Systemic Analgesia and Sedation for Procedures
Procedural sedation and analgesia (PSA) refers to the use of analgesic, dissociative, and sedative agents to relieve the pain and anxiety associated with diagnostic and therapeutic procedures performed in various settings. PSA is an integral element of emergency medicine residency and pediatric emergency medicine fellowship training and curricula, and graduates of these programs are skilled in the practice of PSA. Emergency clinicians are skilled in resuscitation, vascular access, and advanced airway management, which permits them to effectively recognize and manage the potential complications associated with PSA.1
In a recent study of all practitioners, the most common clinical errors associated with PSA were delayed recognition of respiratory depression and arrest, inadequate monitoring, and inadequate resuscitation,2 mistakes that are unlikely to be made by emergency clinicians. The safety of PSA techniques by emergency clinicians has been well documented in numerous series in both children and adults.3–7 Safe and successful application of PSA requires careful patient selection, customization of therapy to the specific needs of the patient, and careful monitoring of patients for adverse events. Emergency clinicians must ensure that all patients receive pain relief and sedation commensurate with their individual needs during any procedure.
Terminology
In 1985, the American Academy of Pediatrics (AAP) and the National Institutes of Health issued guidelines for the management and monitoring of children receiving sedation for diagnostic and therapeutic procedures in response to the growing use of opioids and sedative-hypnotic agents in the outpatient setting and a number of sedation-related deaths.8,9 In these documents, three levels of sedation were defined (conscious sedation, deep sedation, general anesthesia) to create a common language for describing drug-induced alterations in consciousness (Box 33-1).10,11 A key development in the field of PSA has been revision of the original terminology and adoption of clearer descriptions of varying types and degrees of sedation (see Box 33-1). Though historically popular, the widely misinterpreted and misused term “conscious sedation” has fallen into disfavor12; it has been labeled as “confusing,”13 “imprecise,”12 and an “oxymoron”12,13 and has been replaced with the term “moderate sedation.”10
PSA Guidelines
Before the promulgation of PSA guidelines by specialty societies and governmental agencies, clinicians simply administered sedatives in varied clinical settings and used individual judgment to determine the need for specific monitoring devices and supporting personnel. Since 1985, at least 27 sets of PSA guidelines have been published,15 each crafted for the unique and differing settings in which PSA is practiced. Naturally, not all are in agreement.5 The intent of each of these guidelines is to better standardize the manner in which PSA is performed to enhance patient safety. Those most pertinent to emergency clinicians are from the American College of Emergency Physicians,3 the AAP,16 and the American Society of Anesthesiologists (ASA).14,17
In the early 1990s, the Joint Commission on Accreditation of Healthcare Organizations, an independent, not-for-profit organization that evaluates and accredits hospitals in the United States, took a special interest in PSA, with the central theme being that the standard of sedation care provided should be comparable throughout a given hospital. Thus, patients sedated in the emergency department (ED) should not receive a significantly different level of attention or monitoring than those sedated for a similar-level procedure in the operating room or in the endoscopy suite. To ensure this, the Joint Commission requires specific PSA protocols to be applied consistently throughout each institution. These hospital-wide sedation policies will vary from site to site based on the specific needs and expertise available within each institution. In 2001, the Joint Commission released new standards for pain management, sedation, and anesthesia care.10
At each hospital accreditation survey the Joint Commission determines whether practitioners practice PSA consistently with their hospital-wide sedation policy and whether they provide sufficient documentation of such compliance. Clinicians must be familiar with their hospital’s sedation policies and should work with their medical staff to ensure that such policies are suitably detailed, yet reasonable and realistic. Unduly restrictive policies do a disservice to patients by discouraging appropriate levels of analgesia and anxiolysis. Most hospitals pattern their sedation policies after the Joint Commission standards and definitions. It is important to note that the unique ketamine dissociative state does not fit into the existing Joint Commission definitions of sedation and anesthesia.11 A ready solution is to assign a distinct definition for “dissociative sedation” (see Box 33-1).
The Joint Commission requires that PSA practitioners who are permitted to administer deep sedation be qualified to rescue patients from general anesthesia.10 Emergency clinicians typically perform all levels of sedation except general anesthesia. Moderate sedation suffices for the majority of procedures in adults and cooperative children, although it will not be adequate for extremely painful procedures (e.g., hip reduction, cardioversion). Deep sedation can facilitate such procedures, but with greater risk for cardiorespiratory depression than is the case with moderate sedation. Moderate sedation is frequently insufficient for effective anxiolysis and immobilization in younger, frightened children, and deep or dissociative sedation is an appropriate alternative.
Evaluation before PSA
General
Assess the type and severity of any underlying medical problems. This is usually best documented by the standard ED medical record, history, physical examination, and nursing notes. Another tool used for this purpose is the ASA physical status classification for preoperative risk stratification (Table 33-1). Verify current medications and allergies. Inquire about previous adverse experiences with PSA or anesthesia.
TABLE 33-1
ASA Physical Status Classification
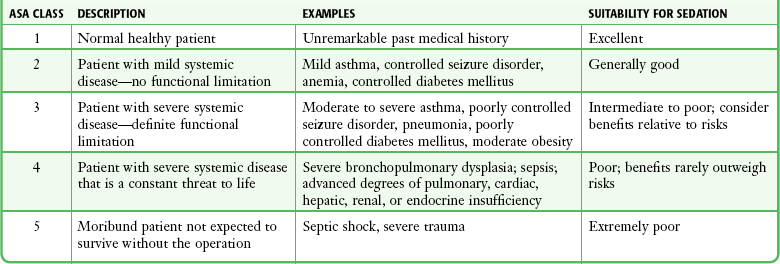
ASA, American Society of Anesthesiologists.
From Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342:938.
Airway
Inspect the airway to determine whether any abnormalities (e.g., severe obesity, short neck, small mandible, large tongue, trismus) are present that might impair airway management. Consider assessments such as Mallampati scoring or the distance between the chin and hyoid bone (see Chapter 4, Fig. 4-3).
Gastrointestinal
Assess the time and nature of the last oral intake because pulmonary aspiration of gastric contents is a dreaded complication of vomiting when protective airway reflexes are impaired. Figure 33-1 shows a four-step assessment tool to stratify the risk for aspiration before sedation and to identify prudent limits of targeted sedation,18 although this tool has not yet been validated.
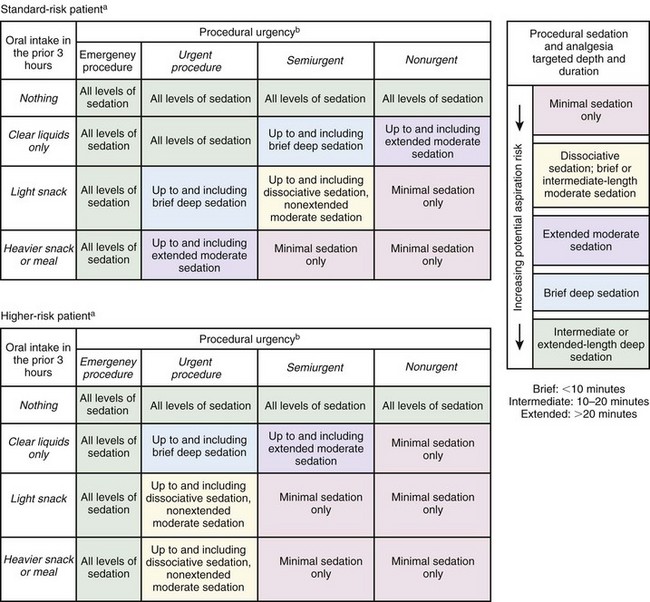
Figure 33-1 Prudent limits of targeted depth and length of emergency department procedural sedation and analgesia based on presedation assessment of aspiration risk.
aHigher-risk patients are those with one or more of the following present to a degree individually or cumulatively judged clinically important by the treating clinician:
(From Green SM, Roback MG, Miner JR, et al. Fasting and emergency department procedural sedation and analgesia: a consensus-based clinical practice advisory. Ann Emerg Med. 2007;49:454.)
More conservative guidelines from the ASA for elective surgery or procedures in healthy patients specify an age-stratified fasting requirement of 2 to 3 hours for clear liquids and 4 to 8 hours for solids and nonclear liquids.19 Nonetheless, they acknowledge that regarding PSA, “the literature provides insufficient data to test the hypothesis that preprocedure fasting results in a decreased incidence of adverse outcomes.”14,17 The concept of preprocedure fasting is logistically difficult or impossible for emergency clinicians, who have no control over patients’ oral intake before arrival at the ED. In actual practice, emergency clinicians routinely perform PSA safely on patients who are noncompliant with the ASA elective-procedure fasting guidelines.18–20 Procedures can sometimes be delayed for a number of hours; however, this must be balanced against prolongation of pain and anxiety in the patient, inconvenience for the patient and family, and expenditure of room space and other finite ED resources. In addition, many ED procedures require urgent if not immediate attention (e.g., débridement and repair of animal bite wounds, acute burn management, arthrocentesis for suspected septic arthritis, reduction of joint dislocations, lumbar puncture in an uncooperative septic patient, hernia reduction, eye irrigation for ocular trauma or chemical burns, cardioversion in a hemodynamically unstable patient). Though uncommon, there may be occasions in which nonfasting patients require urgent procedures with a substantial depth of sedation that may be more safely managed in the operating room with endotracheal intubation to protect the airway.
Personnel and Interactive Monitoring
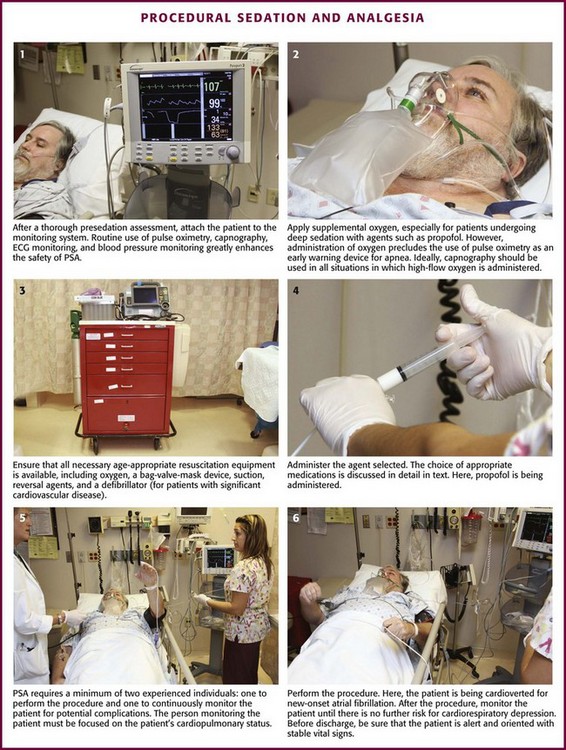
The most important element of PSA monitoring is close and continuous observation of the patient by an individual capable of recognizing complications of sedation (Fig. 33-2). This person must be able to continuously observe the patient’s face, mouth, and chest wall motion. Equipment or sterile drapes must not interfere with such visualization. Such careful observation allows prompt detection of adverse events such as respiratory depression, apnea, partial airway obstruction, emesis, and hypersalivation.
During deep sedation, the individual dedicated to patient monitoring should have experience with this depth of sedation and no other responsibilities that would interfere with the advanced level of monitoring and documentation appropriate for this degree of sedation.16 Individual hospital-wide sedation policies may have additional requirements regarding how and when deep sedation is administered based on the patient’s specific needs and the clinician’s expertise.
Equipment and Mechanical Monitoring
Capnography
Capnography is a very useful tool that provides a continuous, breath-by-breath measure of the respiratory rate and CO2 exchange. Importantly, capnography can detect the common adverse airway and respiratory events associated with PSA.21–33 Capnography is the earliest indicator of airway or respiratory compromise and will show abnormally high or low end-tidal carbon dioxide pressure well before pulse oximetry detects falling oxyhemoglobin saturation, especially in patients receiving supplemental oxygen. Early detection of respiratory compromise is especially important in infants and toddlers, who have smaller functional residual capacity and greater oxygen consumption than older children and adults do.34–36 Capnography provides a non–impedance-based respiratory rate directly from the airway (via an oral-nasal cannula). This is more accurate than impedance-based respiratory monitoring, especially in patients with obstructive apnea or laryngospasm, in whom impedance-based monitoring will interpret chest wall movement without ventilation as a valid breath.
Two recent randomized controlled trials have demonstrated that the use of capnography during procedural sedation decreases the incidence of hypoxic events.37–39 Both studies randomized patients to standard monitoring alone (oximetry, ECG, and blood pressure) or standard monitoring with capnography, with hypoxia being the outcome measure. In both studies, the addition of capnography to standard monitoring alerted clinicians to ventilatory abnormalities before the development of hypoxia, and as a result, capnography significantly decreased the incidence of hypoxic events.37,38
BIS Monitoring
The bispectral index (BIS) is a monitoring modality that uses a processed electroencephalogram signal to quantify the depth of anesthesia or sedation. A BIS value of 100 (unitless scale) is considered complete alertness, 0 represents no cortical activity at all, and the range of 40 to 60 is believed to be consistent with general anesthesia. Although this technology has been used widely to monitor the depth of sedation in the operating room, the ASA has judged that its clinical applicability for this purpose “has not been established.”40 Furthermore, a 2011 study found that patients in whom a modified minimum alveolar concentration protocol (i.e., the inhalational anesthetic concentration needed for 50% of patients to not move with the application of a noxious stimulus) was used had fewer awareness events than did those in whom a BIS protocol was used.41 Even though PSA research has demonstrated statistical associations between BIS and standard sedation scores, these studies have also noted unacceptably wide ranges of BIS values at various depths of sedation.21,42–46 Thus, although BIS is correlated with the depth of sedation in aggregate groups, it lacks sufficient capacity to reliably gauge such depth in individual patients and therefore cannot currently be recommended for ED PSA.
Supplemental Oxygen
Substantial variation in practice exists with regard to the use of supplemental oxygen during PSA. The premise is a logical one—increasing systemic oxygen reserves should naturally delay or perhaps avert hypoxemia should an airway or respiratory adverse event occur. However, the price paid for this well-intentioned safeguard is the loss of pulse oximetry as an early warning device.12,15,21 Hyperoxygenated patients will desaturate only after the apnea is prolonged—indeed, the time required for preoxygenated, apneic, healthy adults and adolescents to desaturate to 90% averages more than 6 minutes.47,48
Deitch and colleagues have shown in a series of randomized controlled trials that high-flow supplemental oxygen decreases the incidence of hypoxia during propofol sedation (number needed to benefit of 4)49 whereas lesser amounts of oxygen (3 L/min) do so only marginally with propofol and not at all with lighter levels of sedation.23,50 Thus, high-flow oxygen is strongly recommended with propofol or other deep sedation, assuming that interactive monitoring includes capnography to promptly identify respiratory depression.51,52 For lighter levels of sedation, supplemental oxygen has no established benefit and may impair detection of respiratory depression when using pulse oximetry without capnography.52
Discharge Criteria
Monitor all patients receiving PSA until they are no longer at risk for cardiorespiratory depression (Table 33-2). Before discharge be sure that patients are alert and oriented (or have returned to an age-appropriate baseline) with stable vital signs. Many hospitals have chosen to use standardized recovery scoring systems similar to those used in their surgical postanesthesia recovery areas (Table 33-3). Although no generally accepted minimum durations for safe discharge have been established, one large ED study found that in children with uneventful sedation, no serious adverse effects occurred more than 25 minutes after final medication administration.53 This suggests that in most cases, prolonged observation beyond  hour is unlikely to be necessary.
hour is unlikely to be necessary.
TABLE 33-2
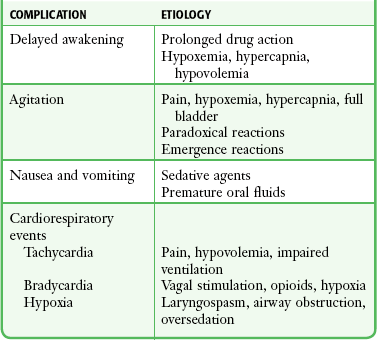
From Krauss B, Brustowicz R, eds. Pediatric Procedural Sedation and Analgesia. Philadelphia: Lippincott, Williams & Wilkins; 1999:145.
TABLE 33-3
Sample Recovery Scoring Systems
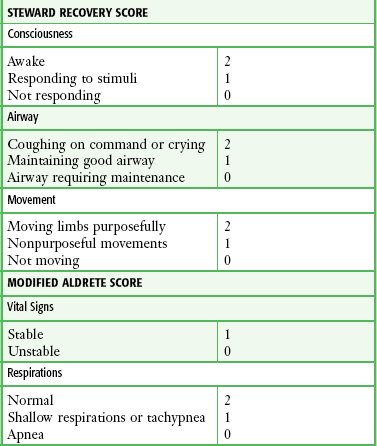
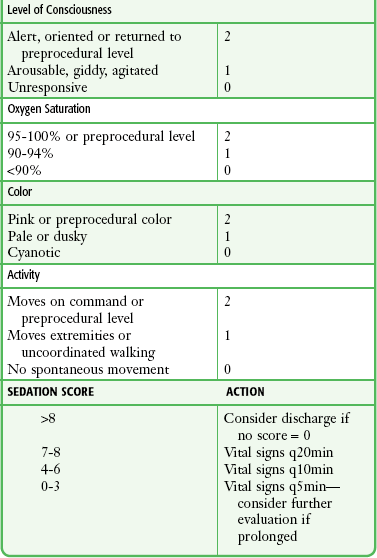
From Krauss B, Brustowicz R, eds. Pediatric Procedural Sedation and Analgesia. Philadelphia: Lippincott, Williams & Wilkins; 1999:157.
Make sure that all patients leave the hospital with a reliable adult who will observe them after discharge for postprocedural complications. Document the name of the individual in the hospital record. Give written instructions regarding appropriate diet, medications, and level of activity (Boxes 33-2 and 33-3). Even though patients may appear awake and able to comprehend instructions, they may not remember details once they leave the ED.
To be eligible for safe discharge, children are not required to walk unaided or demonstrate that they can tolerate an oral challenge because most PSA agents are emetogenic. Forcing fluids after sedation can lead to emesis before or after discharge. The AAP guidelines require only that “the patient can talk (if age-appropriate)” and “the patient can sit up unaided (if age-appropriate).”16 When infants and young children are discharged after their evening bedtime, caution parents to position the child’s head in the car seat carefully. Significant forward flexion might cause airway obstruction if the child falls asleep on the way home.
General Principles
Before drug administration, every effort should be made to minimize a patient’s anxiety and distress, particularly in children. The emotional state of a patient on induction strongly correlates with the degree of distress on emergence and in the days immediately after the procedure.54–57 Avoid being pressured by consultants to cut corners or rush PSA. Incorporating into the presedation preparation a discussion with the consultant about the sedation plan and the length of time required to safely prepare and sedate the patient can avoid the risks associated with hurried sedation.
Routes of Administration
The oral, transmucosal (i.e., nasal, rectal), and IM routes are more convenient means of administration because IV access is not necessary, but they are much less reliable for timely dose titration to a desired response. New drug delivery systems, however, are expanding the effectiveness and ease of use of these routes of administration. The refinement of intranasal drug delivery has significantly increased the efficacy of this route of administration.58,59 Before the development of metered-dose atomizers, the degree of absorption and effectiveness of intranasal drug administration were operator dependent. Furthermore, new drug formulations with concentrations appropriate for intranasal administration are becoming available for study.60,61
Drug Selection Strategies
The majority of nonpainful or minimally painful ED procedures in older children and adults can be performed without systemic sedation and analgesia. Skilled practitioners can frequently combine a calm, reassuring bedside manner with distraction techniques, careful local or regional anesthesia, or both.62–64 Many procedures, however, cannot be technically or humanely performed without PSA. These situations can be divided into three categories.
Insufficient Analgesia.: Despite a cooperative patient, for some procedures it is impossible to achieve effective pain control with local or regional anesthesia. Examples of procedures requiring systemic PSA include fracture reductions, dislocation reductions, incision and drainage of large loculated abscesses, wounds that require scrubbing such as “road rash,” cardioversion, bone marrow aspiration/biopsy, and extensive burn débridement.
Insufficient Anxiolysis.: Despite effective local or regional anesthesia, some patients will be so frightened that procedures cannot be technically or humanely performed without PSA. Young children requiring repair of lacerations are frequently terrified, and older children and adults may be highly anxious in anticipation of such repairs in sensitive or personal regions (e.g., face, genitalia, perineum).
Insufficient Immobilization.: Despite effective local or regional anesthesia and anxiolysis, PSA may be indicated to prevent excessive motion during procedures that require substantial immobilization (e.g., repair of complex facial lacerations, diagnostic imaging studies). Immobilization is most commonly an issue with young children and the mentally challenged.
General Considerations.: Clinicians must therefore base customization of their selection of drugs (e.g., anxiolysis, sedation, analgesia, immobilization) on the unique needs of the patient and their individual level of experience with specific agents (Table 33-4). A risk-benefit analysis should be performed before every sedation (Box 33-4). The benefits of reducing anxiety and controlling pain should be carefully weighed against the risk for respiratory depression and airway compromise. Factors influencing the extent of pharmacologic management are listed in Box 33-5. Some general drug selection strategies are discussed later and shown in Table 33-3.
TABLE 33-4
Indications for PSA and Sedation Strategies*
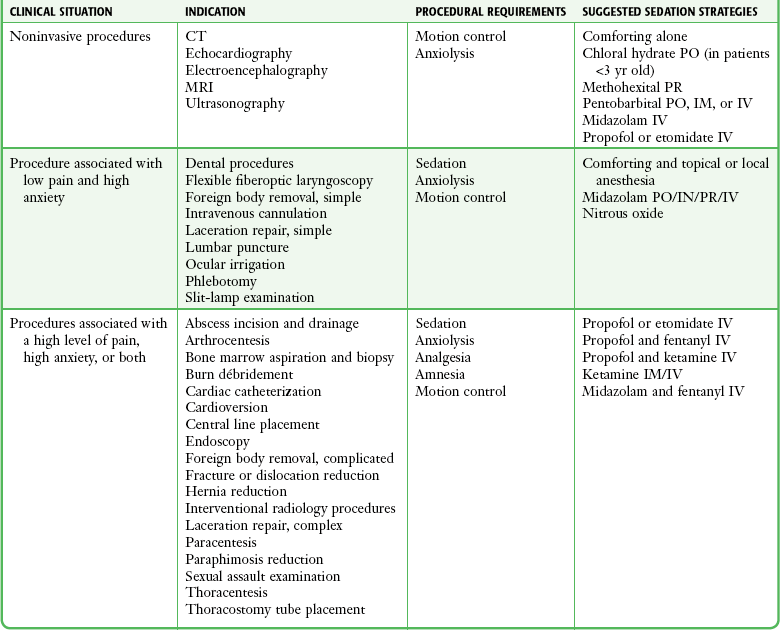
*There is no universally accepted or clinically correct dose, medication, or combination. Many regimens are acceptable. This table is intended as a general overview. Sedation strategies should be individualized. Although the pharmacopoeia is large, clinicians should familiarize themselves with a few agents that are flexible enough to be used for the majority of procedures. In all cases it is assumed that practitioners are fully trained in the technique, appropriate personnel and monitoring are used as detailed in this chapter, and specific drug contraindications are absent.
Modified from Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342:938.
Minor Procedures In Cooperative Adults and Older Children.: Such procedures can usually be managed with topical, local, or regional anesthesia. Systemic PSA is typically unnecessary, although mild anxiolysis (e.g., nitrous oxide, oral midazolam) can make these patients more comfortable.
More Complex Procedures of Longer Duration In Cooperative Adults and Older Children.: Supplementation of topical, local, or regional anesthesia with either nitrous oxide or IV midazolam and fentanyl permits customization of the depth of sedation and pain relief to the specific needs of each patient.
Procedures In Uncooperative Adults or The Mentally Challenged.: Essentially all procedures in uncooperative adult-sized patients are difficult without systemic PSA. Depending on operator experience, IV midazolam, IV propofol, IV etomidate, or IM/IV ketamine or midazolam may be used in these situations. Given that the sedatives midazolam, propofol, and etomidate lack specific analgesic properties, many emergency physicians attempt to control pain with an opioid such as fentanyl before the procedure. Midazolam can be titrated intravenously to a relatively deep level of sedation, although as discussed previously, the risk for adverse effects increases with the depth of sedation. Ketamine (typically with coadministered midazolam when used in adults) can also provide the profound analgesia and immobilization necessary to perform painful procedures. However, in adults there is a risk for unpleasant hallucinatory recovery reactions. Ketamine should be used with extreme caution in older adults because its sympathomimetic properties may aggravate any underlying coronary artery disease or hypertension. Occasionally, procedures in extremely uncooperative adults or the mentally challenged are best managed in the operating room with general anesthesia.
Minor Procedures In Uncooperative Older Children and In Young Children.: Minor procedures (e.g., small lacerations, IV cannulation, venipuncture, removal of superficial foreign bodies) in uncooperative children can frequently be managed by skilled practitioners with a combination of nonpharmacologic techniques (e.g., distraction, guided imagery, hypnosis, comforting, breathing techniques) in conjunction with topical anesthesia, careful local anesthesia, and when necessary, brief forcible immobilization (by personnel or a restraining device). In other cases, supplementing nonpharmacologic techniques with topical or local anesthesia and anxiolysis with oral midazolam may be sufficient to permit successful wound repair. Although oral administration is most popular and least invasive, the nasal or rectal routes can also be used depending on operator experience and preference.
Major Procedures In Uncooperative Children.: Major painful procedures (e.g., fracture reduction, incision and drainage of large loculated abscesses, arthrocentesis of a major joint) require systemic PSA. Options include IM/IV ketamine, IV propofol, IV etomidate, or IV midazolam. Given that the sedatives midazolam, propofol, and etomidate lack specific analgesic properties, many emergency physicians attempt to control pain with an opioid such as fentanyl before the procedure. Ketamine may be the best option in such children because dissociative sedation can consistently provide immobilization and analgesia while maintaining protective airway reflexes and upper airway muscular tone.
Pharmacopeia
There is no universally correct or preferred medication or drug regimen. Many options are acceptable and successful. The best choice is an agent whose pharmacologic properties are familiar to the operator and that is used frequently by the operator, is easily titratable, and has a short duration of action or is readily reversible. All drugs should be given in adequate doses because underdosing of opioids or sedatives provides no useful purpose. Dosing recommendations for PSA drugs are provided in Table 33-5, and specialized protocols for midazolam or fentanyl, propofol, and ketamine are presented in Boxes 33-6, 33-7, and 33-8, respectively. Individual agents are discussed in the following sections.
TABLE 33-5
Drug Dosing Recommendations for PSA*
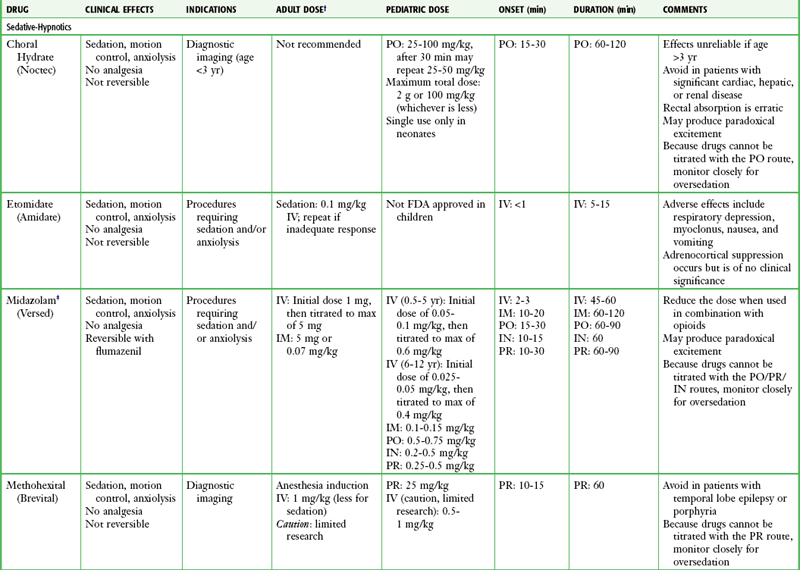
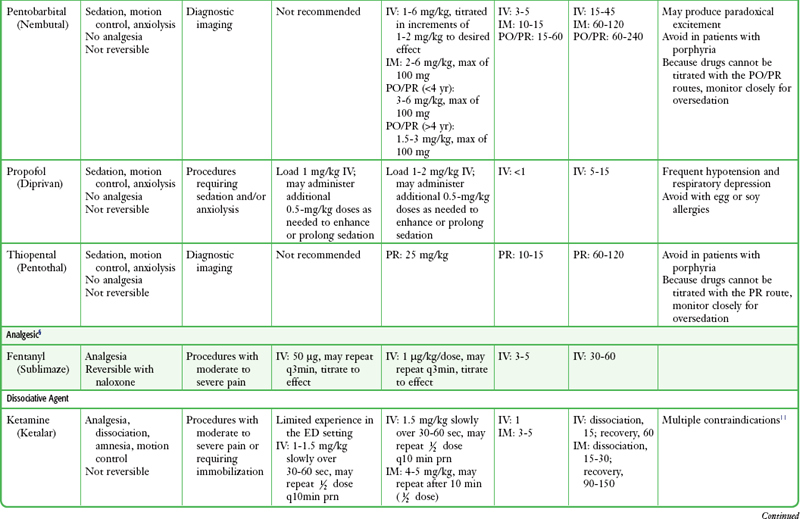
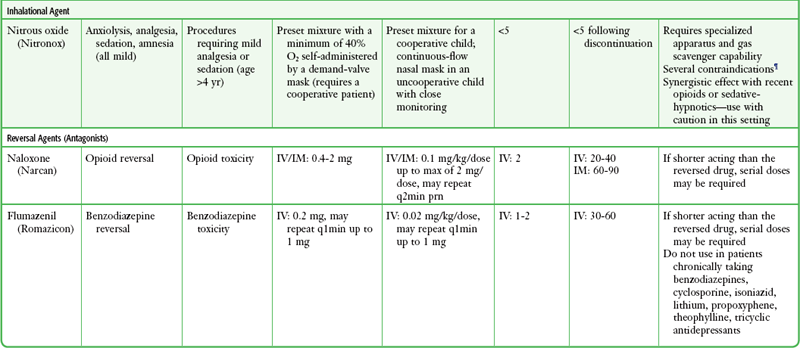
*Alterations in dosing may be indicated depending on the clinical situation and the practitioner’s experience with these agents. Individual dosages may vary when used in combination with other agents, especially when benzodiazepines are combined with opioids.
†Use lower doses in geriatric patients and those with significant cardiopulmonary disease.
‡Midazolam is preferred over other benzodiazepines (e.g., diazepam, lorazepam) for procedural sedation and analgesia because of its shorter duration of action and multiple routes of administration.
§Fentanyl is preferred over other opioids (e.g., morphine, meperidine) for procedural sedation and analgesia because of its faster onset, shorter recovery, and lack of histamine release.
||Generally accepted contraindications to ketamine include age younger than 3 months; history of airway instability, tracheal surgery, or tracheal stenosis; procedures involving stimulation of the posterior pharynx; active pulmonary infection or disease (including active upper respiratory infection); cardiovascular disease, including angina, heart failure, or hypertension; significant head injury, central nervous system masses, or hydrocephalus; glaucoma or acute globe injury; psychosis; porphyria; and thyroid disorder or thyroid medication.
¶Generally accepted contraindications to nitrous oxide include pregnancy (patient or personnel), preexisting nausea or vomiting, trapped gas pockets (e.g., middle ear infection, pneumothorax, bowel obstruction).
Adapted from Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342:938.
Sedative-Hypnotic Agents
Pharmacology.: Chloral hydrate is a pure sedative-hypnotic agent without analgesic properties. When administered orally, the average time to peak sedation is approximately 30 minutes, with a recovery time of an additional 1 to 2 hours.65,66 Residual motor imbalance and agitation may persist for several hours beyond this period.67 Rectal administration is erratically absorbed and therefore not recommended.
Adult Use.: Use of chloral hydrate is limited to diagnostic imaging studies in children. It has no current use in adults.
Pediatric Use.: Chloral hydrate is widely used as a sedative to facilitate nonpainful outpatient diagnostic procedures such as electroencephalography and computed tomography (CT) or MRI.66,68–72 IV pentobarbital appears to be more effective than chloral hydrate for the latter indication,73 although many centers prefer chloral hydrate in younger children (e.g., <18 months) simply to avoid the need for IV access.69,70,73
Adverse Effects.: Despite a wide margin of safety, chloral hydrate can cause airway obstruction and respiratory depression, especially at higher doses (75 to 100 mg/kg).1,66,69,71,72 The incidence was 0.6% in one large series.66 There is no known dosage threshold of chloral hydrate below which this potential complication can be consistently avoided,1,71 and accordingly, standard interactive and mechanical monitoring precautions apply to chloral hydrate as they do to other PSA agents.
Because it is a halogenated hydrocarbon, overdoses of chloral hydrate can be arrhythmogenic and produce ventricular dysrhythmias. β-Blockers may be most effective in terminating ventricular arrhythmias. Despite reports of potential carcinogenicity, the AAP has judged that the evidence is currently insufficient to avoid single doses of chloral hydrate for this reason alone.74
Midazolam
Pharmacology.: Benzodiazepines are a group of highly lipophilic agents that possess anxiolytic, amnestic, sedative, hypnotic, muscle relaxant, and anticonvulsant properties. They lack direct analgesic properties and thus are commonly coadministered with opioids. Caution must be exercised when using benzodiazepines and opioids together because the risk for hypoxia and apnea is significantly greater than when either is used alone.75
Adult Use.: Midazolam can be used effectively for moderate and deep sedation through careful IV titration to effect, typically together with fentanyl (see Box 33-6).
Pediatric Use.: Advantages of midazolam over other benzodiazepines for pediatric PSA are its short duration of action, reversibility, and availability in multiple routes of administration. Midazolam may be used for the same indications and in the same manner as in adults. Some children require larger doses than would be typical for adults on a milligram-per-kilogram basis,76 and paradoxical responses (e.g., hyperexcitability) are not uncommon.67,77,78 Midazolam does not reliably render a child motionless, and therefore methohexital or pentobarbital is generally preferred for neuroimaging.73,79,80
To avoid the need for IV access in frightened children, midazolam has been alternatively administered via the IM,81 oral,77,82–86 intranasal,82,87–90 and rectal routes.91 However, the inability to effectively titrate with these routes dictates that a reliable depth of sedation cannot be predictably or regularly achieved. Consequently, these non-IV routes are primarily reserved for pure anxiolysis or mild sedation (or both) for minimally painful procedures. Respiratory depression can also occur via these routes.86
Adverse Effects.: When administered by skilled practitioners using standard precautions (see Box 33-6), the safety profile for midazolam is excellent.5,6,92 However, when administering benzodiazepines, one must maintain continuous vigilance for respiratory depression.1,67,75,92,93 Such respiratory depression is dose dependent and greatly enhanced in the presence of ethanol or other depressive drugs, especially opioids. These effects are exaggerated in the elderly. Deaths from undetected apnea have occurred,75 thus underscoring the critical role of continuous interactive and mechanical monitoring.
Pentobarbital
Pharmacology.: Pentobarbital is a barbiturate capable of profound sedation, hypnosis, amnesia, and anticonvulsant activity in a dose-dependent fashion. It has no inherent analgesic properties. When carefully titrated intravenously, sedation is evident within 5 minutes with a duration of approximately 30 to 40 minutes.79
Adult Use.: Pentobarbital has no advantage over midazolam for adult PSA and is rarely used for this purpose.
Ultrashort-Acting Sedative-Hypnotic Agents
Substantial controversy surrounded the early administration of these agents for ED PSA.96 Proponents have cited their extremely rapid onset and recovery as enormous advantages over other sedatives. Critics have cited the level of continuous vigilance required to achieve a desired effect while simultaneously avoiding significant cardiopulmonary depression because these agents can result in rapid swings in levels of consciousness. Research that includes thousands of ED patients for propofol and hundreds for etomidate has subsequently demonstrated that the safety profiles of these agents are the same or better than those of other agents in the PSA pharmacopoeia.21 Given the rapid onset and offset of propofol, consider (when available) an additional dedicated clinician (separate from the individual performing the procedure) to oversee administration of medication.97
Pharmacology.: Propofol is becoming an agent of choice for PSA in the ED because of its efficacy and safety profile (Fig. 33-3). When administered by IV bolus, its onset of action is typically within 30 seconds. The half-life for blood-brain equilibration is approximately 1 to 3 minutes, and its clinical effects typically resolve within 5 to 7 minutes. Longer procedures can be facilitated by repeated dosing. Patients are typically awake and alert within 15 minutes after discontinuation. Propofol exhibits inherent antiemetic and perhaps euphoric properties, and patient satisfaction is typically high. Propofol should be avoided in patients with known or suspected allergy to eggs or soy products.22,97–104
Adult and Pediatric Use.: Deep sedation can be achieved reliably in both adults and children with a single IV loading dose of propofol. Repeated bolus dosing is preferred, but an IV drip can be administered as needed to enhance or prolong sedation.22,97–104
Adverse Effects.: Transient apnea and respiratory depression can occur with propofol but typically resolve spontaneously before intervention is necessary. Reported rates of assisted ventilation range from 0% to 4.6%.97 Similarly, transient hypotension (via direct negative inotropy as well as arterial and venous dilation) is common but typically resolves spontaneously without treatment. Injection site pain is noted less frequently in the ED setting than in the operating room, where higher doses are generally administered.22,97–104 Propofol usually causes pain on injection in awake individuals. Injecting slowly through a large vein, not a dorsal hand vein, and administering 2 to 3 mL of 2% lidocaine slowly into the vein before propofol infusion will lessen the pain of injection.
Pharmacology.: When administered by IV bolus, the onset of action of etomidate is typically within 30 seconds, and patients are usually awake and alert within 30 minutes after discontinuation.
Adult Use.: Deep sedation can be achieved reliably with single loading doses.105–108 Dosing can be repeated as needed to enhance or prolong sedation. Etomidate may be somewhat less effective overall than propofol and, given its additional adverse effect of myoclonus, appears to be a less desirable choice than propofol for deep sedation.21,38
Pediatric Use.: There is significantly less published experience with etomidate in children for ED PSA; however, its safety and efficacy profile appears to be similar to that in adults.109,110
Adverse Effects.: The primary adverse effects of etomidate are respiratory depression, myoclonus, nausea, and vomiting.110,111 Respiratory depression has been reported to be less common with propofol PSA than with methohexital, fentanyl/midazolam, or etomidate.112 Myoclonus is common yet generally benign, but it may be disconcerting and can interfere with the procedure. It consists of transient jerking or twitching movements that can be mistaken for seizure activity. Transient adrenal suppression occurs with etomidate in septic patients but appears to lack clinical significance for single doses when used for ED PSA.21,111,113
Pharmacology.: Because of their lipid solubility, barbiturates are rapidly absorbed rectally. When given by the IV route, both thiopental and methohexital produce sedation within 1 minute. Clinical recovery is rapid (≈15 minutes) as a result of rapid redistribution from the central nervous system to the periphery.
Adult Use.: There is limited published experience using these IV barbiturates for ED PSA,114 and propofol or etomidate would appear to be a better choice.
Pediatric Use.: Rectal thiopental and methohexital can reliably produce sedation suitable for CT or MRI.115–120 Respiratory depression is unusual when using typical doses (see Table 33-5) but can occur.115,116,118–120 There is limited published experience using these IV barbiturates for ED PSA,114 and propofol would appear to be a better choice.
Adverse Effects.: Barbiturates cause potent respiratory depression; in one ED report, apnea occurred in 10% of patients.121 Barbiturates also frequently cause hypotension at typical IV doses, so their use should be avoided whenever possible in patients with volume depletion or cardiovascular compromise.
Analgesic Agents
Fentanyl is the most common opioid used for PSA because of its rapid onset, brief duration, rapid reversibility with naloxone, and lack of histamine release.7 It is often combined with other agents such as propofol, etomidate, and midazolam to provide additional effect and pain relief. The effects of fentanyl can be reversed immediately with naloxone should excessive sedation or respiratory depression occur. The longer-duration opioids morphine and meperidine are preferred for nonprocedural or preprocedural pain control and are frequently given initially for acute analgesia followed by fentanyl to facilitate the needed procedure. Although longer-acting opioids can be readily used for analgesia during PSA, they will be associated with longer recovery times and a higher incidence of histamine-related effects (e.g., nausea and vomiting, hypotension, pruritus). Fentanyl lacks these effects and is therefore preferred.
Pharmacology.: Fentanyl is 100 times more potent than morphine and has no intrinsic anxiolytic or amnestic properties. A single IV dose has a rapid onset (<30 seconds) with a peak at 2 to 3 minutes and brief clinical duration (20 to 40 minutes). This increase in potency and onset of action is in part related to its greater lipid solubility, which facilitates passage of the drug across the blood-brain barrier. The effects of fentanyl can be rapidly and completely reversed with opioid antagonists (e.g., naloxone, nalmefene).
Adult Use.: Because of its pharmacokinetics, IV fentanyl is an ideal agent when analgesia is required for painful procedures; it can be easily and rapidly titrated.7 Because anxiolysis and sedation do not occur at low doses (1 to 2 µg/kg), concurrent administration of a pure sedative, most commonly midazolam, is advisable, especially in children (see Box 33-6).
Pediatric Use.: The combination of fentanyl and midazolam remains a popular PSA sedation regimen in children, with a strong safety and efficacy profile when both drugs are carefully titrated to effect.6,92,122,123 Any necessary level of mild to deep sedation can be achieved with these agents.
Fentanyl is also available in an oral transmucosal preparation. Although this novel and noninvasive delivery route obviates the need for IV access, titration is difficult and its efficacy is variable.124 Furthermore, the incidence of emesis is high (31% to 45%),124,125 and consequently this formulation has never become popular for PSA.
Adverse Effects.: Like all opioids, fentanyl can cause respiratory depression.6,7,92,122,123 When used for PSA, standard interactive and mechanical monitoring is required. Because the opioid effect is most pronounced on the central nervous system respiratory centers, apnea precedes loss of consciousness. If apnea should occur, verbal or tactile stimulation should be attempted before the administration of opioid antagonists. As discussed earlier, caution must be exercised when using benzodiazepines and other PSA agents and opioids together because the risk for hypoxia and apnea is significantly greater than when either is used alone.5,75
In the absence of significant ethanol intoxication, hypovolemia, or concomitant drug ingestion, hypotension is rare, even with very large doses of fentanyl (doses of 50 µg/kg are common in adult and pediatric cardiac surgery). Because of its safe hemodynamic profile, fentanyl is an ideal analgesic agent for use in critically ill or injured patients. In addition, nausea and vomiting are rare in comparison to analgesia with morphine or meperidine. A commonly observed reaction to fentanyl is nasal pruritus, and patients frequently attempt to scratch their nose during the procedure.7
A rare side effect of fentanyl with potential for respiratory compromise is chest wall rigidity. This complication has not been problematic in the ED and is related to higher doses (>5 µg/kg as a bolus dose) than those used for PSA and has not been reported in any ED series.6,7,122,123 If it should occur, chest wall rigidity can usually be reversed with opioid antagonists, positive pressure ventilation, or both. Equipment for urgent pharmacologic paralysis should be available if reversal and positive pressure ventilation are unsuccessful.
Diamorphine
Diamorphine is a nasal opioid that is currently available in the United Kingdom, Australia, New Zealand, and Canada, but not in the United States.58,59,126 Diamorphine has an onset and duration of action similar to that of morphine; however, its higher water solubility permits potent doses to be delivered in the small (0.1 mL) volumes necessary for comfortable intranasal administration. In two studies of children and teenagers with fractures, intranasal diamorphine, 0.1 mg/kg, provided a similar level of analgesia with faster onset as IM morphine, 0.2 mg/kg. Intranasal spray administration was better tolerated than the injection, and there were no adverse events.58,59,126 Diamorphine may prove to be a useful initial analgesic for children and teenagers with acute pain, although in practice, an IV line would most likely be established to permit titration to full pain relief and PSA for any procedures needed (e.g., fracture reduction). The role of diamorphine in adults remains to be determined.
Other Short-Acting Opioids
Sufentanil, alfentanil, and remifentanil are other short-acting opioids that have a potential role in PSA. Currently, however, there is insufficient published experience to warrant their routine use. Although intranasal sufentanil, 0.75 µg/kg, appeared promising in one small pediatric trial,127 in another, doses of 1.5 µg/kg resulted in oxygen desaturation in 8 of the 10 children studied.88 This low toxic-to-therapeutic ratio and inability to titrate would appear to limit the utility of intranasal sufentanil. In the one published report of IV remifentanil with midazolam for PSA, there was an unacceptably high incidence of hypoxemia.128 Currently, there does not appear to be a clinically important advantage with these drugs versus fentanyl.
Ketamine
Pharmacology.: Ketamine produces a unique state of cortical dissociation that permits painful procedures to be performed more consistently and effectively than with other PSA agents. This state of “dissociative sedation” is characterized by profound analgesia, sedation, amnesia, and immobilization (Fig. 33-4) and can be rapidly and reliably produced with IV or IM administration.11 Ketamine is widely used worldwide and has demonstrated a remarkable safety profile in a variety of settings.4,6,129–132 Clinicians administering ketamine must be especially knowledgeable about the unique actions of this drug and the numerous contraindications to its use (see Table 33-5).
Ketamine differs from other PSA agents in several important ways. First, it uniquely preserves cardiopulmonary stability. Upper airway muscular tone and protective airway reflexes are maintained. Spontaneous respiration is preserved, although when administered intravenously, ketamine must be given slowly (over a period of 30 to 60 seconds) to prevent respiratory depression. Second, it differs from other agents in that it lacks the characteristic dose-response continuum to progressive titration. At lower doses ketamine produces analgesia and disorientation. However, once a dosage threshold (≈1 to 1.5 mg/kg intravenously or 3 to 4 mg/kg intramuscularly) is achieved, the characteristic dissociative state appears. This dissociation has no observable levels of depth, and thus the only value of ketamine “titration” is to maintain the presence of the state over time. Finally, the dissociative state is not consistent with formal definitions of moderate sedation, deep sedation, or general anesthesia (see Table 33-1) and must therefore be considered from a different perspective than agents that exhibit the classic sedation continuum.11,15
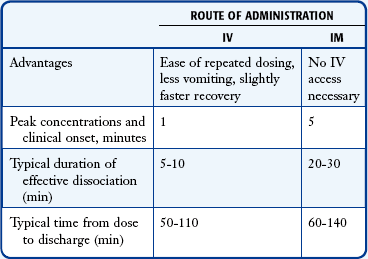
Ketamine is most effective and reliable when given intravenously or intramuscularly. Ketamine has a rapid circulation time when given intravenously, with onset of dissociation noted within 1 minute and effective procedural conditions lasting for about 10 to 15 minutes. When given intramuscularly, the same effect is achieved within 5 minutes, with effective procedural conditions for about 15 to 30 minutes. The typical duration from dosing to dischargeable recovery is 50 to 110 minutes when given intravenously and 60 to 140 minutes when given intramuscularly.129,132
Like the benzodiazepines, ketamine undergoes substantial first-pass hepatic metabolism. As a result, oral and rectal administration results in less predictable effectiveness and substantially higher doses are required. Clinical onset and recovery are considerably longer than when given parenterally, and thus these routes are rarely used in the ED.91,133
Ketamine can occasionally induce excessive salivation, and to combat this, some have historically coadministered atropine or glycopyrrolate. However, there is no evidence that either of these anticholinergic agents diminishes the risk for airway or respiratory adverse events,134–136 and their routine prophylactic use is no longer recommended.134 Instead, they can be reserved for treatment should excessive secretions occur.
Adult Use.: The safety and efficacy of ketamine are well established in ED adults despite less published experience than in children.134,137–143 Such experience is corroborated by the wide and successful use of ketamine in adults throughout the developing world for both minor and major surgery, particularly in areas lacking resources for inhalational anesthesia.129,130,141,142
Ketamine presents potential risk to patients with coronary artery disease because it is sympathomimetic and produces mild to moderate increases in blood pressure, heart rate, and myocardial oxygen consumption. Accordingly, other sedatives are preferred in the setting of known or possible ischemic heart disease, congestive heart failure, or hypertension.134,142 There is no accepted maximum age for ketamine; instead, emergency physicians must weigh the risks and benefits of ketamine in older adults who may have unrecognized coronary artery disease.
Hallucinatory so-called emergence reactions have been reported in up to 30% of adults receiving ketamine (though rare in children) and can be fascinating and pleasurable or, alternatively, unpleasant and nightmarish.129 In adults, coadministered IV midazolam (0.03 mg/kg) can modestly reduce their incidence (number needed to benefit of 6),140 although not all such reactions are clinically important and this therapy should be considered optional.144
Pediatric Use.: Ketamine is an ideal agent to facilitate short, painful procedures in children because of its superbly documented ED safety and efficacy.* The IM route is simple and effective, with venous access being unnecessary. IV administration is attractive because a lower cumulative dose can be used and recovery is faster than with the IM route. The primary caution with this route is that the initial bolus of ketamine must be administered slowly (over a 30- to 60-second period) or respiratory depression and transient apnea can occur.132
Unpleasant recovery reactions are uncommon in children and teenagers and are typically mild when they do occur.146,147 Benzodiazepine coadministration does not measurably reduce the incidence of such reactions in children (unlike adults),134,135,145–147 and these agents should be reserved for treating preprocedural anxiety or unpleasant reactions if they occur.134
Adverse Effects.: In a metaanalysis of ketamine administration in 8282 pediatric patients, the overall incidence of airway and respiratory adverse events was 3.9%—primarily airway malalignment but also including transient laryngospasm (0.3%) and transient apnea (0.8%). None of these patients were intubated or had adverse sequelae.134 The frequency of such events was slightly higher when unusually high doses of ketamine were administered; otherwise, there was no clinically important association with age, doses within the typically recommended range, or other clinical factors.
Vomiting was noted in 8.4% overall in this same metaanalysis, with early adolescence representing the greatest risk and a lower incidence of emesis occurring in younger and older children.145 It occurs more frequently with the IM route than with the IV route, and there is no evidence to support any dose relationship within the usual range of clinically administered doses.145 When emesis occurs, it is typically late during the recovery phase when the patient is alert and can clear the airway without assistance.134 Vomiting does occur in some patients after discharge, including some who do not vomit in the ED.134 Although ondansetron prophylaxis (0.15 mg/kg up to a maximum of 4 mg intravenously) does reduce vomiting with ketamine, the magnitude of this effect is modest (number needed to benefit of 13), and thus it cannot be considered mandatory.148
In 40 years of regular use there have been no documented reports of clinically significant ketamine-associated aspiration in patients without established contraindications.134 Because of its unique preservation of protective airway reflexes, ketamine may be preferred over other agents for urgent or emergency procedures when fasting is not ensured.4,5,129
Mild agitation (whimpering or crying) during recovery was noted in 7.6% of children in the large metaanalysis, with more pronounced agitation occurring in 1.4%. Such recovery reactions are not related to age, dose, or other factors to any clinically important degree, except for a higher incidence with subdissociative (<3 mg/kg intramuscularly) dosing.145 In contrast to traditional thinking, adolescents are not at substantially higher risk.145
Despite the modest impact of prophylactic midazolam in reducing adult recovery agitation, in children, two controlled trials and a large metaanalysis failed to note even a trend toward a measurable benefit.145–147 Children have far fewer recovery reactions than adults do, and their reactions are milder when they do occur. Midazolam is therefore not recommended for routine prophylaxis but is optimally reserved to treat unpleasant ketamine-associated recovery reactions when they do rarely occur.134
Ketamine is relatively contraindicated in patients with central nervous system masses, abnormalities, or hydrocephalus; however, there is no compelling evidence that it needs to be avoided in the setting of acute head trauma.134,149,150 Repeated reports that ketamine can increase intracranial pressure have prompted traditional caution against use of this drug in the setting of real or potential neurologic compromise,1,5,129,134,151–153 and there are case reports of deterioration in patients with hydrocephalus.154,155 However, newer suggestive evidence indicates that in most patients the resulting increases in pressure are minimal, assuming normal ventilation,149,150,154,156 and that the corresponding cerebral vasodilatory effect of ketamine may actually improve overall cerebral perfusion.149,150
Dissociative sedation may represent a risk in patients with acute globe injury or glaucoma given the inconclusive and conflicting evidence of increased intraocular pressure with ketamine.157–161
Ketamine-Propofol Combination (Ketofol) for Procedural Sedation and Analgesia in the ED
The PSA combination of ketamine and propofol, referred to by the portmanteau “ketofol,” is both popular and controversial.162 There is no doubt that ketofol is safe and effective in both adults and children163–170; instead, the dispute is whether the combination exhibits any clinically important advantage over use of either drug alone.162
An important allure to ketofol proponents is how these two completely different sedatives balance each other’s deficits. Propofol is a superb sedative but lacks the analgesia that ketamine can amply provide. Ketamine mitigates propofol-induced hypotension, and propofol mitigates ketamine-induced vomiting and recovery agitation. The drugs exhibit synergistic and perhaps smoother sedation,164 and the combination has the theoretical benefits of minimizing the propofol dose and obviating the need for coadministered opioids.162
Critics note that the existing ketofol literature fails to demonstrate superior procedural conditions or less respiratory depression than occurs with each drug alone163 and question the added complexity of administering two drugs when one is sufficient. They argue that the purported advantages related to hemodynamics and the total propofol dose are not clinically important. Ketofol uses a subdissociative dose of ketamine, and thus ketamine may just be replacing fentanyl as an analgesic rather than as a sedative.162
Ketofol dosing is based on weight for both adults and children. One regimen uses an initial IV loading dose of ketamine of 0.5 mg/kg, followed by a 0.5- to 1.0-mg/kg IV propofol bolus, with subsequent titration of propofol alone.164–166,168 A second strategy mixes propofol and ketamine together 1 : 1 in the same syringe (both at 10-mg/mL concentrations) and then titrates in increments of 0.25 mg/kg of each drug to a usual effective dose of about 0.75 mg/kg.162,167,169,170
Nitrous Oxide
Pharmacology.: Inhaled nitrous oxide provides anxiolysis and mild analgesia. It is commonly dispensed at concentrations between 30% and 50%, with oxygen composing the remainder of the mixture. Nitrous oxide quickly diffuses across biologic membranes and, accordingly, has a rapid onset of action (30 to 60 seconds). Its maximum effect occurs after about 5 minutes, and the clinical effect wears off quickly on discontinuation. At typical PSA concentrations, there is preservation of hemodynamic status, spontaneous respirations, and protective airway reflexes.171–174 Nitrous oxide is widely used in dentistry at higher concentrations.175
Nitrous oxide has an excellent safety profile; however, as a sole agent, it cannot reliably produce adequate procedural conditions.171–174 Given its relatively weak analgesic properties, in many cases nitrous oxide needs to be supplemented with an IV opioid or local or regional anesthesia (or both).
Adult and Cooperative Child Use.: The safest method of administering nitrous oxide is via a self-administered demand-valve mask (Fig. 33-5).172–174 Patients must generate a negative pressure of 3 to 5 cm H2O within the handheld mask or mouthpiece to activate the flow of gas. They can thus self-titrate the dose by inhaling at will through the mask. Naturally, this will be effective only when the patient is cooperative. This technique provides a built-in fail-safe measure in that if patients become somnolent, the mask will fall from their face and gas delivery will cease.
Uncooperative Child Use.: The primary limitation of self-administration is that it is ineffective in uncooperative patients, including most frightened young children. Continuous-flow nitrous oxide via a mask strapped over the nose or over the nose and mouth has been used in this population (Fig. 33-6).176–179 Nitrous oxide can effectively produce moderate or deep sedation when administered in this manner; however, this technique necessitates an additional clinician dedicated to continuous gas titration to avoid oversedation. In addition, it appears that the continuous-flow technique is associated with a higher rate of emesis (10%) than self-administration is (0% to 4%),171–174,176–179 which may be a potential hazard if a mask is strapped tightly over the child’s mouth.177
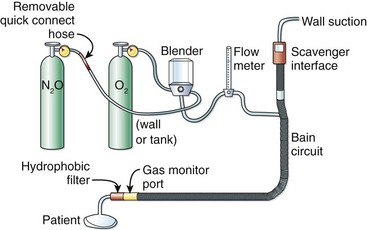
Figure 33-6 Nitrous oxide/oxygen continuous-flow system.
Adverse Effects and Precautions.: A number of generally minor adverse effects may be seen, including nausea, dizziness, changes in voice, euphoria, and laughter.171–174 Nitrous oxide should be avoided in patients with closed-space disease such as bowel obstruction, middle ear disease, pneumothorax, or pneumocephalus. Because of its property of high diffusibility, it has the potential to increase the size of the closed space. This should be unlikely for short-term use in typical PSA concentrations.
Antagonists
Naloxone is an antagonist that competitively displaces opioids from opiate receptors. It rapidly reverses the analgesic and respiratory depressant effects of opioids. It may be administered intravenously, intramuscularly, subcutaneously, or even sublingually if needed,163 and dosing has been standardized for infants and children.180 Naloxone will not induce systemic opioid withdrawal symptoms in a patient without preexisting physiologic dependence. However, some patients will experience nausea with opioid reversal, and patients with persistent pain after their procedure will be quite uncomfortable. Rapid reversal may also lead to return of anxiety and stimulation of the sympathetic nervous system. If the situation permits, careful titration of small amounts of naloxone (0.1-mg aliquots intravenously) may permit partial rather than complete reversal. The only absolute contraindication to the use of naloxone is administration to a neonate born to an opioid-dependent mother because of the risk of precipitating life-threatening opioid withdrawal. The length of observation is dose related, but usually no more than 60 to 90 minutes after reversal will be sufficient if no more than 1 mg of naloxone has been administered intravenously.
Nalmefene
Nalmefene is a long-acting opioid antagonist with a duration of action significantly longer than that of naloxone.181,182 Nalmefene may be given intravenously, intramuscularly, or subcutaneously, although intravenously is the preferred route.182,183 Intravenously, it can be titrated in incremental doses of 0.25 µg/kg every 2 to 5 minutes until the desired effect is attained. Although either naloxone or nalmefene will reverse the analgesia of opioids, naloxone is the preferred agent. For ED PSA, short-acting opioids such as fentanyl are commonly used, and administration of a reversal agent with a duration of action as prolonged as that of nalmefene does not confer any additional benefit. Furthermore, nalmefene would interfere with postprocedure control of pain with opioids.
Flumazenil
Flumazenil is a benzodiazepine antagonist that can promptly reverse benzodiazepine-induced sedation and respiratory depression.1,15,184,185 In the setting of PSA, flumazenil is a safe and effective method of reversing oversedation caused by benzodiazepines. It is not routinely used to reverse PSA because of the potential for resedation, and many clinicians prefer to allow patients to recover on their own. Flumazenil has not been shown to substantially decrease the time of observation in the ED required for a patient undergoing PSA. Flumazenil lowers the seizure threshold and may rarely lead to life-threatening seizures. It should be avoided in patients with known benzodiazepine dependence, seizure disorder, cyclic antidepressant overdose, and elevated intracranial pressure.165 It should also be given cautiously to patients who are taking medications known to lower the seizure threshold (cyclosporine, tricyclic antidepressants, propoxyphene, theophylline, isoniazid, lithium).186 These issues, however, are not usually involved in PSA in the ED. It has not been shown that simply taking therapeutic doses of these medications contraindicates the use of flumazenil, but flumazenil-induced seizures are generally associated only with drug overdose. Rapid reversal may also lead to return of anxiety and sympathetic stimulation. If the situation permits, careful titration of small amounts of flumazenil (0.1- to 0.2-mg aliquots intravenously) will reduce the risk for adverse effects and may permit partial rather than complete reversal. Observation times vary. For example, 1 hour after reversal will allow accurate assessment of residual sedation if less than 1 mg of flumazenil has been used to reverse conscious sedation with midazolam.
References
1. O’Connor, RE, Sama, A, Burton, JH, et al. Procedural sedation and analgesia in the emergency department: recommendations for physician credentialing, privileging and practice. Ann Emerg Med. 2011;58:365–370.
2. Cote, CJ, Karl, HW, Notterman, DA, et al. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633.
3. American College of Emergency Physicians. Clinical policy for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 1998;31:663.
4. Green, SM, Rothrock, SG, Lynch, EL, et al. Intramuscular ketamine for pediatric sedation in the emergency department: safety profile with 1022 cases. Ann Emerg Med. 1998;31:688.
5. Krauss, B, Green, SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342:938.
6. Pena, BMG, Krauss, B. Adverse events of procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 1999;34:483.
7. Chudnofsky, CR, Wright, SW, Dronen, SC, et al. The safety of fentanyl use in the emergency department. Ann Emerg Med. 1989;18:635.
8. American Academy of Pediatrics Committee on Drugs. Guidelines for the elective use of conscious sedation, deep sedation, and general anesthesia in pediatric patients. Pediatrics. 1985;76:317.
9. National Institutes of Health. Consensus conference—anesthesia and sedation in the dental office. JAMA. 1985;8:1073.
10. Joint Commission on Accreditation of Healthcare Organizations, Accreditation Manual for Hospitals, Oakbrook Terrace, IL, JCAHO, 2001. Available at, http://www.jcaho.org.
11. Green, SM, Krauss, B. The semantics of ketamine [editorial]. Ann Emerg Med. 2000;36:480.
12. Cote, CJ. Conscious sedation: time for this oxymoron to go away!. J Pediatr. 2001;139:15.
13. Murphy, MF. Sedation. Ann Emerg Med. 1996;27:461.
14. American Society of Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 1996;84:459.
15. Krauss, B, Green, SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766.
16. American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587.
17. American Society of Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004.
18. Green, SM, Roback, MG, Miner, JR, et al. Fasting and emergency department procedural sedation and analgesia: a consensus-based clinical practice advisory. Ann Emerg Med. 2007;49:454.
19. American Society of Anesthesiologists. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896.
20. Green, SM, Krauss, B. Pulmonary aspiration risk during ED procedural sedation—an examination of the role of fasting and sedation depth. Acad Emerg Med. 2002;9:35.
21. Green, SM. Research advances in procedural sedation and analgesia [editorial]. Ann Emerg Med. 2007;49:31.
22. Anderson, JL, Junkins, E, Pribble, C, et al. Capnography and depth of sedation during propofol sedation in children. Ann Emerg Med. 2007;49:9.
23. Deitch, K, Chudnofsky, CR, Domenici, P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized controlled trial. Ann Emerg Med. 2006;49:491.
24. Krauss, B, Hess, DR. Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;50:172.
25. Bhavani Shankar, K, Moseley, H, Kumar, AY, et al. Capnometry and anesthesia. Can J Anaesth. 1992;39:617.
26. Cote, CJ, Liu, LM, Szyfelbein, SK, et al. Intraoperative events diagnosed by expired carbon dioxide monitoring in children. Can Anaesth Soc J. 1986;33:315.
27. Kaneko, Y. Clinical perspectives on capnography during sedation and general anesthesia in dentistry. Anesth Prog. 1995;42:126.
28. Miner, JR, Heegaard, W, Plummer, D. End-tidal carbon dioxide monitoring during procedural sedation. Acad Emerg Med. 2002;9:275.
29. Poirier, MP, Gonzalez Del-Rey, JA, McAneney, CM, et al. Utility of monitoring capnography, pulse oximetry, and vital signs in the detection of airway mishaps: a hyperoxemic animal model. Am J Emerg Med. 1998;16:350.
30. Swedlow, DB. Capnometry and capnography: the anesthesia disaster early warning system. Semin Anesth. 1986;3:194.
31. Tobias, J. End-tidal carbon dioxide monitoring during sedation with a combination of midazolam and ketamine for children undergoing painful, invasive procedures. Pediatr Emerg Care. 1999;15:173.
32. Weingarten, M. Respiratory monitoring of carbon dioxide and oxygen: a 10-year retrospective. J Clin Monit Comput. 1990;6:217.
33. Williamson, JA, Webb, RK, Cockings, J, et al. The capnograph: applications and limitations—an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:551.
34. Farmery, AD, Roe, PG. A model to describe the rate of oxyhaemoglobin desaturation during apnea. Br J Anaesth. 1996;76:284.
35. Patel, R, Lenczyk, M, Hannallah, RS, et al. Age and the onset of desaturation in apnoeic children. Can J Anaesth. 1994;41:771.
36. Soto, RG, Fu, ES, Vila, H, et al. Capnography accurately detects apnea during monitored anesthesia care. Anesth Analg. 2004;99:379.
37. Deitch, K, Miner, J, Chudnofsky, CR, et al. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55:258–264.
38. Qadeer, M, Vargo, JJ, Dumot, JA, et al. Capnographic monitoring of respiratory activity improves safety of sedation for endoscopic cholangiopancreatography and ultrasonography. Gastroenterology. 2009;136:1568–1576.
39. Green, SM, Pershad, J. Should capnographic monitoring be standard practice during emergency department procedural sedation and analgesia? Pro and con [editorial]. Ann Emerg Med. 2010;55:265–267.
40. Society of Anesthesiologists. Practice advisory for intraoperative awareness and brain function monitoring. Anesthesiology. 2006;104:847.
41. Avidan, MS, Jacobsohn, E, Glick, D, et al. Prevention of intraoperative awareness in a high-risk surgical population. for the BAG-RECALL Research Group. N Engl J Med. 2011;365:591–600.
42. Miner, JR, Danahy, M, Moch, A, et al. Randomized clinical trial of etomidate versus propofol for procedural sedation in the emergency department. Ann Emerg Med. 2006;49:15.
43. Chan, MT, Gin, T. What does the bispectral EEG index monitor? Eur J Anaesthesiol. 2000;17:146.
44. Rosow, C, Manberg, P. Bispectral index monitoring. Anesthesiol Clin North Am. 1998;2:89.
45. Sleigh, J, Andrzejowski, J, Steyn-Ross, A, et al. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88:659.
46. Vissers, R, McHugh, D. Bispectral index monitoring as a continuous, non-invasive measure of sedation during procedures in the ED. Acad Emerg Med. 2000;7:529.
47. Jense, HG, Dubin, SA, Silverstein, PI, et al. Effect of obesity on safe duration of apnea in anesthetized humans. Anesth Analg. 1991;72:89.
48. Patel, R, Lenczyk, M, Hannallah, RS, et al. Age and the onset of desaturation in apnoeic children. Can J Anesth. 1994;41:771.
49. Deitch, K, Chudnofsky, CR, Domenici, P, et al. The utility of high-flow oxygen during emergency department procedural sedation and analgesia with propofol: a randomized, controlled trial. Ann Emerg Med. 2011;58:360–364.
50. Deitch, K, Chudnofsky, CR, Domenici, P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with propofol: a randomized controlled trial. Ann Emerg Med. 2008;52:1–8.
51. Deitch, K, Miner, J, Chudnofsky, CR, et al. Does ETCO2 monitoring during emergency department procedural sedation and analgesia with propofol lower the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55:258–264.
52. Green, SM, Krauss, B. Supplemental oxygen during propofol sedation: yes or no [editorial]? Ann Emerg Med. 2008;52:9–10.
53. Newman, DH, Azer, MM, Pitetti, RD, et al. When is a patient safe for discharge after procedural sedation? The timing of adverse effect events in 1,367 pediatric procedural sedations. Ann Emerg Med. 2003;42:627.
54. Kain, ZN, Mayes, LC, O’Connor, TZ, et al. Preoperative anxiety in children: predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238.
55. Kain, Z, Mayes, L, Caramico, L, et al. Distress during induction of anesthesia and postoperative behavioral outcomes. Anesth Analg. 1999;88:1042.
56. Kain, Z, Mayes, L, Caramico, L, et al. Postoperative behavioral outcomes in children: effects of sedative premedication. Anesthesiology. 1999;90:758.
57. McCann, ME, Kain, ZN. The management of preoperative anxiety in children: an update. Anesth Analg. 2001;93:98.
58. Kendall, JM, Reeves, BC, Latter, VS. Multicentre randomised controlled trial of nasal diamorphine for analgesia in children and teenagers with clinical fractures. BMJ. 2001;322:261.
59. Wilson, JA, Kendall, JM, Cornelius, P. Intranasal diamorphine for paediatric analgesia: assessment of safety and efficacy. J Accid Emerg Med. 1997;14:70.
60. Paech, MJ, Lim, CB, Banks, SL, et al. A new formulation of nasal fentanyl spray for postoperative analgesia: a pilot study. Anaesthesia. 2003;58:740.
61. Borland, M, Jacobs, I, King, B, et al. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. 2007;49:335.
62. Duff, AJA. Incorporating psychological approaches into routine paediatric venipuncture. Arch Dis Child. 2003;88:931.
63. Chen, E, Joseph, MH, Zeltzer, LK. Behavioral and cognitive interventions in the treatment of pain in children. Pediatr Clin North Am. 2000;47:513.
64. Kennedy, RM, Luhmann, JD. The “ouchless emergency department.” Getting closer: advances in decreasing distress during painful procedures in the emergency department. Pediatr Clin North Am. 1999;46:1215.
65. Binder, LS, Leake, LA. Chloral hydrate for emergent pediatric procedural sedation: a new look at an old drug. Am J Emerg Med. 1991;9:530.
66. Olson, DM, Sheehan, MG, Thompson, W, et al. Sedation of children for electroencephalograms. Pediatrics. 2001;108:163.
67. Malviya, S, Voepel-Lewis, T, Prochaska, G, et al. Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics. 2000;105:E42.
68. D’Agostino, J, Terndrup, TE. Chloral hydrate versus midazolam for sedation of children for neuroimaging: a randomized clinical trial. Pediatr Emerg Care. 2000;16:1.
69. Greenberg, SB, Faerber, EN, Aspinall, CL, et al. High-dose chloral hydrate sedation for children undergoing MR imaging: safety and efficacy in relation to age. AJR Am J Roentgenol. 1993;161:639.
70. Hubbard, AM, Markowitz, RI, Kimmel, B, et al. Sedation for pediatric patients undergoing CT and MRI. J Comput Assist Tomogr. 1992;16:3.
71. Malviya, S, Voepel-Lewis, T, Tait, AR. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg. 1997;85:1207.
72. Vade, A, Sukhani, R, Dolenga, M, et al. Chloral hydrate sedation in children undergoing CT and MR imaging: safety as judged by American Academy of Pediatrics (AAP) guidelines. AJR Am J Roentgenol. 1995;165:905.
73. Pereira, JK, Burrows, PE, Richards, HM, et al. Comparison of sedation regiments for pediatric outpatient CT. Pediatr Radiol. 1993;23:341.
74. American Academy of Pediatrics Committee on Drugs. Use of chloral hydrate for sedation in children. Pediatrics. 1993;92:471.
75. Bailey, PL. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826.
76. Karl, HW, Cote, CJ, McCubbin, MM, et al. Intravenous midazolam for sedation of children undergoing procedures: an analysis of age and procedure-related factors. Pediatr Emerg Care. 1999;15:167.
77. Davies, FC, Waters, M. Oral midazolam for conscious sedation of children during minor procedures. J Accid Emerg Med. 1998;15:244.
78. Massanari, M, Novitsky, J, Reinstein, LJ. Paradoxical reactions in children associated with midazolam use during endoscopy. Clin Pediatr (Phila). 1997;36:681.
79. Moro-Sutherland, DM, Algren, JT, Louis, PT, et al. Comparison of intravenous midazolam with pentobarbital for sedation for head computed tomography imaging. Acad Emerg Med. 2000;7:1370.
80. Strain, JD, Campbell, JB, Harvey, LA, et al. IV Nembutal: safe sedation for children undergoing CT. AJR Am J Roentgenol. 1988;151:975.
81. McGlone, RG, Fleet, T, Durham, S, et al. A comparison of intramuscular ketamine with high-dose intramuscular midazolam with and without intranasal flumazenil in children before suturing. J Emerg Med. 2001;18:34.
82. Connors, K, Terndrup, TE. Nasal versus oral midazolam for sedation of anxious children undergoing laceration repair. Ann Emerg Med. 1994;24:1074.
83. Fatovich, DM, Jacobs, IG. A randomized, controlled trial of oral midazolam and buffered lidocaine for suturing lacerations in children (the SLIC trial). Ann Emerg Med. 1995;25:209.
84. Feld, LH, Negus, JB, White, PF. Oral midazolam preanesthetic medication in pediatric outpatients. Anesthesiology. 1990;73:831.
85. Haas, DA, Nenniger, SA, Yacobi, R, et al. A pilot study of the efficacy of oral midazolam for sedation in pediatric dental patients. Anesth Prog. 1996;43:1.
86. Younge, PA, Kendall, JM. Sedation for children requiring wound repair: a randomized controlled double-blind comparison of oral midazolam and oral ketamine. J Emerg Med. 2001;18:30.
87. McGlone, RG, Ranasinghe, S, Durham, S. An alternative to “brutacaine”: a comparison of low-dose intramuscular ketamine with intranasal midazolam in children before suturing. J Accid Emerg Med. 1998;15:231.
88. Abrams, R, Morrison, JE, Villasenor, A, et al. Safety and effectiveness of intranasal administration of sedative medications (ketamine, midazolam, or sufentanil) for urgent brief pediatric dental procedures. Anesth Prog. 1993;40:63.
89. Ackworth, JP, Purdie, D, Clark, RC. Intravenous ketamine plus midazolam is superior to intranasal midazolam for emergency pediatric procedural sedation. J Emerg Med. 2001;18:39.
90. Theroux, MC, West, DW, Corddry, DH, et al. Efficacy of intranasal midazolam in facilitating suturing of lacerations in preschool children in the emergency department. Pediatrics. 1993;91:624.
91. Tanaka, M, Sato, M, Saito, A, et al. Reevaluation of rectal ketamine premedication in children: comparison with rectal midazolam. Anesthesiology. 2000;93:1217.
92. Kennedy, RM, Porter, FL, Miller, JP, et al. Comparison of fentanyl/midazolam with ketamine/midazolam for pediatric orthopedic emergencies. Pediatrics. 1998;102:956.
93. Sievers, TD, Yee, JD, Foley, ME, et al. Midazolam for conscious sedation during pediatric oncology procedures: safety and recovery parameters. Pediatrics. 1991;88:1172.
94. Bloomfield, EL, Masaryk, TJ, Caplin, A, et al. Intravenous sedation for MR imaging of the brain and spine in children: pentobarbital versus propofol. Radiology. 1993;186:93.
95. Egelhoff, JC, Ball, WS, Jr., Koch, BL, et al. Safety and efficacy of sedation in children using a structured sedation program. AJR Am J Roentgenol. 1997;168:1259.
96. Green, SM. Propofol for emergency department procedural sedation—not yet ready for prime time [editorial]. Acad Emerg Med. 1999;6:975.
97. Miner, JR, Burton, JH. Clinical practice advisory: emergency department procedural sedation with propofol. Ann Emerg Med. 2007;32:249.
98. Bassett, KE, Anderson, JL, Pribble, CG, et al. Propofol for procedural sedation in children in the emergency department. Ann Emerg Med. 2003;42:773.
99. Burton, JH, Miner, JR, Shipley, ER, et al. Propofol for emergency department procedural sedation and analgesia: a tale of three centers. Acad Emerg Med. 2006;13:24.
100. Guenther, E, Pribble, CG, Junkins, EP, et al. Propofol sedation by emergency physicians for elective pediatric outpatient procedures. Ann Emerg Med. 2003;42:783.
101. Green, SM, Krauss, B. Propofol in emergency medicine: pushing the sedation frontier [editorial]. Ann Emerg Med. 2003;42:792.
102. Havel, CJ, Strait, RT, Hennes, H. A clinical trial of propofol vs midazolam for procedural sedation in a pediatric emergency department. Acad Emerg Med. 1999;6:989.
103. Lowrie, L, Weiss, AH, Lacombe, C. The pediatric sedation unit: a mechanism for pediatric sedation. Pediatrics. 1998;102:E30.
104. Mallory, MD, Baxter, AL, Yanosky, DJ, et al. Emergency physician–administered propofol sedation: a report on 25,433 sedations from the Pediatric Sedation Research Consortium. Ann Emerg Med. 2011;57:462–468.
105. Burton, JH, Bock, AJ, Strout, TD, et al. Etomidate and midazolam for reduction of anterior shoulder dislocation: a randomized, controlled trial. Ann Emerg Med. 2002;40:496.
106. Ruth, WJ, Burton, JH, Bock, AJ. Intravenous etomidate for procedural sedation in emergency department patients. Acad Emerg Med. 2001;8:13.
107. Vinson, DR, Bardbury, DR. Etomidate for procedural sedation in emergency medicine. Ann Emerg Med. 2002;39:592.
108. Hunt, GS, Spencer, MT, Hays, DP. Etomidate and midazolam for procedural sedation: prospective, randomized trial. Am J Emerg Med. 2005;23:299.
109. DiLiddo, L, D’Angelo, A, Nguyen, B, et al. Etomidate versus midazolam for procedural sedation in pediatric outpatients: a randomized controlled trial. Ann Emerg Med. 2006;48:433.
110. Dickinson, R, Singer, AJ, Carrion, W. Etomidate for pediatric sedation prior to fracture reduction. Acad Emerg Med. 2001;8:74.
111. Yealy, DM. Safe and effective…maybe: etomidate in procedural sedation/analgesia [editorial]. Acad Emerg Med. 2001;8:68.
112. Miner, JR, Biros, MH, Heegaard, W, et al. Bispectral electroencephalographic analysis of patients undergoing procedural sedation in the emergency department. Acad Emerg Med. 2003;10:638.
113. Schenarts, CL, Burton, JH, Riker, RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8:1.
114. Lerman, B, Yoshida, D, Levitt, MA. A prospective evaluation of the safety and efficacy of methohexital in the emergency department. Am J Emerg Med. 1996;14:351.
115. Beekman, RP, Hoorntje, TM, Beek, FJ, et al. Sedation for children undergoing magnetic resonance imaging: efficacy and safety of rectal thiopental. Eur J Pediatr. 1996;155:820.
116. Daniels, AL, Cote, CJ, Polaner, DM. Continuous oxygen saturation monitoring following rectal methohexitone induction in paediatric patients. Can J Anaesth. 1992;39:27.
117. Glasier, CM, Stark, JE, Brown, R, et al. Rectal thiopental sodium for sedation of pediatric patients undergoing MR and other imaging studies. AJNR Am J Neuroradiol. 1995;16:111.
118. Manuli, MA, Davies, L. Rectal methohexital for sedation of children during imaging procedures. AJR Am J Roentgenol. 1993;160:577.
119. O’Brien, JF, Falk, JL, Carey, BE, et al. Rectal thiopental compared with intramuscular meperidine, promethazine, and chlorpromazine for pediatric sedation. Ann Emerg Med. 1991;20:644.
120. Pomeranz, ES, Chudnofsky, CR, Deegan, TJ, et al. Rectal methohexital sedation for computed tomography imaging of stable pediatric emergency department patients. Pediatrics. 2000;105:1110.
121. Sedik, H. Use of intravenous methohexital as a sedative in pediatric emergency departments. Arch Pediatr Adolesc Med. 2001;155:665.
122. Billmire, DA, Neale, HW, Gregory, RO. Use of IV fentanyl in the outpatient treatment of pediatric facial trauma. J Trauma. 1985;25:1079.
123. Pohlgeers, AP, Friedland, LF, Keegan-Jones, L. Combination fentanyl and diazepam for pediatric conscious sedation. Acad Emerg Med. 1995;2:879.
124. Schechter, NL, Weisman, SJ, Rosenblum, M, et al. The use of oral transmucosal fentanyl citrate for painful procedures in children. Pediatrics. 1995;95:335.
125. Schutzman, SA, Liebelt, E, Wisk, M, et al. Comparison of oral transmucosal fentanyl citrate and intramuscular meperidine, promethazine, and chlorpromazine for conscious sedation of children undergoing laceration repair. Ann Emerg Med. 1996;28:385.
126. Goldman, RD. Intranasal drug delivery for children with acute illness. Curr Drug Ther. 2006;1:127.
127. Bates, BA, Schutzman, SA, Fleisher, GR. A comparison of intranasal sufentanil and midazolam to intramuscular meperidine, promethazine, and chlorpromazine for conscious sedation in children. Ann Emerg Med. 1994;24:646.
128. Litman, RS. Conscious sedation with remifentanil and midazolam during brief painful procedures in children. Arch Pediatr Adolesc Med. 1999;153:1085.
129. Green, SM, Johnson, NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19:1033.
130. Green, SM, Clem, KJ, Rothrock, SG. Ketamine safety profile in the developing world—survey of practitioners. Acad Emerg Med. 1996;3:598.
131. Green, SM, Kupperman, N, Rothrock, SG, et al. Predictors of adverse events with ketamine sedation in children. Ann Emerg Med. 2000;35:35.
132. Green, SM, Rothrock, SG, Harris, T, et al. Intravenous ketamine for pediatric sedation in the emergency department: safety and efficacy with 156 cases. Acad Emerg Med. 1998;5:971.
133. Qureshi, F, Mellis, PT, McFadden, MA. Efficacy of oral ketamine for providing sedation and analgesia to children requiring laceration repair. Pediatr Emerg Care. 1995;11:93.
134. Green, SM, Roback, MG, Kennedy, RM, et al. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57:449–461.
135. Green, SM, Roback, MG, Krauss, B, et al. Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: an individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:158–168.
136. Green, SM, Roback, MG, Krauss, B. Anticholinergics and ketamine sedation in children: a secondary analysis of atropine versus glycopyrrolate. Acad Emerg Med. 2010;17:157–162.
137. Newton, A, Fitton, L. Intravenous ketamine for adult procedural sedation in the emergency department: a prospective cohort study. Emerg Med J. 2008;25:498–501.
138. Strayer, RJ, Nelson, LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26:985–1028.
139. Vardy, JM, Dignon, N, Mukherjee, N, et al. Audit of the safety and effectiveness of ketamine for procedural sedation in the emergency department. Emerg Med J. 2008;25:579–582.
140. Sener, S, Eken, C, Schultz, CH, et al. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med. 2011;57:109–114.
141. Green, SM, Li, J. Ketamine in adults: what emergency physicians need to know about patient selection and emergence reactions [editorial]. Acad Emerg Med. 2000;7:278.
142. Li, J, Ketamine: emergency applications. Plantz SH, ed. Emergency Medicine Text. Boston Medical Publishing: Boston, 1999. Available at, http://www.emedicine.com/emerg/topic802.htm.
143. Chudnofsky, CR, Weber, JE, Stoyanoff, PJ, et al. A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients. Acad Emerg Med. 2000;7:228.
144. Green, SM, Krauss, B. The taming of ketamine—40 years later. Ann Emerg Med. 2011;57:115–116.
145. Green, SM, Roback, MG, Krauss, B, et al. Predictors of emesis and recovery agitation with emergency department ketamine sedation: an individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:171–180.
146. Sherwin, TS, Green, SM, Khan, A, et al. Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2000;35:239.
147. Wathen, JE, Roback, MG, Mackenzie, T, et al. Does midazolam alter the clinical effects of intravenous ketamine sedation in children? A double-blind, randomized, controlled emergency department trial. Ann Emerg Med. 2000;36:579.
148. Langston, WT, Nathen, JE, Roback, MG, et al. Effect of ondansetron on the incidence of vomiting associated with ketamine sedation in children: a double-blind, randomized, placebo controlled trial. Ann Emerg Med. 2008;52:30.
149. Bar-Joseph, G, Guilburd, Y, Tamir, A, et al. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. J Neurosurg Pediatr. 2009;4:40–46.
150. Himmelseher, S, Durieux, ME. Revising a dogma: ketamine for patients with neurological injury? Anesth Analg. 2005;101:524–534.
151. Tjaden, RJ, Ethier, R, Gilbert, RG, et al. The use of CI-581 (Ketalar) for pediatric pneumoencephalography. J Can Assoc Radiol. 1969;20:155–156.
152. Takeshita, H, Okuda, Y, Sari, A. The effects of ketamine on cerebral circulation and metabolism in man. Anesthesiology. 1972;36:69–75.
153. Shapiro, HM, Wyte, SR, Harris, AB. Ketamine anaesthesia in patients with intracranial pathology. Br J Anaesth. 1972;44:1200–1204.
154. Yehuda, YB, Watemberg, N. Ketamine increases opening cerebrospinal pressure in children undergoing lumbar puncture. J Child Neurol. 2006;21:441–443.
155. Lockhart, CH, Jenkins, JJ. Ketamine induced apnea in patients with increased intracranial pressure. Anesthesiology. 1972;37:92–93.
156. Mayberg, TS, Lam, AM, Matta, BF, et al. Ketamine does not increase cerebral blood flow velocity or intracranial pressure during isoflurane/nitrous oxide anesthesia in patients undergoing craniotomy. Anesth Analg. 1995;81:84–89.
157. Yoshikawa, K, Murai, Y. The effect of ketamine on intraocular pressure in children. Anesth Analg. 1971;50:199–202.
158. Harris, JE, Letson, RD, Buckley, JJ. The use of CI-581, a new parenteral anesthetic, in ophthalmic practice. Trans Am Ophthalmol Soc. 1968;66:206–213.
159. Ausinsch, B, Rayburnx, RL, Munson, ES, et al. Ketamine and intraocular pressure in children. Anesth Analg. 1976;55:773–775.
160. Adams, A. Ketamine in paediatric ophthalmic practice. Anaesthesia. 1973;28:212–213.
161. Peuler, M, Glass, DD, Arens, JF. Ketamine and intraocular pressure. Anesthesiology. 1975;43:575–578.
162. Green, SM, Andolfatto, G, Krauss, B. Ketofol for procedural sedation? Pro and con [editorial]. Ann Emerg Med. 2011;57:444–448.
163. Andolfatto, G, Abu-Laban, RB, Zed, PJ, et al. Ketamine-propofol combination (Ketofol) versus propofol alone for emergency department procedural sedation and analgesia: a randomized double-blind trial. Ann Emerg Med. 2012;59:504–512. [e1-e2].
164. David, H, Shipp, J. Combined ketamine/propofol for emergency department procedural sedation. Ann Emerg Med. 2011;57:435–441.
165. Messenger, DW, Murray, HE, Dungey, PE, et al. Subdissociative-dose ketamine versus fentanyl for analgesia during propofol procedural sedation: a randomized clinical trial. Acad Emerg Med. 2008;15:877–886.
166. Sharieff, GQ, Trocinski, DR, Kanegaye, JT, et al. Ketamine-propofol combination sedation for fracture reduction in the pediatric emergency department. Pediatr Emerg Care. 2007;23:881.
167. Andolfatto, G, Willman, E. A prospective case series of single-syringe ketamine-propofol (ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med. 2011;18:237–245.
168. Shah, A, Mosdossy, G, McLeod, S, et al. A blinded, randomized controlled trial to evaluate ketamine-propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med. 2011;57:425–433.
169. Andolfatto, G, Willman, EV. A prospective case series of pediatric procedural sedation and analgesia in the emergency department using single-syringe ketamine-propofol combination (ketofol). Acad Emerg Med. 2010;17:194–201.
170. Willman, EV, Andolfatto, G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23.
171. Annequin, D, Carbajal, R, Chauvin, P, et al. Fixed 50% nitrous oxide oxygen mixture for painful procedures: a French survey. Pediatrics. 2000;105:e47.
172. Burton, JH, Auble, TE, Fuchs, SM. Effectiveness of 50% nitrous oxide/50% oxygen during laceration repair in children. Acad Emerg Med. 1998;5:112.
173. Hennrikus, WL, Shin, AY, Klingelberger, CE. Self-administered nitrous oxide and a hematoma block for analgesia in the outpatient reduction of fractures in children. J Bone Joint Surg Am. 1995;77:335.
174. Wattenmaker, I, Kasser, JR, McGravey, A. Self-administered nitrous oxide for fracture reduction in children in an emergency room setting. J Orthop Trauma. 1990;4:35.
175. Wilson, S. A survey of the American Academy of Pediatric Dentistry membership: nitrous oxide and sedation. Pediatr Dent. 1996;18:287.
176. Gamis, AS, Knapp, JF, Glenski, JA. Nitrous oxide analgesia in a pediatric emergency department. Ann Emerg Med. 1989;18:177.
177. Krauss, B. Continuous-flow nitrous oxide: searching for the ideal procedural anxiolytic for toddlers. Ann Emerg Med. 2001;37:61.
178. Luhmann, JD, Kennedy, RM, Jaffe, DM, et al. Continuous-flow delivery of nitrous oxide and oxygen: a safe and cost-effective technique for inhalation analgesia and sedation of pediatric patients. Pediatr Emerg Care. 1999;15:388.
179. Luhmann, JD, Kennedy, RM, Porter, FL, et al. A randomized clinical trial of continuous-flow nitrous oxide and midazolam for sedation of young children during laceration repair. Ann Emerg Med. 2001;37:20.
180. American Academy of Pediatrics Committee on Drugs. Naloxone dosage and route of administration for infants and children: addendum to emergency drug doses for infants and children. Pediatrics. 1990;86:484.
181. Barsan, WG, Seger, D, Danzl, DF, et al. Duration of antagonistic effects of nalmefene and naloxone in opiate-induced sedation for emergency department procedures. Am J Emerg Med. 1989;7:155.
182. Nalmefene—a long-acting injectable opioid antagonist. Med Lett. 1995;37:97.
183. Glass, PSA, Jhaveri, RM, Smith, R. Comparison of potency and duration of action of nalmefene and naloxone. Anesth Analg. 1994;78:536.
184. Chudnofsky, CR, for the Emergency Medicine Conscious Sedation Study Group. Safety and efficacy of flumazenil in reversing conscious sedation in the emergency department. Acad Emerg Med. 1997;4:944.
185. Shannon, M, Albers, G, Burkhart, K, et al. Safety and efficacy of flumazenil in the reversal of benzodiazepine-induced conscious sedation. J Pediatr. 1997;131:582.
186. Sugarman, JM, Paul, RI. Flumazenil: a review. Pediatr Emerg Care. 1994;10:37.