Chapter 149 Systemic Evaluation and Management of Patients with Uveal Melanoma
Introduction
In developed nations, patients with metastatic uveal melanoma rarely present with the classic “distended abdomen and artificial eye.” Occurring in up to 50% of cases, most metastases are asymptomatic and found by hematologic and radiographic screening.1–3 The rationale for screening is that early detection allows for both palliative treatment or enrollment in clinical trials. Furthermore, early detection offers patients more time to plan future medical and personal care. Indeed, Eskelin and associates demonstrated that largest tumor dimension of metastasis and serum alkaline phosphatase levels could be correlated to median patient survival.4 Survival extends from 2.5 months to 14 months in patient subgroups with the lowest tumor burden at diagnosis.4,5 Screening aims at uncovering smaller, less numerous and even solitary metastases. Though the liver is typically involved in over 80% of cases, alternative common sites include bone and subcutaneous skin.2,3,5,6 Since the liver is most commonly involved, periodic radiographic abdominal evaluations are widely recommended for early detection of metastases.7 Unfortunately, there exist no standardized practice guidelines nor comparative studies to determine the relative efficacy of current staging and screening methods for metastatic uveal melanoma.
Physical examination
Most retinologists and eye cancer specialists will not disrobe patients for examination. However, they can play an integral role during their periodic patient interactions. For example, a history of weight loss, subcutaneous nodularity or abdominal pain should raise suspicion. The eye cancer specialist should ensure or coordinate systemic patient care including: a physical examination looking for subcutaneous nodularity, organomegaly and periodic clinical testing. (Additionally, use of the 7th edition of the American Joint Committee on Cancer’s (AJCC) site-specific universal staging system enables analysis of risk factors for metastasis and multicenter cooperative studies.8)
Serology: liver function tests
Liver function tests (LFTs) include gamma-glutamyl transpeptidase, lactate dehydrogenase, alkaline phosphatases, aminotransferases and bilirubin. When considered individually, they have demonstrated reported sensitivities ranging between 0.27 and 0.67 for metastases detection.9 However, in studying the 2320 patients enrolled in the Collaborative Ocular Melanoma Study (COMS), the authors concluded that sensitivity, specificity, positive predictive value and negative predictive value associated with at least one abnormal LFT before diagnosis of metastatic disease were 14.7%, 92.3%, 45.7% and 71.0%, respectively.9 Elevated LFTs are thought to be less sensitive for and specific to the diagnosis of hepatic metastases than radiographic imaging. Therefore, LFTs should be used for metastases screening only as a complement to radiographic imaging.9
Radiologic assessment: computerized tomography, magnetic resonance imaging and ultrasonography
The liver is easily visualized through various radiographic imaging procedures. In general, ultrasonography (US) can uncover metastases not detected by LFTs.7,10 Contrast enhanced US will demonstrate increased sensitivity and specificity over standard US.10 Triphasic computed tomography (CT) has shown excellent sensitivity, but has a low positive predictive value due to misleading benign lesions found on imaging.11 Contrast-enhanced magnetic resonance imaging (MRI) is considered to be the most sensitive hepatic imaging tool.12 However, there exists concern about gadolinium-associated renal toxicity and is contraindicated for patients with metallic implants. Consequently, without a preferred practice pattern, the choice of imaging is largely governed by cost, radiation exposure and physician preferences.
Positron emission tomography/computed tomography
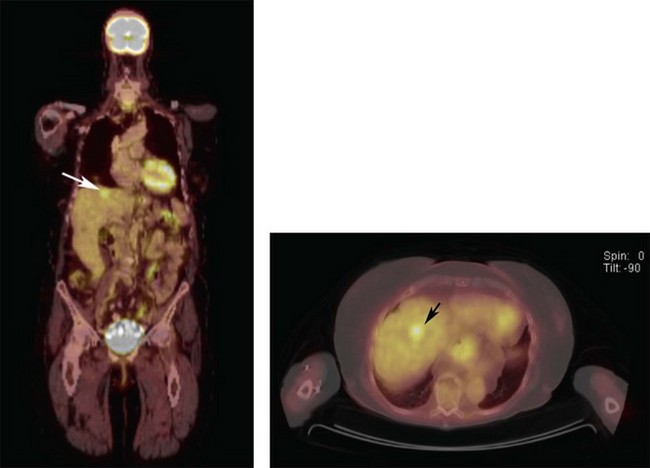
Unlike the previously mentioned radiographic imaging modalities, positron emission tomography/computed tomography (PET/CT) was the first to add PET-function to CT-form on the same diagnostic page (Fig. 149.1).13,14 This enabled discrimination between inflammatory and neoplastic tumors. In contrast to abdominal CT, MRI and US, PET/CT routinely allows for whole body scanning and staging. In practice, we have found PET/CT to yield a remarkably high positive predictive value for uveal melanoma metastases detection.2,3 In addition, PET/CT has been used for initial and follow-up tumor staging as well as detection of synchronous cancers, determination for risks for metastasis, evaluation of post-irradiation tumor viability and for clarification of suspicious CT or MRI findings.13–18 The potential benefits of PET/CT screening have been downplayed due to radiation risks and cost. In our opinion, though most patients prefer initial total body scanning, those at high risk for metastatic uveal melanoma (e.g., T3 and T4 tumors) may be the subgroups to most benefit from PET/CT imaging.3
Pathology, genetics, and molecular biology
Histopathological features of primary uveal melanoma (cellular subtype, extracellular vascular matrix patterns and mitotic index) have been related to the risk for metastasis.19 Further, cytogenetic changes within tumors (e.g., loss of a chromosome 3) were described during the 1990s and are the most relevant risk factor for metastatic spread.20,21 Moreover, gain of chromosome 8q increases the risk of spreading whereas gain of chromosome 6p seems protective.21 Recently, genetic mutations have been identified within primary ocular melanomas, which may offer potential therapeutic targets for treatment.22–24
First, mutations in GNAQ/GNA11 are considered early events in tumorigenesis and may or may not be prognostic for tumor stage or metastatic spread.22,23 In contrast, mutations in the BRCA1-associated protein-1, BAP1, have been strongly linked to metastatic spread and patient survival.24 Inactivation of BAP1 most often occurs through mutation of one allele and subsequent loss of an entire copy of chromosome 3 (monosomy 3) to unmask the mutant copy.24 In an effort to better understand the relationships between these clinical, histopathological and cytogenetic factors, biomarker expression profiles are being investigated to understand how melanoma cells gain the ability to metastasize during tumor development.
Ethical considerations of screening and biopsy
Patients who agree to a metastatic work-up should benefit from a more favorable outcome. While LFTs and abdominal ultrasonography are relatively non-invasive, CT, MRI and PET/CT carry aforementioned collateral health risks. There are also risks related to intraocular biopsy often required to gain tissue for pathology and genetic analysis.25,26 These risks should be outweighed by benefits for preservation of life, vision and eye retention. Though there may be no evidence-based proof of a cure for metastatic uveal melanoma; in our experience intervention has been associated with tumor shrinkage and extended periods of tumor control.
For example, periodic surveillance by radiographic imaging may allow for surgical resection of a solitary hepatic metastasis.27 However, discovery of multiorgan metastatic uveal melanoma may allow for enrollment into a clinical trial and allow for end-of-life planning.3,4 Clearly, the risks of radiographic imaging must be tailored to tumor factors and the patient’s willingness to accept that risk.
Treatment of metastatic disease
Liver metastases
The liver is the most common initial site of metastatic spread.3,6 However, most patients are found to have multifocal hepatic or multiorgan metastasis untreatable by surgical metastectomy.3,7,27 Patients with the longest relapse-free interval after this procedure present with a solitary metastasis that occurs more than 5 years after initial ocular treatment.27 Local control or palliation of liver metastases can be achieved with a wide range of procedures (hepatic artery chemoembolization, hepatic perfusion or radiofrequency ablation). Procedure selection is made in consideration of the extent of hepatic disease, the location of the tumors, the patient’s performance status and the institution’s interventional capability. Multifocal liver metastases not amenable to local therapy and those with multiorgan disease should be treated within clinical research studies.28
1 Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–4659.
2 Finger PT, Kurli M, Reddy S, et al. Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol. 2005;89:1270–1274.
3 Freton AC, Chin KJ, Raut R, et al. Initial PET/CT Staging for Choroidal Melanoma: AJCC Correlation and Second Non-Ocular Primaries in 333 Patients. Eur J Ophthalmol. 2011 Sep;23:21959680.
4 Eskelin S, Pyrhonen S, Hahka-Kemppinen M, et al. A prognostic model and staging for metastatic uveal melanoma. Cancer. 2003;97:465–475.
5 Rajpal S, Moore R, Karakousis CP. Survival in metastatic ocular melanoma. Cancer. 1983;52:334–336.
6 Lorigan JG, Wallace S, Mavligit GM. The prevalence and location of metastases from ocular melanoma: imaging study in 110 patients. Am J Roentgenol. 1991;157:1279–1281.
7 Eskelin S, Pyrhonen S, Summanen P, et al. Screening for metastatic malignant melanoma of the uvea revisited. Cancer. 1999;85:1151–1159.
8 AJCC. Cancer Staging Manual, 7th edn. Available at http://www.cancerstaging.org/staging/index.html
9 Diener-West M, Reynolds SM, Agugliaro DJ, et al. Screening for metastasis from choroidal melanoma: the Collaborative Ocular Melanoma Study Group Report 23. J Clin Oncol. 2004;22:2438–2444.
10 Larsen LP. Role of contrast enhanced ultrasonography in the assessment of hepatic metastases: A review. World J Hepatol. 2010;2:8–15.
11 Feinstein EG, Marr BP, Winston CB, et al. Hepatic abnormalities identified on abdominal computed tomography at diagnosis of uveal melanoma. Arch Ophthalmol. 2010;128:319–323.
12 Servois V, Mariani P, Malhaire C, et al. Preoperative staging of liver metastases from uveal melanoma by magnetic resonance imaging (MRI) and fluorodeoxyglucose-positron emission tomography (FDG-PET). Eur J Surg Onc. 2009;36:189–194.
13 Finger PT, Kurli M, Wesley P, et al. Whole body PET/CT imaging for detection of metastatic choroidal melanoma. Br J Ophthalmol. 2004;88:1095–1097.
14 Freudenberg LS, Schueler AO, Beyer T, et al. Whole-body fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) in staging of advanced uveal melanoma. Surv Ophthalmol. 2004;49:537–540.
15 Chin K, Finger PT, Kurli M, et al. Second cancers discovered by (18)FDG PET/CT imaging for choroidal melanoma. Optometry. 2007;78:396–401.
16 Klingenstein A, Haug AR, Nentwich MM, et al. Whole-body F-18-fluoro-2-deoxyglucose positron emission tomography/computed tomography imaging in the follow-up of metastatic uveal melanoma. Melanoma Res. 2010;20:511–516.
17 Finger PT, Chin K, Iacob C. 18-flourine-labelled 2-deoxy-2-fluoro-D glucose positron emission tomography/computed tomography standardized uptake values: a non-invasive biomarker for the risk of metastasis from choroidal melanoma. Br J Ophthalmol. 2006;90:1263–1266.
18 Finger PT, Chin KJ. 18F fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) physiologic imaging of choroidal melanoma: Before and after ophthalmic plaque radiation therapy. Int J Radiat Oncol Biol Phys. 2011;79:137–142.
19 McLean IW, Foster WD, Zimmerman LE. Uveal melanoma: location, size, cell-type and enucleation as risk factors in metastasis. Hum Pathol. 1982;13:123–132.
20 Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225.
21 Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res. 2010;16:6083–6092.
22 Onken MD, Worley LA, Long MD, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:5230–5234.
23 Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199.
24 Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. 3
25 Petousis V, Finger PT, Milman T. Anterior segment tumor biopsy using an aspiration cutter technique: clinical experience. Am J Ophthalmol. 2011;152:771–775.
26 Milman T, Petousis V, McCormick SA, et al. Anterior segment tumor aspiration cutter assisted biopsy: Experience with pathology. Am J Ophthalmol. 2011;152:771–775.
27 Aoyama T, Mastrangelo MJ, Berd D, et al. Protracted survival after resection of metastatic uveal melanoma. Cancer. 2000;89:1561–1568.
28 National Cancer Institute. Clinical Trials Search Results. http://www.cancer.gov/clinicaltrials/search/results?protocolsearchid=7978738&vers=1. (accessed September 4, 2011)