Chapter 37 T-Cell Lymphomas
Treatment of Peripheral T-Cell Lymphoma
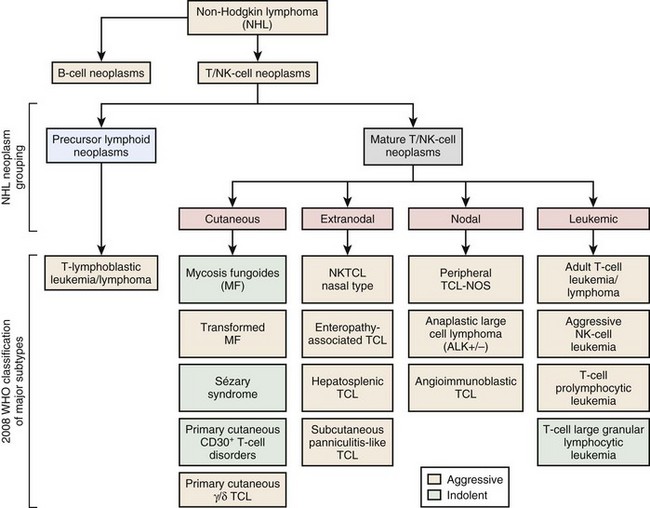
Figure 37-1 WHO CLASSIFICATION OF THE MATURE T-CELL NEOPLASMS.
NK, Natural killer; NOS, not otherwise specified; TCL, T-cell lymphoma.
Table 37-1 WHO Classification of the Mature T-Cell Lymphomas
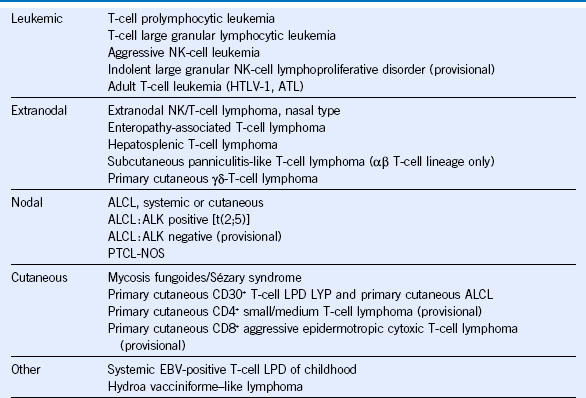
ALCL, Anaplastic large cell lymphoma; ATL, adult T-cell leukemia/lymphoma; EBV, Epstein-Barr virus; HTLV-1, human T-lymphotropic virus-1; LPD, lymphoproliferative disease; LYP, lymphomatoid papulosis; NK, natural killer; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma; WHO, World Health Organization.
Table 37-2 Recent FDA-Approved and Emerging New Drugs in Peripheral T-Cell Lymphoma
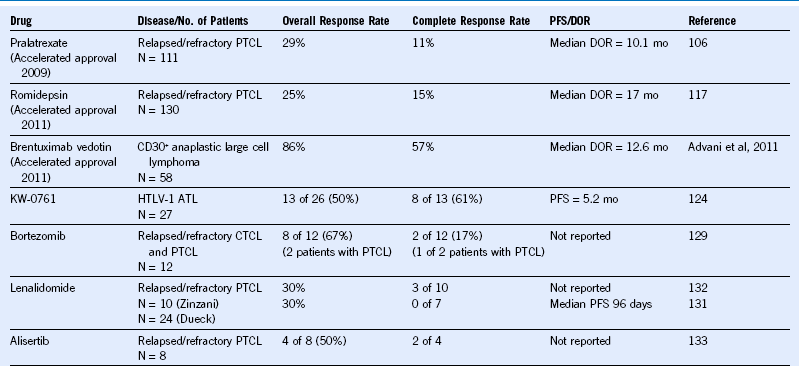
ATL, Adult T-cell leukemia/lymphoma; CTCL, cutaneous T-cell lymphoma; DOR, duration of response; FDA, Food and Drug Administration; HTLV-1, human T-lymphotropic virus-1; PFS, progression-free survival; PTCL, peripheral T-cell lymphoma.
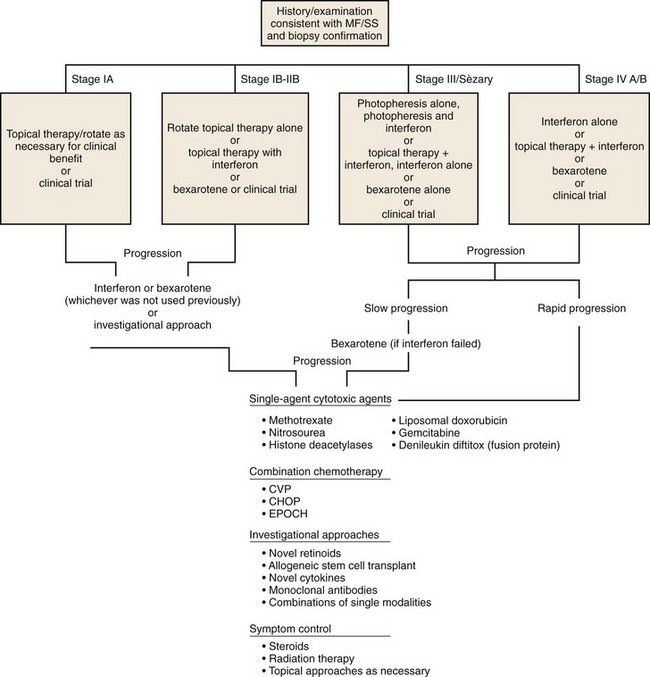
Algorithm for Care of Patients With Mycosis Fungoides or Sézary Syndrome
Table 37-3 TNM Staging System for Cutaneous T-Cell Lymphomas
| Classification | Description |
|---|---|
| T | Skin |
| T0 | Clinically or histopathologically suspicious lesions |
| T1 | Limited plaques, papules, or eczematous patches covering 10% of the skin surface |
| T2 | Generalized plaques, papules, or erythematous patches covering 10% of the skin surface |
| T3 | Tumors (one or more) |
| T4 | Generalized erythroderma |
| Pathology of T1 to 4 is diagnostic of a cutaneous T-cell lymphoma. When more than one T stage exists, both are recorded and highest is used for staging. Record other features if appropriate (e.g., ulcers, poikiloderma, scale) | |
| N | Lymph nodes |
| N0 | No clinically abnormal peripheral lymph nodes |
| N1 | Clinically abnormal peripheral lymph nodes (record number of sites) |
| NP0 | Biopsy performed, not CTCL |
| NP1 | Biopsy performed, CTCL |
| PB | Peripheral blood |
| PB0 | Atypical circulating cells not present (≤5%) |
| PB1 | Atypical circulating cells not present (>5%), record total white blood cell count, total lymphocyte count, and percentage of abnormal cells |
| M | Visceral organs |
| M0 | No visceral organ involvement |
| M1 | Visceral involvement (must have pathologic confirmation), record organ involved |
| Staging | |
| Stage IA | T1, N0 NP0, M0 |
| Stage IB | T2, N0 NP0, M0 |
| Stage IIA | T1-2, N1 NP0, M0 |
| Stage IIB | T3, N0 NP0, M0 |
| Stage III | T4, N0 NP0, M0 |
| Stage IVA | T1-4, N0,1 NP1, M0 |
| Stage IVB | T1-4, N0,1 NP0,1, M1 |
CTCL, Cutaneous T-cell lymphoma; TNM, tumor-node-metastasis.
Table 37-4 Therapeutic Options for Mycosis Fungoides
| TOPICAL THERAPY |