Chapter 6 Systemic Inflammation
Numerous advances in perioperative care have allowed increasingly high-risk patients to safely undergo cardiac surgery. Although mortality rates of 1% are quoted for “low-risk” cardiac surgery, results from large series of patients older than 65 years suggest that mortality rates are actually more substantial.1 Postoperative morbidity is common and complications include atrial fibrillation, poor ventricular function requiring inotropic agents, and non–cardiac-related causes such as infection, gastrointestinal dysfunction, acute lung injury, stroke, and renal dysfunction.2
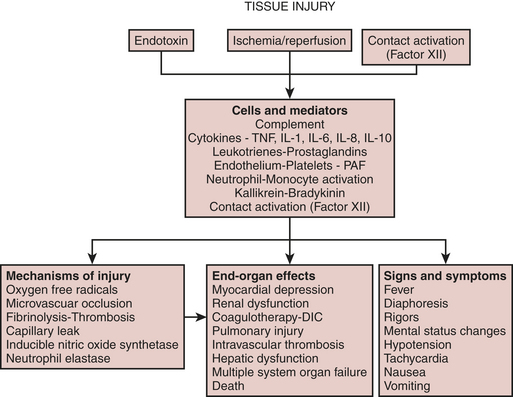
Many postoperative complications appear to be caused by an exaggerated systemic proinflammatory response to surgical trauma.3 The most severe form of this inflammatory response leads to multiple organ dysfunction syndrome and death. Milder forms of a proinflammatory response cause less severe organ dysfunction, which does not lead to admission to an intensive care unit but nevertheless causes suffering, increased hospital length of stay, and increased cost. The etiology and the clinical relevance of systemic inflammation after cardiac surgery are poorly understood. Systemic inflammation is a multifactorial process and has profound secondary effects on both injured and normal tissues. Proinflammatory mediators can have beneficial as well as deleterious effects on multiple organ systems. According to most theories, tissue injury, endotoxemia, and contact of blood with the foreign surface of the cardiopulmonary bypass (CPB) circuit are some of the major factors postulated to initiate a systemic inflammatory response. Nevertheless, there is controversy surrounding the etiology as well as pathogenesis of inflammation in the perioperative period.
SYSTEMIC INFLAMMATION AND CARDIAC SURGERY
The systemic inflammatory response after cardiac surgery is multifactorial. A schematic of the inflammatory process is depicted in Figure 6-1. There does not appear to be much disagreement with the statement that all of these processes may happen and may be responsible for causing complications in cardiac surgical patients. Tissue injury, endotoxemia, and contact of blood with the foreign surface of the CPB circuit are thought to initiate a systemic inflammatory response after cardiac surgery. What is least understood and most controversial is the issue of which of these many processes is the most clinically relevant. It appears as if major surgery is an important cause of systemic inflammation and that CPB further exacerbates the elaboration of proinflammatory mediators.
Mechanisms of Inflammation-Mediated Injury
Activation of neutrophils and other leukocytes is central to most theories regarding inflammation-induced injury.4 Neutrophil activation leads to the release of oxygen radicals, intracellular proteases, and fatty acid (e.g., arachidonic acid) metabolites. These products, as well as those from activated macrophages and platelets, can cause or exacerbate tissue injury.
In localized areas of infection, oxygen free radicals liberated by activated neutrophils aid in the destruction of pathogens.5 Complement, in particular C5a, results in activation of leukocytes and oxygen free radical formation. These activatedneutrophils liberate toxic amounts of oxygen free radicals such as hydrogen peroxide, hydroxyl radicals, and superoxide anion. Oxygen free radicals are thought to cause cellular injury ultimately through damage to the lipid membrane.
A related mechanism of injury results from the degranulation of neutrophils. Activated neutrophils release granules containing myeloperoxidase, as well as other toxic digestive enzymes such as neutrophil elastase, lactoferrin, β-glucuronidase, and N-acetyl-β-glucosaminidase.6 Release of these intracellular enzymes not only causes tissue damage but also reduces the number of cells that can participate in bacterial destruction.
Physiologic Mediators of Inflammation
Cytokines
Cytokines are believed to play a pivotal role in the pathophysiology of acute inflammation associated with cardiac surgery.7 Cytokines are proteins released from activated macrophages, monocytes, fibroblasts, and endothelial cells that have far-reaching regulatory effects on cells. They are small proteins that exert their effects by binding to specific cell surface receptors. Many of these proteins are called interleukins because they aid in the communication between white blood cells (leukocytes).
Cytokines are an important component of the acute-phase response to injury or infection. The acute-phase response is the host’s physiologic response to tissue injury or infection and is intended to fight infection as well as contain areas of diseased or injured tissue. Cytokines mediate this attraction of immune system cells to local areas of injury or infection. They also help the host through activation of the immune system, thus providing for an improved defense against pathogens. Most cytokines are proinflammatory, whereas others appear to exert an anti-inflammatory effect, suggesting a complex feedback system designed to limit the amount of inflammation. Excessive levels of cytokines, however, may result in an exaggerated degree of systemic inflammation, which may lead to greater secondary injury. Numerous cytokines (e.g., tumor necrosis factor [TNF], interleukin [IL]-1 to IL-16) and other protein mediators have been described and may play an important role in the pathogenesis of postoperative systemic inflammation (Box 6-1).8,9
Complement System
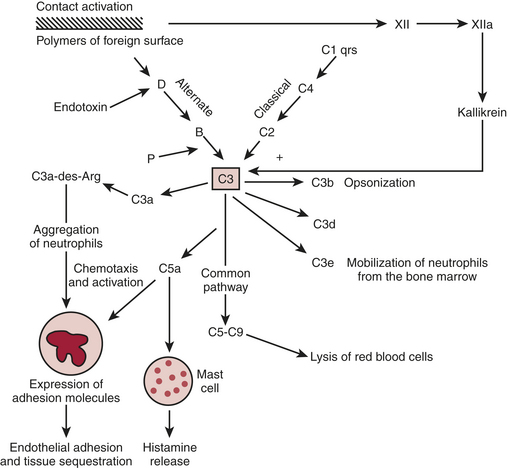
The complement cascade is illustrated in Figure 6-2. The complement cascade can be triggered by either the classical pathway or the alternate pathway. In the alternate pathway, C3 is activated by contact of complement factors B and D with complex polysaccharides, endotoxin, or exposure of blood to foreign substances such as the CPB circuit. Contact activation (Fig. 6-3) describes contact of blood with a foreign surface with resulting adherence of platelets and activation of factor XII (Hageman factor). Activated factor XII has numerous effects, including initiation of the coagulation cascade through factor XI and conversion of prekallikrein to kallikrein. Kallikrein leads to generation of plasmin, which is known to activate the complement as well as the fibrinolytic systems. Kallikrein generation also activates the kinin-bradykinin system.
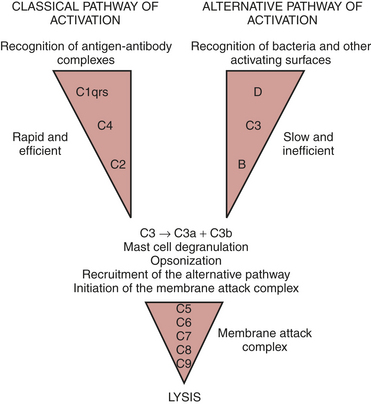
Figure 6-2 Simplified components of the complement system.
(Paul WE: Introduction to the immune system IN Paul WE: Fundamental Immunology. New York, Roven, 1989.)
The classical pathway involves the activation of C1 by antibody-antigen complexes. In the case of cardiac surgery, there are two likely mechanisms for the activation of the classical pathway. Endotoxin can be detected in the serum of almost all patients undergoing cardiac surgery. Endotoxin forms an antigen-antibody complex with antiendotoxin antibodies normally found in serum, which can then activate C1. The administration of protamine after separation from CPB has been reported to result in heparin/protamine complexes, which can also activate the classical pathway10 Contact activation leads to activation of factor XII, which results in the generation of plasmin. Plasmin is capable of activating complement factors C1 and C3.
ENDOTOXEMIA
Endotoxemia refers to the presence of endotoxin in the blood. It is common in cardiac surgical patients.11,12 It is not surprising that some investigators have failed to detect endotoxemia during cardiac surgery given its transient and intermittent nature, although differences in endotoxin-assaying techniques used may also contribute to this discrepancy.
SPLANCHNIC PERFUSION
Splanchnic hypoperfusion appears to be an important cause of systemic inflammation. The gut is one of the most susceptible organs to hypoperfusion during conditions of trauma or stress.13,14 Studies suggest that during periods of hypovolemia, the gut vasoconstricts, thus shunting blood toward “more vital organs” such as the heart and brain. In addition to hypovolemia, endogenously released vasoconstrictors during CPB, such as angiotensin II, thromboxane A2, and vasopressin, may also result in decreased splanchnic perfusion. Vasoconstrictors such as phenylephrine are routinely administered by anesthesiologists and perfusionists to increase blood pressure and are likely to further reduce gut perfusion.
POSTOPERATIVE COMPLICATIONS ATTRIBUTABLE TO INFLAMMATION
Potential Therapies for the Prevention of Inflammation-Related Complications
Numerous strategies and pharmacologic agents have been postulated to reduce the severity and incidence of systemic inflammation. Many studies have demonstrated reductions in intermediate endpoints, such as laboratory indices of complement activation and cytokinemia. At the present time, there are no therapies in widespread clinical use for the prevention or treatment of organ dysfunction resulting from systemic inflammation, although several approaches have been studied (Box 6-2).
Corticosteroid Administration
Several attempts have been made to prevent elevations in proinflammatory cytokines and complement activation during cardiac surgery with corticosteroid administration.17 The overall data suggest that corticosteroids are probably of limited benefit and may, in fact, be harmful.18
Role of Cardiopulmonary Bypass Technique
The role of membrane oxygenators as a means of reducing systemic inflammation-related complications is also controversial. Less complement activation has been observed with the use of membrane oxygenators, although other studies have found no difference.19 There is also controversy as to whether hypothermia during CPB worsens systemic inflammation. Hypothermia has been shown to reduce markers of complement activation. Finally, current data suggest that the use of CPB for cardiac surgery may not in and of itself be more deleterious than cardiac surgery without the use of CPB. Results from randomized clinical trials do not suggest that outcomes are substantially different in patients undergoing on-pump versus off-pump CABG.20–22
SUMMARY
1. Hammermeister K.E., Burchfiel C., Johnson R., Grover F.L. Identification of patients at greatest risk for developing major complications at cardiac surgery. Circulation. 1990;82:IV-380.
2. Rady M.Y., Ryan T., Starr N.J. Perioperative determinants of morbidity and mortality in elderly patients undergoing cardiac surgery. Crit Care Med. 1998;26:225.
3. Bone R.C., Balk R.A., Cerra F.B., et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644.
4. Miller B.E., Levy J.H. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:355.
5. Weiss S.J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365.
6. Royston D., Fleming J.S., Desai J.B., et al. Increased production of peroxidation products associated with cardiac operations: Evidence for free radical generation. J Thorac Cardiovasc Surg. 1986;91:759.
7. Tonnesen E., Christensen V.B., Toft P. The role of cytokines in cardiac surgery. Int J Cardiol. 1996;53(suppl):S1.
8. Rothenburger M., Soeparwata R., Deng M.C., et al. Prediction of clinical outcome after cardiac surgery: The role of cytokines, endotoxin, and antiendotoxin core antibodies. Shock. 2001;16(suppl 1):44.
9. Rothenburger M., Tjan T.D., Schneider M., et al. The impact of the pro- and anti-inflammatory immune response on ventilation time after cardiac surgery. Cytometry. 2003;53B:70.
10. Kirklin J.K., Chenoweth D.E., Naftel D.C., et al. Effects of protamine administration after cardiopulmonary bypass on complement, blood elements, and the hemodynamic state. Ann Thorac Surg. 1986;41:193.
11. Oudemans-van Straaten H.M., Jansen P.G., Hoek F.J., et al. Intestinal permeability, circulating endotoxin, and postoperative systemic responses in cardiac surgery patients. J Cardiothorac Vasc Anesth. 1996;10:187.
12. Watarida S., Mori A., Onoe M., et al. A clinical study on the effects of pulsatile cardiopulmonary bypass on the blood endotoxin levels. J Thorac Cardiovasc Surg. 1994;108:620.
13. Deitch E.A. Bacterial translocation of the gut flora. J Trauma. 1990;30:S184.
14. Mythen M.G., Webb A.R. The role of gut mucosal hypoperfusion in the pathogenesis of postoperative organ dysfunction. Intensive Care Med. 1994;20:203.
15. Mythen M.G., Webb A.R. Intraoperative gut mucosal hypoperfusion is associated with increased postoperative complications and cost. Intensive Care Med. 1994;20:99.
16. Mythen M.G., Webb A.R. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423.
17. Tabardel Y., Duchateau J., Schmartz D., et al. Corticosteroids increase blood interleukin-10 levels during cardiopulmonary bypass in men. Surgery. 1996;119:76.
18. Chaney M.A. Corticosteroids and cardiopulmonary bypass: A review of clinical investigations. Chest. 2002;121:921.
19. Videm V., Fosse E., Mollnes T.E., et al. Complement activation with bubble and membrane oxygenators in aortocoronary bypass grafting. Ann Thorac Surg. 1990;50:387.
20. Puskas J.D., Williams W.H., Mahoney E.M., et al. Off-pump vs conventional coronary artery bypass grafting: Early and 1-year graft patency, cost, and quality-of-life outcomes: A randomized trial. JAMA. 2004;291:1841.
21. Khan N.E., De Souza A., Mister R., et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21.
22. Racz M.J., Hannan E.L., Isom O.W., et al. A comparison of short- and long-term outcomes after off-pump and on-pump coronary artery bypass graft surgery with sternotomy. J Am Coll Cardiol. 2004;43:557.