Chapter 39
Systemic Complications
Cardiac
William Scott Beattie, Giora Landesberg
In an editorial in 2004, Mosucuci and Eagles wrote, “If one carefully screens vascular patients and excludes patients with symptoms of unstable angina, left main disease, aortic stenosis, and severe left ventricular dysfunction, and if one provides excellent medical care to those remaining, then coronary artery revascularization does not appear to provide additional benefit.”*
Epidemiology
Prevalence of Coronary Disease
Atherosclerosis is ubiquitous in vascular surgical patients. Hertzer drew attention to this by showing the small percentage of vascular surgery patients (8%) who were free of coronary artery disease (CAD).1 This study, conducted over 30 years ago, revealed that 5% of vascular surgical populations at the Cleveland Clinic had left main CAD and that 11% had significant three-vessel disease. Notably, in our opinion, the most important finding was that 15% of patients had severe, yet unsuspected, coronary disease.
The intervening 30 years have seen seismic changes in both the primary and secondary prevention of CAD, including smoking cessation, the adoption of aggressive lipid-lowering therapies, and a 30% decrease in the incidence of myocardial infarction and cardiac death in nonsurgical populations.2 Conversely, patients presenting for vascular surgery in 2013 are on average a decade older and have more comorbidities, and the incidence of major coronary pathology in vascular patients may be higher. Thus despite clear advances in the treatment of CAD, it is conceivable that more patients presenting for vascular surgery have silent yet significant coronary artery disease. This paradox is illustrated in the Coronary Artery Revascularization Prophylaxis (CARP) trial, in which 17% of all patients who underwent angiography had three-vessel disease and an additional 4.6% had left main disease.3 In the intervening years, our attempts to identify patients with significant coronary disease, through a variety of processes of care, have apparently been inadequate. This was demonstrated by Monaco et al, who screened patients using the American Heart Association/American College of Cardiology (AHA/ACC) guidelines and identified fewer patients with three-vessel or left main coronary disease than a randomized cohort having routine preoperative coronary angiograms.4
Our current processes of care fail to identify many patients with significant CAD, and when we do correctly identify them, few effective treatments are available. In this chapter we will outline and define the pathology, incidence, diagnostic criteria, risk stratification, and treatment options designed to limit the effect of postoperative cardiac adverse events.
Cardiac Complications
Definition of Adverse Cardiac Events
We suggest that only the terms myocardial infarction (MI), congestive heart failure (CHF), cardiac arrest, and death have utility in the modern lexicon of adverse cardiac events.
Myocardial Infarction
MI is the leading cause of death after surgical procedures. The Third Universal Definition of MI,5 as endorsed by all major cardiology organizations, is a rise and fall in a cardiac biomarker, mainly cardiac troponin, with at least one measurement above the 99th percentile of the upper reference limit (for the specific troponin assay), associated with at least one of the following:
2. Electrocardiographic evidence of ischemia, ST-T wave changes or left bundle branch block.
3. Development of pathologic Q waves.
4. Imaging showing loss of viable myocardium or a new abnormal LV wall motion.
The universal classification of myocardial infarction is outlined in more detail in Box 39-1.
If this low cutoff level of troponin elevations is used to define MI, postoperative myocardial injury occurs in as many as 25% of vascular surgery patients. Myocardial infarction comprises two distinct electrocardiographic entities. The first subgroup, those patients who may have ST-elevation MI (STEMI), accounts for less than 1% of surgical patients.6 The second, and largest, subgroup sustain a biomarker rise with ST-segment depression or without transient ST-T changes, which is analogous to a non–ST-elevation myocardial infarction (NSTEMI).
Congestive Heart Failure
CHF is ill defined in older investigations and is frequently the result of fluid overload or transfusion-associated cardiac overload (TACO) (as prime examples). However, the inability to handle excess fluids is a sign of a failing left ventricle. We would submit, therefore, that in this modern era of advanced echocardiographic technology and training, postoperative CHF should be more thoroughly investigated with biomarkers, chest radiography, and cardiac imaging (i.e., echocardiography).
Cardiac Death
Death sometimes occurs before biomarkers are measured (or appear), and therefore the following situations have been defined as cardiac deaths: (a) death that is sudden and unexpected, (b) cardiac arrest with symptoms suggestive of myocardial ischemia, (c) death that is accompanied by new ST elevation or new left bundle branch block (but before a biomarker can be obtained), and (d) autopsy evidence of fresh thrombus in a coronary artery.
Perioperative Myocardial Injury
A discussion on myocardial injury is necessary since many patients with postoperative troponin elevations do not fulfill the additional diagnostic criteria necessary for the diagnosis of postoperative MI. A secondary analysis of POISE trial makes clear that postoperative MI is predominately clinically silent.6 ST-T changes are captured in less than 30% of the patients, and many patients have cardiac biomarkers detected that are totally asymptomatic. Levy and colleagues conducted a meta-analysis of seven studies where a biomarker has been used to routinely screen all patients, which draws attention to the association between elevated cardiac biomarkers and postoperative death.7 In the intervening 2 years there have been three more evaluations in which vascular patients have had universal biomarkers drawn postoperatively.8–10 These 10 studies found that 1,032 of the 6,083 patients had significant troponin elevations (16.3%). Similarly, in FOCUS, a randomized trial assessing transfusion triggers in elderly orthopedic patients with universal biomarker monitoring, only 33% of patients with detectable biomarker had MI.11 Landesberg et al showed essentially the same results in vascular surgical patients (Table 39-1).12
Table 39-1
Association of Longest Ischemia Duration with Biochemical Markers of MI
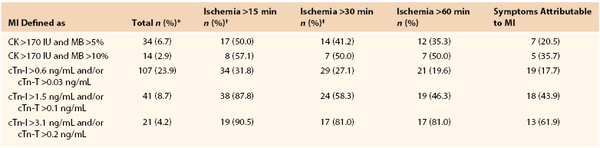
* Percentage of all 501 vascular operations.
† All four biochemical criteria of infarction were significantly associated (P < 0.0001) with both >60 min ischemia duration using chi-square analysis.
‡ Prolonged chest pain, congestive heart failure, or new onset arrhythmia.
CK, Creatine kinase; cTn-I, cardiac troponin-I; cTn-T, cardiac troponin-T; IU, international units; MB, MB fraction; MI, myocardial infarction.
The reasons for this lack of symptomatology remains speculative but may include the concomitant use of narcotics, diabetes mellitus, competing somatic sensations, and a high incidence of symptoms such as postoperative nausea and vomiting, which coexist with both surgery and acute cardiac ischemia. The lack of ECG changes may be due to the transient nature of postoperative ST-T changes and the lack of routine cautious monitoring of postoperative ECG with ST-segment analysis.15 Furthermore, ST-segment monitoring may be complicated by preexisting left or right bundle branch blocks, baseline ST-T changes, drug effects, acid/base alterations, and electrolyte abnormalities.16,17 In addition, without regular surveillance, as many as two thirds of biomarker elevations will be missed, which underlines the importance of ordering troponin in high-risk patients in the postoperative period.
Definition of Myocardial Infarction
The universal classifications of myocardial infarction (see Box 39-1) distinguish between two main types of MI10:
The other types of MI in the universal definition are less relevant to this discussion and include sudden cardiac death before biomarkers are available (type 3) or as a result of coronary interventions (post-PCI—type 4; post CABG—type 5).
Type 1 MI
This type typically occurs as a result of an unstable or “vulnerable” coronary plaque and is characterized by a large lipid core and a thin, weakened fibrous cap, which suddenly undergoes acute disruption, leading to acute coronary thrombosis. Type 1 MI, together with unstable angina pectoris or sudden cardiac death, is defined as acute coronary syndrome (ACS).
When plaque rupture and coronary thrombosis occur in a major coronary artery, supplying an area that was not significantly stenosed before the acute occlusion and therefore lacks sufficient collaterals, the result is often ST-segment MI (STEMI). As a rule, STEMI, is a medical emergency causing severe chest pain, dyspnea, and other related symptoms, requiring immediate intervention to reopen the occluded coronary artery, preferably by percutaneous coronary intervention (PCI) or by thrombolysis. Even with immediate coronary intervention, STEMI is associated with up to 10% early mortality.
Other patients may have similar symptoms but no ST-segment elevations or rather ST-segment depression on ECG; these infarctions are defined as non–ST-segment MI (NSTEMI). Pathologically, these may be plaque ruptures and coronary thromboses in coronary arteries supplying smaller myocardial territories or territories supplied also by collaterals or thromboses causing nontotal coronary occlusions. Patients with NSTEMI usually do not require—and do not benefit from—immediate coronary interventions, unless hemodynamic instability or progression of symptoms occurs.
In the past, in nonsurgical Western populations, the proportions of STEMI and NSTEMI were approximately 50% each. More recently, the absolute incidence of STEMI is decreasing in the West while the incidence of NSTEMI is consistently increasing. Reasons for this shift include the older average age of the Western population, increased availability of primary and secondary prevention strategies, and better detection of NSTEMI through high-sensitivity troponin assays. Hence STEMIs currently account for 30% or less of all myocardial infarctions.
Type 2 MI
In contrast, type 2 MI typically occurs in patients with severe yet stable CAD, usually the result of extracardiac causes leading to a prolonged imbalance between myocardial oxygen supply and demand. The most common triggers for type 2 MI are prolonged tachycardia, hypotension or hypertension, anemia, and possibly, emotional or physical stress causing coronary vasospasm.
Type 2 MI is almost uniformly associated with ST-depression or non–ST-elevation ischemia and is therefore indistinguishable from type 1 NSTEMI. The circumstances (i.e., spontaneous in type 1 versus stress-induced in type 2) should help differentiate between the two types of MI. Current data suggest that the vast majority of perioperative myocardial infarctions are NTSEMI and most likely, type 2.
Cardiac Biomarkers
Several biomarkers have been developed to measure myocardial injury. Currently, the most frequently used biomarker is cardiac troponin cTn (T or I), followed by the myocardial band of creatinine kinase (CK-MB). Other less frequent tests include lactate dehydrogenase (LDH), aspartate transaminase (AST), myoglobin ischemia modified albumin, glycogen phosphorylase isoenzyme BB and pro-brain natriuretic peptide. Since troponin assays are based on monoclonal antibodies specific to the cardiac troponins (I and T), they are the most sensitive and specific tests for myocardial injury, whereas CK-MB is specific only when there is no skeletal muscle damage.
The remaining tests lack specificity since other causes of tissue damage can elevate the marker. LDH is a late occurring marker of injury (normalizing in 10-14 days) that can also be elevated in cancer, meningitis, or human immunodeficiency virus (HIV). AST is primarily a liver function test. Myoglobin, the main intracellular oxygen transport molecule in myocytes, lacks specificity since it is released in all forms of muscle degradation; yet it has the advantage of the earliest detection after injury (2 hours) and is sometimes used to monitor reperfusion.
Postoperative Troponin Elevations and Mortality.
Troponin elevation in the first 3 days after major vascular surgery is incrementally associated with higher 5-year mortality.14 Recently, VISION trial reported that myocardial injury, as measured by a fourth generation high-sensitivity troponin T assay in the first 3 days after major general surgery, shows “dose response” association between the degree of troponin detected and 30-day postoperative mortality.13 A single-center retrospective study showed that when troponin measurement is not a standard of care, myocardial injury is missed in as many as 66% of patients.9 This study also showed that isolated cardiac troponin release is associated with increased mortality in a “dose-dependent” manner. Levy et al conducted a meta-analysis of seven studies in which cardiac troponin has been used to routinely screen all patients and demonstrated the association between elevated cardiac troponins and postoperative death.7 The incidence of increased cardiac troponins in these studies ranged from 12.4% to 26.5%. It is recommended that serum troponin concentrations be measured in high-risk vascular surgery patients (we suggest that this be defined as a Revised Cardiac Risk Index ≥2) for the first 3 postoperative days.
Electrocardiography
The electrocardiogram occupies a central position in the diagnosis of myocardial infarction, as is seen in the recently revised consensus statement, the Third Universal Definition of Myocardial Infarction.5 Acute ST-segment changes indicate MI and, in conjunction with troponin elevations, can indicate evolving infarction. However, in the presence of preexisting changes, such as left or right bundle branch block, pacemaker rhythms, or baseline ST-T wave changes, these diagnostic criteria become less specific and less sensitive. This is particularly problematic in vascular surgery, in which more than 30%18 of patients demonstrate such changes. This becomes even more troublesome when one considers that the incidence of these preexisting electrocardiographic changes increases with the underlying cardiac risk.
Objective measurements such as continuous 12-lead ECG recording in the perioperative period, combined with rigorous screening of cardiac biomarkers, might give a more accurate estimation of the true incidence of perioperative MI. A few studies have used this modality, all of them in patients undergoing major vascular procedures. One of these studies found that in 447 patients scheduled for elective open abdominal aortic repairs and monitored by continuous 12-lead ECG recording, cardiac troponin, and CK/CK-MB for the first 3 postoperative days, 23.9% of patients experienced cardiac troponin release above the lowest cutoff level. Remarkably, fewer than a third (31.8%) of these patients had perioperative ST-segment changes (see Table 39-1).12
Cardiac Imaging
We are unaware of any perioperative studies that have evaluated either left ventricular function, new onset regional wall motion abnormailties, or mitral insufficiency to aid in the diagnosis of MI. Many imaging modalities are difficult to conduct postoperatively. However, with the advent of bedside ultraound and limited cardiac echocardiography, this may change in the near future. Focused cardiac ultrasound examinations have been show to improve the diagnosis of MI in Emergency Departments.19
Pathogenesis of Perioperative Myocardial Infarction
A schematic diagram of proposed perioperative myocardial injury mechanisms is seen in Figure 39-1. The perioperative surgical stress response includes a catecholamine surge with associated hemodynamic stress, vasospasm, reduced fibrinolytic activity, platelet activation, and consequent hypercoagulability. However, despite the common occurrence of CAD in vascular surgery patients, STEMI is rare compared with the common occurrence of NSTEMI, suggesting that perioperative MI is most commonly caused by a sustained imbalance between myocardial oxygen supply and demand, usually as a result of the tachycardia and increase in myocardial load.20
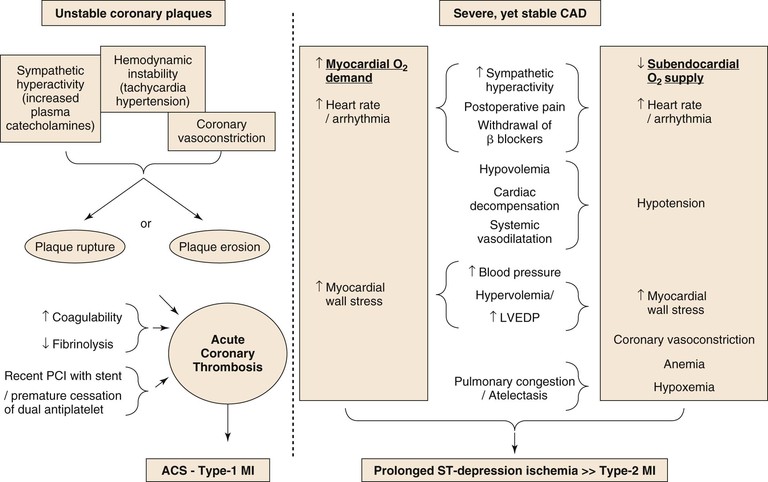
Figure 39-1 The two distinct mechanisms of PMI.
Episodes of perioperative ST-segment depression, indicative of subendocardial myocardial ischemia, have been described in up to 41% of vascular surgery patients, mostly occurring within the first 2 days after surgery,21–23 whereas Q wave infarction is rare.6,24 Although plaque disruption fissure or hemorrhage can be found in postmortem analyses in up to half of patients who died after postoperative MI,25,26 similar findings are also common in patients with CAD who die from other, noncoronary causes (see Fig. 39-1).
Incidence of MI after Vascular Surgery
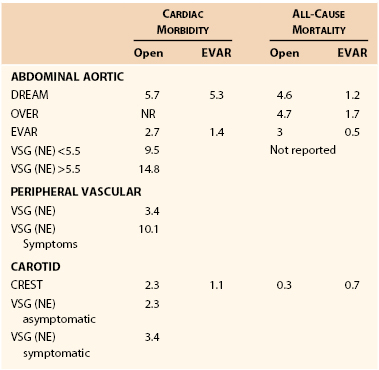
The incidence of MI after vascular surgery has been reported in various formats, including prospective randomized trials, prospective observational trials, and retrospective reports. The incidence of major cardiac complications is dependent on patient characteristics, symptomatology, and type of surgery. The incidence of major cardiac complications is displayed in Table 39-2.
Aortic surgery has the highest incidence of cardiac morbidity, ranging from 1.4% in the OVER trial to more than 14% in the Vascular Study Group of New England registry.27 Generally, the event rates in randomized trials are half of those in registries. In addition, the cardiac event rate is higher in patients with larger aneurysms. The acute in-hospital infarctions rates are lower for endovascular compared to open procedures. 28–31 The cardiac event rate in peripheral vascular surgery is dependent on symptoms; patients with peripheral ischemic symptoms have more than a 10% cardiac event rate, which is three times that of patients without rest ischemia.
Carotid surgery is associated with approximately 2% to 3% perioperative cardiac morbidity.32–34 The results from randomized trials are similar to those from registry data. Finally, patients with symptomatic carotid disease have similar rates of MI based on the registry data. Carotid endarterectomy is associated with a doubling in cardiac morbidity for surgery as opposed to a carotid stent procedure; however, this was more than offset by an increased stroke rate in patients having stenting procedures.35,36
Preoperative Cardiac Evaluation
Three decades ago, based on their observations of the high incidence of severe CAD in patients undergoing major vascular surgery, Hertzer et al established the philosophy that the mere fact that a patient has atherosclerosis and requires vascular surgery justifies a high index of suspicion, which may lead to coronary investigation and possible revascularization.1
Since then, tremendous changes have occurred in the field, which have led to our current practices based on patient risk,37 in which coronary investigation and revascularization are selectively performed before major vascular surgery. The changes in this philosophy and the approach to the cardiac patient undergoing major surgery include the following.
2. Numerous advances in cardiology have tempered the enthusiasm for prophylactic revascularization.
• Percutaneous coronary intervention does not improve survival in patients with stable CAD.38
The following paragraphs outline the major historic developments in the field in the last decades, along with the most important tools that are currently available for preoperative cardiac investigation. A careful attempt will be made to provide general recommendations for preoperative cardiac assessment and intervention. Mosucuci and Eagle, in an editorial accompanying the CARP trial in 2004, commented,
“The issue of whom to screen and how to screen preoperative patients beyond history taking, physical examination and preoperative electrocardiogram is far from settled.”1
Preoperative cardiac risk assessment affects perioperative decision making in three ways. (1) Given the patient’s anatomical and/or symptomatic indication for vascular surgery, a high cardiac risk may influence the perioperative or long-term risk-benefit ratio of the elective surgery and may therefore lead to its deferral. (2) Effective cardiac risk assessment may enable preoperative optimization by prompting medical therapy for cardiac failure (if present); in specific cases, risk assessment may lead to preoperative coronary interventions to reduce perioperative and long-term cardiac risk. (3) Accurate preoperative knowledge of the patient’s cardiac function and risk allow better intraoperative and postoperative treatment and resource utilization, such as intraoperative monitoring and postoperative ICU monitoring, when needed, for the patient.
Cardiac Risk Indices
As a general rule any risk index must be simple, accurate, and generalizable across populations. Numerous clinical risk indices have been proposed, including the seminal Goldman cardiac risk index,39 the Detsky’s risk index,40 l’Italien,41 the Glasgow Aneurysm Score,42 and others. However, no index has yet gained wide and lasting acceptance.
In 1999 Lee et al constructed a simplified revised cardiac risk index (RCRI).37 In a derivation cohort of 2893 consecutive patients, six independent risk factors were identified: (1) a history of heart disease, (2) a history of congestive heart failure, (3) cerebral vascular disease, (4) insulin therapy, (5) chronic renal failure (defined as a preoperative creatinine of 177 mmol/L, or 2 g/L), and (6) high-risk surgery (major vascular, thoracic, and upper abdominal surgery). The validation cohort of 1422 patients showed that patients with none, 1, 2, and 3 of these factors had cardiac complications of 0.4%, 0.9%, 7%, and 11%, respectively. The overall accuracy ranged between 0.75 and 0.80 and was superior in this population to the other cardiac risk indices and the American Society of Anesthesiologists (ASA) classification. Since its publication, the RCRI has been widely used and adopted by the ACC/AHA guidelines committee for the preoperative stratification of patients’ perioperative cardiac risk.43 The index has also been shown to be generalizable across numerous populations.
A recent meta-analysis of 24 studies in over 750,000 patients shows that the RCRI discriminated moderately well in mixed surgical populations, with a sensitivity of 0.65 and specificity of 0.76.44 When this meta-analysis was limited strictly to studies of vascular surgical populations, the RCRI had an aggregate sensitivity and specificity of 0.70 and 0.55, respectively. The vascular surgery group has also published a modification of RCRI that purports to improve the prediction of major cardiac events.27 However, this analysis suffers from the fact that not all patients were screened for a biomarker elevation. This methodologic defect has been shown to underestimate the incidence of MACE by up to threefold.9 A recent analysis of the accuracy of the RCRI in vascular surgical patients, who also had universal biomarker screening, had calculated receiver operating characteristic of 0.75.45 Therefore it is our opinion that the RCRI alone is inadequate to reliably screen vascular surgery patients; it was proposed that further information be gained through laboratory testing to increase the accuracy of the preoperative cardiac investigation.
Preoperative Laboratory Testing
Complete Blood Count
Laboratory findings are used routinely for preoperative risk assessment, and the most frequent lab test in the preoperative assessment is a complete blood cell count. Preoperative anemia has been linked to increasing cardiac morbidity.45 Preoperative anemia is also linked to increased transfusion requirements, which have been secondarily shown to increase cardiac morbidity. In particular, surgical patients who regularly take beta blockers do not tolerate acute anemia, which may lead to increased cardiac morbidity.46 We are unaware of any study that has incorporated anemia into a risk index.
Creatinine
The RCRI shows that chronic renal failure is independently associated with increased cardiac morbidity. However, creatinine is closely related to lean muscle mass. Thus many elderly patients with normal creatinine levels have chronic renal failure. The conversion of creatinine to glomerular filtration rate, through either the Modification of Diet in Renal Disease Study or the Cockcroft-Gault equations may increase the sensitivity and specificity of the measurement.47
Blood Glucose
Diabetes is a common comorbidity found on preoperative screening, seen in up to 50% of vascular surgical patients. Diabetes is strongly associated with most other risk factors, including coronary disease, renal disease, and cerebral vascular disease. It is interesting to note that, although insulin therapy was independently associated with cardiac morbidity in the derivation set of the RCRI, it was not significant in the validation set. Further, when both the derivation and validation sets were combined, insulin therapy achieved marginal statistical significance. Several observational trials have suggested that elevated levels of glycosylated hemoglobin (HbgA1c) are related to perioperative cardiovascular outcomes.48,49 Certainly, an HbgA1c higher than 6.7% has been shown to be associated with increased morbidity after cardiac surgery and percutaneous coronary interventions.50 However, no reliable prospective data in vascular surgery are available to suggest that elevated HbgA1c improves diagnostic accuracy over a history of diabetes alone. Clearly, this is an area in which further research is needed. Tight glucose control in the perioperative period, the focus of several critical care randomized trials, failed to show any improvement in outcomes.51
Preoperative Biomarkers
Increased preoperative levels of plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) and B-type natriuretic peptide (BNP) have been associated with increased postoperative adverse outcomes. Rodseth et al have completed a meta-analysis of six prospective observational trials of 850 vascular surgical patients.52 Using a composite outcome of cardiovascular death and nonfatal myocardial infarction, this meta-analysis shows that a BNP below 30 pg/mL has a low likelihood ratio of 0.11 for a postoperative cardiac event. For levels between 30 and 116 pg/mL, the positive likelihood ratio was 3.6. For levels above 116 pg/mL, the positive likelihood ratio was found to be 6.4. Although these results need confirmation in larger prospective trials, in the interim, preoperative screening with BNP would seem to significantly improve the performance of the RCRI in vascular surgery.
The advent of high-sensitivity (third- and fourth-generation) troponin assays has led to the awareness that patients with many cardiovascular anomalies have elevated troponin levels. Recently, one study has evaluated preoperative troponin levels as a prognostic indicator. In this multicentered observational study assessing RCRI, BNP, and high-sensitivity troponin, preoperative troponin above the 99th percentile for a healthy population was associated with a fivefold increase in mortality. In this study preoperative troponin outperformed both RCRI and BNP as a prognostic indicator. These data suggest that low-level troponin elevation existing before surgery represents preexisting cardiac abnormality or dysfunction. These changes increase the likelihood of postoperative cardiac complications. As of yet, no definitive determination has been made of the best biomarker or of the levels that signify increases complications. Several investigations are currently underway, which should give a clearer idea of the utility of preoperative biomarkers.
Resting Electrocardiogram
The resting ECG is an elementary and mandatory tool for screening patients with suspected cardiac disease. ST-segment changes, either ST-elevation or ST-depression, should always be suspected for acute or recent ischemia and must be confronted with symptoms and compared with previous ECGs.
Most other ECG changes represent chronic patterns of injury or conduction abnormalities. Pathologic Q waves represent old MIs; the more ECG leads with pathologic Q waves, the wider the territory of infracted myocardium. Tall R waves and wide QRS complexes, with or without ST-T changes, and inverted T waves represent myocardial hypertrophy or dilatation. ECG also detects and helps define abnormal rates and rhythms. These chronic pathologic ECG changes are associated with increased risk for perioperative events.37 In patients undergoing noncardiac surgery, Van Klei et al found that although preoperative bundle branch blocks, Q waves, and ST-T changes were significant predictors of postoperative cardiac events, these ECG abnormalities did not remain predictive in a multivariable logistic regression that included the six independent risk factors of the revised cardiac risk index.16
ECG changes are common in vascular surgery patients. A study in these patients showed that 33% had pathologic Q waves, 22% had baseline ST-segment changes, 38% had T wave inversions, and 16% had voltage criteria for left ventricular hypertrophy (LVH). Patients with pathologic Q waves or LVH are more likely to have perioperative ischemia or infarction.53
Chest Radiography
The chest radiograph is also an essential qualitative tool in screening cardiac patients, and its main value is to supplement the history and physical examination in identifying and assessing the severity of cardiac failure. Signs of cardiac failure on chest radiograph, recognizable by abnormal cardiac size and increased pulmonary vasculature, should trigger further examination to gain better understanding of the causes of cardiac dysfunction.
Echocardiography
Routine preoperative echocardiography is not recommended and must be justified by a specific clinical question arising from the patient’s medical history, physical examination, or chest radiograph. Echocardiography is justified before major surgery to verify or evaluate significant valvular disease, in particular aortic stenosis. Severe aortic valvular stenosis is common in elderly patients and has been long shown to correlate with perioperative complications and death. Severe aortic stenosis is also a relative contraindication for regional anesthesia. Significant mitral valvular disease predisposes the patient to congestive failure in the perioperative period, and its recognition before surgery may alter the approach to the patient and the perioperative clinical management.
Other important indications for preoperative echocardiography are to evaluate significantly reduced left and/or right ventricular functions, evaluation of cardiomyopathies (in particular, hypertrophic obstructive cardiomyopathy), and the presence and severity of pulmonary hypertension.
Resting echocardiography is not a reliable method to evaluate the severity of CAD and the risk of perioperative MI.
Ambulatory ST-Segment Monitoring
Silent myocardial ischemia is defined as episodes of ST-segment depression on ambulatory ECG with ST-segment trend analysis that are not accompanied by chest pain. Silent MI is a well-known phenomenon in patients with CAD and has been shown to predict worse prognosis. Similarly, the detection of silent preoperative ischemia on preoperative Holter monitoring has been associated with increased postoperative morbidity. Raby et al used Holter monitoring for 24 hours before vascular surgery and found that preoperative ischemia predicted postoperative cardiac complications with a sensitivity of 75% and specificity of 83%.54 In contrast, however, meta-analysis of eight studies of more than 800 patients found a cumulative sensitivity of 52% and a specificity of 70%.55 One major reason for this relative insensitivity is thought to be related to the fact that as many as 30% of vascular patients are taking a digitalis-like compound or have preexisting ST-T changes, bundle branch block, or pacemakers, all of which make the interpretation of ST segments difficult. In addition, Holter monitoring uses only two, rarely three modified ECG leads and is susceptible to motion and other artifacts during normal daily activity.
Concept of Preoperative Cardiac Investigations
Although coronary angiography is the gold standard for determining the existence and severity of coronary artery disease, its invasiveness and cost make it unfeasible for preoperative screening. Coronary angiography provides information on the major coronary anatomy but less so on the downstream minor vessels or on functional consequences of that anatomy. Nevertheless, Hertzer et al1 performed routine coronary angiographies on all patients scheduled for major vascular surgery in the early 1980s, and patients with significant left main and triple vessel disease were treated with CABG.
In a retrospective analysis of these 1522 patients, Ellis and Hertzer56 found that 81% of the patients with postoperative MI or cardiac death had at least one total coronary artery occlusion with collaterals, compared with 29% in match-controlled patients without cardiac complications. In every other respect, patients with postoperative complications also had severe CAD before surgery as compared with the control group. Based on this observation, it is possible to conclude that postoperative cardiac complications most likely occur in patients with preoperative long-standing severe CAD. This is also consistent with the previously described observations that postoperative MI and cardiac complications are the result of prolonged stress-induced ischemia in the presence of severe yet stable CAD. Therefore identifying patients with severe and often clinically silent coronary disease should be the focus of our investigations.
Preoperative Noninvasive (Stress) Testing
The ACC/AHA guidelines suggest that patients who report limited functional capacity (i.e., inability to climb at least two flights of stairs without chest pain or shortness of breath, the equivalent of four metabolic equivalents) are candidates for noninvasive cardiac stress testing before major noncardiac surgery. Limited functional capacity is indeed a marker of higher perioperative risk, and although the limited exercise tolerance may be due to numerous causes (cardiac, pulmonary, orthopedic, neurologic, or peripheral vascular factors; obesity; older age; poor physical fitness), the noninvasive cardiac testing can help sort out the cardiac from other causes. The ACC/AHA guidelines recommend preoperative cardiac exercise or pharmacologic stress testing to patients (1) with an intermediate pretest probability of CAD, (2) undergoing initial evaluation for suspected or proven CAD, (3) with a significant change in clinical status, (4) with demonstration or proof of myocardial ischemia before coronary revascularization, (5) upon evaluation of the adequacy of medical treatment, and (6) upon prognostic assessment after an acute coronary syndrome.43
Exercise Stress Testing
The most common physiologic stress test for detecting myocardial ischemia uses a treadmill or cycle ergometer. Among its advantages, this test provides an estimate of functional capacity and hemodynamic response and detects myocardial ischemia through ST-segment changes. The accuracy of an exercise ECG varies widely among studies. A meta-analysis by Kertai et al for the detection of myocardial ischemia with treadmill testing in vascular surgery patients showed a rather low sensitivity (74%) and specificity (69%).55 The extremely low effort capacity in some vascular surgical patients limits the utility of this test and often leads to the use of pharmacologic stress testing.
Myocardial Perfusion Imaging
Single-photon emission computed tomography (SPECT) with thallium-201 or technetium-based agents has been available since the 1970s and is still the most widely used noninvasive test. The test can be done with physiologic stress (treadmill/cycle ergometer) or pharmacologic stress (dipyridamole). When the cardiac images acquired in a resting state are compared with the stressed state, the investigation is able to differentiate fixed from reversible filling defects in myocardial tracer uptake. These differences are suggestive of areas of infarct versus ischemia.
A recent meta-analysis of 114 articles showed that the sensitivity and specificity of SPECT for detecting CAD with ≥50% stenosis are 88% and 61%, respectively.56 SPECT has a time-honored and important value in predicting long-term risk in patients with suspected CAD. A meta-analysis of 14 articles on 12,000 patients showed that negative and positive stress SPECT are associated with 0.6% and 7.4% annualized rate of hard events (nonfatal MI or death), respectively.57 SPECT can also provide calculation of left ventricular ejection fraction during stress and rest. Transient left ventricular dilatation and increased lung uptake during stress are additional parameters used to evaluate higher cardiac risk. A meta-analysis by Etchells et al investigated the prognostic value of semiquantitative dipyridamole MPI for determining perioperative cardiac risk in patients undergoing vascular surgery58 and found that reversible ischemia in fewer than 20% of the myocardial segments did not change the likelihood of perioperative complications, whereas greater extents of reversibility of myocardial perfusion defects were associated with increased risk of perioperative cardiac complications. In contrast, however, a normal SPECT scan may occur in up to 15% of patients (false negative rate) with left main disease on account of balanced ischemia in multivessel disease. False positive tests are also common in obese patients as a result of elevated diaphragm or breast artifacts.
Positron Emission Tomography
Positron emission tomography (PET) is a newer, more expensive modality that is not widely available. PET includes both perfusion (by Ru-82, N-13 ammonia, or O-15 water) and functional metabolic (F-18 fluorodeoxyglucose, or FDG) imaging. Mismatch between perfusion and metabolism suggests viable ischemic area, whereas matched reduction in both blood flow and metabolism suggests an area of infarct. A meta-analysis of 19 studies showed that PET had a sensitivity of 92% and specificity of 85% for diagnosing significant CAD.59
Cardiac MRI
Cardiac MRI (CMR) is another new technology tool that may become the standard preoperative screening test in the future. Unlike SPECT and PET, CMR has excellent spatial resolution and can provide accurate information on transient (ischemia) or fixed (infarction) hypoperfusion and scar tissue, even in small areas of the subendocardium. Myocardial perfusion is imaged during the first pass of a bolus of gadolinium during stress (achieved by adenosine or dipyridamole), rest, and late gadolinium enhancement. In a meta-analysis, CMR was shown to have better sensitivity (89%) and specificity (76%) than SPECT for detecting significant CAD.1 Patients with contraindications to the CMR may be the key limitations to this test. These include the high magnetic field in patients with implanted metal, patients with hypersensitivity to gadolinium, patients with renal insufficiency, and patients with severe asthma or chronic obstructive pulmonary disease (who may have contraindications to vasodilatating agents, adenosine, and dipyridamole).
Dobutamine Stress Echocardiography
Dobutamine stress echocardiography (DSE) is a relatively low-cost, widely available test in which stress is induced by increasing doses of dobutamine. This test assesses new or worsening global and regional myocardial wall motion abnormalities in patients. Meta-analysis of 62 studies showed that the sensitivity of DSE is 83% in patients without prior MI but decreases to 74% when patients with prior MI were included. The specificity of DSE for detecting significant CAD is also approximately 80%. The long-term prognosis of a negative DSE test is 0.5% to 0.8% annualized risk of death or MI. A recent meta-analysis by Beattie et al compared the value of DSE versus MPI in predicting postoperative MI or in-hospital death.60 This report included 25 studies (3373 patients) of mainly DSE, as well as 50 MPI studies with marked variability in results among the studies. The likelihood ratio for positive test was greater for DSE than for MPI, 4.1 versus 1.8, respectively, and the area under the ROC curve for the tests was 0.8 and 0.75, respectively.
Computed Tomography Coronary Angiography
Computed tomography coronary angiography (CTCA) is a novel noninvasive method for evaluating coronary anatomy, as well as myocardial function. Studies show that CTCA has a high diagnostic accuracy for detecting the presence of coronary artery stenosis. A meta-analysis of 27 studies showed that sensitivity and specificity of PPV and NPV were 97%, 91%, 93%, and 96% compared with conventional coronary angiography.61 A more recent study showed that although the sensitivity of CTCA remains strong, the specificity drops significantly down to 66% in patients with coronary calcifications. Recently, a prospective study in 290 consecutive patients undergoing CTCA, using a 64-row detector, found that a higher RCRI, a high coronary artery calcium score, the presence of significant coronary artery stenosis, and multivessel coronary artery disease were significantly associated with postoperative cardiovascular events. However, 7 of the 18 postoperative cardiac events defined as cardiac death, acute coronary syndrome, pulmonary edema, ventricular fibrillation, ventricular tachycardia with hemodynamic compromise, and complete heart block within 30 days after surgery occurred in patients with normal or “nonsignificant coronary disease.”62 The promise of this technology may be realized with higher resolution scans.
Preoperative Coronary Angiography and Revascularization
The detection of significant CAD by noninvasive testing is only the first step in investigating cardiac patients undergoing vascular surgery. The decision to proceed to coronary angiography based on the noninvasive testing includes an assessment of whether benefits of an intervention (prophylactic preoperative coronary revascularization or medical therapy) outweigh the risks associated with the intervention and delaying surgery. These decisions continue to represent a major therapeutic dilemma.
Until a decade ago, several retrospective studies suggested that preoperative coronary revascularization in vascular surgery patients was associated with better postoperative and long-term survival.23,63 However, these studies suffered from the limitations of retrospective studies, among which was the failure to include all patients who died or had serious complications from the preoperative revascularization and therefore could not undergo the expected vascular surgery. In 2004, McFalls et al published the randomized coronary artery prophylaxis (CARP)3 trial, in which 510 candidates for major vascular surgery (i.e., after screening, they had coronary angiography showing one or more major coronary vessels with ≥70% stenosis) were randomized to either coronary revascularization (by PCI or CABG) before vascular surgery or no coronary intervention before surgery. Patients were followed for a mean period of 2.5 years after surgery. There was no difference in survival between the groups. This trial had two important limitations. First, the screening process did not rigorously follow the ACC/AHA guidelines, and only a minority of the patients had evidence of severe ischemia on noninvasive testing. The study was underpowered to assess this key aspect. Second, patients with left main coronary artery disease or severely ill patients with reduced left ventricular ejection fraction were excluded.
This trial has had a strong impact worldwide on perioperative patient management by shifting the paradigm toward fewer coronary investigations and interventions before major noncardiac and vascular surgery. This trial coincided with the COURAGE trial,64 which showed that PCI does not confer better survival than optimal medical treatment in patients with stable CAD, creating evidence that prophylactic revascularization was unwarranted in vascular surgery.
It is important to note, however, that subsequent publications from the CARP trial and registry reported that (1) patients who had CABG with more complete coronary revascularization had better survival than those revascularized by PCI,65 (2) patients with left main disease, excluded from the trial, had markedly better survival with coronary revascularization than without it,66 and (3) in a subset of 109 patients with ischemia on preoperative MPI, patients who were randomized to preoperative coronary revascularization had significantly better long-term survival free of MI than patients without revascularization. In particularly, anterior wall ischemia was associated with increased mortality if not revascularized.67
We believe this evidence supports the notion that preoperative coronary revascularization before major vascular surgery in selected high-risk patients is still appropriate.
In 2009 Monaco et al4 randomized 208 consecutive patients scheduled for elective abdominal aortic surgery, who had RCRI of ≥2 to either a “selective strategy” in which coronary angiography was conducted if the stress test was positive or to a “systematic strategy” in whom coronary angiography was performed to all 105 patients. The systematic strategy discovered 50% more patients with significant CAD than the selective strategy, had subsequently more coronary revascularizations (58% versus 40%, respectively) and was associated with significantly better long-term survival and freedom from major adverse cardiac events. Although this small study again supports the concept of prophylactic preoperative coronary angiography and revascularization to patients at high clinical risk (RCRI ≥ 2), the routine use coronary angiography without prior noninvasive screening deserves further investigation.
The latest ACC/AHA update on evaluation and care for noncardiac surgery (2009) recommends:
4. Coronary revascularization is useful in patients with high-risk unstable angina or NSTEMI.
5. Coronary revascularization is useful in patients with STEMI.
All of these criteria are given level-of-evidence grade A. Although we agree with these criteria, the problem of identifying these high-risk patients remains. To date, the only strategy that has been shown to accurately identify significant coronary anatomy in these high-risk patients is preoperative coronary angiography. These points are outlined in the section of preoperative workup.
Medical Prophylaxis
Beta Blockade
As many as 25% of patients assessed for vascular surgery are taking a beta blocker. Beta blockade is indicated for the treatment of various comorbidities, including benign essential tremor, hypertension, coronary artery disease (both stable angina and after acute MI), and congestive heart failure. Studies in stable angina show that beta blockers provide excellent symptomatic improvement. Patients who are maintained on beta blockers after MI show moderately improved long-term survival. In patients with congestive failure beta blockers also improve long-term survival.
Nevertheless, a growing body of evidence shows that chronic beta blockade may also be harmful. A meta-analysis comparing beta blockade to other forms of antihypertensive therapies (ACE inhibitors, calcium channel blockers) suggests that beta blockers are associated with an increase in cerebral vascular accidents.68,69 A recent large, prospective observational trial has shown again that although chronically administered beta blockers, given to patients with known prior myocardial infarction, are associated with improved long-term survival70 in patients with known coronary disease but without MI, there was no discernible benefit after 4 years of follow-up. Finally, in patients with no coronary disease, beta blockers were associated with an 18% (95% CI 2%-36%) increase in morbidity after 4 years compared with all other forms of therapy.
Perioperative myocardial infarction is predominately NSTEMI. Postoperative MI is preceded by prolonged ST-depression ischemia, and notably, the ischemia itself is associated with a significant increase in heart rate. These findings gave impetus to the theory that perioperative beta blockade would reduce perioperative MI and subsequently lead to numerous clinical trials. In a high-quality meta-analysis of 33 trials involving more 12,000 patients, Bangalore et al demonstrated that perioperative beta blockade was indeed associated with a 35% (95% CI 54%-79%) reduction of nonfatal MI.71 This was achieved, however, at the expense of a doubling in the stroke rate (NNH, 293). The meta-analysis could not show a significant effect on all cause mortality.
The largest and highest-quality trial (POISE) in the meta-analysis shows that the beneficial effect on nonfatal MI comes at the expense of increased stroke incidence and a 30% increase in all-cause mortality. The POISE trial also showed that mortality was significantly higher in patients receiving beta blockade if they developed serious infection or sepsis postoperatively. Most of the trials in the meta-analysis have several features in common: first, these trials usually administered metoprolol; second, the beta blockers were generally started on the day of surgery; and third, patients were given a fixed dose of drug. On the basis of no improvement in mortality and a doubling in postoperative strokes, prophylactic acute (day of surgery) perioperative beta blockade cannot be recommended.
The obvious question, then, becomes why, if the most frequent morbid event and the leading cause of postoperative mortality is reduced by beta blockade, can this therapy ultimately lead to harm? There are numerous possible explanations. Lindenauer, in an analysis of the Premier Prospective database, showed that patients with no risk factors had a 30% increase in mortality.72 In the same analysis, beta blockade was associated with reduced postoperative mortality only in patients with RCRI of ≥2. Redelmeier et al showed, in a cohort of Medicare patients over the age of 65, that atenolol, as compared with metoprolol, was associated with less postoperative mortality.73 The theory advanced by these authors suggests that metoprolol results in more morbid events because metoprolol has a shorter half-life and therefore patients are being withdrawn from the drug. This explanation loses some credibility however, when one considers that the two largest beta blocker trials, both finding an increase in mortality, used metoprolol succinate, a long-acting formulation. Other observational studies, have shown that chronic dosing is associated with superior outcomes compared with the acute beta blockade.74,75 Chronic dosing may be superior since it allows for (1) elimination of those patients who cannot tolerate the drug and (2) controlled titration of the dose.
Finally, two independent investigations have now shown that major blood loss (or acute anemia) is associated with increased morbidity in beta blocked patients.46,76 Hence the increased mortality and stroke rate caused by prophylactic beta blockade, despite the decrease in nonfatal MI as suggested by the larger studies, is most likely related to the potential detrimental effects of beta blockade (i.e., slowing heart rate and decreasing myocardial contractility in patients with postoperative anemia or infection who are at the greatest need for tachycardia and high cardiac output after surgery).
Statins
A recent meta-analysis in patients at risk of cardiovascular events, combining 27 prospective trials and more than 174,000 patients, has shown that for each 1 mmol decrease in low-density lipoprotein (LDL), stains reduced the long-term relative risk of a cardiovascular event 24% (95% CI 21%-27%).77 This reduction was seen across all risk populations and reduced the need for revascularization, along with the incidence of stroke and other vascular events. Furthermore, these benefits far exceed the risks of elevated liver enzyme, myopathy, or rhabdomyolysis. This study suggests that all vascular patients, who by definition have atherosclerosis, should receive statins if tolerated, which should be continued long term for management of their cardiovascular disease, irrespective of the need for surgery. Large observational trials have convincingly shown that perioperative statins are safe.78 Taken together it is our opinion that there is no need to conduct a statin trial, all vascular surgical patients should be taking lipid-lowering medication chronically. In the spirit of providing “excellent medical care,” the number of patients taking stains should be increased.
Aspirin
Although primary prevention of cardiovascular events is controversial,79 the utility of aspirin in secondary prevention of cardiovascular events is well established.80 The benefits of ASA in terms of reduction of reinfarction, stroke, and ischemia far outweigh the increased risks of bleeding. The Anti-Thrombotic Trialist’s Collaboration conducted a meta-analysis of 287 trials in more than 130,000 patients compared with a control group; myocardial infarction was reduced by one third, nonfatal stroke by one quarter, and vascular death by one sixth. The absolute reduction in cardiovascular morbidity in high-risk patients was 22 per 1000 patients after 2 years of therapy.80 These results confirm that patients at risk of recurrent vascular morbidity should be treated with ASA.
We are aware of only one RCT evaluating antiplatelet therapy in vascular surgery. The ACE (Aspirin in Carotid Endarterectomy) trial compared four doses of aspirin in patients having elective carotid endarterectomy.81 The trial was conducted in response to the NASCET trial, which suggested that aspirin reduced the perioperative stroke rate. Unfortunately, the ACE trial did not have a placebo control, but low-dose ASA (75-360 mg) was superior to higher doses. In an efficacy evaluation of patients who were not on prior ASA or NSAIDS, the efficacy of the low-dose regimen was more than twice that of patients who had prior ASA.
The largest perioperative trial to date is the Pulmonary Embolism Prevention trial (PEP), which randomized more than 12,000 patients with either fractured hips or elective arthroplasty and undefined cardiac risk to a daily dose of 160 mg of ASA.82 The primary outcome was death after 35 days. Aspirin was associated with an absolute reduction of 9 pulmonary embolisms per 1000 patients, a reduction of 36%. Myocardial infarctions were increased (hazard ratio 1.23; 95% CI 0.87-1.75). The need for transfusion was increased by an absolute value of 1 per 1000 patients.
Despite the clear advantages of secondary prevention, the effectiveness of low-dose ASA in CEA, and excellent evidence of no excessive bleeding, low dose ASA continues to be withdrawn before surgery. A meta-analysis of retrospective studies, conducted by Burger et al, assessed 41 studies in almost 15000 patients.83 This analysis showed that aspirin withdrawal precedes 10% of acute coronary syndromes, and MI occurred an average of 7 days after cessation. The major reason for withdrawal was an upcoming elective surgical procedure. The authors concluded that ASA should be maintained in all patients except for those who had the highest risk of bleeding. There are no prospective studies assessing withdrawal of ASA in any surgical population.
The continuation of aspirin is particularly relevant in patients with intracoronary stents. A recent prospective observational study in over 1000 patients with PCI shows that complete interruption of antiplatelet agents is associated with a twofold increased rate of major acute coronary events.84 In a smaller multicentered prospective observational study, Vincenzi et al found that the patients who received unfractionated heparin were more likely to sustain cardiac morbidity.85 Webster had previously shown that unfractionated heparin, given intraoperatively to vascular surgery patients, induced an acute resistance syndrome.86 Aspirin resistance is common in high-risk cardiac patients, and this resistance is associated with increased morbidity. A meta-analysis of 20 studies in 2900 patients showed that ASA resistance occurred in over 27% of patients. In this population, in which all patients were chronically taking ASA, documented resistance, by any standard testing method, resulted in increased cardiovascular morbidity (OR 3.8, 95% CI 3.2-4.8).87 It is unclear at this time if ASA resistance is amenable to treatment.
Antiplatelet Therapy after Coronary Stenting
More than 1 million PCI procedures are conducted annually in North America88 alone, and more than 5% of these patients will undergo elective surgery within 1 year.88,89 These patients, who have been recently revascularized using PCI and stents, are treated initially with dual-antiplatelet therapy for a variable period of time, usually aspirin and clopidogrel, to prevent stent thrombosis.
These patients require special consideration. Kaluza et al90 drew attention to the increased mortality in patients having elective surgical procedures shortly after PCI. Mortality was related either to excessive bleeding due to continuation of the dual-antiplatelet therapy through surgery or to stent thrombosis after the abrupt discontinuation of the antiplatelet medications. This case series was then quickly followed by a myriad of other case series showing similar increased morbidity when procedures were carried out within 90 days of PCI.91,92 A retrospective analysis of 8116 stent patients from large a comprehensive stent registry, linked to a Medicare administrative database, confirmed that surgical procedures conducted within 45 days of a PCI are associated with very high mortality.93 Patients with drug eluting stents were at especially high risk of cardiac morbidity, which was found to be greater than 20%. However, after 45 days the risk was similar to patients with an RCRI of equal to or greater than 2 for both patients with bare metal or drug eluting stents. After 180 days, the risk of major cardiac morbidity is approximately 1% (Fig. 39- 2). It is strongly recommended that these patients have one antiplatelet therapy continued throughout the perioperative period; normally, the aspirin is continued and clopidogrel is restarted as soon as possible after surgery.84 These patients also require increased surveillance for an adverse cardiac event after surgery.
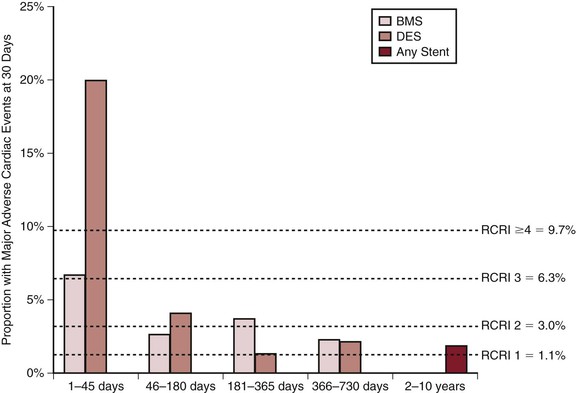
Figure 39-2 Proportion of patients with major adverse cardiac events with 30 days of elective surgery based on the interval after most recent stent insertion and subsequent surgery. The horizontal dashed lines represent the event rates in individuals without stent insertion stratified by the RCRI. (From Circulation; copyright Wolters Kluwer.)
Treatment
STEMI is a medical emergency, irrespective of the setting, and the more so if it occurs after surgery. The indications for therapy are described in ACC guidelines,94 and a cardiologist should be consulted. Immediate reperfusion strategies, with percutaneous transluminal coronary angioplasty with stenting, should be considered.
In general, for patients with NSTEMI, medical therapy to relieve ischemia may be sufficient. Whereas aggressive anticoagulation or antiplatelet therapy is a standard,95 this can be problematic postoperatively, and a delicate balance between bleeding and thrombotic complications must be found. Figure 39-3 outlines an algorithm for managing patients with suspected postoperative ischemia, troponin elevation, or MI. It is notable that although managing patients with myocardial ischemia and normal or high blood pressure is relatively easy, management of patients with ischemia in face of hypotension and hypoperfusion is more complicated and requires the assistance of an intensive care unit, additional monitoring, and imaging devices and consultation with the anesthesiologists, intensivists, and cardiologists. Continued longer-term follow-up should be ensured after discharge from hospital.
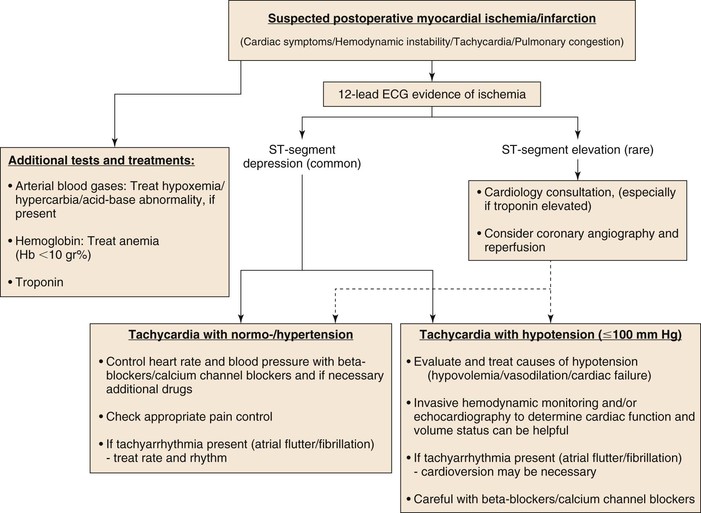
Figure 39-3 Suggested perioperative MI treatment algorithm.
Selected Key References
Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet. 2008;372:1962–1976.
The definitive work to date regarding the cardioprotective effects of beta adrenergic antagonists..
Beattie WS, Karkouti K, Tait G, Steel A, Yip P, McCluskey S, Farkouh M, Wijeysundera DN. Use of clinically based troponin underestimates the cardiac injury in non-cardiac surgery: a single centre cohort study in 51,701 consecutive patients. Can J Anesth. 2012.
Devereaux PJ, Chan MT, onso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati BG, Botto F, Guyatt G, Heels-Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ, VanHelder T, Bhandari M, Bosch J, Kurz A, Polanczyk C, Malaga G, Nagele P, Le MY, Leuwer M, Yusuf S. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304.
Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26–35.
Monaco M, Stassano P, Di TL, Pepino P, Giordano A, Pinna GB, Iannelli G, Ambrosio G. Systematic strategy of prophylactic coronary angiography improves long-term outcome after major vascular surgery in medium- to high-risk patients: a prospective, randomized study. J Am Coll Cardiol. 2009;54:989–996.
Rodseth RN, Lurati Buse GA, Bolliger D, Burkhart CS, Cuthbertson BH, Gibson SC, Mahla E, Leibowitz DW, Biccard BM. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol. 2011;58:522–529.
Wijeysundera DN, Wijeysundera HC, Yun L, Wasowicz M, Beattie WS, Velianou JL, Ko DT. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation. 2012;126:1355–1362.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Hertzer NR. Clinical experience with preoperative coronary angiography. J Vasc Surg. 1985;2:510–514.
2. Roger VL, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197.
3. McFalls EO, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804.
4. Monaco M, et al. Systematic strategy of prophylactic coronary angiography improves long-term outcome after major vascular surgery in medium- to high-risk patients: a prospective, randomized study. J Am Coll Cardiol. 2009;54:989–996.
5. Thygesen K, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598.
6. Devereaux PJ, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154:523–528.
7. Levy M, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2011;114:796–806.
8. Marston N, et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long-term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27:66–72.
9. Beattie WS, et al. Use of clinically based troponin underestimates the cardiac injury in non-cardiac surgery: a single centre cohort study in 51,701 consecutive patients. Can J Anesth. 2012.
10. van Waes JA, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013.
11. Carson JL, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462.
12. Landesberg G, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–1554.
13. Devereaux PJ, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304.
14. Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth. 2003;17:90–100.
15. Devereaux PJ, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847.
16. van Klei WA, et al. The value of routine preoperative electrocardiography in predicting myocardial infarction after noncardiac surgery. Ann Surg. 2007;246:165–170.
17. Landesberg G. Monitoring for myocardial ischemia. Best Pract Res Clin Anaesthesiol. 2005;19:77–95.
18. Landesberg G, et al. A clinical survival score predicts the likelihood to benefit from preoperative thallium scanning and coronary revascularization before major vascular surgery. Eur Heart J. 2007;28:533–539.
19. Labovitz AJ, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23:1225–1230.
20. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153–184.
21. Mangano DT, et al. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–1788.
22. Landesberg G, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet. 1993;341:715–719.
23. Landesberg G, et al. Preoperative thallium scanning, selective coronary revascularization, and long-term survival after major vascular surgery. Circulation. 2003;108:177–183.
24. London MJ, et al. Intraoperative myocardial ischemia: localization by continuous 12-lead electrocardiography. Anesthesiology. 1988;69:232–241.
25. Dawood MM, et al. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol. 1996;57:37–44.
26. Cohen MC, et al. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol. 1999;8:133–139.
27. Bertges DJ, et al. The Vascular Surgery Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately that the Revised Cardiac risk Index in vascular surgical patients. J Vasc Surg. 2010;52:674–683.
28. Greenhalgh RM, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843–848.
29. Prinssen M, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618.
30. Lederle FA, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535–1542.
31. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2187–2192.
32. Stoner MC, et al. Defining the high-risk patient for carotid endarterectomy: an analysis of the prospective National Surgical Quality Improvement Program database. J Vasc Surg. 2006;43:285–295.
33. LaMuraglia GM, et al. Significant perioperative morbidity accompanies contemporary infrainguinal bypass surgery: an NSQIP report. J Vasc Surg. 2009;50:299–304.
34. Ederle J, et al. Percutaneous transluminal angioplasty and stenting for carotid stenosis: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81:477–478.
35. Bonati LH, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–1073.
36. Brott TG, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23.
37. Lee TH, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
38. Shaw LJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291.
39. Goldman L, et al. Cardiac risk factors and complications in non-cardiac surgery. Medicine (Baltimore). 1978;57:357–370.
40. Detsky AS, et al. Predicting cardiac complications in patients undergoing non-cardiac surgery. J Gen Intern Med. 1986;1:211–219.
41. L’Italien GJ, et al. Development and validation of a Bayesian model for perioperative cardiac risk assessment in a cohort of 1,081 vascular surgical candidates. J Am Coll Cardiol. 1996;27:779–786.
42. Samy AK, et al. Prospective evaluation of the Glasgow Aneurysm Score. J R Coll Surg Edinb. 1996;41:105–107.
43. Fleisher LA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13–e118.
44. Ford MK, et al. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26–35.
45. Davis C, et al. The revised Cardiac Risk index in the new Millenium: a single centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Aneshtesia. 2013 [DOI 10.1007/s12630-013-9988-5] .
47. Wijeysundera DN, et al. Evaluating surrogate measures of renal dysfunction after cardiac surgery. Anesth Analg. 2003;96:1265–1273.
48. O’Sullivan CJ, et al. Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg. 2006;32:188–197.
49. Marchant MH Jr, et al. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91:1621–1629.
50. Szekely A, et al. Impact of hyperglycemia on perioperative mortality after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2011;142:430–743.
51. Griesdale DE, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827.
52. Rodseth RN, et al. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol. 2011;58:522–529.
53. Landesberg G, et al. Perioperative myocardial ischemia and infarction: identification by continuous 12-lead electrocardiogram with online ST-segment monitoring. Anesthesiology. 2002;96:264–270.
54. Raby KE, et al. Usefulness of Holter monitoring for detecting myocardial ischemia in patients with nondiagnostic exercise treadmill test. Am J Cardiol. 1993;72:889–893.
55. Kertai MD, et al. A meta-analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery. Heart. 2003;89:1327–1334.
56. Jaarsma C, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59:1719–1728.
57. Iskander S, et al. Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol. 1998;32:57–62.
58. Etchells E, et al. Semiquantitative dipyridamole myocardial stress perfusion imaging for cardiac risk assessment before noncardiac vascular surgery: a meta-analysis. J Vasc Surg. 2002;36:534–540.
59. Nandalur KR, et al. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol. 2008;15:444–451.
60. Beattie WS, et al. A meta-analytic comparison of preoperative stress echocardiography and nuclear scintigraphy imaging. Anesth Analg. 2006;102:8–16.
61. Abdulla J, et al. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28:3042–3050.
62. Ahn JH, et al. Risk stratification using computed tomography coronary angiography in patients undergoing intermediate-risk noncardiac surgery. J Am Coll Cardiol. 2013;61:661–668.
63. Eagle KA, et al. Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study. Circulation. 1997;96:1882–1887.
64. Boden WE, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516.
65. Ward HB, et al. Coronary artery bypass grafting is superior to percutaneous coronary intervention in prevention of perioperative myocardial infarctions during subsequent vascular surgery. Ann Thorac Surg. 2006;82:795–800.
66. Garcia S, et al. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am J Cardiol. 2008;102:809–813.
67. Garcia S, et al. Preoperative coronary artery revascularization and long-term outcomes following abdominal aortic vascular surgery in patients with abnormal myocardial perfusion scans: a subgroup analysis of the coronary artery revascularization prophylaxis trial. Catheter Cardiovasc Interv. 2011;77:134–141.
68. Webb AJ, et al. Effects of beta-blocker selectivity on blood pressure variability and stroke: a systematic review. Neurology. 2011;77:731–737.
69. Webb AJ, et al. The effect of antihypertensive treatment on headache and blood pressure variability in randomized controlled trials: a systematic review. J Neurol. 2012;259:1781–1787.
70. Bangalore S, et al. Beta-blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349.
71. Bangalore S, et al. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet. 2008;372:1962–1976.
72. Lindenauer PK, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361.
73. Redelmeier D, et al. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ. 2005;331:932.
74. Ellenberger C, et al. Chronic beta blockade is associated with a better outcome after elective noncardiac surgery than acute beta blockade: a single-center propensity-matched cohort study. Anesthesiology. 2011;114:817–823.
75. Wallace AW, et al. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology. 2011;114:824–836.
76. Le MY, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology. 2012;117:1203–1211.
77. Mihaylova B, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590.
78. Lindenauer PK, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291:2092–2099.
79. Berger JS, et al. Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: a meta-analysis of randomized trials. Am Heart J. 2011;162:115–124.
80. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
81. Taylor DW, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet. 1999;353:2179–2184.
82. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355:1295–1302.
83. Burger W, et al. Low-dose aspirin for secondary cardiovascular prevention—cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation—review and meta-analysis. J Intern Med. 2005;257:399–414.
84. Albaladejo P, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart. 2011;97:1566–1572.
85. Vicenzi MN, et al. Coronary artery stenting and non-cardiac surgery–a prospective outcome study. Br J Anaesth. 2006;96:686–693.
86. Webster SE, et al. Anti-platelet effect of aspirin is substantially reduced after administration of heparin during carotid endarterectomy. J Vasc Surg. 2004;40:463–468.
87. Krasopoulos G, et al. Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336:195–198.
88. Gurevich Y, et al. Health indicators 2009: a focus on cardiac care in Canada. Healthcare Q. 2010;13:18–21.
89. Berger PB, et al. Frequency of major noncardiac surgery and subsequent adverse events in the year after drug-eluting stent placement results from the EVENT (Evaluation of Drug-Eluting Stents and Ischemic Events) Registry. JACC Cardiovasc Interv. 2010;3:920–927.
91. Wilson SH, et al. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42:234–240.
92. Nuttall GA, et al. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology. 2008;109:588–595.
93. Wijeysundera DN, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation. 2012;126:1355–1362.
94. Antman EM, et al. 2007 focused update of the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329.
95. Anderson JL, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579.