Chapter 40
Systemic Complications
Respiratory
Leigh Ann Slater, Pamela A. Lipsett
Based on a chapter in the seventh edition by Jayme E. Locke and Pamela A. Lipsett
A major contributor to perioperative morbidity and mortality is postoperative pulmonary complications.1–5 The incidence ranges from 5% to 80%, depending on the population studied and the criteria used to define complications.2,5 Comprehensively defined, atelectasis, bronchospasm, bronchitis, pneumonia, aspiration pneumonitis and pneumonia, exacerbation of chronic obstructive pulmonary disease (COPD), pulmonary edema, and various forms of upper airway obstruction are considered postoperative pulmonary complications2 (Box 40-1). This chapter discusses identification and prevention of pulmonary complications with particular emphasis on amelioration of modifiable risk factors and newer treatments of respiratory failure.
Risk Factors
Preoperative risk factors for pulmonary complications include both patient-related and procedure-related risks (Table 40-1).7–12 Procedure-related risk factors may be more important, but except in the choice of procedure, they may not be as readily modifiable. Perioperative and postoperative risk factors include those associated with aspiration, poor inspiratory effort, and overfeeding. Many of these risk factors are modifiable.
Table 40-1
Potential Patient-Related and Procedure-Related Risk Factors for the Development of Postoperative Pulmonary Complications
| Patient-Related Risk Factors | Procedure-Related Risk Factors |
| Chronic obstructive lung disease | Surgical site |
| Asthma | Duration of surgery (>3 hours) |
| Tobacco use | Type of anesthesia |
| Health status (American Society of Anesthesiologists class >2) | Endotracheal intubation |
| Obesity | Long acting nondepolarizing blockade |
| Age | Large tidal volumes (10-12 mL/kg) |
| Upper respiratory infection | High FIO2 |
| Metabolic factors | |
| Heart failure | |
| Poor exercise tolerance | |
| Functional dependence | |
| Abnormal lung scan |
Patient-Related Risk Factors
Chronic Lung Disease
Preexisting lung disease is the most important patient-related risk factor for postoperative pulmonary complications. Reported unadjusted relative risks for patients with underlying pulmonary disease range from 2.7 to 6.6. Preoperative findings consistent with COPD include decreased breath sounds, prolonged expiratory phase, rales, wheezes, rhonchi, and chest radiographic evidence of hyperinflation. Studies demonstrate postoperative pulmonary complications in up to 26% of patients with these findings versus only 8% of patients without these findings. Nevertheless, there is no identified lower threshold of pulmonary function below which surgery is absolutely contraindicated.13–20 Even extremely high risk patients may proceed to surgery if the indication is sufficiently compelling and the patient and surgeon are willing to assume the added risks.
Asthma
Despite early reports of higher than expected rates of postoperative pulmonary complications, it has since been shown that patients with well-controlled asthma and peak flow measurements better than 80% of predicted can proceed to surgery at average risk.6,14–15,17 Patients who have active use of asthma medications and emergency department or office visits within 30 days before anticipated surgery are at increased risk of postoperative pulmonary complications and should have their surgery delayed to permit adequate control of asthma before proceeding.
Smoking
Current smoking increases the risk of postoperative pulmonary complications threefold (see Chapter 27 for further discussion).1,2,21–24 Whereas textbooks and older literature suggested that recent discontinuation of smoking increased risk of postoperative pulmonary complications purportedly through increased sputum production, a recent systematic review found nine studies examining the effect of timing of discontinuation of smoking on perioperative complications. One of the studies demonstrated a beneficial effect of recent quitting compared with continued smoking. Meta-analysis demonstrated that quitting smoking within 8 weeks before surgery was not associated with either an increase or a decrease in postoperative pulmonary complications for four studies examining this specific group of complications (relative risk, 1.18; 95% CI, 0.95-1.46).22,24 Thus patients should be strongly encouraged to stop smoking whenever they present for medical care.
General Health Status
The commonly used American Society of Anesthesiologists classification considers any systemic disease that affects activity or is a threat to life, including chronic lung disease, and it generally correlates well with pulmonary risk. An American Society of Anesthesiologists class higher than 2 confers a 1.5-fold to 3.2-fold increased risk of postoperative pulmonary complications.2
Obesity
Physiologic changes in morbid obesity include reduced lung volumes (functional residual capacity [FRC]), ventilation-perfusion mismatch, and relative hypoxemia.25,26 As similar changes are seen under anesthesia, the specific contribution of obesity to the incidence of postoperative pulmonary complications is difficult to quantify. Calligaro et al studied 128 aortic surgery patients, 26 of whom were obese, and demonstrated a pulmonary complication rate of 27% in obese patients versus 17% in nonobese patients, with an unadjusted risk ratio of 1.6.27 A careful analysis would suggest that patients with both comorbidities and obesity are at increased risk, and identification and treatment of these associated diseases is important for preoperative optimization to minimize complications.
Age
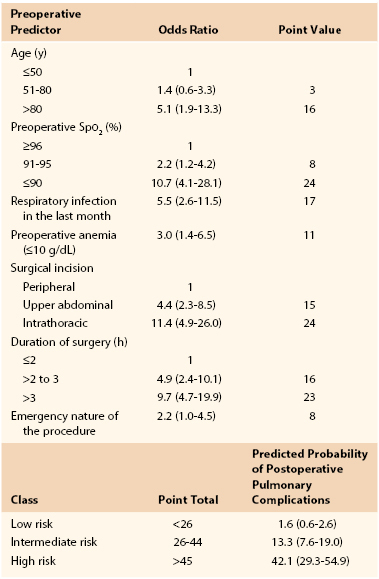
Early studies suggested an increased risk of pulmonary complications with advanced age,28 but they were not adjusted for overall health status or preexisting pulmonary disease. Canet’s straightforward risk model9 for estimating risk of postoperative pulmonary complications assigns 3 risk score points for age of 51 to 80 years and 16 points for age older than 80 years. Overall, low risk is assigned for less than 26 points (rate of 1.6), intermediate risk for 26 to 44 points (rate of 13.2), and high risk for more than 45 points (rate of 42.1) (Table 40-2).9
Upper Respiratory Infection
Patients who have had a respiratory infection within the month before surgery are at increased risk of postoperative pulmonary complications. In a study of 1624 patients, the odds ratio was 5.5 (2.6-11.5) if a recent infection had occurred. It would seem wise to avoid elective surgery in this setting.2,10
Metabolic Factors
A multifactorial risk index for postoperative respiratory failure identified two metabolic risk factors.29 Albumin level of less than 3 g/dL and blood urea nitrogen concentration of more than 30 mg/dL each predicted increased risk with odds ratios of 2.53 and 2.29, respectively. Etz et al30 examined outcomes after thoracoabdominal aneurysm repair and also found an association between preoperative renal insufficiency (blood urea nitrogen concentration >24 mg/dL) and the development of pulmonary complications, with incidence of 42% versus 25% (P = .03).
Procedure-Related Risk Factors
Table 40-1 summarizes procedure-related risk factors for perioperative pulmonary risk. These include operative site, duration and method of anesthesia, and choice of neuromuscular blocker.2,10
Surgical Site
Surgical site is the most important procedural risk factor for postoperative pulmonary complications, with the incidence of complications inversely related to distance of the incision from the diaphragm. Choice of incision may adversely influence respiratory muscle and diaphragmatic function.6,31,32 Complication rates for thoracic surgery range from 19% to 59%; for upper abdominal surgery, from 17% to 76%; and for lower abdominal surgery, from 0% to 5%.30–33
Duration of Surgery
Operative duration of more than 2 to 3 hours confers a higher risk of postoperative pulmonary complications.6,10,33 A study of 520 patients demonstrated such complications in 8% of surgeries lasting less than 2 hours versus 40% of procedures lasting more than 4 hours. When one is available, it is prudent to pursue a less ambitious, briefer procedure in the high-risk patient.
Type of Anesthesia
Whereas the data are conflicting and generally of low quality, two large meta-analyses as well as retrospective and randomized trials suggest that postoperative pulmonary complication rates are lower for patients who have spinal or epidural anesthesia for surgery or epidural analgesia postoperatively.34–39 In addition, a meta-analysis by Block et al34 demonstrated that both static and dynamic postoperative pain relief is improved with epidural analgesia. A classic clinical trial performed at our institution by Norris et al39 randomized 168 patients undergoing abdominal aortic surgery to receive either thoracic epidural anesthesia with light general anesthesia or full general anesthesia alone with either intravenous or epidural patient-controlled analgesia postoperatively. Epidural patient-controlled analgesia correlated with a significantly shorter time to extubation. All treatment groups had otherwise comparable results, however, including time to intensive care unit (ICU) discharge, ward admission, first bowel sounds, first flatus, tolerance of clear liquids and regular diet, independent ambulation, and postoperative pain scores.
Type of Neuromuscular Blockade
The choice of neuromuscular blockade has a significant influence on postoperative pulmonary complications (see Box 40-1).1,2,15 In one prospective nonrandomized study, the difference in postoperative pneumonia between pancuronium and atracurium was 13% versus 5%.33 Evidence suggests that use of short-acting or intermediate-acting neuromuscular blockers is preferred. A prospective randomized study showed that patients treated with longer acting neuromuscular blockers were four times as likely to develop postoperative pulmonary complications.40
Perioperative and Postoperative Risk Factors
Perioperative and postoperative risk factors for postoperative pulmonary complications include those associated with aspiration, poor inspiratory effort, prolonged intubation, and overfeeding. Many of these risk factors are within our power to control and to remedy. Factors associated with aspiration and pneumonia include insufficient elevation of the head of the bed, immobility, overaggressive use of restraints, presence of nasogastric or other tubes that stent open both upper and lower esophageal sphincters, and oversedation or other toxic or metabolic causes of altered level of consciousness.41,42 In patients who must remain intubated, longer duration is associated with higher complication rates, and every effort should be made to extubate expeditiously, including protocols for daily weaning of sedation and trials of spontaneous respiration and readiness for extubation. Subglottic suctioning endotracheal tubes minimize risk of aspiration of microorganisms around the cuff. Patients who have been intubated for longer than 3 days should be properly assessed after extubation and before feeding with the expertise of speech-language pathologists to assess swallow function and supplemental cine-esophagography when bedside evaluation is equivocal. Attention should be paid to high gastric residuals and other signs of postoperative ileus or bowel obstruction. When gastric emptying is in question, a jejunal feeding access would be more appropriate.
Poor inspiratory effort is also multifactorial and may be associated with location of incision, poor pain control, suboptimal patient positioning and immobility, placement of chest tubes or braces or binders that impede full chest wall excursion, oversedation, neuromuscular defects, and poor nutrition. Each of these factors can be improved with appropriate attention to detail.
Conversely, overfeeding produces a surfeit of carbon dioxide that may be beyond the patient’s capacity to effectively exhale and can be demonstrated by a respiratory quotient greater than 1. Hypophosphatemia limits the patient’s available energy (adenosine triphosphate) for muscle contractility. Other electrolyte deficiencies, including hypokalemia, hypocalcemia, and hypomagnesemia, may also have an adverse impact. All may contribute to failure to be weaned from the ventilator and should be aggressively sought and corrected when present.
Preoperative Evaluation
A complete history and physical examination form the basis of preoperative risk assessment. Significant risk factors should be identified. Evidence suggesting underlying chronic lung disease should be sought, including a history of exercise intolerance, unexplained dyspnea, and cough. Physical examination should seek evidence of obstructive lung disease, such as decreased breath sounds, wheezes, rhonchi, or prolonged expiratory phase.14,15 Adjunctive studies including pulmonary function tests, arterial blood gas measurements, and chest radiography may be warranted but are no substitute for appropriate clinical evaluation and should not be done routinely.43,44
Consensus guidelines for preoperative practice emphasize general health and fitness and state that patients who demonstrate more than four metabolic equivalents (climbing two flights of stairs or running a short distance) are considered safe to proceed to surgery from a cardiac standpoint. However, patients with vascular disease are often unable to undergo exercise testing because of claudication or other symptoms. Cardiopulmonary exercise testing has been suggested as an objective measure of functional reserve that can be performed safely in such high-risk populations.45,46 Cardiopulmonary exercise testing is a ramped exercise test during which the electrocardiogram, blood pressure, and oxygen saturation are monitored and respiratory variables, including oxygen uptake and carbon dioxide excretion, are measured. A number of parameters can be derived, including the peak oxygen uptake (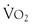 -peak), which is effort dependent, and oxygen uptake at the anaerobic threshold, which is effort independent. Whereas this test may be of future value in assessing perioperative risk, a review by Young et al44 suggests that currently there is insufficient evidence to justify its routine use in vascular surgery patients.
-peak), which is effort dependent, and oxygen uptake at the anaerobic threshold, which is effort independent. Whereas this test may be of future value in assessing perioperative risk, a review by Young et al44 suggests that currently there is insufficient evidence to justify its routine use in vascular surgery patients.
Pulmonary Function Testing
Except in pulmonary resection, there is considerable debate about the role of preoperative pulmonary function tests (PFTs) for perioperative risk stratification.2,43,47–50 Two reasonable goals justify preoperative PFTs in nonthoracic surgery: (1) identification of patients for whom the risk of the proposed open vascular surgical procedure outweighs the benefit or in whom a percutaneous or endoluminal approach would be better tolerated; and (2) identification of patients at higher risk, for whom more aggressive perioperative management is warranted. PFTs should not be obtained routinely for patients having abdominal surgery and should not be used as the primary reason for denying a patient an operation. PFTs might be useful for patients with COPD or asthma if clinical evaluation cannot determine whether the patient is at baseline with optimal reduction of airflow obstruction. Similarly, in patients with dyspnea or exercise intolerance that remains unexplained after clinical evaluation, the differential diagnosis may include cardiac disease or deconditioning, and the results of PFTs may change preoperative management and suggest a more aggressive treatment approach, such as perioperative corticosteroids, to achieve the best possible baseline functional level.50
PFTs provide an estimate of forced expiratory volume (FEV1) and forced vital capacity (FVC). An FEV1 or FVC of less than 70% and an FEV1/FVC ratio of less than 65% of predicted are associated with an increased risk of postoperative pulmonary complications.50 It again must be emphasized that most patients identified by PFTs as high risk for postoperative pulmonary complications can be identified equally well by astute history and physical examination.15,50
Arterial Blood Gas Analysis and Chest Radiography
No data suggest that hypercapnia identifies high-risk patients beyond those who would already be identified on the basis of established clinical criteria. In addition, abnormalities on chest radiographs are seen with increasing frequency with age, with uncertain significance. Chest radiographs also add little to the clinical evaluation of otherwise healthy patients, as was demonstrated in a meta-analysis of studies of routine preoperative chest radiography.51 Of 14,390 preoperative radiographs, there were only 140 unexpected abnormalities and only 14 cases in which the chest radiographic finding was abnormal and influenced management. The available literature does not allow an evidence-based determination of which patients would benefit from preoperative chest radiography. Chest radiography should, however, be performed if there has been a recent significant change in cardiac or pulmonary status.
Pulmonary Risk Indices
Cardiac risk indices have been used widely since 1977 to stratify the risk of perioperative cardiac complications.52 Several studies have attempted to formulate analogous pulmonary risk indices, but each study has significant limitations, often due to the complexity of the proposed index.53–57 The most simple and easily applied risk index is that proposed by Canet et al.9 In a study of 2464 patients, 252 postoperative pulmonary complication events were identified in 123 patients (5%), with higher 30-day mortality in those who suffered a postoperative pulmonary complication (19.5% [95% CI, 12.5%-26.5%] vs 0.5% [95% CI, 0.2%-0.8%]).9 The seven identified risk factors are listed in Table 40-2, with an area under the receiver operating curve of 88% (95% CI, 84%-93%). Aside from delaying or changing the magnitude of the planned procedure, most of the risk factors are not modifiable preoperatively. Whether more aggressive treatment of underlying pulmonary disease could improve oxygen saturation and therefore decrease risk is unproven. As a result, this index is more valuable to predict risk and to determine candidacy for surgery than as a tool for risk reduction strategies.
Perioperative Pulmonary Physiology
In a normal unstressed individual, respiratory rate is 10 to 12 breaths/min with a tidal volume of approximately 500 mL and a total minute ventilation of 5 to 6 L/min. Total minute ventilation consists of alveolar ventilation and the anatomic dead space, which is the portion of a breath not available for gas exchange. Anatomic dead space is roughly 130 to 180 mL per breath for a normal-size adult. Gas exchange depends on alveolar ventilation rather than on total minute ventilation.
Reduction in lung volumes resulting from anesthesia and surgery contributes to the development of atelectasis and other postoperative pulmonary complications.32,58,59 Patients typically experience a significant postoperative decrement in pulmonary function, especially after upper abdominal and thoracic incisions.31 The mechanisms causing the reduced lung volumes include decreased phrenic nerve activity and resultant decreased diaphragmatic function along with increased intercostal and abdominal muscle tone in response to postoperative pain and spinal reflex arcs. The most important physiologic change observed is a decreased FRC, the volume of air left in the lungs after normal expiration. Without intervention, FRC reaches a nadir at 24 to 48 hours postoperatively and returns to normal in uncomplicated low- to moderate-risk patients in approximately 1 week.
The relationship between FRC and closing capacity, the volume left in the lungs as the midsize and small airways begin to collapse, is a major determinant of postoperative pulmonary function and the resultant pulmonary morbidity.59,60 Normally, FRC is well above closing capacity, and tidal volumes result in air distribution throughout all alveoli. In the postoperative period, although FRC decreases dramatically, closing capacity remains unchanged. Patients in severe pain often cannot generate sufficient tidal volumes to overcome the closing capacity, resulting in lack of airflow to most alveoli with consequent atelectasis. Similarly, patients with mild pain may only be able to overcome closing capacity sufficiently to oxygenate alveolar units when at peak inspiration. This leads to ventilation-perfusion mismatch and consequent postoperative hypoxemia. Adequate pain control is essential to enable patients to breathe more deeply (increased tidal volume and FRC) and to cough more effectively (increased FEV1). Lower abdominal surgery is associated with similar changes but to a lesser degree, and reductions in lung volumes are not seen with extremity surgery.
Oxygen delivery is equal to the oxygen content of arterial blood multiplied by the cardiac output. The oxygen content of arterial blood is determined by the concentration of oxygen-saturated hemoglobin and dissolved oxygen in the blood (Table 40-3). Thus, it is evident that oxygen kinetics depend heavily on ventilation and perfusion. Oxygen is consumed peripherally at a rate of 200 mL/min in the average adult. Many factors affect oxygen consumption, including catecholamines and thyroid hormones. Oxygen delivery can be increased by improving oxygenation, correcting anemia, and optimizing cardiac output. Although optimizing oxygen delivery seems a worthy goal, studies using a pulmonary artery catheter to set a specified goal (>500 mL/min) thus far have not convincingly demonstrated improved outcomes, although these studies were primarily of unselected rather than specifically ill populations.61,62 Vascular surgery patients with abnormally low oxygen delivery or high oxygen consumption have not been definitively studied. As a practical matter, patients with abnormal oxygen delivery and low mixed venous saturations should have cardiac output or hemoglobin concentration or both optimized.
Table 40-3
Oxygen Measurements
| Measurement | Equation |
| Arterial oxygen content (CaO2) | CaO2 = (1.36 × [Hb] × SaO2) + (PaO2 × 0.0031) |
Mixed venous oxygen content (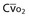 ) ) |
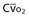 = (1.36 × [Hb] × = (1.36 × [Hb] × 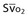 ) + ( ) + (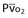 × 0.0031) × 0.0031) |
| Arteriovenous oxygen difference (a-vDO2) | a-vDO2 = CaO2 − CvO2 |
| Oxygen delivery (DO2) | DO2 = ([1.36 × [Hb] × SaO2] + [PaO2 × 0.0031]) × CO |
Mixed venous oxygen saturation (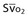 ) ) |
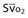 = (1.36 × [Hb] × SaO2) + ( = (1.36 × [Hb] × SaO2) + (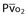 × 0.0031) × 0.0031) |
Oxygen consumption ( ) ) |
VO2 = CO × (a-vDO2) |
CO, Cardiac output; Hb, hemoglobin.
Clinical Presentation
Atelectasis
Clinically significant atelectasis has been reported in 20% of patients undergoing upper abdominal surgery and in 30% of patients undergoing thoracic surgery.59,60,63 Altered compliance of lung tissue, impaired regional ventilation, and retained airway secretions contribute to the development of atelectasis.2
Nosocomial Pneumonia
Nosocomial pneumonia is the leading cause of death due to hospital-acquired infections. Estimates of associated mortality range from 5% to 50% and depend greatly on the degree of case-matching and controls.64–66 The greatest risk factor is duration of mechanical ventilation; an average risk of ventilator-associated pneumonia is 1% per day, although the daily risk of acquisition of ventilator-associated pneumonia is highest during the first week of intubation (Box 40-2). Arozullah et al8,67 developed an index to predict the risk of postoperative pneumonia using a large Veterans Administration database and modeled it after their pulmonary risk index. They assigned each factor points on the basis of its strength in a multivariate analysis (Table 40-4). Again, procedure-related risk factors dominated the index; however, it also did identify several modifiable risk factors, including functional status, weight loss, cigarette use, and alcohol consumption.
Table 40-4
Multivariate Risk Factors for Postoperative Pneumonia
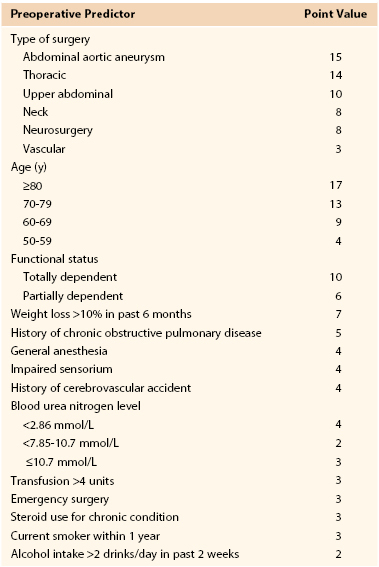
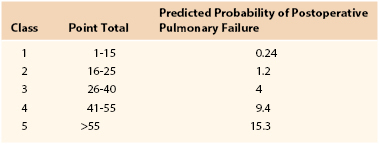
From Arozullah AM, et al: Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 135:847-857, 2001.
Microaspiration is thought to play a central role in the pathogenesis of nosocomial pneumonia and is discussed further in a separate section.41,42 Nosocomial pneumonias are frequently polymicrobial, with gram-negative bacilli predominating in 60% of nosocomial pneumonias and constituting six of the seven most frequently identified pathogens.68–70 In some institutions, Acinetobacter species can be important pathogens. Large variations in pathogens may be seen within and between institutions, and clinicians should be familiar with the specific pathogens and sensitivities in their own institutions and especially in ICUs.
Diagnostic strategies and controversies regarding nosocomial pneumonia are beyond the scope of this chapter. Suffice it to say that nosocomial pneumonia is often diagnosed on uncertain clinical grounds and treated although not clearly present by histologic grounds.71 Whether an aggressive invasive strategy employing bronchoalveolar lavage should be used for diagnosis remains a subject of active debate. A study from France evaluated an invasive diagnostic approach using protected brush specimens or bronchoalveolar lavage versus clinical criteria in 413 ICU patients with a clinical suspicion of ventilator-associated pneumonia and noted significantly lower mortality at 14 days (16% vs 26%) and 28 days as well as fewer antibiotic days and lower number of antibiotics administered.71 However, a study by the Canadian Clinical Trials Group suggested that there are no significant differences in outcomes based on whether an invasive or noninvasive method of diagnosis of ventilator-associated pneumonia is used.72
Aspiration and Aspiration Pneumonia
Aspiration is defined as the inhalation of foreign material into the airways beyond the vocal cords.41,73 The content of aspirated material can vary substantially, containing secretions, bacteria, lipids, or even food particles. Aspiration can be silent with repeated episodes of microaspiration without acute symptoms versus a single symptomatic episode of macroaspiration. Aspiration events can also be categorized as aspiration pneumonitis (chemical pneumonitis) versus aspiration pneumonia (infectious process secondary to an aspiration event), although distinguishing between these two processes can be difficult.42
Patients with altered swallowing mechanics (e.g., neurobulbar dysfunction) are at significant risk of aspiration. An altered level of consciousness is also a major risk factor for aspiration and thus general anesthesia is associated with some risk of this complication. In modern anesthesia, the risk of aspiration is about 1 : 3000, with a mortality of approximately 1 : 125,000. Additional populations at higher risk of aspiration include elderly and nursing home residents,74,75 patients with gastrointestinal and esophageal abnormalities,76 and patients with neurologic trauma and neuromuscular diseases.77
As noted before, aspiration may be silent or may present with a mild cough, dysphonia, or even a life-threatening and fulminant pneumonitis that evolves into the acute respiratory distress syndrome (ARDS). In a major aspiration event, clinical signs of distress and hypoxemia often exceed radiographic findings early in the disease. As the chemical pneumonitis improves, radiologic signs may actually worsen while the clinical picture improves.
Prevention of aspiration principally involves eliminating or modifying risk factors. For example, effective pain control with minimal sedation or delirium would likely decrease aspiration risk. Although some authors believe that an endotracheal tube is itself protective against aspiration, in fact the presence of an endotracheal tube can actually facilitate the aspiration of oropharyngeal or gastric pathogens. An endotracheal tube with a subglottic suction port has been shown to reduce the incidence of ventilator-associated pneumonia.78–81 If aspiration has occurred, depending on the number and virulence of the organisms reaching the lung, pneumonia may ensue during the hours and days after a chemical event.
Gram-negative bacteria colonize the oropharynx within several days of hospital admission, 75% of the time within 48 hours.82 In addition, the near-sterility of the normal stomach and upper gastrointestinal tract may be disrupted by alterations in gastric pH secondary to illness, medications such as proton pump inhibitors, or enteric feedings. Less frequently, pneumonia can result from inhalation of infectious aerosols or from bacteremia originating in a distant focus.
Aspiration pneumonitis must be distinguished from aspiration pneumonia to avoid unnecessary antibiotic use and selective pressure toward multidrug-resistant flora.41,42 Whereas this may seem straightforward, in fact it is a most challenging distinction, especially when no witnessed aspiration event is identified. Aspiration is often a disease of exclusion in which other causes of hypoxia, such as pulmonary edema, pulmonary embolism, and community- or hospital-acquired bacterial pneumonia, have been eliminated. The diagnosis of aspiration pneumonitis is typically entertained with a combination of hypoxemia and radiographic findings in dependent portions of the lung, although the exact location of infiltrates depends on the position of the patient during aspiration. A number of biomarkers have been proposed to differentiate aspiration pneumonitis from aspiration pneumonia, but none has yet been adopted for widespread clinical practice.42
The treatment of aspiration pneumonitis is essentially supportive, with oxygen supplementation and intubation and mechanical ventilation as needed. If intubation is not required, alternative lung expansion modalities should be considered. Whereas routine bronchoscopy and lavage after aspiration is controversial, it can be considered in patients who have aspirated particulate matter and have had alveolar or lobar collapse. The lavage fluid can be sent for quantitative culture to determine whether antibiotics are indicated.
Although it is common practice, prophylactic use of antibiotics in patients with suspected or witnessed aspiration is not recommended.41,42,73 Because aspiration is associated with neutrophil infiltration and cytokine production, fever and leukocytosis are common. Routine antibiotic use in this setting is discouraged as it may select for more resistant organisms in a patient with an uncomplicated chemical pneumonitis. However, empirical antimicrobial therapy may be appropriate in patients who aspirate gastric contents in the setting of small bowel obstruction or in other circumstances associated with colonization of gastric contents. Antimicrobial therapy should be considered in patients with aspiration pneumonitis that fails to resolve within 48 hours. Empirical therapy with broad-spectrum agents is recommended; anaerobic coverage is not routinely required.83
Acute Respiratory Failure
Acute respiratory failure includes many pulmonary pathologic processes.84–87 In general, it implies profound ventilation, perfusion, or gas exchange abnormalities. Acute respiratory failure is considered hypoxic, hypercapnic, or both. The lungs commonly display markedly decreased compliance and require higher inspiratory pressures to achieve the same level of inflation, with increased work of breathing that results in further oxygen debt or mechanical ventilatory failure.
Shallow breathing secondary to poorly controlled pain in the postoperative patient, aspiration, bronchospasm, absorption atelectasis from anesthesia, or pneumothorax or hemothorax from central line placement can cause alveolar collapse and decrease FRC. Alveolar collapse affects gas exchange by creating a ventilation-perfusion mismatch. Acutely, compensation occurs through pulmonary artery spasm and shunting of blood to ventilated areas. Volume loss secondary to alveolar collapse decreases lung compliance, making it difficult to ventilate. Positive end-expiratory pressure (PEEP) is often used in this setting to “recruit” alveoli, increasing the size of the functional lung and improving compliance, decreasing dead space, increasing oxygenation, and decreasing shunt. In the attempt to recruit collapsed alveoli, however, normal alveoli may become overdistended and subject to volutrauma from shear forces.88 Normal lung is vulnerable to this phenomenon because it is the most compliant. To avoid this side effect, plateau inspiratory pressures should be kept at less than 30 to 35 cm H2O, typically with tidal volumes of 6 mL/kg and not more than 8 mL/kg of predicted body weight, even in patients who do not have acute pulmonary disease.
Pulmonary edema also decreases FRC. There are three main causes for pulmonary edema: increased hydrostatic pressure from left ventricular failure and fluid overload, decreased plasma oncotic pressure from liver failure, and increased capillary permeability as seen in burns and septic shock. Patients often require positive-pressure ventilation to improve gas exchange. This mode of ventilation limits alveolar collapse, increases surface area for water accumulation, and overcomes the effects of bronchial occlusion. Pulmonary edema is a more serious cause of acute respiratory failure than alveolar collapse alone because the protein-rich fluid makes the patient highly susceptible to infection and fibrosis.
Acute Respiratory Distress Syndrome
Originally defined in 1994, ARDS is a diffuse inflammatory process involving both lungs that arises as a complication of diseases that produce a severe form of systemic inflammatory response.86 The 1994 definition stated that the disease must have an acute onset; the arterial partial pressure of oxygen–to–fraction of inspired oxygen ratio (PaO2:FIO2 or P : F ratio) must be less than 200; the chest radiograph must have bilateral infiltrates, without a clinical suspicion of pneumonia; and the left atrial pressure must be normal, or if elevated left atrial pressure is suspected, a pulmonary artery catheter must be placed to assess wedge pressure. A pulmonary capillary wedge pressure of less than 18 mm Hg combined with the previously stated findings would be consistent with ARDS. However, given issues with reliability and validity of this definition, in 2011 a consensus conference reevaluated the definition of ARDS, proposed draft definitions, and evaluated their effectiveness with actual ICU patients.85 The new definition elements are listed in Table 40-5. With use of this 2011 Berlin Definition of ARDS, escalating stages of mild, moderate, and severe ARDS were found to be associated with increasing mortality (27% [95% CI, 24%-30%], 32% [95% CI, 29%-34%], and 45% [95% CI, 42%-48%], respectively; P < .01) as well as extended duration of mechanical ventilation in surviving patients.85
Table 40-5
The Berlin Definition of Acute Respiratory Distress Syndrome
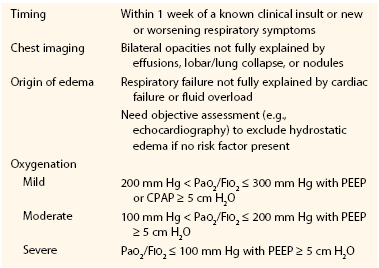
CPAP, Continuous positive airway pressure; PEEP, positive end-expiratory pressure.
From ARDS Definition Task Force: Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526-2533, 2012.
Epidemiologic data suggest that ARDS may account for 36,000 deaths per year in a country the size of the United States.84,87,89 Although there is some evidence that the mortality of ARDS may be declining over time, it remains high (30% to 40%), and ARDS is also an important cause of morbidity in patients who leave the hospital. Herridge et al90 found that survivors of ARDS had persistent functional disability 1 year after discharge from the ICU. Despite this bleak outlook, research in the field of critical care medicine has produced new treatment strategies, discussed in a later section of this chapter.
Predisposing conditions for ARDS include sepsis, blood product transfusion, catheter sepsis, pneumonia, pulmonary contusion, cardiopulmonary bypass, and pancreatitis.87 ARDS is often progressive and characterized by distinct stages with different clinicopathologic and radiographic stigmata. The acute or exudative phase is manifested by the rapid onset of respiratory failure in a patient at risk. Arterial hypoxemia refractory to supplemental oxygen is characteristic. Computed tomography has shown that alveolar filling, consolidation, and atelectasis occur predominantly in dependent lung zones, whereas other areas may be relatively spared. However, lavage of the spared areas does reveal substantial inflammation, especially in the cytokines interleukin-1, interleukin-6, and tumor necrosis factor. Although the disease may resolve in some patients after this acute phase, it progresses in other patients to fibrosing alveolitis with persistent hypoxemia, increased alveolar dead space, and further decrement in pulmonary compliance.
The cellular constituents of the lung change greatly during ARDS.87 The alveolar epithelium has extensive necrosis of type I cells, which slough from the alveolar surface to be replaced by proteinaceous deposits (hyaline membranes). Type II cells, in addition to secreting surfactant, provide a population of cells capable of replication and differentiation to replace type I cells. Type II cells are more vulnerable to the effects of stretch and more susceptible to high levels of inspired oxygen (>60%). Type II cells replace type I cells, thereby creating fibrotic noncompliant lungs that are more susceptible to stress/stretch–related trauma.87,90
Medical Treatment
Patient Optimization and Prevention of Respiratory Complications
Comorbid Conditions
The treatment and preparation of patients who have COPD before elective surgery are the same as those in nonoperative settings.91–95 Clinicians should not employ treatments simply for upcoming surgery unless these treatments are otherwise indicated. Multimodality treatment programs, including chest physical therapy, bronchodilators, smoking cessation, and antibiotics and corticosteroids (when indicated), reduce the risk of postoperative pulmonary complications in patients with COPD.1,15,92 Standard therapy for COPD includes use of ipratropium for patients who have daily symptoms plus the addition of inhaled beta agonists as needed for symptoms.2,92 Theophylline should be reserved for patients not responsive to other maximal therapies. Approximately 20% to 30% of patients with COPD respond to systemic corticosteroids.94 Unless a patient has been shown previously to be a nonresponder, clinicians should attempt systemic corticosteroids preoperatively in patients with COPD if airflow is not at the optimal baseline despite other therapies, as determined by symptoms and peak flow.94,95 There is no role for routine antibiotics before surgery. Clinicians should recommend antibiotics only if a change in the chest radiograph or the character or amount of sputum suggests lower respiratory tract infection, and elective surgery should be canceled until the patient has returned to baseline.
Physicians should prepare patients who have asthma for surgery with optimization of airway obstruction and reactivity just as in the nonsurgical setting.17,20 A 1-week course of systemic corticosteroids (e.g., prednisone, 40 to 60 mg daily) can be used safely as needed without an increase in the risk of respiratory infection or wound complications.17,96,97 The goals of treatment are to render the patient wheeze free and asymptomatic if possible and to achieve a peak flow rate of greater than 80% of predicted or personal best. Patients with a history of cigarette use should be encouraged to quit at least 8 weeks before surgery.
Lung Expansion
Lung expansion maneuvers range from simple but effort-dependent strategies, such as incentive spirometry and deep breathing, to more complex, expensive, and effort-independent strategies, such as continuous positive airway pressure (CPAP). In a meta-analysis of different lung expansion techniques, Thomas and McIntosh98 reported odds ratios for pulmonary complications for incentive spirometry and deep breathing exercises of 0.44 and 0.43. In a study of preoperative intensive muscle training for cardiac surgical patients at high risk for pulmonary complications, patients trained daily for 20 minutes for 2 weeks before surgery. Pulmonary complications were reduced from 35% in the usual care group to 18% in the intensive training group; pneumonia rates decreased by nearly 10%.99
Using a rigorous study selection strategy that excluded all studies with a methodologic flaw, Overend et al100 found 35 of 46 retrieved articles on incentive spirometry to be flawed. Of the remaining 11 studies, only 5 used pulmonary complications as an endpoint, and 2 of these showed a benefit of incentive spirometry. Nevertheless, incentive spirometry and deep breathing exercises are low risk, inexpensive, and therefore still recommended as a strategy to reduce risk. The combination of these two interventions is no more effective than either one alone.
Postoperative CPAP is an effective strategy. Stock et al101 randomly assigned patients after upper abdominal surgery to postoperative CPAP, incentive spirometry, or coughing and deep breathing. Atelectasis was less common in the CPAP group than in either of the other two groups (23% vs 42%). Because CPAP is more costly and carries a small risk of barotrauma, it is reasonable to reserve this intervention for patients who are unable to cooperate with effort-dependent strategies.
Intermittent positive pressure breathing (IPPB) was used commonly in the 1960s and 1970s, but it is associated with more complications than other methods of lung expansion are. This association was illustrated in a prospective study that found postoperative pulmonary complication rates in an IPPB-treated group to be similar to the rates in patients receiving incentive spirometry or voluntary deep breathing exercises102; however, 18% of the IPPB-treated group required discontinuation of therapy because of abdominal distention. In addition, IPPB is more costly than other methods of lung expansion. IPPB should not be used for routine prophylaxis.
Antibiotic Prophylaxis
Systemic antibiotic prophylaxis does not reduce pulmonary complications and should be avoided. However, several studies have demonstrated that decontamination of the nasopharynx with chlorhexidine gluconate can significantly reduce the risk of lower respiratory tract infections.103,104 This should be considered in all patients who remain mechanically ventilated for more than 1 day.
Pain Control
Pain control strategies, including epidural analgesia and intercostal nerve blocks, reduce splinting and promote the ability to take deep breaths after thoracic, aortic, and upper abdominal surgeries. Although pain control is improved with epidural analgesia and intercostal nerve blocks compared with systemic opioids, studies of their ability to reduce postoperative pulmonary complications have shown conflicting results.34–39 In a systematic review and meta-analysis, Ballantyne et al35 helped determine the benefit of specific pain control strategies. Epidural opioids and intercostal nerve blocks showed a nonsignificant trend toward reduced pulmonary complications, whereas epidural local anesthetics significantly reduced both pulmonary infection rates (relative risk, 0.36; 95% CI, 0.21-0.65) and total pulmonary complications (relative risk, 0.58; 95% CI, 0.42-0.80).
Pneumonia
The most significant risk factor for nosocomial pneumonia is mechanical ventilation. Many authors use the terms nosocomial pneumonia and ventilator-associated pneumonia interchangeably. Intubation increases the risk of nosocomial pneumonia 6-fold to 21-fold.65,66 Other risk factors are listed in Table 40-4. Some of these risk factors are easily modifiable and should be modified in all patients. A patient on mechanical ventilation should be formally assessed for suitability for weaning and extubation on a daily basis.105 This assessment is best performed by a daily spontaneous breathing trial, usually as a T-piece trial for 30 to 120 minutes. The longer time interval may be best for marginal patients, although both time intervals have been studied and successfully predict success or failure of extubation in most patients. In addition to daily breathing trials, patients should be kept in an upright position unless there is a specific contraindication. Finally, results from a randomized controlled trial among patients undergoing thoracoabdominal aneurysm repair demonstrate that prophylactic application of nasal CPAP immediately after extubation may significantly reduce pulmonary morbidity.106,107
Several studies have suggested an increased incidence of nosocomial pneumonia when the gastric pH was increased with the use of H2 blockers or antacids. A meta-analysis of studies published before 1990 failed to corroborate this at a statistically significant level.108 In the randomized controlled trial of ranitidine versus sucralfate, which was designed to assess specifically the efficacy of prophylaxis versus the nosocomial pneumonia rate, no significant difference in pneumonia was seen, but surprisingly, ranitidine was more efficacious in preventing stress ulcer bleeding.109
Selective decontamination of the digestive tract is another strategy that aims to decrease the incidence of nosocomial pneumonia by preventing oropharyngeal and gastric colonization with aerobic gram-negative bacilli and Candida species, without disrupting the anaerobic flora. Although the data are convincing that selective decontamination of the digestive tract prevents nosocomial pneumonia, it has not found widespread favor in North America. Reasons for this are many, but most compelling is the fear of encouraging widespread resistant organisms.110
Several studies have shown that patients in the supine position are predisposed to microaspiration of gastric contents compared with patients in the semirecumbent position.111–113 A randomized trial of positioning in 90 intubated patients was stopped early when interim analysis found a significantly lower incidence of clinically suspected and microbiologically confirmed nosocomial pneumonia in semirecumbent versus supine patients.111 Although no effect of positioning on mortality has been shown, it seems prudent to place intubated patients preferentially in the semirecumbent position unless it is contraindicated.
Subglottic suctioning tubes have been demonstrated to significantly reduce the risk of pneumonia in patients mechanically ventilated for more than 2 or 3 days.78–81,114–116
Medical Management of Complications
Acute Respiratory Failure
The approach to and management of respiratory failure can be broken down simply into a four-step process:
Ultimately, if the patient’s underlying disease process is not corrected and a systemic inflammatory response persists, acute respiratory failure does not reverse and culminates in ARDS.
Acute Respiratory Distress Syndrome
Historically, traditional ventilator strategies have relied on tidal volumes in the range of 10 to 12 mL/kg.117,118 Because of the amount of atelectasis present, delivery of a tidal volume of 10 to 12 mL/kg into the smaller actual lung volume has deleterious consequences. Dependent portions of the lung that are atelectatic do not open with this tidal volume; the remaining lung of necessity accommodates the entire large tidal volume, causing higher peak and plateau inspiratory pressures. Portions of the lung in the midzone have alveoli that open but become overdistended with this tidal volume, whereas alveoli that are partially collapsed may be recruited. Alveoli that are overdistended develop shear force stress, increased vascular permeability, loss of surfactant function, cytokine production, and further local and systemic end-organ injury. This increase in lung inflammation is believed to perpetuate the systemic inflammatory response syndrome. Strategies for management with a smaller tidal volume during ARDS were developed out of these concepts. Studies using smaller tidal volumes (4-6 mL/kg of predicted body weight) have demonstrated decreased cytokine production and temporally related reduction of organ failure.
Other authors have suggested that the high mortality rates associated with ARDS are due to direct alveolar injury from cyclic alveolar closing and reopening and overdistention. Use of low tidal volume with higher levels of PEEP is referred to as protective mechanical ventilation118: elevated PEEP, tidal volume less than 6 mL/kg of predicted body weight (not actual body weight), permissive hypercapnia, and use of pressure-limited ventilator modes. Patients receiving protective mechanical ventilation compared with conventional ventilation had lower 28-day mortality, higher rate of weaning, and decreased rate of barotrauma. It is likely that this strategy maximizes alveolar recruitment and aeration, minimizing shear stress and decreasing parenchymal inflammation.
In a seminal multicenter randomized trial involving 517 critically ill patients, the ARDS Network compared traditional ventilation tidal volumes (12 mL/kg) with lower tidal volumes (6 mL/kg) and found that the use of lower tidal volumes in patients with ARDS resulted in fewer ventilator-dependent days and decreased mortality (40% with conventional versus 31% with protective lung ventilation).117,118 The ARDS Network subsequently reported on the ALVEOLI trial,119 which confirmed the benefit of a strategy that lowers tidal volume and limits plateau pressure in patients with ARDS. The mortality of patients ventilated with low versus conventional end-expiratory pressure strategies was less than 30% and did not differ significantly between the two groups. Specifically, the mortality did not improve with a high PEEP strategy versus a lower PEEP strategy, in which tidal volumes were low in both groups.119 It is evident from this work that patients with ARDS should be managed with a protective lung ventilation strategy incorporating a tidal volume of 4 to 6 mL/kg with limitation of plateau pressure to 35 cm H2O. Because such low tidal volumes are used in this protective strategy, traditional goals of gas exchange also must be modified, with tolerance of permissive hypercapnia.
Pneumonia
The Centers for Disease Control and Prevention recently collaborated with experts in diagnosis and treatment of ventilator-associated pneumonia to consider a change in surveillance definitions.120 These new definitions, effective January 2013, attempt to clarify and to provide more objective data about ventilator complications, whether they are infectious or noninfectious in nature. Many patients are incorrectly thought to have pneumonia, leading to overtreatment with the attendant risks of superinfection, promotion of resistance, and antibiotic toxicity. For patients who do have pneumonia, prompt initiation of the appropriate antibiotic therapy significantly affects survival.121 If treatment is indicated, the choice of antibiotic should be influenced by the patient’s recent antibiotic therapy (if any), the resident flora in the hospital or ICU, the presence of underlying diseases, and the available culture data (interpreted with care).122,123 In addition, duration of hospitalization and severity of illness should influence the choice of empirical coverage. Because increasing data suggest that initial appropriate empirical antibiotic therapy is essential to survival, the current recommendation for the treatment of nosocomial pneumonia is initially broad spectrum with de-escalation of therapy as information and clinical status change.
Alternative Modes of Mechanical Ventilation
Noninvasive Positive Pressure Ventilation
Acute respiratory failure unresponsive to conservative medical therapy often requires mechanical ventilation via an endotracheal tube. Endotracheal intubation poses a risk of morbidity including upper airway trauma, nosocomial pneumonia, and sinusitis. In more recent years, noninvasive positive pressure ventilation (NPPV, the combination of pressure support and PEEP delivered by facemask or nasal mask) has been used increasingly to avoid endotracheal intubation in patients with acute respiratory failure.107,124–127 The effectiveness of NPPV depends on the etiology or indication for its use. For acute exacerbations of COPD, clinical trial evidence strongly suggests that NPPV not only brings about rapid symptomatic and physiologic improvements but also reduces the need for intubation, mortality rates, and, in some studies, hospital length of stay. Although NPPV may be associated with nasal bridge ulceration in less than 46% of patients, and gastric distention occasionally occurs, major complications such as aspiration are infrequent. A consensus opinion recommended that NPPV be considered the ventilatory mode of first choice in selected patients with exacerbations of COPD.107
For asthmatic patients, the data supporting NPPV are inconclusive.107 A reasonable approach to an asthmatic patient who does not respond promptly to initial medical therapy but who has not developed a contraindication to NPPV may be a trial of this therapy. Asthmatic patients may deteriorate rapidly, however, and delay of needed intubation is a risk mandating very close surveillance. NPPV also may be used in patients with upper airway edema after extubation and in patients with acute respiratory failure resulting from obstructive sleep apnea.126,127
For patients with pulmonary edema, CPAP (10 cm H2O) should be used initially, with NPPV reserved for patients with substantial hypercapnia or unrelenting dyspnea.125–127 This recommendation is based on the fact that CPAP is effective and that the sole randomized trial comparing CPAP and NPPV for patients with acute pulmonary edema showed an increase in myocardial infarction rate in the NPPV patients. A study of high-flow oxygen versus NPPV did not show any differences in myocardial ischemia rates, however. For selected groups of patients with severe community-acquired pneumonia, chest wall restrictive diseases, or immunocompromise, NPPV has been administered successfully.
Advanced or Nontraditional Ventilatory Modes
A number of ventilatory modes have been proposed128 to treat severe ARDS, including high-frequency oscillatory ventilation129,130 and even extracorporeal membrane oxygenation in selected patients.131 A recent trial of high-frequency oscillatory ventilation for early ARDS has been terminated for lack of efficacy and possible harm associated with its use.130 For adults with severe ARDS, transfer to an extracorporeal membrane oxygenation center early in ARDS disease progression, whether or not extracorporeal membrane oxygenation is eventually employed, appears to produce better outcomes.131 Not only do patients have access to extracorporeal membrane oxygenation therapy if they need it, but additional resources are often available at the centers where this therapy is provided. These newer modalities remain available in most centers focusing on specialized treatment of patients with severe ARDS, and such centers often offer entry into randomized clinical trials.
Selected Key References
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533.
Provides updated, more reliable definition of acute respiratory distress syndrome and stratifies the severity of the oxygenation deficit, which may guide clinician management strategies..
Arozullah AM, Khuri SF, Henderson WG, Daley J. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–857.
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher vs. lower positive end-expiration pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336.
Canet J, Gallart L, Gomar C, Paluzie G, Vallès J, Castillo J, Sabaté S, Mazo V, Briones Z, Sanchis J, ARISCAT Group. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350.
Chiumello D, Chevallard G, Gregoretti C. Non-invasive ventilation in postoperative patients: a systematic review. Intensive Care Med. 2011;37:918–929.
MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ, American College of Chest Physicians; American Association for Respiratory Care; American College of Critical Care Medicine. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(Suppl):375S–395S.
Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D, VAP Guidelines Committee and the Canadian Critical Care Trials Group. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23:126–137.
National Asthma Education and Prevention Program. Expert Panel report 3: guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute: Bethesda, Md; 2007 www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, MacDonald R, Shekelle P, American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Sachdev G, et al. Postoperative pulmonary complications: pneumonia and acute respiratory failure. Surg Clin North Am. 2012;92:321–344.
2. Johnson DC, et al. Perioperative pulmonary complications. Curr Opin Crit Care. 2011;17:362–369.
3. Agostini P, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65:815–818.
4. Lawrence VA, et al. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1995;10:671–678.
5. Shander A, et al. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med. 2011;39:2163–2172.
6. Qaseem A, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575–580.
7. Grigorakos L, et al. Preoperative pulmonary evaluation (PPE) as a prognostic factor in patients undergoing upper abdominal surgery. Hepatogastroenterology. 2008;55:1229–1232.
8. Johnson RG, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1188–1198.
9. Canet J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350.
10. Scholes RL, et al. Duration of anaesthesia, type of surgery, respiratory co-morbidity, predicted VO2max and smoking predict postoperative pulmonary complications after upper abdominal surgery: an observational study. Aust J Physiother. 2009;55:191–198.
11. Von Ungern–Sternberg BS, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773–783.
12. Smetana GW, et al. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–595.
13. Tarhan S, et al. Risk of anesthesia and surgery in patients with chronic bronchitis and chronic obstructive pulmonary disease. Surgery. 1973;74:720.
14. Stein M, et al. Preoperative pulmonary evaluation and therapy for surgery patients. JAMA. 1970;211:787.
15. Sweitzer BJ, et al. Identification and evaluation of the patient with lung disease. Anesthesiology Clin. 2009;27:673–686.
16. Spieth PM, et al. Chronic obstructive pulmonary disease. Curr Opin Anaesthesiol. 2012;25:24–29.
17. Woods BD, et al. Perioperative considerations for the patient with asthma and bronchospasm. Br J Anaesth. 2009;10(Suppl. 1):i57–i65.
18. Panaretou V, et al. Postoperative pulmonary function after open abdominal aortic aneurysm repair in patients with chronic obstructive pulmonary disease: epidural versus intravenous analgesia. Ann Vasc Surg. 2012;26:149–155.
19. Compton CN, et al. Is abdominal aortic aneurysm repair appropriate in oxygen-dependent chronic obstructive pulmonary disease patients? J Vasc Surg. 2005;42:650–653.
20. National Asthma Education and Prevention Program. Expert Panel report 3: guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute: Bethesda, Md; 2007 www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
21. Bluman LG, et al. Preoperative smoking habits and postoperative pulmonary complications. Chest. 1998;113:883–889.
22. Nakagawa M, et al. Relationship between the duration of the preoperative smoke-free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest. 2001;120:705–710.
23. Shi Y, et al. Brief preoperative smoking abstinence: is there a dilemma? Anesth Analg. 2011;113:1348–1351.
24. Myers K, et al. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis. Arch Intern Med. 2011;171:983–989.
25. Pasulka PS, et al. The risks of surgery in obese patients. Ann Intern Med. 1986;104:540–546.
26. Thomas EJ, et al. Body mass index as a correlate of postoperative complications and resource utilization. Am J Med. 1997;102:277–283.
27. Calligaro KD, et al. Pulmonary risk factors of elective abdominal aortic surgery. J Vasc Surg. 1993;18:914–920.
28. Djokovic JL, et al. Prediction of outcome of surgery and anesthesia in patients over 80. JAMA. 1979;242:2301–2306.
29. Arozullah AM, et al. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232:242–253.
30. Etz CD, et al. Pulmonary complications after descending thoracic and thoracoabdominal aortic aneurysm repair: predictors, prevention, and treatment. Ann Thorac Surg. 2007;83:S870–S876.
31. Ford GT, et al. Diaphragm function after upper abdominal surgery in humans. Am Rev Respir Dis. 1983;127:431–436.
32. Xue FS, et al. The influence of surgical sites on early postoperative hypoxemia in adults undergoing elective surgery. Anesth Analg. 1999;88:213–219.
33. Pedersen T, et al. A prospective study of risk factors and cardiopulmonary complications associated with anaesthesia and surgery: risk indicators of cardiopulmonary morbidity. Acta Anaesthesiol Scand. 1990;34:144–155.
34. Block BM, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290:2455–2463.
35. Ballantyne JC, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86:598–612.
36. Rodgers A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anesthesia; results from overview of randomized trials. BMJ. 2000;321:1493.
37. Park WY, et al. Effect of epidural anesthesia and analgesia on perioperative outcome: a randomized, controlled Veterans Affairs co-operative study. Ann Surg. 2001;234:560–569.
38. Rigg JR, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomized trial. Lancet. 2002;359:1276–1282.
39. Norris EJ, et al. Double-masked randomized trial comparing alternate combinations of intraoperative anesthesia and postoperative analgesia in abdominal aortic surgery. Anesthesiology. 2001;95:1054–1067.
40. Berg H, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–1103.
41. Marik PE. Pulmonary aspiration syndromes. Curr Opin Pulm Med. 2011;17:148–154.
42. Raghavendran K, et al. Aspiration-induced lung injury. Crit Care Med. 2011;39:818–826.
43. Bernstein WK. Pulmonary function testing. Curr Opin Anaesthesiol. 2012;25:11–16.
44. Young EL, et al. A systematic review of the role of cardiopulmonary exercise testing in vascular surgery. Eur J Vasc Endovasc Surg. 2012;44:64–71.
45. Struthers R, et al. Assessing fitness for surgery: a comparison of questionnaire, incremental shuttle walk, and cardiopulmonary exercise testing in general surgical patients. Br J Anaesth. 2008;101:774–780.
46. Ridgway ZA, et al. Cardiopulmonary exercise testing: a review of methods and applications in surgical patients. Eur J Anaesthesiol. 2010;27:858–865.
47. Preoperative pulmonary function testing. American College of Physicians. Ann Intern Med. 1990;112:793–794.
49. Lawrence VA, et al. Preoperative spirometry before abdominal operations. A critical appraisal of its predictive value. Arch Intern Med. 1989;149:280–285.
50. Zibrak JD, et al. Indications for pulmonary function testing. Ann Intern Med. 1990;112:763–771.
51. Archer C, et al. Value of routine preoperative chest x-rays: a meta-analysis. Can J Anaesth. 1993;40:1022–1027.
52. Goldman L, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850.
53. Brooks-Brunn JA. Validation of a predictive model for postoperative pulmonary complications. Heart Lung. 1998;27:151–158.
54. Castillo R, et al. Chest physical therapy: comparative efficacy of preoperative and postoperative in the elderly. Arch Phys Med Rehabil. 1985;66:376–379.
55. Epstein SK, et al. Predicting complications after pulmonary resection. Preoperative exercise testing vs a multifactorial cardiopulmonary risk index. Chest. 1993;104:694–700.
56. Melendez JA, et al. Cardiopulmonary risk index does not predict complications after thoracic surgery. Chest. 1998;114:69–75.
57. Johnson RG, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1188–1198.
58. Rosenberg J, et al. Ventilatory pattern and associated episodic hypoxaemia in the late postoperative period in the general surgical ward. Anaesthesia. 1999;54:323–328.
59. Tusman G, et al. Atelectasis and perioperative pulmonary complications in high-risk patients. Curr Opin Anaesthesiol. 2012;25:1–10.
60. Wahba RM. Airway closure and intraoperative hypoxaemia: twenty-five years later. Can J Anaesth. 1996;43:1144–1149.
61. Barone JE, et al. Routine perioperative pulmonary artery catheterization has no effect on rate of complications in vascular surgery: a meta-analysis. Am Surg. 2001;67:674–679.
62. Sandham JD, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14.
63. Mavros MN, et al. Atelectasis as a cause of postoperative fever: where is the clinical evidence? Chest. 2011;140:418–424.
64. Bouadma L, et al. Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis. 2012;25:395–404.
65. Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344:e3325.
66. Fagon JY, et al. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288.
67. Arozullah AM, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–857.
68. Wunderink RG, et al. Update in respiratory infections 2011. Am J Respir Crit Care Med. 2012;185:1261–1265.
69. Magill SS, et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol. 2012;33:283–291.
70. Gastmeier P, et al. Early- and late-onset pneumonia: is this still a useful classification? Antimicrob Agents Chemother. 2009;53:2714–2718.
71. Fagon JY, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132:621–630.
72. Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630.
73. Marik PE. Aspiration pneumonitis and pneumonia: a clinical review. N Engl J Med. 2001;344:665–672.
74. Mylotte JM, et al. Validation and application of the pneumonia prognosis index to nursing home residents with pneumonia. J Am Geriatr Soc. 1998;46:1538–1544.
75. Mylotte JM, et al. Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51:17–23.
76. Ravelli AM, et al. Pulmonary aspiration shown by scintigraphy in gastroesophageal reflux-related respiratory disease. Chest. 2006;130:1520–1526.
77. Daniels SK, et al. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79:14–19.
78. Muscedere J, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med. 2011;39:1985–1991.
79. Dezfulian C, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118:11–18.
80. Shorr AF, et al. Continuous subglottic suctioning for the prevention of ventilator-associated pneumonia: potential economic implications. Chest. 2001;119:228–235.
81. Ramirez P, et al. Prevention measures for ventilator-associated pneumonia: a new focus on the endotracheal tube. Curr Opin Infect Dis. 2007;20:190–197.
82. Scheld WM, et al. Nosocomial pneumonia: pathogenesis and recent advances in diagnosis and therapy. Rev Infect Dis. 1991;13(Suppl 9):S743–S751.
83. Kwong JC, et al. New aspirations: the debate on aspiration pneumonia treatment guidelines. Med J Aust. 2011;195:380–381.
84. Vincent JL, et al. Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31(Suppl):S296–S299.
85. ARDS Definition Task Force, Ranieri VM, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533.
86. Bernard GR, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(Pt 1):818–824.
87. Matthay MA, et al. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740.
88. Pinhu L, et al. Ventilator-associated lung injury. Lancet. 2003;361:332–340.
89. Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003;31(Suppl):S276–S284.
90. Herridge MS, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693.
91. Qaseem A, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. [American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society] Ann Intern Med. 2011;155:179–191.
92. Ryan C, et al. Pharmacist-led interventions for adults with asthma or COPD. Cochrane Database Syst Rev. 2013;(4).
93. Walters JA, et al. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;(1).
94. Walters JA, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10).
95. Yang I, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7).
96. Kabalin CS, et al. Low complication rate of corticosteroid-treated asthmatics undergoing surgical procedures. Arch Intern Med. 1995;155:1379–1384.
97. Pien LC, et al. Minimal complications in a surgical population with severe asthma receiving prophylactic corticosteroids. J Allergy Clin Immunol. 1988;82:696–700.
98. Thomas JA, et al. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Phys Ther. 1994;74:3–10.
100. Overend TJ, et al. The effect of incentive spirometry on postoperative pulmonary complications: a systematic review. Chest. 2001;120:971–978.
101. Stock MC, et al. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest. 1985;87:151–157.
102. Celli BR, et al. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing pulmonary complications after abdominal surgery. Am Rev Respir Dis. 1984;130:12–15.
103. Gastmeier P, et al. Prevention of ventilator-associated pneumonia: analysis of studies published since 2004. J Hosp Infect. 2007;67:1–8.
104. Segers P, et al. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296:2460–2466.
105. MacIntyre NR, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. [American College of Chest Physicians; American Association for Respiratory Care; American College of Critical Care Medicine] Chest. 2001;120(Suppl):375S–395S.
106. Kindgen-Milles D, et al. Nasal-continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest. 2005;128:821–828.
107. Bagan P, et al. Prevention of pulmonary complications after aortic surgery: evaluation of prophylactic noninvasive perioperative ventilation. Ann Vasc Surg. 2011;25:920–922.
108. Cook DJ, et al. Nosocomial pneumonia and the role of gastric pH. A meta-analysis. Chest. 1991;100:7–13.
109. Cook D, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791–797.
110. van Nieuwenhoven CA, et al. Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA. 2001;286:335–340.
111. Drakulovic MB, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851–1858.
112. Orozco-Levi M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med. 1995;152(Pt 1):1387–1390.
113. Torres A, et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med. 1992;116:540–543.
114. Smulders K, et al. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest. 2002;121:858–862.
115. Kollef MH, et al. A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients. Chest. 1999;116:1339–1346.
116. Shorr AF, et al. Continuous subglottic suctioning for the prevention of ventilator-associated pneumonia: potential economic implications. Chest. 2001;119:228–235.
117. Amato MB, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354.
118. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308.
119. Brower RG, et al. Higher vs. lower positive end-expiration pressures in patients with the acute respiratory distress syndrome. [National Heart, Lung, and Blood Institute ARDS Clinical Trials Network] N Engl J Med. 2004;351:327–336.
120. Klompas M, et al. Objective surveillance definitions for ventilator-associated pneumonia. [CDC Prevention Epicenters Program] Crit Care Med. 2012;40:3154–3161.
121. Luna CM, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest. 1997;111:676–685.
122. Muscedere J, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. [VAP Guidelines Committee and the Canadian Critical Care Trials Group] J Crit Care. 2008;23:126–137.
123. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
124. Cereda M, et al. Noninvasive respiratory support in the perioperative period. Curr Opin Anaesthesiol. 2013;26:134–140.
125. Chiumello D, et al. Non-invasive ventilation in postoperative patients: a systematic review. Intensive Care Med. 2011;37:918–929.
126. Kindgen-Milles D, et al. Nasal continuous positive airway pressure: a method to avoid endotracheal reintubation in postoperative high-risk patients with severe nonhypercapnic oxygenation failure. Chest. 2000;117:1106–1111.
127. Liesching T, et al. Acute applications of noninvasive positive pressure ventilation. Chest. 2003;124:699–713.
128. Slinger P, et al. Perioperative lung protection strategies in cardiothoracic anesthesia: are they useful? Anesthesiol Clin. 2012;30:607–628.
129. Young D, et al. High-frequency oscillation for acute respiratory distress syndrome. [OSCAR Study Group] N Engl J Med. 2013;368:806–813.
130. Ferguson ND, et al. High-frequency oscillation in early acute respiratory distress syndrome. [OSCILLATE Trial Investigators; Canadian Critical Care Trials Group] N Engl J Med. 2013;368:795–805.
131. Brodie D, et al. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–1914.