly done. The images are of suboptimal quality, making accurate quantitation difficult. Instead, estimates are made for non–gamma emitters from tracer studies using a gamma emitter that has a chemical makeup similar to that of the therapeutic radionuclide. Because animal studies have shown that the biodistribution is similar, although not usually identical, for 111In and 90Y, tracer/dosimetry studies with 111In-labeled antibody have frequently been performed in conjunction with 90Y-labeled antibody therapy to estimate the subsequent biodistribution/dosimetry of the 90Y immunoconjugate.73,561 Quantitative immuno-positron emission tomography (PET) with 89Zr-labeled antibody has shown good correlation with 111In-labeled antibody biodistribution and may be useful as a positron-emitting surrogate for 90Y-labeled antibody therapy.562 PET scanning with 124I-labeled antibody or other agents may prove to be useful.563 Thus far, PET images from 89Zr-cG250 antibody have been superior to those of 111In-cG250 antibody in an animal model.564 Other examples include use of 99mTc antibody for imaging/dosimetry studies in conjunction with 186Re antibody therapy.565 Another variable in estimating the expected dose from therapeutic RIT using a preliminary biodistribution/dosimetry is that even when a small amount of a therapeutic agent is used (trace-labeled study using the same antibody and radionuclide that will be used for therapy), there can be either very good correlation between doses predicted and later delivered or a significant discrepancy in the range of approximately 30% between estimated absorbed doses for the two procedures in the same patient.436,512,566,567
To estimate the dose, conjugate views of the whole body and regions of interest (ROI) (e.g., tumors and normal organs such as liver) are obtained. The activity for each ROI is measured from the counts in that region at multiple time points. The counts in each ROI are corrected for background. Attenuation correction factors are calculated for each patient, and a sample of the administered radionuclide is used for calibration so that counts per minute can be converted to units of radioactivity (mCi or MBq). Scatter correction may also be applied. SPECT (single-photon emission computed tomography) for ROIs may provide superior definition and quantitation, but this has not yet become the standard for quantitation and is more time-consuming than planar conjugate-view methodology.568,569 Absorbed radiation doses can be calculated from radioactivity quantitation and the specific absorbed fraction for the target. The specific absorbed fraction takes into account the type and energy of radiation emissions, the fraction of energy from the source absorbed by the target, and the mass of the target.570 The mass of each ROI is usually determined by estimating volumes from CT, MRI, or other methods such as 99mTc-sulphur colloid liver scans and then converting volume to mass by assuming a unit density of 1 g/mm3. Accounting for excretory routes is needed in quantitating changes in radioactivity distribution over time. For radionuclides such as 131I, where the major route of excretion is renal, urinary activity measurements can provide an estimate of total body clearance if it is not feasible to obtain whole body counts.
The MIRD Committee of the Society of Nuclear Medicine provides guidance for methods to calculate radiation absorbed-dose estimates for the whole body and organs.571 This methodology has been adapted for TaRT, with continuing effort by this committee and others to further improve the models and methods available.528,529,570–574 MIRD 16, other informational publications, web sites, and computer programs have been developed to assist with these calculations.575,576 Among web sites available, www.doseinfo-radar.com was developed to provide information in a number of areas, including standardized dose estimates, decay data, and absorbed fractions. With fusion of anatomic and physiologic images, dose estimates can be calculated in three dimensions at the voxel level, taking into account heterogeneity of radioactivity distribution within an organ.577,578
Biopsy results are infrequently used for dosimetry. Although biopsy provides a direct measurement and can be used for autoradiography, it is invasive and is not practical for most tumor and/or organ dosimetry. Although biopsy data are of interest for correlation with other methods of dosimetry, they are of limited value alone because of the small sample size, which may not be representative and is usually only studied once. Therefore biopsy dosimetry does not allow a time-activity curve to be generated. Implanted thermoluminescent dosimeters (TLDs) and other devices have been used for quantitation in experimental preclinical studies and in limited clinical trials.579 When used in patients with ovarian cancer treated with intraperitoneal RIT, TDL-obtained dose measurements were in the range of those estimated with other methods.565 As with biopsy, TLDs provide a direct measurement of absorbed dose but also usually require an invasive procedure for placement that has the disadvantage of potentially introducing a sampling error. Like most other dosimetry methods, TLDs do not account for dose rate, which may be an important determinant of dose-response relationships. Another implantable dosimeter has been developed and applied to external beam radiation that can give real time wireless feedback to a computer. This technology should be applicable to radionuclides but, similar to TLDs, will only provide data from a small area.580
The relationship between outcome and radiation dose-related factors is variable, with some studies showing a strong correlation and others no correlation.581–583 Some of the factors making it more difficult to establish a dose-response relationship for TaRT, as compared with external beam radiation therapy, may include the relative uncertainty associated with TaRT dose calculations, the heterogeneity of dose deposition that occurs with TaRT, dose rate effects, and agent/patient variation in excretion/clearance. Whereas most normal organ tolerance levels for external beam radiation have been established using high-dose-rate radiation, and vary as a function of fraction size, the dose rate is often low and variable with TaRT, limiting the validity of extrapolating from high-dose-rate tolerance levels to those expected with TaRT. Fractionation of TaRT, as with external beam radiation therapy, increases the total radionuclide dose tolerated.48,525 Several studies have demonstrated how biologic factors affect tolerance, but they are not accounted for in standard dose/toxicity reporting.501 To date, one of the strongest dose/toxicity correlations has been with the adjustment of the calculated absorbed marrow dose for levels of a biomarker involved in hematopoiesis.503 Although data suggest the presence of a relationship between the TaRT radiation dose and the tumor response, analyses to date with relatively small numbers of patients receiving a limited dose range fail to show a strong correlation between these two factors.569,584 Despite the progress and improving accuracy of dosimetry, most studies are still being done without required organ or tumor dosimetry in the nontransplant setting. Activity is usually given per kilogram or per square meter, as is common with chemotherapy. For one of the FDA-approved agents (131I-tositumomab), the whole body effective half-time is used to individualize administered activity, but no organ or tumor dosimetry is required. As better correlation is achieved between dosimetry results and clinical outcome, additional tumor dosimetry may be required, as has usually been needed for high-dose therapy followed by stem cell rescue.585–587 Individualized kidney dosimetry has been important in predicting toxicity of PRRT.588
Optimization of TaRT
Encouraging results have been obtained in TaRT studies using a variety of targeting agents, radionuclides, and study designs.14 The success with lymphomas has spurred much investigation to further optimize this form of therapy in non-Hodgkin’s lymphoma and other cancers, including use as consolidation.6,589–592 Multiple presentations from recent meetings, including ASTRO, ASCO, ASH, and SNM, have reported encouraging results from attempts to further optimize TaRT. Although much progress has been made, especially in reducing immunogenicity of antibody-derived targeting, adding nonantibody targeting agents, and improving radionuclide conjugate chemistry, many challenges remain.57,453 Information about overcoming some of the challenges has been included in a previous section on solid nonhematologic tumors.
One of the main limitations of systemic TaRT has been the low uptake of radioactivity (generally <0.001 %ID/g) in tumors. This low uptake is affected by such factors as tumor vascularity and size; charge, affinity, and avidity of the targeting agent; and interstitial pressure in the tumor.593 The use of small targeting agents may improve tumor penetration with more rapid tumor uptake, but it is generally limited by a relatively short retention time/half-life.594,595 Other approaches for increasing tumor uptake of radiolabeled antibody include the use of external beam therapy. In early studies, external beam radiation therapy was used to increase vascular permeability.596 More recent studies show that it may also increase the expression of some target antigens that can enhance efficacy.597 In patients the combination of external beam therapy with TaRT increased the objective response rate of liver tumors to RIT. This combined approach has used both local irradiation as well as total body irradiation in stem cell rescue studies during which radiolabeled antibody was still circulating.598,599 In head and neck cancer, RIT has been combined with proton therapy in preclinical studies.600 The use of hyperthermia and vasoactive cytokines such as interleukin-2 and tumor necrosis factor has also resulted in higher tumor uptake of radiolabeled antibody in early studies.601–603 Many studies have been preclinical; however, hyperthermia and interleukin-2 have been used clinically in conjunction with RIT with some success.604 Of note, hyperthermia can have multiple effects, including increased antigen expression, direct cytotoxic activity, inhibition of radiation repair at medium and low dose rates, and reduced interstitial fluid pressure.605–607 Hyperthermia enhances both antibody uptake in tumor via vascular mechanisms and low-dose-rate radiation effects.605,608 Neovascular targeting is also a promising approach to use in combination with TaRT (e.g., up-regulated receptors on new tumor vessels).52,609–611 Antiangiogenic agents can be combined with TaRT.55,612 For example, a selective peptide agonist of human C5a, given before and after radiolabeled antibody therapy to increase vascular permeability of tumor capillaries, improved the therapeutic outcome of TaRT in xenograft tumors.613 Fractionation of TaRT dosing has generally been favorable in many preclinical investigations and in limited clinical experience.27,525,614,615 However, more study is needed because some preclinical studies have shown decreased vascular permeability with fractionation, which could have a deleterious effect.616 As previously discussed, high-affinity antibodies have been reported to improve antibody uptake but not necessarily intratumor distribution, and in some cases high-affinity antibodies have produced a more favorable therapeutic ratio. Clinical trials using high-affinity antibodies have been performed for cancer of the colon, breast, prostate, lung, and ovary.510,567,617–619 Targeting may also be enhanced by increasing antigen expression on target cells.620 Several studies show that interferon can increase the expression of some common tumor-associated antigens, including carcinoembryonic antigen (CEA), TAG-72, and an antigen expressed on melanomas.621,622 Applications of this approach in vivo using human tumor xenografts in mice have shown greater tumor growth inhibition with interferon administration before RIT compared with similarly treated mice without interferon.623 Human tumor biopsy material has also confirmed increased antigen expression following interferon administration. Benefit from adjuvant interferon in clinical trials has been difficult to demonstrate. Potential benefit may be inferred because increased tumor antigen expression was demonstrated by biopsy, and imaging of antibody localization in known sites of disease was improved by interferon compared with results obtained in other trials with similar radiolabeled antibody that did not use interferon.624,625 Targeting of two antigens with separate antibodies is also promising. Studies of unlabeled veltuzumab, a humanized anti-CD20 antibody, combined with 90Y-epratuzumab, an anti-CD22 antibody, show that it enhances the therapy response of lymphoma in mouse studies. Use of the nonidentical unlabeled antibody may promote apoptosis in conjunction with the radiolabeled antibody but eliminate the potential for competition between additional unlabeled antibody when using the same antibody for an unlabeled predose and as a radionuclide conjugate.626 Although not a mechanism to increase uptake, removal of unbound radiolabeled agent decreases toxicity and can allow dose escalation.627
Several pretargeting methods have been proposed to overcome some of the limitations of conventional one-step TaRT where prolonged radiation exposure of the bone marrow results in dose-limiting marrow suppression. As previously mentioned, pretargeting approaches dissociate the delivery of unlabeled antibody, or other targeting entity, from the delivery of the radionuclide. Ideally, this approach delays the delivery of the radioactivity until the ratio of tumor-bound to non–tumor-bound targeting agent has reached its maximum. Because the radionuclide is usually given conjugated to a small hapten that interacts with the targeting entity, any nonbound radioactivity will clear quickly because of the small molecular weight of the radionuclide conjugate. Therefore, the pretargeting approach has the potential to improve the tumor to normal tissue ratios several-fold over the ratios achieved with directly labeled antibodies by minimizing circulation time of unbound radionuclide. Proof of principle of the pretargeting approach has been established in multiple preclinical and early-phase clinical trials, and studies continue for further optimization.44,628,629 Application of pretargeted radioimmunotherapy in a high-risk group of medullary thyroid cancer patients improved survival rates.630
A number of pretargeting designs have been devised and others are in development.631–633 To date, the most popular are based on the use of bifunctional antibodies or biotin-streptavidin interactions, with these components complexed to a targeting agent and radionuclide.8,614,628 Less common pretargeting approaches include the use of antibody-oligonucleotide conjugates, a prodrug/enzyme strategy, and DNA/DNA systems.634,635 Other mechanisms that allow for amplification/enhancement of targeting are under study.633,636 Common to all methods is the desire for (1) high specificity and retention of the targeting agent, without shedding or internalization; (2) high specificity of the radionuclide conjugate for the bound targeting agent; and (3) rapid clearance of radionuclide that does not localize to target.
Bifunctional antibodies have been constructed by cross-linking Fab’ fragments from two antibodies that have different specificities.637–639 For RIT, one end of a bifunctional antibody contains an unlabeled fragment directed toward a tumor-associated antigen/receptor, whereas the other binding site reacts with a hapten linked to a radionuclide. Sharkey and colleagues have designed a “universal” system by making one Fab’ fragment specific for the chelating agent that is conjugated to the radionuclide.637,638,640 Therefore different radionuclides can be used as long as they are complexed to that chelating agent. Bivalent nonantibody peptides have also been developed.637,641
Advantages of the biotin-streptavidin system include not only the small molecular weight of biotin, which can quickly circulate throughout the body, but also the high binding affinity between biotin and avidin or streptavidin (1015 M−1). Streptavidin is tetravalent (i.e., has four biotin binding sites) and can therefore increase the amount of radionuclide localization in tumor. On the other hand, a disadvantage of the biotin/streptavidin system is the immunogenicity of streptavidin or avidin. Polyethylene glycol modification can reduce this immunogenicity but may also decrease the binding capacity of biotin.637,642
Although the concept of pretargeting does not require a clearing step between administration of the targeting agent and radionuclide, use of a clearing agent can further enhance the tumor to normal tissue ratio. Theoretical and experimental pharmacokinetic modeling studies suggest that pretargeting approaches using a two-step method will result in a twofold to threefold enhancement of efficacy compared with conventional one-step RIT.637,643 Use of a clearing agent can further improve tumor to normal tissue ratios by several-fold. A variety of clearing mechanisms have been studied to date, including an antibody that reacts with the targeting agent, plasmapheresis/extracorporeal immunoadsorption, and high-molecular-weight agents that react with the targeting agent in the circulation and/or other agents that are primarily confined to the vasculature.644–647 The primary objective of clearing strategies is to remove unbound targeting agent from the circulation without affecting that bound to tumor. Clearing designs for biotin-streptavidin systems have usually used three to five steps to promote clearance of unbound targeting agent to which the radionuclide complex would otherwise bind.648 By using a three-step approach, a mean tumor to marrow ratio of 19 was achieved in glioma patients. Using this three-step pretargeting approach, 12 of 48 glioma patients with residual or recurrent disease after conventional therapy achieved a greater than 25% reduction in tumor, with 8 of 48 patients in remission at 12 months.542,649 Efficacy has been reported by Paganelli and his European Institute of Oncology colleagues using this biotinylated antibody in a three-step approach for therapy in patients with other solid tumors as well.649,650 One of the most efficient clearing agents is a complex of galactosamine-biotin molecules that bind streptavidin antibody and are extracted via the liver. After optimal tumor targeting, antibody complexes were efficiently removed so that blood concentrations of streptavidin were reduced by more than 95% within 6 hours of administration of a synthetic monobiotin poly-N-acetyl-galactosamine compound developed by NeoRx Corporation investigators.651,652 Extraction of the clearing agent from the blood occurs via interaction with Ashwell receptors in the liver. Use of clearing agents that complex with the targeting agent and are trapped in the liver has allowed dose escalation as high as 140 mCi/m2 90Y, whereas the maximum tolerated dose for conventional RIT of 90Y antibody conjugates is usually less than 30 mCi/m2 because of myelosuppression.44,510,653 This synthetic monobiotin poly-N-acetyl-galactosamine compound has been used with antibodies conjugated chemically with streptavidin and more recently with fusion proteins.651,654 The genetically engineered fusion proteins have been produced by fusing single-chain antibody fragments with streptavidin. This has provided tetrameric products with four binding sites for the bivalent antibody and four binding sites for biotin.38 Early study of an anti-CD20/streptavidin fusion protein in a pretarget approach with the synthetic biotin-poly-N-acetyl-galactosamine compound has resulted in an average tumor to whole body radiation dose ratio of 49, with most patients having no significant hematologic toxicity at 15 mCi/m2 of 90Y.651
The pretargeting approach is very promising for treating solid tumors where efficacy has been limited primarily by the inability to deliver sufficient tumor doses with acceptable toxicity. Meaningful responses in a variety of nonmyelogenous solid tumors as well as lymphomas have been achieved with pretargeting.44,647,655,656 Several reviews provide additional details of pretargeting development.5,8,628,630,637,640 A new technique for partial breast treatment involves pretargeting of the tumorectomy cavity by intraoperative injection with avidin, followed later by intravenous radiolabeled biotin.657,658
Use of regional or direct injection of TaRT into tumors or tumor cavities has allowed dose escalation for treatment of relatively radioresistant tumors, with encouraging results as discussed in the solid tumor section.109,509,535,659–667 Other approaches that may improve uptake of radiolabeled antibody in tumors include the use of unlabeled antibody to bind circulating antigen and nonspecific binding sites and the use of “cocktails” or mixtures of antibodies reactive with different tumor-associated antigens.626,668–670 An advantage of antibody mixtures has been confirmed in animal studies that showed superior detection and localization to smaller tumors and greater tumor growth inhibition with the combination of two 131I-labeled antibodies than either antibody alone using the same total amount of radionuclide.671,672 Several human trials have used two or more antibodies simultaneously. However, it has not been possible to make a direct comparison of one versus two or more antibodies in patients, because most trials did not test one antibody alone compared with a combination of antibodies in the same study.625 When optimized, antibody cocktails may include the combination of whole antibodies with smaller constructs and/or use of different radionuclides. Radionuclides are chosen on the basis of their physical half-life, radiation emission characteristics, and pharmacokinetics in a manner that would complement one another and will be matched with the half-life of the antibody or antibody constructs. In addition, the use of antibodies with different affinities and/or the use of genetically engineered antibodies with greater avidity may also increase the targeting and retention of radioimmunoconjugates in tumors. Use of two radionuclides, even with the same carrier, shows improved results in a rat model of PRRT 90Y and 177Lu and in initial clinical experience.64,63 In an effort to increase the efficacy of TaRT in relatively radioresistant cancers, TaRT is being studied in conjunction with other agents or therapies, such as external beam radiation therapy, which may increase targeting by affecting tumor vasculature while causing G2/M arrest in a radiosensitive phase of the cell cycle. With G2/M arrest during this radiosensitive phase of the cell cycle, tumors will receive prolonged low-dose-rate radiation from radionuclide emissions associated with TaRT. Approaches for improving the therapeutic ratio and/or efficacy of TaRT include the use of radiosensitizers and radioprotectors.673 TaRT is now being studied in combination with chemotherapy and a variety of other therapeutic agents as previously described, but these combined-modality approaches have generally not been optimized (e.g., dose sequence and timing).674 Optimization of these regimens may vary with tumor/radionuclide systems.675–677 Polyethylene glycol (PEG) modification of targeting agents is an example of an optimization strategy under study that provides multiple carrying sites for molecules such as radiosensitizers.678,679 TaRT has been studied in combination with a variety of agents, including the radiosensitizer SR2508, intercalating agents, and the hypoxic cytotoxin SR4233 (tirapazamine).605 A variety of chemotherapy agents have also been studied as radiosensitizing agents in conjunction with TaRT.680–684
The taxanes radiosensitize tumors by arresting cells in the G2/M cell cycle phase and have demonstrated synergistic effects in preclinical TaRT studies. Subsequently, they have been studied in clinical trials in combination with RIT.535,674 Cisplatin has also been shown to have a supra-additive effect in combination with low-dose-rate radiation.685 Buchsbaum and colleagues287 have reported increased growth inhibition in human tumor xenografts in nude mice when bromodeoxyuridine, which is incorporated into the DNA in place of thymidine, was given for 4 days with a 1-day washout before RIT. Tirapazamine potentiated cytotoxicity by RIT in mice bearing human tumor xenografts.605,686 Caffeine has also been shown to modulate sensitivity to RIT in lymphoma cells.89 Sensitization may be achieved with low doses of adjuvant agents, and it has been feasible to use nearly full doses of each agent with acceptable toxicity in some trials.510,535 Patients with recurrent glioblastoma were given the combination of locoregional pretargeted TaRT with temozolomide without significant neurologic toxicity or severe hematologic toxicity.687
At the preclinical level, gene therapy can enhance the efficacy of TaRT. Gene transfer methods have been used to genetically induce tumor cells to express enhanced levels of cell surface tumor-associated antigens or receptors, which have resulted in increased tumor uptake of radiolabeled antibody or peptides in animal models.688 This approach could potentially result in enhanced therapeutic efficacy.689 There is also the potential to use gene transfer techniques to radiosensitize tumors by knocking out genes involved in DNA repair, signal transduction, and control of the cell cycle that would result in sensitization of tumors to TaRT. Alternatively, gene therapy may be used to provide radioprotection of bone marrow. The combination of toxin-gene delivery and TaRT also offers the potential for improved therapeutic efficacy.688 For example, specific genes can be targeted with short-range radionuclides such as Auger emitters, as has been demonstrated by 125I-labeled triplex-forming oligonucleotides.690
Future Considerations
TaRT is a promising therapeutic modality. Ongoing work in this area will result in expansion of indications and improved efficacy. In addition to acceptance of TaRT as a component of the standard armamentarium for lymphohematopoietic malignant tumors, the efficacy of TaRT for selected nonhematologic entities is continually being improved. It is expected that for many diseases, TaRT will be most useful as a component of multimodality therapy.408,464,682 Future studies should define how to optimally combine TaRT with other therapeutic modalities. More investigation is needed to determine agents/schedules that result in synergistic antitumor activity without significantly greater toxicity. TaRT has been useful both as a component of early therapy and as a salvage therapy (e.g., improved survival of glioma patients). It is also being studied in lymphoma as an adjunct to other agents in an effort to further improve therapeutic outcome.464,691 Active areas of investigation include studies of TaRT using alpha particles, small nonantibody targeting agents, gene therapy, and other approaches for increasing tumor uptake of radionuclide and enhancing efficacy.613