CHAPTER 93 SYSTEMIC INFLAMMATORY RESPONSE SYNDROME AND MULTIPLE-ORGAN DYSFUNCTION SYNDROME: DEFINITION, DIAGNOSIS, AND MANAGEMENT
In 1973, Tilney et al.1 described 18 patients who developed “sequential system failure” following surgery for ruptured abdominal aneurysms. It was at this time that the idea that severe physiologic insults could lead to multiple-organ failure (MOF) was first established. Several decades later, MOF (or multiple organ dysfunction syndrome [MODS]) remains a major source of postinjury morbidity and a leading cause of death in surgical intensive care units (SICUs). Although the pathogenesis of this syndrome remains to be fully defined, it is evident that sepsis, systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS), and MODS are closely related phenomena. Consequently, the goal of this chapter is to review SIRS and MODS, focusing on current strategies for diagnosing, managing, and (most importantly) preventing these syndromes.
INCIDENCE
The concept that death from trauma has a trimodal distribution (with these deaths being caused by hemorrhage, head injury, and sepsis/organ failure) is well established. Because MODS is the most common cause of late trauma deaths, it has been the subject of intense investigation. It is now clear that certain clinical risk factors can be used to predict the likelihood of a patient developing MODS. These include age, injury severity score (ISS), number of blood transfusions, and lactate/base deficit levels.2 However, it is only over the last decade that the incidence of MODS in high-risk trauma patients appears to have decreased. This decrease appears to be due to a better knowledge of the factors predisposing patients to its development, as well as to the immunoinflammatory response to shock and trauma. For example, a 12-year prospective study examining 1344 trauma patients noted that the actual incidence of MODS (25%) was lower than its predicted rate.3
The authors concluded that this decrease was likely due to the concomitant drop in the liberal use of blood transfusions, which have been shown to be an independent predictor of MODS, SIRS, and mortality.4,5 Not only does the incidence of MODS appear to be decreasing but there is emerging data to suggest that the mortality rate of patients with MODS is also declining—as reflected in a retrospective study of MODS-related death after blunt multiple trauma during a 25-year period. This study revealed an approximately 50% reduction in MODS-related mortality, from 29% to 14% over this time period.6 As will be discussed later in this chapter, several therapeutic interventions have been developed that have been shown to reduce mortality or to attenuate organ dysfunction, which would help explain this decline in mortality. In spite of these improvements, once MODS has become established the risk of death is significant—with the patient’s prognosis being more closely related to the number of organs that have failed than to any other variable, including the underlying processes that initiated the MODS.7
MECHANISMS OF MODS
The clinical picture of MOF is indicative of a generalized systemic inflammatory response, which typically occurs as a result of infection or uncontrolled inflammation in the patient with severe trauma. Several distinct and often conflicting hypotheses have been proposed to explain the mechanisms underlying MODS.7 Nonetheless, MOF can be viewed as a systemic process involving the excessive stimulation of certain inflammatory responses mediated by circulating factors whose effects contribute to injury or dysfunction in organs not involved in the initial insult. To a large extent, the cascade of events culminating in MOF is likely to be mediated by the same factors irrespective of the exact nature of the triggering insult. In fact, it is the host’s inflammatory response to injury or infection that is probably more important in the genesis of SIRS, ARDS, and MODS than the microbial agent or the initiating insult. Thus, an appreciation of the role of the inflammatory response of the host in the pathogenesis of MOF is vital in order to develop new and effective modalities for the prevention and treatment of this syndrome.
Another mechanism by which hemorrhagic shock and trauma could predispose to the developments of MODS is through an ischemia-reperfusion injury and/or damage to the microcirculation. Because shock is essentially a total-body ischemia-reperfusion insult and the microcirculation of various tissues and organs are highly susceptible to ischemia-reperfusion–mediated insults, this process has been termed the microcirculatory hypothesis of MODS.7 Physiologically, circulatory shock could contribute to MOF through inadequate global oxygen delivery, the ischemia-reperfusion phenomenon, and/or the promotion of deleterious endothelial-leukocyte interactions.
Although prolonged tissue hypoxia leads to inadequate ATP generation and potentially irreversible cell damage, under most clinical conditions the shock period is not long enough for this process to occur. Thus, in clinical situations it appears that most of the tissue damage occurs after ischemia is relieved by reperfusion and that this damage is due to the production of reperfusion-induced oxygen radicals and proinflammatory factors (such as oxidants, nitric oxide, chemokines, and cytokines). In fact, recent studies show that the combination of reperfusion-induced increased levels of nitric oxide and superoxide anion synergistically increase cell injury via the production of peroxynitrite, which is a long-lasting and potent oxidant that causes direct cell injury through lipid peroxidation. This notion that increased nitric oxide production is important in the pathogenesis of MODS is supported by clinical studies showing that serum nitrate levels (an index for the systemic production of nitric oxide) correlated well with MOF scores in critically ill patients.8
Endothelial-leukocyte interactions leading to tissue injury also seem to be a key step in the pathogenesis of SIRS, ARDS, and MODS. Many factors related to shock and tissue injury, including cytokines, necrotic tissue, endotoxins, and oxidants, can convert endothelial cells from a quiescent state to a proinflammatory procoagulant one and can activate neutrophils. The combination of these changes in endothelial cell phenotype and neutrophil activation has been documented to lead to increased neutrophil adherence to the microcirculatory endothelium, thereby promoting neutrophil-mediated microvascular injury.7 Experimentally, inhibition of neutrophil-endothelial interactions has been shown to limit shock- and sepsis-induced injury to a number of organs, including the lung. Furthermore, neutrophil activation in trauma patients has been identified as a predictor of the development of SIRS, ARDS, and MODS. Therefore, endothelial cell–neutrophil interactions, whether induced by shock, sepsis, or an augmented inflammatory response, appear to be an important effector mechanism in the development of ARDS and MODS.
The gut hypothesis of MOF has been used to explain why no identifiable focus of infection can be found in as many as 30% of bacteremic patients who die from MOF.9 An extensive body of experimental as well as clinical studies supports this hypothesis. For example, clinical studies indicate that intestinal permeability is increased in patients with sepsis after major thermal injury or trauma and that loss of intestinal barrier function correlates with the development of systemic infection, ARDS, and MODS.10 Likewise, studies in intensive care unit (ICU) and trauma patients indicated that gut ischemia, as measured by gastric tonometry, is a better predictor of the development of ARDS and MODS than global indices of oxygen delivery.11 Although both clinical and experimental studies implicated intestinal injury and bacterial translocation in the development of SIRS and MODS, a study by Moore et al.12 began to cast doubt on the clinical relevance of bacterial translocation.
These investigators failed to find bacteria or endotoxin in the portal blood of severely injured patients, including a subgroup of patients developing MODS. One potential explanation for this failure to find endotoxin or bacteria in the portal blood was that the gut-derived factors contributing to SIRS, ARDS, and MODS were exiting the gut via the lymphatics. Studies testing this possibility have documented that nonbacterial factors exiting the ischemic gut contribute to acute ARDS, MODS, neutrophil activation, and endothelial cell injury/activation in both rodent and primate models of trauma-hemorrhagic shock and have led to the gut-lymph hypothesis of MODS.10 This gut lymph hypothesis of MODS proposes that nonbacterial noncytokine factors released from the stressed gut via the lymphatic system activate neutrophils and endothelial cells, thereby leading to organ dysfunction. Thus, over the last several years the gut hypothesis has expanded beyond bacterial translocation and now also implicates gut-derived nonbacterial proinflammatory and tissue-injurious factors in the pathogenesis of SIRS, ARDS, and MODS.
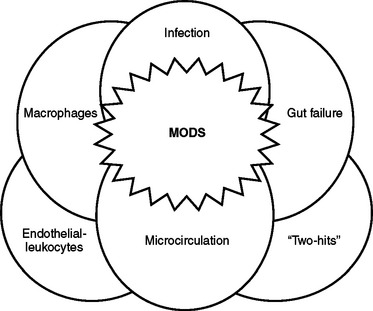
This clinical observation has led to the “two-hit” hypothesis of MODS, where potentially clinically modest events prime the host so that the host’s response to subsequent secondary events becomes exaggerated, culminating in SIRS, ARDS, and MOF. Although this two-hit theory needs to be further understood, it is a feasible explanation of how trauma or burn injury can convert a nonlethal infectious or hypoxic challenge into a lethal insult. In fact, as illustrated in Figure 1 it is clear that the difficulty in finding an effective therapy to prevent or treat MODS relates to the overlapping nature of the multiple systems activated by shock and trauma as well as the ability of one system to prime other systems for an exaggerated physiologic response to secondary insults. Nonetheless, the knowledge gained from these basic studies of the physiology of inflammation and MODS have provided important therapeutic insights. For example, they highlight the importance of prompt and adequate volume resuscitation and microcirculatory blood flow to prevent organ ischemia, the need for early excision of nonviable tissue to limit systemic inflammation, and the need for therapies to better preserve gut barrier dysfunction and limit uncontrolled inflammation.
DIAGNOSIS
A key step in the treatment of a disease process is the establishment of an accurate diagnosis. To that end, a number of consensus conferences have been held in an attempt to provide classification schemes that allow SIRS, ARDS, and MODS to be accurately diagnosed. Based on these conferences, SIRS is defined as the response to a variety of severe clinical insults, which is manifested by two or more of the four conditions listed in Table 1.13 Furthermore, SIRS should be viewed as an evolved dynamic process that has adaptive survival value for the host under most circumstances because it signals the body to respond to injury or to an external threat such as a bacterial infection. However, if this protective inflammatory response becomes uncontrolled or excessive it has maladaptive consequences due to its potential to injure the host’s own tissues.
Table 1 Definition of Systemic Inflammatory Response Syndrome
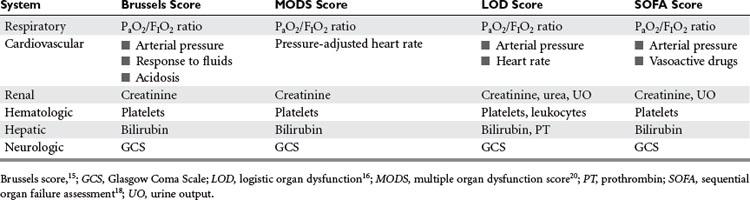
The term “MODS” was introduced by a consensus conference of the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) in 1991.14 Prior to that time, this syndrome had many different names, including sequential organ dysfunction syndrome and multiple-organ failure syndrome. Several MODS scoring systems have been established that grade the severity of MODS and emphasize the concept that there exists a continuous spectrum from mild to full-blown dysfunction that correlates, on a patient population level, with mortality and morbidity (Table 2).15–18
These systems, much like the sequential organ failure assessment (SOFA) score developed by Vincent et al.,18 score organ failure by assigning a numerical scale in which more points are given to the higher degree of organ dysfunction in several organ systems (Table 3).16,19 Although not developed to predict mortality in individual patients, there are several areas where the use of such scoring systems can be beneficial in critically ill patients. This includes their use in the daily clinical evaluation of a patient’s response, in research involving epidemiologic studies, and in the assessment of new therapies in clinical trials. Although these scoring systems use slightly different parameters to grade organ failure, most studies have found that the clinical utility of these scoring systems is comparable.20,21
MANAGEMENT
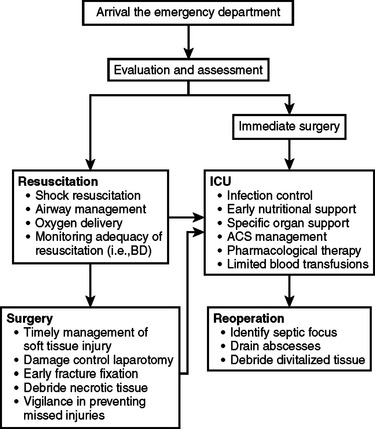
At the current time, the treatment of patients with established MODS is largely symptomatic and dedicated to supporting organs and systems that have failed. Because there is no “cure” for MODS, once it is present—and because the mortality rate of patients with established MODS is high—prevention becomes a key strategy in the care of the high-risk trauma patient. Therefore, it is important to understand and utilize certain strategies and approaches that have been shown to reduce the risk for developing MODS. In trauma patients, prevention begins in the field, with rapid transport to a medical facility, and extends throughout the resuscitative, operative, and ICU phases of care (Table 4). Because the approaches and strategies used at different phases of patient care may vary to some extent, each of these phases is discussed individually—although in actual clinical practice these phases often overlap.
| Resuscitative Phase |
ICU, Intensive care unit.
Resuscitative Phase
This is because blood pressure and urine output may not reflect the adequacy of volume resuscitation in the severely injured trauma patient. In this setting, arterial blood base deficit and serum lactate levels have been shown to be useful markers with which to monitor the response to resuscitation. A worsening base deficit or serum lactate has been shown to correlate with ongoing blood loss or inadequate volume resuscitation, whereas improvements in these parameters are indicative of adequate volume resuscitation. Because in severely injured patients the period of volume resuscitation may last up to 48 hours, serial measurements are important. Based on prospective studies demonstrating that patients who cleared their base deficient or lactate levels within 48 hours had a reduced incidence of ARDS and MODS plus a higher survival rate than those who did not,22,23 the resuscitative goal should be to reduce and keep the base deficit below −2 mmol/l and/or the serum lactate less than 1.5 mEq/l.
The choice of resuscitative fluid has become a more controversial subject with the recognition that Ringers lactate is proinflammatory and thus may exacerbate the inflammatory response and contribute to the development of organ injury in shock states.24–27 Given these concerns, plus the recent recognition that large-volume resuscitation with crystalloid solutions contributes to the development of the abdominal compartment syndrome (ACS),28 attention has refocused on the early resuscitation of trauma patients with hypertonic (7.5%) saline. The largest clinical trial comparing hypertonic saline versus Ringer’s lactate when administered in the field demonstrated similar survival between the two groups.29 However, there were decreased complications (such as renal failure and ARDS) in the hypertonic saline group.29
Nonetheless, at the current time due to the paucity of clinical trials there is not enough data to determine whether or not initial hypertonic saline resuscitation is superior to standard crystalloid resuscitation of the trauma patient. Another encouraging approach is the use of resuscitation fluids containing antioxidants, with three clinical trials, including a recent prospective randomized trial, showing that splanchnic-directed antioxidant therapy helps prevents MODS in trauma patients.30 As investigations into novel resuscitation fluids with pharmacologic actions (i.e., gut-protective, immune modulatory) continues, it is likely that the initial resuscitative approach of the trauma patient will evolve from Ringer’s lactate to include new fluid formulas.
The role of blood transfusions in trauma patients has also undergone an intense reevaluation based on clinical studies showing that blood is immune-suppressive and that blood transfusions are an independent predictor of MODS, especially when blood older than 2 weeks is administered.31 These observations, plus the fact that ICU patients as well as trauma patients can be safely managed with hemoglobin levels in the range of 7 g/dl, has led to the emergence of a selective transfusion policy in which prophylactic transfusions for anemia are no longer routinely administered. In fact, the TRICC trial demonstrated a significant reduction in the severity of new organ dysfunction in a critical care setting when transfusion was withheld unless the hemoglobin concentration was less than 7 g/dl.32,33
Operative Intervention
In an early article on multiple organ failure, Eiseman et al. described a series of 42 surgical patients with MODS, 24 of whom developed MODS as a result of intraoperative error or postoperative mismanagement.34 This study emphasizes one of the key elements in the perioperative care of trauma patients: missed injuries are not uncommon in trauma patients and they can have dire consequences, including the development of ARDS, MODS, and death.35–37 Although the specifics of the operative care of the trauma patient are covered elsewhere, certain aspects are important in the context of MODS. An example is the judicious use of damage-control laparotomy to limit both acute and delayed MODS. The rationale behind a damage-control laparotomy is the clinical observation that prolonged attempts at definitive control of intra-abdominal injuries can result in hemodynamic instability, acidosis, and coagulopathy.
If the patient survives the operation, the incidence of postoperative MODS is high. In contrast, a planned reoperation is safer and easier in patients who have been warmed and fully resuscitated and have had their acidosis and coagulopathy corrected. Although the morbidity and mortality rate of patients undergoing damage-control laparotomies is significant, the incidence of MODS appears to be reduced and survival increased.36 A second example of operative intervention to reduce the incidence of ARDS and MODS is early fixation of long-bone fractures.38 In fact, beginning as early as 1985, numerous prospective and retrospective clinical trials have documented that early fixation of long-bone fractures compared with delayed fracture fixation is associated with lower rates of renal, respiratory, and liver failure and lower rates of death.
Intensive Care Unit Management Phase
The incidence of postoperative and postinjury MOF can be prevented through strategies such as continued resuscitation, management of infectious complications, and early nutritional and specific organ support. Although some organs (such as the pulmonary system) have randomized prospective data supporting certain therapies that improve outcome, other systems (such as the hepatic system) rarely require specific treatment. In this section we focus on preventive and therapeutic strategies that appear to have reduced the incidence of MODS and/or improved outcome in patients with MODS (Tables 4 and 5).
Table 5 ICU Interventions That Reduce Mortality or Attenuate Organ Dysfunction
| Objective | Intervention |
|---|---|
| Resuscitation | Early goal-directed resuscitation |
| Prophylaxis | SDD |
| ICU support | Restrictive transfusion strategy |
| Low tidal volume ventilation | |
| Daily awakening | |
| Tight glucose control | |
| Enteral feeding | |
| Mediator-targeted therapy | Activated protein C |
| Low-dose corticosteroids |
ICU, Intensive care unit; SDD, selective decontamination of digestive tract.
Because infections can contribute to the development of MODS and can increase mortality, several key concepts must be kept in mind to limit infection-related MODS. The use of early empiric antibiotics in patients suspected of having pneumonia is important because the use of early adequate empiric antibiotic has been shown to reduce pneumonia-related mortality.39 Interestingly, although not used much in the United States it appears that selective decontamination of the digestive tract (SDD) reduces infectious complications as well as mortality in critically ill trauma and other surgical patients.40
In contrast, a prospective randomized trial documented that the recombinant form of activated protein C improved 28-day survival and led to a more rapid resolution of cardiovascular, respiratory, and hematologic dysfunction in patients with severe sepsis.41 The reason activated protein C was effective where other agents were not may relate to the fact that it has both anticoagulant and anti-inflammatory activity, thereby protecting the microcirculation as well as limiting the inflammatory response. Last, the use of low-dose steroids has emerged as an effective therapy in patients with pressor-refractory septic shock and an impaired response to ACTH stimulation because controlled trials have documented that in this patient group the administration of 50 mg of hydrocortisone every 6 hours and 50 mcg of fludrocortisone improves survival.42
In addition to infectious issues, other non organ-specific therapies that appear to be beneficial include early enteral alimentation, glucose control, elevation of the head of the bed, and daily cessation of sedative infusions in ventilated patients. The notion of early enteral feeding is based on the concept of limiting gut-origin sepsis because the fed gut is more resistant to stress-induced injury and parenteral alimentation is associated with gut atrophy, increased permeability, and loss of barrier function.7 Based on the results of multiple prospective randomized trials, early enteral nutrition has been found to effectively reduce infectious complications, ICU, and total hospital length of stay, although it does not appear to improve survival.43 Thus, in an attempt to further improve the beneficial effects of enteral feedings a number of immune-enhancing enteral formulas were produced and tested in trauma and ICU patients.
Although some studies comparing standard to immune-enhancing enteral formulas suggested that immune-enhancing diets are associated with a further decrease in infectious complications, others did not.44 Thus, at the current time the institution of early enteral feeding seems to be the key factor in reducing infectious complications—with the composition of the enteral formula being of secondary importance. A second metabolic approach has been the institution of tight glucose control in which insulin is liberally used to keep the serum glucose less than 120 mg/dl.
Since the original prospective randomized controlled study showing that tight glucose control (<120 mg/dl) was associated with a survival advantage compared to a more liberal glucose control regimen (<215 mg/dl),45 numerous other studies (including several in trauma patients) have validated the concept that elevated serum glucose levels are associated with an increased incidence of infectious complications and poorer clinical outcomes.46 Other easily instituted ICU therapies have been shown to reduce complications. For example, daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation reduces ICU length of stay and morbidity,47 whereas elevation of the head of the bed of ventilated patients reduces the incidence of pneumonia and helps to preserve pulmonary function.48
In addition to elevating the head of the bed and daily sedative cessation, other advances in the care of the patient with respiratory failure have been made over the last several years. The most important of these was the recognition that high tidal volumes and increased airway pressures cause, rather than prevent, lung injury by inducing lung inflammation. This process has been termed ventilator-induced lung injury (VILI). Consistent with this physiologic concept, multicenter randomized controlled trials confirmed that mechanical ventilation of patients with acute lung injury and ARDS with a lower tidal volume (i.e., 6 ml/kg) than traditionally used results in decreased mortality and attenuates the local and systemic release of proinflammatory mediators.49,50 In addition, further clinical trials documented that outcomes in patients with acute lung injury or ARDS are similar whether lower or higher PEEP levels are used when an end-inspiratory plateau-pressure limit of 30 cm of water is maintained.51
While oxygenation is maintained with low tidal volumes, permissive hypercapnia and increased CO2 levels may develop as a result of decreased ventilation, but this does not appear to be harmful.52,53 Thus, the use of low-tidal volume ventilation that maintains the inspiratory plateau pressure below 30 cm of water is effective both in the prevention and treatment of acute lung injury and ARDS. A number of other ventilatory strategies have either failed to show consistent benefit (such as inhaled nitric oxide) or remain to be proven beneficial (such as prone ventilation or high-frequency ventilation).
Renal replacement therapy has been effective in critically ill patients with MODS by allowing regulation of fluid and electrolytes. Renal replacement therapy also has the potential to remove toxins and circulating mediators of inflammation. Methods of supporting renal function, such as the prophylactic use of low-dose dopamine, have not been found to be effective.54 Thus, currently the best way to limit renal failure is to avoid underresuscitation and to promptly diagnose and treat infectious complications. Once renal failure has occurred, continuous venovenous hemodialysis appears to be superior to hemodialysis because it avoids the need for systemic anticoagulation and is less likely to cause hypotensive episodes in the fragile patient.55
A recently recognized and important treatable cause of MODS is the abdominal compartment syndrome (ACS). The ACS can be viewed as a reversible mechanical cause of MODS that is related to increased intra-abdominal pressure.56,57 As the intra-abdominal pressure rises, abdominal visceral perfusion decreases, ventilation is impaired, and cardiac output declines. Clinically, the ACS is manifested as a decreasing urine output, inadequate ventilation associated with elevated peak airway pressures, and hypotension. Patients at highest risk of developing ACS are those suffering from multiple trauma, massive hemorrhage, and prolonged operations with massive volume resuscitation, as well as those requiring intra-abdominal packing to control bleeding.
CONCLUSIONS AND ALGORITHM
The development of SIRS and MODS in trauma patients remains relatively common. However, due to advances in understanding the biology of the host’s immunoinflammatory system as well as the mechanisms involved in the pathogenesis of SIRS, ARDS, and MODS progress in the treatment and prevention of these syndromes has occurred. This progress is reflected both as a decrease in the incidence of MODS and an improvement in the survival of patients with MODS. The strategies used to accomplish these goals involve both preventive and therapeutic approaches that begin in the resuscitative phase of the operative care of these patients and continue through the operative and ICU phase (Figure 2).
1 Tilney NL, Bailey GL, Morgan AP. Sequential system failure after rupture of abdominal aortic aneurysms: an unsolved problem in postoperative care. Ann Surg. 1973;178:117-122.
2 Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39-45.
3 Ciesla DJ, Moore EE, Johnson JL, et al. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140:432-439.
4 Dunne JR, Malone DL, Tracy JK, Napolitano LM. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect. 2004;5:395-404.
5 Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620-625.
6 Nast-Kolb D, Aufmkolk M, Rucholtz S, et al. Multiple organ failure still a major cause of morbidity but not mortality in blunt multiple trauma. J Trauma. 2001;51:835-842.
7 Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117-134.
8 Groeneveld PH, Kwappenberg KM, Langermans JA, et al. Nitric oxide (NO) production correlates with renal insufficiency and multiple organ dysfunction syndrome in severe sepsis. Intensive Care Med. 1996;22:1197-1202.
9 Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrere JS. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985;120:1109-1115.
10 Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92-98.
11 Ivatury RR, Simon RJ, Islam S, et al. A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg. 1996;183:145-154.
12 Moore FA, Moore EE, Poggetti R, et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629-638.
13 Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481-1483.
14 Bone RC, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Chest. 1992;101:1644-1655.
15 Bernard G. The Brussels Score. Sepsis. 1997;1:43-44.
16 Le Gall JR, Klar J, Lemeshow S, et al. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996;276:802-810.
17 Marshall JC. Multiple organ dysfunction syndrome. ACS Surgery: Principles and Practice. November 3, 2003.
18 Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793-1800.
19 Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638-1652.
20 Peres Bota D, Melot C, Lopes Ferreira F, et al. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002;28:1619-1624.
21 Pettila V, Pettila M, Sarna S, et al. Comparison of multiple organ dysfunction scores in the prediction of hospital mortality in the critically ill. Crit Care Med. 2002;30:1705-1711.
22 Davis JW, Kaups KL, Parks SN. Base deficit is superior to pH in evaluating clearance of acidosis after traumatic shock. J Trauma. 1998;44:114-118.
23 Davis JW, Shackford SR, Mackersie RC, Hoyt DB. Base deficit as a guide to volume resuscitation. J Trauma. 1988;28:1464-1467.
24 Rhee P, Wang D, Ruff P, et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;28:74-78.
25 Rhee P, Burris D, Kaufmann C, et al. Lactated Ringer’s solution resuscitation causes neutrophil activation after hemorrhagic shock. J Trauma. 1998;44:313-319.
26 Koustova E, Stanton K, Gushchin V, et al. Effects of lactated Ringer’s solutions on human leukocytes. J Trauma. 2002;52:872-878.
27 Alam HB, Sun L, Ruff P, et al. E- and P-selectin expression depends on the resuscitation fluid used in hemorrhaged rats. J Surg Res. 2002;94:145-152.
28 Balogh Z, McKinley BA, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138:637-643.
29 Mattox KL, Maningas PA, Moore EE, et al. Prehospital hypertonic saline/dextran infusion for post-traumatic hypotension. The U.S.A. Multicenter Trial. Ann Surg. 1991;213:482-491.
30 Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814-822.
31 Moore EE. Blood substitutes the future is now. J Am Coll Surg. 2003;196:1-17.
32 Hebert P, Wells G, Blajchman M, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-413.
33 Hebert PC, McDonald BJ, Tinmouth A. Clinical consequences of anemia and red cell transfusion in the critically ill. Crit Care Clin. 2004;20:225-235.
34 Eiseman B, Beart R, Norton L. Multiple organ failure. Surg Gynecol Obstet. 1977;144:323-326.
35 Brooks A, Holroyd B, Riley B. Missed injury in major trauma patients. Injury. 2004;35:407-410.
36 Hirshberg A, Wall MJJr, Mattox KL. Planned reoperation for trauma: a two year experience with 124 consecutive patients. J Trauma. 1994;37:365-369.
37 Houshian S, Larsen MS, Holm C. Missed injuries in a level I trauma center. J Trauma. 2002;52:715-719.
38 Carlson DW, Rodman GHJr, Kaehr D, et al. Femur fractures in chest-injured patients: is reaming contraindicated? J Orthop Trauma. 1998;12:164-168.
39 Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, et al. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742-2751.
40 De Jonge E. Effects of selective decontamination of digestive tract on mortality and antibiotic resistance in the intensive-care unit. Curr Opin Crit Care. 2005;11:144-149.
41 Vincent JL, Angus DC, Artigas A, et al. Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med. 2003;31:834-840.
42 Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
43 Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2003;29:2264.
44 Kieft H, Roos AN, van Drunen JD, et al. Clinical outcome of immunonutrition in a heterogeneous intensive care population. Intensive Care Med. 2005;31:524-532.
45 Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
46 Laird AM, Miller PR, Kilgo PD, et al. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058-1062.
47 Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272-1276.
48 Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-1858.
49 Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54-61.
50 The Acute Respiratory Distress Syndrome N. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
51 Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327.
52 Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22:1568-1578.
53 Laffey JG, Tanaka M, Engelberts D, et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000;162:2287-2294.
54 Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510-524.
55 Swartz RD, Bustami RT, Daley JM, et al. Estimating the impact of renal replacement therapy choice on outcome in severe acute renal failure. Clin Nephrol. 2005;63:335-345.
56 Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848-861.
57 Burch JM, Moore EE, Moore FA, Franciose R. The abdominal compartment syndrome. Surg Clin North Am. 1996;76:833-842.