Neurologic system
A Arteriovenous malformation neurosurgery
Arteriovenous malformations (AVMs) are congenital, intracerebral networks in which arteries flow directly into veins. Patients with these malformations generally are younger than those with aneurysms. Patients may have bleeding or seizures or, less commonly, ischemia resulting from “steal” from normal areas or occurring with high-output congestive heart failure.
The anesthetic problems parallel those associated with patients undergoing aneurysm surgery. Notably, AVMs do not autoregulate their blood flow. The operation is likely to be longer and bloodier than that of aneurysm clipping. Surgery may be preceded by an attempt at embolization by the neuroradiologist to diminish the risk of surgery. The neurologic examination should be repeated after embolization to document new deficits that otherwise might be attributed to anesthesia and surgery.
B Awake craniotomy
In a small percentage of patients (those in whom a seizure focus may be suppressed during general anesthesia or may be adjacent to an area of eloquent cortical function), awake craniotomy may be necessary. Awake craniotomy is the most reliable method to ensure neurologic integrity in cerebral gliomas that infiltrate or come close to the eloquent areas of the brain. It allows for the localization of eloquent cortical areas by electrical stimulation and epileptic foci through cortical recordings. Continuous monitoring of the functional integrity of the brain in awake patients is inherently protective while surgical removal of the gliomatous tissue is performed.
a) Patient selection: To minimize the risk of intraoperative complications, contraindications for awake craniotomy include developmental delay, lack of maturity, an exaggerated or unacceptable response to pain, a significant communication barrier, and a failure to obtain patient consent. Only patients who have the ability to clearly understand risks and benefits and, in the opinion of the neurosurgeon, will cooperate during surgery should be considered as candidates for an awake craniotomy. Seizure management should be optimized with acceptable levels of antiepileptic medications verified.
b) Patient teaching: The single most important element in successful awake craniotomy is a highly motivated, well-informed patient. Each step of the procedure is discussed with the patient and family. Special emphasis is paid to prolonged surgical procedure, positioning, head immobility, pain anxiety, monitoring, noise, seizure management, and any individual considerations.
a) Upon arrival to the holding area, an intravenous (IV) line is established.
b) Preanesthesia medications (antibiotics, steroids, antiemetic prophylaxis, and anticonvulsants as indicated) are administered.
c) In the operating room suite, the application of noninvasive monitoring is completed.
d) The patient is induced with propofol. Some sources use either dexmedetomidine singly or in combination with propofol.
e) After satisfactory general anesthesia is established, a laryngeal mask airway (LMA) is placed with patient ventilation controlled using a continuous propofol infusion.
f) Invasive monitoring is established (arterial line, central line), and a urinary catheter is placed.
g) The scalp is anesthetized with 0.5% bupivacaine, and the head is placed in a pinion head holder.
h) The patient is carefully positioned with all bony surfaces padded.
i) The patient is carefully secured to the table to minimize a sense of falling when the table is moved during the awake phase of the surgery. Frameless stereotaxis registration is accomplished.
j) Depending on the preoperative radiographic edema findings, hypertonic saline or mannitol is given.
k) During the draping, an area is constructed around the patient’s face such that the face may be clearly seen and accessed.
l) A light is introduced under the drapes to keep the patient from darkness.
m) During the scalp opening, spontaneous ventilation is established. Before bone flap removal, the LMA is removed and verbal contact established.
(1) All sedation is stopped. All issues regarding patient comfort and concerns are addressed before the incision of the dura.
(2) Conversation with the patient is confined to the surgeon and one member of the anesthesia team.
(3) Stimulation of eloquent areas is carried out with results noted.
(4) Any seizures are controlled with propofol. After the stimulation and mapping, volumetric surgical removal of the tumor or seizure focus is accomplished with interval monitoring.
(5) Upon completion of the surgical removal and requisite monitoring, propofol sedation may be restarted and titrated to patient preference. Sedation is discontinued upon conclusion of surgery.
(6) The most common complications associated with awake craniotomy are pain, seizures, nausea, and confusion.
C Cerebral aneurysm
Cerebral aneurysms are abnormal, localized dilations of the intracranial arteries. They are classified as berry or saccular, mycotic, traumatic, fusiform, neoplastic, or atherosclerotic. Rupture of a saccular aneurysm is a leading cause of subarachnoid hemorrhage (SAH).
Approximately 5 million people in North America have cerebral aneurysms, with approximately 30,000 new cases of SAH occurring annually. The peak age for rupture of a cerebral aneurysm is 55 to 60 years. Aneurysmal ruptures are more common in women, occurring in three women for every two men.
More than one-third of patients with SAH die or develop significant and lasting neurologic disabilities before they receive any treatment. A small bleed occurs in approximately 50% of patients and is often tragically ignored or misdiagnosed. Even in patients who receive prompt care, only half remain functional survivors; the other half of patients die or develop serious neurologic deficits.
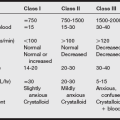
Aneurysms may arise at any point in the circle of Willis. The most common locations of aneurysms are shown in the following table. Most aneurysms are broad based and located in the middle cerebral system. Traumatic aneurysms develop as a result of direct trauma to an artery with injury to the wall.
Mirror aneurysms of the internal carotid system are common, and other combinations of locations occur (see table below). The site of the bleeding aneurysm is best located by computed tomography (CT) studies, evidence of vasospasm in the immediate vicinity, and lobulation of the aneurysm wall on angiographic studies.
Location and Occurrence of Cerebral Aneurysms
| Location | Occurrence (%) |
| Internal carotid | 38 |
| Anterior cerebral system | 36 |
| Anterior communicating junction | 30 |
| Internal carotid at posterior communicating junction | 25 |
| Middle cerebral system | 21 |
| Vertebrobasilar system | 5 |
From Frost AEM: Management of neurosurgical anesthesia: aneurysms. Curr Rev Clin Anesth 1991; 11:125-132.
b) Diagnosis of subarachnoid hemorrhage: Subarachnoid hemorrhage produces an abrupt intense headache in 85% of patients, and transient loss of consciousness may be seen in up to 45% of patients. Nausea and vomiting, photophobia, fever, meningismus, and focal neurologic deficits are common. The severity of an SAH can be graded clinically with the use of classifications listed in the table on pg. 372. Although surgical mortality rates vary somewhat among institutions, patients with a neurologic grade I SAH generally undergo surgical clipping with a low mortality rate (less than 5%), but grade V patients generally do not survive.
Hunt’s Classification of Patients with Intracranial Aneurysms According to Surgical Risk
| Grade | Perioperative Criterion | Mortality Rate (%) |
| I | Asymptomatic or minimal headache and slight nuchal rigidity | 0-5 |
| II | Moderate to severe headache, nuchal rigidity, no neurologic deficit, possible cranial nerve palsy | 2-10 |
| III | Drowsiness, confusion, or mild focal deficit | 10-15 |
| IV | Stupor, moderate to severe hemiparesis, possibly early decerebrate rigidity and vegetative disturbances | 60-70 |
| V | Deep coma, decerebrate rigidity, moribund appearance | 70-100 |
From Hunt WE, Hess RM: Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968; 28:14.
Hypertension often accompanies acute SAH and is postulated to develop secondary to autonomic hyperactivity, which may increase transmural pressure in the aneurysmal sac. Transmural pressure is defined as the differential pressure between mean arterial pressure (MAP) and intracranial pressure (ICP) and represents the stress applied to the aneurysm’s wall.
Increases in blood pressure directly increase the transmural pressure and the likelihood of bleeding; conversely, reductions in blood pressure reduce transmural pressure. Caution should be exercised when purposefully reducing transmural pressure because cerebral autoregulation may be impaired after SAH, and a reduction in blood pressure may induce or aggravate cerebral ischemia, particularly if vasospasm is present. To balance these opposing concerns, many neurosurgeons attempt to maintain systolic blood pressure between 120 and 150 mmHg before clipping the aneurysm.
Electrocardiographic (ECG) changes are common after SAH and have been reported to occur in 50% to 80% of patients. The most common changes involve the T wave or the ST segment, but other changes such as the presence of a U wave, QTc-interval prolongation, and dysrhythmias may be present. Whether such changes in the ECG represent myocardial injury has long been debated. In the majority of patients, these changes do not appear to be associated with adverse neurologic or cardiac outcomes.
Rebleeding from a previously ruptured aneurysm is a life-threatening complication. The incidence of rebleeding is approximately 50% in the first days after SAH, and rebleeding is associated with an 80% mortality rate. The chance of rebleeding from an unsecured aneurysm declines over time, and by 6 months, the risk stabilizes at approximately 3% per year. Approaches used to decrease the risk of rebleeding include early surgical clipping, the use of antifibrinolytic agents, and blood pressure control.
(a) Vasospasm is reactive narrowing of cerebral arteries after SAH. Although arterial narrowing may be detected with angiography in 60% of patients, only half of these patients develop clinical symptoms. The accompanying neurologic deterioration, arising from impaired cerebral perfusion, ischemia, and secondary infarction of the brain, peaks between the fourth and ninth days after SAH and resolves over the following 2 to 3 weeks.
(b) Successful treatment of vasospasm depends on the maintenance of adequate cerebral perfusion pressure (CPP). This is accomplished with expansion of the intravascular volume, which augments blood pressure and cardiac output, avoidance of hyponatremia, and preservation of relative hemodilution (hematocrit approximately 32%). Because of the risk of rebleeding, both hypertension and hypervolemia are used with caution in the period preceding surgical correction.
(c) Pharmacologic vasodilatation of spastic vessels has been ineffective because vasospasm involves a structural alteration in the vessel wall rather than just a spastic contracture or failure of relaxation of the smooth muscle cells in the media of the vessels. Nimodipine and nicardipine (calcium channel blockers) are currently in wide use for prevention of delayed neurologic deficit after SAH. They diminish the level of myoplastic calcium in smooth muscle cells and impede the entry of extracellular calcium necessary for the contraction of the smooth muscle. Recent literature supports a role for magnesium as possible vasospasm prophylaxis.
The presence or absence of vasospasm on angiographic studies has frequently determined the timing of aneurysmal surgery. Current neurosurgical practice suggests that a good outcome is achieved with early operation (within 24 to 48 hours) in patients who are neurologically intact (grade I or II) regardless of whether vasospasm has been demonstrated. Such emergency intervention decreases the likelihood of rebleeding. Only 53% of grade III patients achieve a good outcome after early surgery; this indicates that the gross neurologic condition preoperatively is the best prognostic indicator of intact survival. In the first few days after hemorrhage, the brain is swollen, soft, hyperemic, and prone to contusion and laceration. Impaired autoregulation may decrease cerebral tolerance to brain retraction. Although removal of a subarachnoid clot probably decreases the incidence and severity of delayed arterial narrowing, operative management may be hazardous. In more severely injured patients (grades III through V), surgery is often delayed in anticipation of resolution of vasospasm and improvement in neurologic status.
Endovascular coiling has been used increasingly as an alternative to neurosurgical clipping for treating SAH secondary to aneurysm rupture. The risk of late rebleeding is low. However, there is an increased risk of bleeding after endovascular coiling than after neurosurgical clipping.
a) The baseline neurologic status must be ascertained. The level of consciousness may vary from perfect alertness to deep coma and is an important prognostic factor for the postoperative state. Evidence of increased ICP should be elicited preoperatively so that it can be managed appropriately intraoperatively. Focal motor and sensory signs may indicate intracerebral extension of SAH, vasospasm, or cerebral edema.
b) Pulmonary complications, such as pneumonia, neurogenic pulmonary edema, and atelectasis, are not uncommon and are potentially treatable. Patients often have an increased risk of aspiration because of their depressed level of consciousness, and measures should be taken to reduce gastric acidity and volume preoperatively. The use of prophylactic hypervolemia also increases the likelihood of pulmonary edema.
c) The hemodynamic status of the patient should be assessed, with particular attention paid to the relationship between neurologic deterioration and blood pressure changes. Continuous arterial blood pressure monitoring is essential. Serious dysrhythmias or evidence of ventricular dysfunction should be diagnosed preoperatively so that appropriate monitoring and management can be instituted.
d) The syndrome of inappropriate antidiuretic hormone (SIADH) and diabetes insipidus (DI) can occur in patients with SAH. Preoperative electrolyte studies should be examined to facilitate intraoperative management.
e) The presence of blood in the subarachnoid space may produce a 1° C to 2° C elevation of body temperature. Temperature elevation increases cerebral oxygen requirements and therefore should be treated to prevent an increase of cerebral ischemia.
f) Preoperative sedation is rarely necessary in these patients. Depression of ventilation associated with opioids, barbiturates, and benzodiazepines may result in hypercapnia with resultant increases in cerebral blood flow (CBF) and ICP. Additionally, the reduced level of consciousness preoperatively and postoperatively may make clinical assessment difficult. If preoperative sedation is considered necessary, a small dose of a benzodiazepine (midazolam) with continued observation after its administration is probably the best choice.
g) A type and cross with blood readily available to administer is necessary.
a) The maintenance of adequate intravascular volume requires two large-bore IV cannulas.
b) Intraoperative monitoring includes continuous ECG (V5), arterial pressure monitoring, peripheral nerve stimulator, central venous pressure (CVP) monitoring, electroencephalogram (EEG), end-tidal CO2 (etco2) monitoring, pulse oximetry, and monitoring of temperature and fluid balance.
c) The anesthetic induction should be slow and deliberate. The anesthetic depth should be sufficient to avoid the hypertensive responses that accompany laryngoscopy and endotracheal intubation.
d) Anesthesia is induced with titrated doses of either thiopental or propofol. The addition of an opioid (5 to 10 mcg/kg of fentanyl or 1 to 2 mcg/kg of sufentanil) and IV lidocaine (1.5 mg/kg) further blunts the patient’s response to sympathetic stimulation of laryngoscopy and intubation.
e) An additional dose of opioid or propofol is required for the placement of the three-point pin head holder.
f) Prior injection of local anesthetic minimizes the associated sympathetic stimulation. Isoflurane may be introduced after hyperventilation before laryngoscopy to increase the depth of anesthesia.
g) Ventilation is controlled with administration of 100% oxygen to achieve a Paco2 of 35 to 40 mmHg with normal intracranial compliance.
h) Mild hyperventilation (Paco2 of 25 to 30) is instituted when intracranial compliance is decreased and ICP is increased. Long-term maintenance of hypocarbia results in poor neurologic outcomes in patients with increased ICP.
i) Intubation can be accomplished with 1 mg/kg of rocuronium.
j) The patient is placed in one of several positions, depending on the site of the aneurysm.
(1) Aneurysms that arise from the anterior part of the circle of Willis require that the patient be supine for a frontotemporal approach.
(2) The lateral position for a temporal approach is required for aneurysms that arise from the posterior aspect of the basilar artery. Aneurysms that arise from the vertebral artery or from the lower basilar artery require a sitting or prone position for a suboccipital approach.
(3) Aneurysms that arise from the anterior communicating artery are usually approached from the right; those from the middle cerebral and posterior communicating arteries are approached from the side on which the aneurysm is located.
k) Anesthesia is maintained with air and oxygen or N2O and oxygen, with incremental titrated dosages of an opioid (fentanyl, alfentanil, or sufentanil) or an infusion of remifentanil and a muscle relaxant. Isoflurane may also be added in inspired concentrations not to exceed 1%.
l) Controlled hypotension is commonly used intraoperatively to make aneurysms softer and more pliable at the time of clipping, as well as to minimize blood loss should aneurysmal rupture occur at this time. Sodium nitroprusside and an inhalation anesthetic agent are the drugs most widely used for induction of hypotension.
m) The safe limit of controlled hypotension has not been definitively established. Because autoregulation is maintained to a MAP of 50 to 60 mmHg, some argue that this limit should not be exceeded. In addition, because patients with poor-grade aneurysms may not have intact autoregulation, some argue that a lower limit of 60 mmHg should be adopted. Limits of autoregulation are shifted to higher pressures in patients with preexisting hypertension, so MAP should probably be kept within 40% of baseline. Higher blood pressure maintenance is necessary to avoid cerebral hypoxia if the patients head is elevated.
n) Many neurosurgeons now routinely use temporary proximal occlusion of the parent vessel rather than induce hypotension to facilitate clip ligation of the neck of the aneurysm.
o) The use of mild intraoperative hypothermia has been advocated for cerebral protection during periods of temporary occlusion.
p) At the conclusion of the anesthetic procedure, patients with low-grade aneurysms may be extubated in the operating room, although care must be exercised so that coughing, straining, hypercarbia, and hypertension are avoided. Propofol, lidocaine, or small doses of fentanyl may be used for short-term anesthesia as the procedure is being finished and for reducing the hemodynamic responses to extubation.
q) Although the residual depressant effects of opioids may be reversed with judicious titrated dosages of naloxone, larger doses of naloxone can be hazardous because they may cause sudden, violent awakening of the patient and marked increases in systemic blood pressure.
r) Endotracheal tubes (ETTs) should be retained in patients with high-grade aneurysms and in those who have had intraoperative complications because these patients will probably require postoperative ventilation.
a) Postoperative care is directed at the prevention of vasospasm via the maintenance of intravascular volume expansion and moderate hypertension (MAP of 80 to 120 mm Hg).
b) Changes in the level of consciousness and development of focal neurologic deficits are usually early signs of vasospasm. These clinical signs should be aggressively managed with hypertension, hypervolemia, and hemodilution. Dopamine may be used for blood pressure support. CT should be used for ruling out other causes of neurologic deterioration, including rebleeding, infarction, and hydrocephalus.
Intraoperative aneurysmal rupture can be catastrophic. An abrupt increase in blood pressure during or after induction of anesthesia may indicate that an aneurysm has bled.
a) The use of propofol or 0.5 to 1 mcg/kg of sodium nitroprusside decreases the transmural pressure of the aneurysm, although hypotension can be detrimental at this juncture.
b) Intraoperative aneurysmal rupture necessitates maintenance of the MAP between 40 to 50 mm Hg or lower to facilitate surgical control of the neck of the aneurysm or the parent vessel.
c) Alternatively, one or both carotid arteries may be compressed for up to 3 minutes to produce a bloodless field.
d) Blood that is lost should be continuously replaced with blood, blood products, or colloid solution so that intravascular volume is maintained.
e) A propofol drip or small bolus may be administered immediately before the aneurysm clipping to decrease the cerebral metabolic rate of oxygen consumption.
D Cranioplasty
Cranioplasty can be performed for a bony tumor resulting from traumatic injury (e.g., depressed skull fracture) or, more rarely, from a condition resulting from a congenital malformation (e.g., fused suture lines). These defects may occur anywhere on the head, so the surgical procedure may take place with the patient in varying positions such as supine, sitting, prone, or supine with the head turned. Patients range widely in age from newborn to elderly.
These are individualized according to the patient’s need.
Complete blood count (CBC), electrolytes, blood urea nitrogen, creatinine, glucose, prothrombin time, and partial thromboplastin time (D-dimer or fibrin split products if disseminated intravascular coagulation needs to be ruled out) are used. Type and crossmatch (for at least 2 units). Arterial blood gases are measured if the patient is being ventilated.
a) Monitoring equipment: Standard. An arterial line and central line are used if suggested by the patient’s history. Foley catheter is indicated if surgery is scheduled for more than 2 hours. Some patients may have an ICP monitor in place, and ICP monitoring should be continued intraoperatively.
b) Additional equipment: Determine the patient’s position during surgery. If the patient is supine or supine with the head turned, a foam support aids in positioning the head. Longer ventilation tubing is often needed because the table will be rotated 90 to 180 degrees. When the patient is in the sitting position, a Doppler device and a central line (with a 60-mL syringe attached) are needed to assess and treat venous air embolism (VAE). With the prone position, use prone foam rest shoulder rolls and multiple pads. In all cases, a nasal ETT assists in clearing the surgical field and in stabilizing the ETT. However, if traumatic injury to the facial structures or sinus has occurred, nasal instrumentation should be avoided.
c) Drugs and tabletop: Thiopental and etomidate are useful in cranioplasty because of their cerebral protective properties. Propofol is known to decrease ICP. Most surgeons desire to administer antibiotics during surgery, and they may be helpful.
d) Blood and fluid requirements: Glucose-containing solutions are best avoided in neurologic surgery. It is better to err on the side of underhydration. Fluid is usually replaced with normal saline or lactated Ringer solution at 2 to 4 mL/kg/hr. Blood loss may be substantial, and blood should be immediately available to avoid hypotension or crystalloid overload.
a) Induction is IV, with one of the agents known to decrease ICP. In severe trauma, one may wish to use only oxygen, narcotic, and muscle relaxant.
b) For maintenance, keep the patient’s mean arterial blood pressure slightly below the baseline and maintain normocarbia to slight hypocarbia.
c) A constant infusion of thiopental, etomidate, or propofol with or without inhalation of isoflurane will help to maintain cerebral perfusion and will minimize the cerebral oxygen consumption.
d) Muscle relaxation is not necessary if the procedure is confined to the skull, and the head is immobilized with tongs or some other type of fixator.
e) Most practitioners leave the ETT in place until the neurologic status is certain to allow for regular respiration. Lidocaine is useful in minimizing cough.
Assess postoperative neurologic functions. Pain in patients with altered neurologic status is usually controlled with parenteral agents. Avoid hypercarbia in neurologic patients receiving opiates because it increases CBF and ICP.
E Craniotomy
Intracranial masses may be congenital, neoplastic (benign, malignant, or metastatic), infectious (abscess or cyst), or vascular (hematoma or malformation). Most, but not all, anesthetics can be used safely in patients with cerebral lesions. The effects of the agent on ICP, CPP, CBF, CMRo2 (cerebral metabolic rate of oxygen), promptness of return of consciousness, drug-related protection from cerebral ischemia or edema, blood pressure control, and compatibility with neurophysiologic monitoring techniques are important considerations.
Most craniotomy surgery in the United States today is performed after a propofol induction of anesthesia with intubation of the trachea after a nondepolarizing relaxant. Maintenance of anesthesia is commonly accomplished with a combination of an inhalation agent (usually isoflurane) and narcotic such as fentanyl, sufentanil, or alfentanil in various combinations during maintenance of low normocarbia.
a) The clinical signs of a supratentorial mass include seizures, hemiplegia, and aphasia. The clinical signs of infratentorial masses include cerebellar dysfunction (ataxia, nystagmus, dysarthria) and brainstem compression (cranial nerve palsies, altered consciousness, abnormal respiration). When ICP increases, frank signs of intracranial hypertension can also develop.
b) Preanesthetic evaluation should attempt to establish the presence or absence of intracranial hypertension. CT or magnetic resonance imaging (MRI) data should be reviewed for evidence of brain edema, midline shift greater than 0.5 cm, and ventricular size. A neurologic assessment should evaluate the current mental status and any existing neurologic deficits.
c) Medications prescribed for the control of ICP (corticosteroids, diuretics) and anticonvulsant therapy should be reviewed. Laboratory evaluation should rule out corticosteroid-induced hyperglycemia and electrolyte disturbances (such as SIADH or DI) that may develop secondary to diuretic therapy. Anticonvulsants, dosage time of last dose, and blood levels should be noted.
d) The decision regarding the amount and timing of the premedication administration should be made only after a thorough patient evaluation. Benzodiazepines produce respiratory depression and hypercapnia. Premedication should be omitted in patients with a large mass lesion, a midline shift, and abnormal ventricular size. Opioids are universally avoided in the preoperative period. If premedication is desired in patients deemed appropriate, careful titration of IV midazolam may begin when the patient has been delivered to the preoperative holding area. In an attempt to help control ICP in patients with mass lesions, the head of the bed should be elevated 15 to 30 degrees during transport to the preoperative holding area and the operating room.
e) Due diligence to all existing hospital recommendations for prophylactic antibiotics given at the appropriate time and in the appropriate amount should be performed.
(1) Routine monitors for supratentorial procedures include continuous ECG, cuff measurement of blood pressure, precordial stethoscope, monitoring of the fraction of inspired oxygen, pulse oximetry, temperature, peripheral nerve stimulation, etco2 monitoring, and indwelling urinary catheterization.
(2) For patients with ischemic heart disease, use of a modified V5 ECG lead is recommended. An arterial line placed either before or immediately after anesthetic induction provides for uninterrupted blood pressure monitoring and easy access for blood sampling for laboratory analysis.
(3) Somatosensory evoked potentials (SSEPs) may be assessed and may warrant the need for half an alternative anesthetic of 0.5 minimum alveolar concentration (MAC) and propofol infusion.
(1) Preoperative fluid deficits and intraoperative blood and fluid losses must be adequately replaced during neurosurgical procedures. Judicious fluid administration minimizes the occurrence of cerebral edema and increased ICP, reduced CPP, and worsened cerebral ischemia.
(2) In most neurosurgical patients, fluids that contain osmolarity similar to that of serum (e.g., lactated Ringer solution or 0.9% saline) are administered in a volume that is sufficient for the maintenance of peripheral perfusion but that avoids hypervolemia (0.5 to 1 mL/kg/hr).
(3) Traditionally, less fluid is given than would be administered for nonneurologic surgery, although new recommendations indicate that patients should be kept isovolemic, isotonic, and iso-oncotic. No single IV solution is best suited for neurosurgical patients at risk for intracranial hypertension, but the use of iso-osmolar crystalloids is widely accepted and can be justified on a scientific basis.
(4) Emerging evidence questions the rationale for routine administration of glucose. Hyperglycemia induces marked cerebrovascular changes during both ischemia and reperfusion. Multiple studies have demonstrated that hyperglycemia before and during an episode of global cerebral ischemia will exacerbate the neurologic injury.
(5) Fluid therapy is most challenging during prolonged surgical procedures or in the surgical management of multiple traumas. If tissue trauma is severe or if hemorrhage has been prolonged, patients develop a marked reduction in functional extracellular volume as a result of the internal redistribution of fluids (third-space losses).
(6) Although the extent of tissue manipulation in most routine neurosurgical procedures is small, third-space fluid losses during prolonged surgery and in patients with severe associated systemic trauma can be sufficient to decrease intravascular volume, reduce peripheral perfusion, and impair renal function. The sequestered extracellular fluid can be cautiously replaced with lactated Ringer solution or with 0.9% saline.
(7) In the absence of diuretic therapy, a urinary output of 0.5 to 1 mL/kg/hr suggests adequate replacement as do hemodynamic stability and cardiac filling pressures within the normal range. Although some clinicians prefer to use colloid-containing solutions in neurosurgical patients, such solutions appear to exert negligible effects on brain water and ICP.
a) Although induction of anesthesia for patients undergoing craniotomy can be performed with various agents, a smooth and gentle induction of general anesthesia is more important than the drug combination used. No evidence indicates that one technique or set of drugs is better than another.
b) A reasonable induction sequence would combine preoxygenation, propofol (1-2 mg/kg), and a nondepolarizing muscle relaxant. No evidence suggests that any of the induction agents (midazolam, etomidate, propofol, and methohexital) is superior to thiopental.
c) The hemodynamic response to intubation may be blunted with the administration of fentanyl (10-15 mcg/kg total dose) or lidocaine (1.5 mg/kg) administered 3 minutes before laryngoscopy. The dose of these induction agents may need to be adjusted according to the patient’s age and physical status. Whatever agents are selected, the induction should be accomplished without the development of sudden hypertension or hypotension.
d) The head typically is elevated from 15 to 30 degrees to facilitate venous and cerebrospinal fluid (CSF) drainage. The head may also be turned to the side to facilitate exposure. Excessive neck flexion may impede jugular venous drainage and increase ICP. Because of the flexion-extension-rotation of the head in combination with head fixation in a pinion headrest, the use of an armored or reinforced ETT is recommended to avoid kinking of the tube after positioning is accomplished.
e) The anesthesia circuit connections must be firmly secured by simultaneously pushing and twisting to seat the plastic connectors. The risk of unrecognized disconnections may be increased because the operating table is usually turned 90 to 180 degrees away from the anesthesia provider, and both the patient and the breathing circuit are almost completely covered by surgical drapes.
f) Maintenance of anesthesia may be accomplished with an oxygen–air–opioid technique, a selected potent inhalation agent, or oxygen–air and a continuous infusion of propofol.
g) After endotracheal intubation, mechanical hyperventilation may be initiated, decreasing etco2 to 30 to 35 mmHg, confirmed through arterial blood gas analysis. The patient should be covered with blankets or a forced air warming blanket to maintain core body temperature.
h) An opioid-based anesthetic technique with air in oxygen with low-dose (less than 1%) isoflurane is a popular choice. Incremental administration of fentanyl, sufentanil, alfentanil, or an infusion of remifentanil is acceptable. Alternatively, sufentanil, 0.5 to 1 mcg/kg loading dose followed by either incremental boluses (not to exceed 0.5 mcg/kg/hr) or an IV infusion of 0.25 to 0.5 mcg/kg/hr in combination with less than 1% isoflurane in oxygen may be used. Sufentanil administration should be discontinued approximately 45 minutes before the end of surgery to ensure that the patient awakens promptly. The primary advantage of remifentanil is rapid awakening. If the patient experiences hypertension or tachycardia near the end of surgery, the anesthesia provider should consider giving either labetalol or esmolol, not additional opioids.
i) An inhalation agent (isoflurane, desflurane, or sevoflurane) with little or no opioid supplementation can also be used for maintenance of anesthesia. If isoflurane is used, the concentration should remain less than 1%. Hyperventilation in combination with less than 1% isoflurane generally results in stable intracranial dynamics.
j) N2O may be used in an anesthetic regimen if it is deemed desirable. However, if the patient is suspected to have a pneumocephalus or if the potential for air embolism exists, N2O use is contraindicated. N2O expands both the pneumocephalus and the air embolus.
k) Hyperventilation is an important adjunct to any neuroanesthetic technique. Hypocapnia decreases ICP before opening of the dura and attenuates the vasodilatation produced by the volatile anesthetic agents. Optimal hyperventilation during surgery would yield a Paco2 of 25 to 30 mmHg and is indicated for short periods when ICP is dramatically increased or brain compliance is decreased. Diuretics, when indicated, may be timed just before or after the cranial vault is opened to facilitate surgical exposure.
l) In choosing an agent for muscle relaxation, the length of the procedure and the impact of the drug on ICP should be considered.
(1) Careful attention to appropriate planning for the emergence from anesthesia is important. Sudden emergence from anesthesia can result in uncontrolled hypertension. Delirium with coughing and straining on the endotracheal tube should be avoided. In a patient with a compromised blood-brain barrier, this stormy emergence can produce devastating consequences.
(2) Late emergence from anesthesia can result in a confusing diagnostic picture with possible intracranial hematoma, acute hydrocephalus, or other diagnoses masked by the residual anesthesia.
(3) The goal for emergence is control. A controlled emergence focuses on regulation of blood pressure, ICP, and CBF. Controlled emergence also accounts for the preexisting pathophysiology, the surgical trauma, the length of the procedure, and appropriate management of the airway.
(4) Emergence from anesthesia begins when the surgical pathology has been addressed. Collaboration with the surgeon is essential. The Paco2 should be allowed to return to a normal level. At the surgeon’s request, blood pressure can then be raised to 120% of the normal baseline level before closing of the dura. Hypertension is considered a frequent occurrence of the postoperative period. Tachycardia associated with hypertension “invariably” results from emergence excitement. By raising the blood pressure and the Paco2 before dural closure, the surgeon can directly assess the ability of the brain to withstand such challenges.
(5) After the dura has been closed, the blood pressure is maintained at baseline levels throughout the remainder of the closure. There is strong support for the notion that sympatholytic drugs should be used to decrease blood pressure during emergence. Studies have shown that during the first hour after craniotomy for supratentorial lesions, the arteriovenous oxygen content difference is low, suggesting a state of cerebral luxury perfusion. This event coincides with a high level of mean arterial blood pressure. Accordingly, it is supposed that this correlation is caused by changes in the mean blood pressure and impaired autoregulation. This may be deleterious because it enhances blood-brain barrier leakage, provoking edema and hemorrhage.
(6) A relationship between hypertension and postoperative hematoma formation exists. The parameters for these events for each individual patient are unknown. Normal autoregulation of CBF maintains adequate perfusion at mean blood pressures ranging from 50 to 150 mmHg. Labile hypertension and unstable blood pressure during the perisurgical period may be contributory to intracerebral hemorrhage remote from the site of the initial neurosurgical procedure.
(7) Judicious titration of antihypertensives (esmolol, labetalol) has great clinical utility in controlling the blood pressure during emergence. When access to the patient is regained, the use of anesthetic gases is discontinued and the muscle relaxant is reversed. IV lidocaine (1.5 mg/kg) can be given just before suctioning for cough suppression before extubation.
(8) Delayed awakening may result from residual opioid or remaining end-tidal concentrations of potent inhalation agent. After extubation, the patient is transported to the intensive care unit postoperatively for continued monitoring of neurologic function.
F Electroconvulsive therapy
Electroconvulsive therapy (ECT) is the intentional inducement of a generalized seizure of the central nervous system (CNS) for an adequate duration of time to treat patients with certain severe neuropsychiatric disorders. Antidepressant medication administration, along with ECT, is well tolerated by patients, and both therapies can be beneficial to the patient. Most patients receive three treatments per week and can undergo a total of 6 to 12 treatments total. Clinical improvement is seen within the first three to five treatments, and positive response to treatment is seen in 50% to 90% of patients (even those who had been treatment resistant). ECT treatments exceed the total numbers of coronary revascularizations, herniorrhaphy, and appendectomy procedures performed in the United States. Death from ECT itself is possible but is rare. ECT also is used in certain patients who experience mania, catatonia, vegetative states, dysregulation, inanition, suicidal drive, and schizophrenia with affective disorders.
a) Procedures: Treatment involves placement of electrodes with a conducting gel either right-sided unilaterally or bitemporally bilaterally; an alternating current of electricity is passed through the electrodes. Theories for the mechanism of ECT are related to profound changes in brain chemistry, such as enhancement of dopaminergic, serotonergic, and adrenergic neurotransmission. Another theory postulates the release of hypothalamic or pituitary hormones, which have antidepressant effects. Finally, ECT produces anticonvulsant effects that raise the seizure threshold and decrease seizure duration, exerting a positive effect on the brain.
Anesthesia for ECT involves the administration of an ultra-brief general anesthetic to provide lack of consciousness to the patient for the procedure (see table below).
Anesthetic Medications Used for Electroconvulsive Therapy
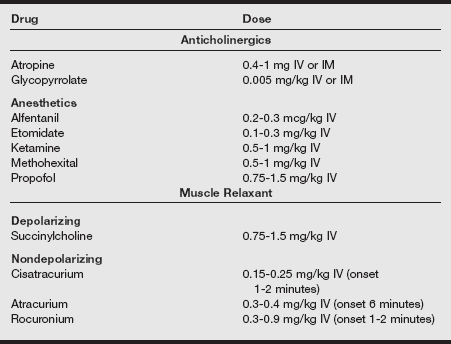
IM, Intramuscular; IV, intravenous.
Adapted from Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg 2002; 94:1351-1364; White PF. Perioperative Drug Manual. 2nd ed. Philadelphia: Saunders. 2005; Wagner KJ, Möllenberg O, Rentrop M, et al. Guide to anaesthetic selection for electroconvulsive therapy. CNS Drugs 2005;19:745-758.
a) A thorough preanesthetic assessment must be performed, with consideration given to the possible great physiologic hemodynamic responses generated by the induced seizure activity. The box on pg. 384 lists the possible physiologic effects as a result of ECT.
b) Adult patients about to undergo ECT should follow fasting guidelines of at least 6 hours for solids and 2 hours for liquids. Necessary bronchodilators may be taken. Oral medications, such as antihypertensives, cardiac medications, anticoagulants, and thyroid medications, may be taken with a sip of water up to 1 hour before the procedure.
c) Patients may have results of laboratory studies, a pharmacologic regimen, and ECG readily available because of their psychiatric hospitalization. Informed consent is obtained whenever possible from the patient or legal guardian. An IV catheter is inserted in a peripheral vein.
d) The patient is monitored with a pulse oximeter, ECG, noninvasive blood pressure monitor, temperature-monitoring device, and peripheral nerve stimulator. Use of etco2 monitoring has been suggested because hypercarbia and hypoxia shorten seizure duration.
e) Suction, oxygen, a positive-pressure Ambu bag and face mask, and rubber bite protectors must be present, as well as necessary airway and cardiovascular resuscitation equipment (including a laryngoscope and ETT with stylet), medications, and supplies because ECT is usually performed in a dedicated psychiatric suite or special treatment room.
f) The patient is preoxygenated before induction. Anticholinergics may be administered as an antisialagogue or to prevent asystole.
g) The induction agent is administered intravenously. Propofol, etomidate, and ketamine have also been used, although an enhanced hemodynamic response and increased ICP is possible after using ketamine. Succinylcholine (0.25-0.5 mg/kg) is usually given and is the drug of choice because of its short duration.
h) Positive-pressure ventilation is applied to the patient via the positive pressure breathing bag and a face mask and is continued until after treatment is completed and spontaneous respirations resume.
i) To assess the duration of the induced convulsion, the psychiatrist usually applies a tourniquet or a manual blood pressure cuff (inflated to slightly greater than the systolic blood pressure) above a lower extremity so that the muscle relaxant cannot reach the skeletal muscle in the extremity.
j) A rubber bite block is gently placed in the patient’s mouth to prevent biting down on the teeth, lips, and tongue at the end of the procedure.
k) A nerve stimulator must be used, and appropriate neuromuscular blockade reversal agents should be administered if necessary. The electrodes are applied, the proper waveform and current level are selected, and the electroconvulsive seizure is induced.
l) The seizure lasts from 30 to 90 seconds; the motor seizure is shorter than the seizure duration as seen on an EEG.
m) Use of an anesthesia awareness, level of consciousness, and depth of sedation monitor correlates with the EEG and can be a useful tool for the anesthesia provider and the psychiatrist.
n) The level of sedation displayed by this monitor correlates with the proper point to induce seizure, the duration of seizure, and the potential for awareness during the ECT procedure.
o) At the end of the seizure, spontaneous respirations resume, the patient is transferred to a recovery area, and vital signs are continually monitored until the patient is determined to be stable and able to be safely discharged.
p) Certain anesthetic medications and techniques such as hyperventilation can affect seizure duration.
The intentional creation of CNS convulsions has profound effects on the patient’s physiology. Patients usually experience temporary cognitive and memory impairment after ECT.
The first type of impairment that may be seen is postictal confusion, in which the patient is transiently restless, confused, and agitated immediately after the convulsive episode and for approximately 30 minutes. A second type of cognitive impairment that may be seen later is anterograde memory dysfunction, in which the patient may rapidly forget new information. A third cognitive dysfunction is retrograde memory dysfunction, which is the forgetting of memories from several weeks to several months before the ECT treatment. The cognitive effects described vary depending on the frequency and the number of ECT treatments the patient has received. The quantities of energy used to elicit the convulsions and the placement of the electrodes are also factors. Type of anesthetic drugs used may also be implicated.
Cardiovascular stimulation also occurs with ECT. The sympathetic and parasympathetic nervous systems are stimulated sequentially. Therefore, the patient may experience an increase in heart rate and blood pressure followed by a period of bradycardia or even asystole. Transient cardiac changes can be managed before ECT with anticholinergics, IV local anesthetics such as lidocaine, or IV narcotics such as remifentanil. Changes after ECT can be managed with β-blockers such as esmolol or labetalol, calcium channel blockers such as verapamil or nifedipine, or other antihypertensives such as nitroprusside or nitroglycerin.
Patients may also experience headache, muscle aches, or postoperative nausea and vomiting (PONV). Symptoms of headache or muscle ache respond well to acetaminophen, aspirin, or nonsteroidal antiinflammatory agents such as IV or intramuscular ketorolac or oral ibuprofen. Nausea can be caused by the stress and anxiety before the ECT treatment, the anesthetic agents used, the seizure itself, or air in the stomach from assisted ventilation. Nausea can be treated with agents such as ondansetron, dolasetron, or granisetron.
New therapies for major depressive disorders
Other therapies for severe major depressive disorders are now available, which require anesthetic treatment: repetitive transcranial magnetic stimulation (rTMS) and vagus nerve stimulation (VNS). Neuroanatomic studies have suggested that patients with major depressive disorder (MDD) have dysfunction within the frontal cortical–subcortical–brainstem neural network, specifically the dorsolateral prefrontal cortices (DLPFC). ECT and antidepressant medications do not act in these discrete areas of the brain, but new therapies stimulating these focal areas of the brain are now approved and in use in the United States.
Repetitive transcranial magnetic stimulation or magnetic seizure therapy
rTMS uses electrical current passing through an electromagnetic coil that has been placed on the scalp. The coil delivers brief, rapidly changing magnetic field pulses to specific areas of the brain. These bursts of pulses are called a “train” of stimuli. Multiple trains of rTMS may be delivered in one session. The scalp and skull are transparent to magnetic fields, which is an advantage over ECT, where the scalp and skull are resistors to the electrical stimulation. A convulsion must be initiated by trains of rTMS in order to produce antidepressant effects because subconvulsive trains of rTMS are ineffective.
Convulsive magnetic energy levels are determined by the use of motor threshold (MT). MT is the point where a single pulse of magnetic energy begins to elicit an electromyographic response, (i.e., a twitch, usually of the abductor pollicis brevis muscle of the thumb or first dorsal interosseous muscle of the index finger). rTMS treatment is safe and well tolerated, with reduced cognitive side effects compared with ECT. Patients are found to recover much more rapidly from rTMS or MST therapy compared with ECT.
MST uses a higher intensity, more frequent, and longer duration magnetic seizure-inducing dose compared with the magnetic dose required for rTMS. MST can stimulate tonic-clonic seizures in more localized and focal regions of the prefrontal cerebral cortex when compared with ECT, as well as generalized tonic-clonic seizures that resemble ECT. MST does not produce the rigid bilateral masseter muscle contractions noted during ECT but can produce elevations in blood pressure and heart rate similar to those of ECT. After rTMS, some patients experience mild headache, disorientation, and inattention (although patients become reoriented much more quickly than with ECT), retrograde amnesia, some anterograde amnesia, transient auditory threshold increases caused by the high-frequency clicking sound heard during coil discharge (which can be alleviated with the use of foam earplugs), and rarely generalized seizure. A single TMS treatment may be all that is necessary for treating certain severe MDD nonpsychotic patients along with their medications, although rTMS may be necessary.
a) Anesthesia for rTMS or MST may not be required, or a short general anesthetic may be required. The decision on type of anesthesia should be made based on the type of procedure, duration, and the patient’s requirements or comorbidities.
b) A patent and secure IV catheter is established, and full monitors are applied.
c) Glycopyrrolate 2.5 to 5 mcg/kg is administered as an antisialagogue along with ketorolac 0.4 mg/kg 2 to 3 minutes before induction of ultra-brief general anesthesia.
d) Etomidate 0.15 to 0.2 mg/kg can be used for induction, as well as methohexital 1 to 2 mg/kg or propofol 1 to 2.5 mg/kg.
e) Succinylcholine 0.5 to 1 mg/kg can be used as the muscle relaxant after isolation of a lower extremity for observation of seizure duration.
f) Use the smallest amount of muscle relaxant necessary to enable recovery from paralysis before the return of consciousness.
g) The anesthesia provider can then manually hyperventilate the patient’s lungs with a face mask to an etco2 value of 30 to 34 torr.
Vagus nerve stimulation
VNS requires surgical implantation of a programmable battery-powered electrical stimulator that connects with the patient’s left vagus nerve (cranial nerve X). The stimulator is usually implanted in the patient’s chest with minimal sedation, moderate sedation and analgesia, deep sedation and analgesia, or under general anesthesia. Patient movement should be limited because of the intricacy of the surgery and proximity of vital structures. Although originally approved for treatment-resistant epilepsy, the VNS is now approved for major depressive episodes that have not responded to four antidepressant medication trials.
G Epilepsy surgery
Surgery is recommended for patients with epilepsy when seizure control is intractable to conventional medical treatment. The goal of epilepsy surgery is to remove a focal area of epileptogenesis without causing neurologic deficits. Epilepsy surgery consists of two types: intracranial electrode placement and surgical resection. Intracranial electrode placement and testing may be required to localize epileptogenic foci. After localization, surgical resection may be performed. Extensive testing is required to define the focal area and its physiologic activity.
2. Preoperative assessment and patient preparation
a) History and physical examination are required.
(1) Neurologic: The patient should be assessed for history of uncontrollable focal or generalized seizures. Note the date of the last seizure. Obtain a description of seizure and prodromal symptoms. Obtain list of antiepileptic drugs. A Wada test (intracarotid injection of a barbiturate) may be performed to determine the dominance or speech function in the area of the surgery. A list of medications should be reviewed and the time of last antiepileptic drug noted.
(1) CBC: A low hematocrit can be found in patients taking phenytoin or phenobarbital. A low white blood cell count can be found in patients taking carbamazepine or primidone. A low platelet count can be found in patients taking carbamazepine, valproate, ethosuximide, or primidone.
(2) Gastrointestinal: Antiepileptic drugs may cause liver damage.
(3) Obtain laboratory levels of antiepileptic drugs as necessary (e.g., phenytoin [Dilantin]).
a) Monitoring equipment: A standard monitors such as ECG, pulse oximeter, temperature, and noninvasive blood pressure cuff and required arterial line and a CVP catheter are used for general anesthesia. A Foley catheter is used to monitor urine output.
b) Additional equipment: Determine the position of the patient during surgery (supine, lateral, prone, semisitting, or table turned 90 or 180 degrees). A foam support aids in positioning the head. A long circuit, long IV tubing will be needed if the table is turned 90 to 180 degrees. Appropriate padding should be used according to the patient’s position.
c) Drugs: Short-acting benzodiazepines, narcotics, or a propofol infusion are acceptable for providing conscious sedation. If intraoperative electrocorticography is used, benzodiazepines and anticonvulsants should be avoided. It is best to avoid anesthetics that may trigger seizure activity (e.g., ketamine). Higher drug doses (e.g., muscle relaxants and narcotics) may be needed because of enzyme induction by anticonvulsant therapy. If a semisitting position is used, the anesthesia provider should be prepared for VAE, and N20 should be avoided.
a) Induction: Local anesthesia with sedation and general anesthesia with an oral ETT are the anesthetic techniques of choice. Local anesthesia with sedation is used when the seizure focus is in the dominant hemisphere or if neurologic injury may be caused by temporal lobectomy. Screening is necessary to determine patients who will be able to tolerate the procedure. Standard induction is used for general anesthesia. If a stereotactic frame is used, fiberoptic awake intubation may be necessary.
b) Maintenance: If general anesthesia is used, low-dose isoflurane, nitrous oxide, and an opioid infusion are generally used. Isoflurane must be turned off during intraoperative electrocorticography and can be resumed if resection follows. The anesthesia provider may be asked to elicit a seizure by inducing hyperventilation or administering methohexital. Muscle relaxants generally are not readministered after induction to facilitate evaluation of motor response.
c) Emergence: The patient should be emerged smoothly and quickly from anesthesia to allow assessment for any neurologic deficits.
Seizure, bleeding, and cerebral edema are postoperative complications. The patient must be monitored carefully for altered mental status. If the patient shows any signs of delayed emergence or altered mental status, a CT scan should be performed.
H Posterior fossa procedures
Neuropathology within the posterior fossa may impair control of the airway, respiratory function, cardiovascular function, autonomic function, and consciousness. The major motor and sensory pathways, primary cardiovascular and respiratory centers, reticular activating system, and nuclei of the lower cranial nerves are all concentrated in the brainstem. All of these vital structures are contained in a tight space with little room for accommodating edema, tumor, or blood.
2. Preoperative assessment and patient preparation
a) History and physical examination
(1) Neurologic: The history and physical examination should include a thorough neurologic evaluation with documentation. Pay special attention to signs and symptoms of brainstem involvement, such as focal neurologic deficits, depressed respiration, and cranial nerve palsies. Changes in level of consciousness may be secondary to increased ICP resulting in headaches, nausea, vomiting, visual changes, and seizures from obstructive hydrocephalus of the fourth ventricle.
(2) Cardiovascular: Evaluate for cardiovascular disease and hypertension. Increased ICP may result in brainstem herniation and manifest as Cushing triad (hypertension and irregular respirations).
(3) Pulmonary: Assess for a coexisting disease process.
(4) Renal: Correct fluid and electrolyte abnormalities, if present.
(5) Gastrointestinal: Infratentorial tumors may involve the glossopharyngeal and vagus nerves. This may impair the gag reflex, increasing the chance for aspiration.
(6) Endocrine: Patients maybe on high-dose steroid therapy to decrease cerebral edema. Preoperative steroid administration would therefore be warranted.
b) Laboratory tests: CBC, electrolytes, blood urea nitrogen, creatinine, glucose, prothrombin time, and partial thromboplastin time are obtained.
c) Diagnostic tests: CT and MRI are used.
d) Preoperative medications: Anxiolytics may be given to alert and anxious patients. Patients who are lethargic or have an altered level of consciousness should not receive premedication.
e) IV therapy: Central line is used if significant blood loss is anticipated or if there is a high risk for VAE; two 16- to 18-gauge IV catheters; consider placement of a pulmonary arterial catheter. Estimated blood loss is 25 to 500 mL.
a) Monitoring equipment: Standard monitors; arterial line; urinary catheter; with possible CVP; ICP monitoring; precordial Doppler; EEG; electromyography; and sensory, somatosensory, and brainstem auditory–evoked potential monitoring are used.
b) Additional equipment: Depending on the position of the patient, have appropriate padding available (i.e., prone pillow, doughnut, chest and axillary rolls); a fluid warmer is used.
(1) Miscellaneous pharmacologic agents: Vasoconstrictors, vasodilators, inotropes, adrenergic antagonists, steroids, osmotic and loop diuretics, thiopental, lidocaine, fentanyl, nondepolarizing muscle relaxants, and antibiotics are used.
(2) IV fluids: Use isotonic crystalloid solutions. Avoid glucose-containing solutions. Limit normal saline to less than or equal to 10 mL/kg/hr plus replacement of urinary output. If volume is required, administer 5% albumin or hetastarch and limit to less than or equal to 20 mL/kg. Maintain hematocrit at 30 to 35. Transfuse to keep hematocrit greater than 30.
(3) Blood: Type and cross match for 2 units of packed red blood cells.
a) Although most posterior fossa explorations may be performed with the patient in either the lateral or the prone position, the sitting position is occasionally preferred because the enhanced CSF and venous drainage facilitates surgical exposure. The use of this position, however, has declined dramatically because of the potential for serious complications.
b) The patient is semirecumbent in the standard sitting position with the back elevated to 60 degrees and the legs elevated (with the knees flexed) to the level of the heart. The latter is important for prevention of venous pooling and to decrease the risk of venous thrombosis.
c) The head is fixed in a three-point head holder with the neck in flexion, and the arms remain at the sides with the hands resting on the lap.
d) Pressure points such as the elbows, ischial spines, and forehead must be protected with foam padding.
e) Excessive neck flexion has been associated with swelling of the upper airway (venous obstruction) and, rarely, quadriplegia resulting from compression of the cervical spinal cord and decreased cervical cord perfusion with elevation of the neck above the heart. Preexisting cervical spinal stenosis probably predisposes to the latter injury.
a) Increased ICP, although common in patients with supratentorial lesions, is less common in patients with posterior fossa lesions. Obstructive hydrocephalus is more typical because CSF outflow is occluded at the level of the aqueduct of Sylvius or fourth ventricle. This can be readily identified preoperatively by MRI or CT. This may be corrected before definitive surgical intervention with the placement of a ventricular catheter.
b) Sedation is contraindicated in patients with obstructive hydrocephalus.
c) Induction should be slow and deliberate to avoid changes in cerebral perfusion and increased ICP. Because the head is generally flexed and fixed in this position, a wire-reinforced ETT may prevent intraoperative kinking. These tubes may become permanently kinked if the patient is lightly anesthetized and bites the tube.
d) Intravenous fluid administration during posterior fossa surgery should be limited to the infusion of deficit and maintenance quantities of a balanced salt solution. Major volume resuscitation can be accomplished with the infusion of blood, colloid, or crystalloid solutions.
e) Emergence from anesthesia should be as smooth and gentle as possible. The intraoperative use of opioids facilitates a smooth emergence without significant coughing and bucking. The administration of lidocaine 1.5 mg/kg intravenously decreases the airway irritation of the ETT.
f) The decision to remove the ETT should be made after the anesthetic course and the surgical procedure are reviewed.
g) Intraoperative air embolism may be followed by the development of pulmonary edema. Although this condition is self-limiting, continued mechanical ventilation is the treatment of choice.
h) Consideration should also be given to the possibility of cranial nerve damage during the operative procedure. Provided the patient is safely extubated, continued vigilant observation is essential because airway compromise may develop after injury to cranial nerves IX, X, and XI.
I Stereotactic surgery
Stereotactic surgery is a neurosurgical technique that makes detailed use of the relationship between the three-dimensional space occupied by intracranial structures or lesions and an extracranial reference system to guide instruments to such targets accurately and precisely. This type of technique is used when the lesion is small and located deep within brain tissue or as a means of obtaining a biopsy of a lesion for diagnosis.
Stereotactic procedures can be frame based or image guided (frameless). If the frame-based procedure is used, the frame is anchored to the skull with either four pins or four screws. Application typically takes place outside the operating room using a local anesthetic. In a cooperative adult, frame application takes only 5 to 10 minutes. For children, general anesthesia is used. If the image-guided procedure is used, small markers called fiducials are placed on the head with adhesive. An imaging study is then performed to provide a system of reference.
a) History and physical examination: Neurologic symptoms vary, depending on the site and size of the lesion; they should be carefully documented. In addition to the routine test, CT is performed preoperatively with the frame in place to determine stereotactic coordinates. After the coordinates are established, airway access is restricted because the frame should not be moved on the head until the operation is complete unless an emergency occurs.
b) Laboratory tests: CBC and other tests are as indicated by the history and physical examination.
c) Diagnostic tests: CT of the head and other tests are as indicated by the history and physical examination.
a) General endotracheal anesthesia or monitored anesthesia care is used.
b) In adults, the stereotactic frame or fiducials are placed the morning of the operation, and the patient is taken to the radiologic suite for CT to determine stereotactic coordinates. The patient is then brought to the operating room with the frame or fiducials still in place. If the operation is to be a biopsy, it is generally done using monitored anesthesia care.
c) If a complete resection is planned, such as in the removal of an AVM, general endotracheal anesthesia is used. In children, it is usually necessary to induce general anesthesia before placing the frame, thus necessitating the maintenance of general anesthesia during the CT. The child is then moved to the operating room still anesthetized, and the operation is completed.
(1) If monitored anesthesia care is planned, oxygen is administered by nasal prongs, and the patient is lightly sedated with combinations of propofol 25 to 50 mg/kg/min, midazolam to provide amnesia, and remifentanil 0.02 to 0.05 mg/kg/min or fentanyl to provide analgesia. The patient must be able to communicate with the surgeon as needed throughout the operation.
(2) Stereotactic neurosurgery for movement disorders such as Parkinson’s disease requires the anesthesia provider to limit sedation to ensure that the patient will be able to cooperate with the surgeon. If general endotracheal anesthesia is needed with frame-based stereotaxy, fiberoptic laryngoscopy is necessary before inducing anesthesia because the frame precludes intubation by direct laryngoscopy. After endotracheal intubation is established, anesthesia may be induced with propofol, followed by a nondepolarizing blocking drug to facilitate positioning of the patient.
(1) If general anesthesia is used, the ideal drug is one that decreases ICP and the cerebral metabolic rate of oxygen, maintains cerebral autoregulation, redistributes flow to the potentially ischemic areas, and provides protection of the brain from focal ischemia.
(2) If children are to be transported from the site of placement of the stereotactic frame to the radiologic suite and then the operating room, it is best to use inhalational anesthesia with isoflurane and 100% oxygen with spontaneous ventilation to ensure adequate ventilation during transport and study. Opiates and neuromuscular blocking drugs should not be administered until the child is in the operating suite.
(3) Hyperventilation and diuresis should be avoided in image-guided stereotaxy because they may cause the brain to shift. If a frame is used, emergent removal from the patient’s head must always be available.
c) Emergence: No special consideration is reported. The patient is extubated awake and after the return of airway reflexes. If the surgeon suspects that the patient may have a slow recovery or a neurologic injury or if the anesthesia provider believes that recovery from the anesthesia may be delayed, it is advisable to leave the ETT in place at least overnight.
Focal bleeding may occur postoperatively, causing the onset of a neurologic deficit.
J Transsphenoidal tumor resections
Approximately 10% of intracranial neoplasms are found in the pituitary gland and are diagnosed because of their mass effects or the hypersecretion of pituitary hormones. These tumors are rarely metastatic and produce local symptoms via bone invasion, hydrocephalus, and compression of a cranial nerve (most often the optic nerve). Frontal-temporal headache and bitemporal hemianopsia are the most common nonendocrine symptoms of enlarging pituitary lesions. Nonsecreting pituitary tumors account for approximately 20% to 50% of lesions in this area and are classified as chromophobe adenomas.
Tumors that secrete excess growth hormone produce acromegaly. Increased growth hormone increases the size of the skeleton, particularly the bones and soft tissues of the hands, feet, and face. The enlarged facial structures may increase the likelihood of difficult intubation. Excess growth hormone may also contribute to the development of coronary artery disease, hypertension, and cardiomyopathy. Hyperglycemia is also a common finding, reflecting growth hormone–induced glucose intolerance.
a) Surgical approach: Medical and surgical therapies exist for both functional and nonfunctional pituitary tumors. Transsphenoidal surgery offers several advantages over the intracranial approach. Statistically, morbidity and mortality rates are reduced because of a decrease in blood loss and less manipulation of brain tissue. In addition, the risk of inducing panhypopituitarism and the incidence of permanent DI are both reduced. For patients with large tumors (>10 mm), tumors of uncertain type, and tumors that have substantial extrasellar extension, the transsphenoidal approach is inadequate and a bifrontal intracranial approach is required for successful removal. Current trends are moving toward endoscopic approaches to the pituitary tumor. Less invasive approaches, such as the transnasal approach combined with endoscopic resection of tumor, have been performed. The endoscopic technique has less morbidity and a shorter hospital stay than the traditional approach.
a) Patients undergo transsphenoidal operations for the treatment of hypersecreting pituitary tumors or nonsecretory tumors that cause visual complications because of their size and location. Clinical symptoms of secretory pituitary tumors include amenorrhea, galactorrhea, Cushing disease, and acromegaly.
b) Each preoperative condition has its own constellation of systemic disorders and accompanying effects on intracranial dynamics that should be considered when an anesthetic technique is selected.
c) Pituitary tumors can damage decussating optic fibers, producing blindness in the temporal half of the visual field of both eyes (bitemporal heteronymous hemianopsia).
d) Occasionally, an aneurysm of one of the internal carotid arteries may produce nasal hemianopsia on the affected side.
e) Patients who have Cushing disease may also be affected by hypertension, diabetes, osteoporosis, obesity, and friability of skin and connective tissue.
f) Patients who have acromegaly may have hypertension, cardiomyopathy, diabetes, and osteoporosis, as well as cartilaginous and soft tissue hypertrophy of the larynx and enlargement of the tongue, complicating intubation of the trachea.
g) Patients who have panhypopituitarism may exhibit hypothyroidism, requiring preoperative thyroid supplementation.
h) Laboratory tests: CBC, electrolytes, BUN, creatinine, as indicated from history and physical. Hyponatremia may indicate DI; hypercalcemia may indicate multiple endocrine neoplasia type 1.
a) The transsphenoidal approach usually necessitates the head and back to be elevated 10 to 20 degrees. The patient’s head is supported by a three-point pin head holder and centered within a C-arm fluoroscopy unit for radiographic control during surgery. The patient’s arms are placed at the sides and padded so that injury to the ulnar nerves is avoided.
b) The patient’s airway is shared with the surgeon; therefore, great attention must be directed to the proper securing of the ETT and anesthesia circuit to prevent unintended extubation and anesthesia circuit disconnection.
c) Hyperventilation is avoided after anesthetic induction because reductions in ICP result in retraction of the pituitary into the sella, making surgical access difficult.
d) The anesthesia provider should also consider the potential for massive hemorrhage because the carotid arteries lie adjacent to the suprasellar area and may be inadvertently injured.
e) Postoperative endocrine dysfunction may occur, namely DI, when the resection involves the suprasellar area.
(1) DI that occurs after most transsphenoidal procedures is usually self-limited and resolves within 1 week to 10 days.
(2) Although the onset is usually on the first or second postoperative day, DI may develop during the perioperative period or in the immediate recovery period.
(3) Intraoperative diagnosis is made with the sudden onset of diuresis. The diagnosis may be confirmed with concurrent urine and serum osmolalities.
(4) If DI persists or if it becomes difficult to match urinary losses, the patient may receive aqueous vasopressin (Pitressin) or desmopressin (DDAVP).
(5) IV DDAVP is longer acting and is not associated with the coronary vasoconstriction that follows administration of aqueous vasopressin.
f) Anesthetic induction, maintenance, and emergence
(1) After anesthetic induction and intubation, the ETT is typically moved to the left corner of the patient’s mouth and secured to the chin with tape to maximize exposure to the surgical field. A right-angled ETT may be effective because such tubes are prebent and curve along the mandible when exiting the mouth.
(2) The esophageal stethoscope and temperature probe are inserted and secured on the lower left as well, leaving the upper lip totally exposed. An orogastric tube is placed, aspirated, and then put to gravity drainage during the procedure.
(3) The oropharynx is then packed with moist cotton gauze.
(4) The eyes are first taped closed and then covered with cotton-padded adhesive patches or Tegaderm or to prevent corneal abrasion and seepage of cleansing solution and blood into the eyes.
(5) Propofol, an opioid (fentanyl, sufentanil, alfentanil, or remifentanil), and a neuromuscular relaxant (either succinylcholine or a nondepolarizing neuromuscular relaxant for intubation followed by a selected nondepolarizing agent) with a combination of air and oxygen is a commonly used anesthetic combination for this procedure. Isoflurane may be added in low concentrations for blood pressure control; alternatively, it may be used as the primary anesthetic drug.
(6) The topical use of cocaine and the oral and nasal submucosal injection of local anesthetic solutions containing epinephrine help constrict gingival and mucosal vessels and dissect the nasal mucosa away from the cartilaginous septum.
(a) Epinephrine use may produce hypertension of dysrhythmias or both; cocaine interferes with the intraneuronal uptake of catecholamine and can augment both the hypertensive and dysrhythmogenic properties of epinephrine.
(b) The use of epinephrine is relatively safe if concentrations of 1:100,000 to 1:200,000 are used and the total dose does not exceed 10 mL of 1:100,000 solution in 10 minutes for a 70 kg adult.
(c) A total dose of 200 mg of cocaine should not be exceeded. Persistent dysrhythmias may require treatment with lidocaine or possibly a β-blocker. Hypertension may be controlled with an increased concentration of the selected inhalation agent or with small IV doses of hydralazine, labetalol, or esmolol.
g) In some cases, it may be necessary to insert a catheter into the lumbar subarachnoid space to facilitate the injection of preservative-free saline to delineate the suprasellar margins or for prevention of CSF leak postoperatively. If air is injected, N2O must be discontinued from the anesthetic mixture because of rapid diffusion into the air present in the closed cranial vault.
h) Emergence from anesthesia should be conducted as described for craniotomy procedures.
(1) IV lidocaine, 1.5 mg/kg given approximately 3 minutes before suctioning and extubation, decreases coughing, straining, and hypertension.
(2) Postoperatively, patients should be responsive to commands in the recovery room.
(3) Steroid therapy is continued throughout this period and is tapered over time, if appropriate.
K Venous air embolism
In addition to the previously mentioned monitoring modalities, monitoring during posterior fossa surgery requires consideration of patient position and the potential for VAE. Air may also be entrained from the cranial pin sites of the Mayfield head holder from improperly connected vascular lines (arterial, central, and IV and high-pressure mechanical ventilation).
The occurrence of VAE depends on the development of a negative pressure gradient between the operative site and the right side of the heart. As the gradient between the cerebral veins and the right atrium increases, the potential for air entry increases. The estimated incidence of VAE during neurosurgical procedures ranges from 5% to 50%, with an increased incidence in the sitting position. The physiologic consequences of VAE depend on both the volume and the rate of air entrainment.
(1) Paradoxical air embolism (PAE) develops with the entry of air into the systemic circulation. Individuals with an existing anatomic connection between the right and left sides of the heart (atrial or ventricular septal defect, probe-patent foramen ovale) are at risk.
(2) If right-sided heart pressures exceed left-sided pressures (a situation that may occur in fluid-restricted neurosurgical patients), systemic air may embolize and enter the arterial circulation through a probe-patent foramen ovale.
(3) Patients who require the sitting position should be carefully evaluated with echocardiograms if the history suggests the presence of an intracardiac defect (presence of heart murmur) or probe-patent foramen ovale. It is estimated that or many as 20% of adults and 50% of children have a probe-patent foramen ovale.
(4) The presence of a probe-patent foramen ovale may be elicited with the injection of contrast material before, during, and after the patient produces a Valsalva maneuver. If a probe-patent foramen ovale is identified, the surgical procedure should be accomplished in an alternative position.
(1) The entrainment of air into the vascular system is usually of little consequence because the lungs serve as effective blood filters. Small bubbles of air are absorbed into the blood or enter the alveoli, where they are eliminated.
(2) The efficient filtering capacity of the lung may be breached by a large bolus of air or after the administration of a pulmonary vasodilator (e.g., aminophylline), which acts to widen the venous-arterial barrier. Air enters the venous circulation as small bubbles that pass through the right side of the heart, entering the pulmonary arterioles.
(3) A reflexive sympathetic pulmonary vasoconstriction is produced after the release of endothelial mediators, which are ultimately responsible for the clinical manifestations (pulmonary hypertension, hypoxemia, CO2 retention, increased dead-space ventilation, and decreased etco2).
(4) The continued entry of air produces an airlock within the right ventricle, producing right ventricular failure and decreased cardiac output.
(5) Altered ventilation-perfusion relationships parallel the hemodynamic changes.
(6) Obstructed pulmonary blood flow increases dead-space ventilation, resulting in decreased etco2. The entry of a large volume of air in the alveoli may be detected by the sudden appearance of end-tidal nitrogen. However, detection of nitrogen requires use of a mass spectrometer.
(7) The selection of appropriate monitoring for the detection of VAE is based on the various sensitivities of the available monitoring modalities.
(8) Precordial Doppler monitoring can detect air entrainment at rates as small as 0.0021 mL/kg/min.
(a) The Doppler probe is affixed over the right side of the heart along the right sternal border between the third and sixth intercostal spaces.
(b) Proper positioning over the right atrium is confirmed if a change in Doppler signal is elicited when a 10-mL bolus of saline is injected rapidly into a previously placed right central venous catheter.
(c) The placement of a right atrial catheter affords the means for diagnosis and recovery of IV air and also reflects cardiac preload.
(d) When a right atrial catheter is placed, it is recommended that either radiographic confirmation or ECG confirmation of proper placement of the catheter tip be obtained.
(9) Capnography complements the capabilities of the Doppler device because small, hemodynamically insignificant air emboli detected with the Doppler device can be differentiated from emboli that may produce arterial hypotension.
(10) Transesophageal echocardiography (TEE) is the most sensitive method of air embolism detection, but it is also the most expensive. With TEE, it is possible to observe both cardiac contractility and air bubbles as they pass through the heart. TEE is also capable of detecting PAE in the heart.
(11) The detection of a “mill-wheel” murmur via precordial or esophageal stethoscope is a late sign of air entrainment.
(12) The use of nitrous oxide is contraindicated when developing a venous embolism is a possibility.
Advantages and disadvantages of selected monitors for detection of VAE are noted in the following table.
Monitors for Detection of Venous Air Embolism
| Monitor | Advantages | Disadvantages |
| Precordial Doppler | Noninvasive Most sensitive noninvasive monitor Earliest detector (before air enters pulmonary circulation) |
Nonquantitative May be difficult to place in obese patients, patients with chest wall deformity, or patients in the prone or lateral position False-negative result if air does not pass beneath ultrasonic beam (approximately 10% of cases) Useless during electrocautery IV mannitol may mimic intravascular air |
| Pulmonary artery (PA) catheter | Quantitative slightly more sensitive than etco2 Widely available Placed with minimum difficulty in experienced hands Can detect right atrial pressure more easily than PCWP |
Small lumen, less air aspiration than with right atrial catheter Placement for optimal air aspiration may not allow PCWP measurement Nonspecific for air |
| Capnography (etco2) | Noninvasive Sensitive Quantitative Widely available |
Nonspecific for air Less sensitive than Doppler, PA catheter Accuracy affected by tachypnea, low cardiac output, COPD |
| End-tidal nitrogen (etn2) | Specific for airDetects air earlier than etco2 | May not detect subclinical air embolism May indicate air clearance from pulmonary circulation prematurely Accuracy affected by hypotension |
| Transesophageal echocardiography (TEE) | Most sensitive detector of air Can detect air in left side of heart and aorta |
Invasive, cumbersome Expensive Monitor must be observed continuously Not quantitative May interfere with Doppler |
COPD, Chronic obstructive pulmonary disease; ETCo2, end-tidal carbon dioxide; IV, intravenous; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure.
Modified from Smith DS, Osborne I. Posterior fossa: anesthetic considerations. In Cottrell JE, Smith DS. Anesthesia and Neurosurgery. 4th ed. St. Louis: Mosby; 2001:343.
a) Treatment of venous air embolus: Detection of VAE should prompt the steps listed in the box on pg. 399.
L Ventriculoperitoneal shunt
A ventriculoperitoneal shunt is placed to relieve increased CSF pressure. The hydrocephalus may be caused by a congenital defect, cyst, tumor, trauma, infection, or CBF absorption abnormality. Therefore, patients undergoing this procedure range in age from newborn to elderly. Besides a conduit to the peritoneum, the ventricle can also be drained into the pleura or right atrium.
a) Neurologic; ICP monitoring is routine.
b) Headaches are the most common symptom. Assess for level of consciousness, headaches, nuchal rigidity, seizures, nausea or vomiting, and papilledema as indications of severe hydrocephalus (ICP >15 mmHg). Presurgical neurologic defects such as stroke, spina bifida, and focal defects should also be assessed.
c) Review results of CT of the head. Review blood pressure and heart rate trends in relation to ICP. Cushing triad is present with increased ICP and includes increased blood pressure, and decreased heart rate, and irregular respirations.
a) CBC, electrolytes (especially if the patient is receiving diuretics), glucose (especially if the patient is taking steroids such as dexamethasone), prothrombin time, partial thromboplastin time, and type and screen are obtained.
b) Other diagnostic tests are as indicated. Communicate with the neurologist if steroids and diuretic therapy are anticipated during surgery. Usually, preoperative medication is not given to patients with increased ICP.
a) Monitors: Standard. An ICP monitor is also used.
b) Position: Supine with the head turned to the contralateral side. A shoulder roll may be used. A foam headrest aids positioning.
c) Additional equipment: The table will probably be turned, thus requiring an adequate length of ventilation tubing. Because much of the patient will be exposed, it is helpful to have a fluid warmer and a higher room temperature.
d) Drugs and tabletop: Standard. Fluids can be administered at approximately 4 to 6 mL/kg/hr. Overzealous fluid administration can exacerbate increased ICP.
5. Anesthetic technique and perioperative management
a) Use general anesthesia with endotracheal intubation. With exception of ketamine, all induction agents are good choices because of their cerebroprotective properties by depressing cerebral metabolism. Muscle relaxation is desirable. Succinylcholine is relatively contraindicated for patients with increased ICP.
b) Normocarbia aids the surgeon in cannulating the ventricles. It is wise to have the propofol or etomidate immediately available during the “tunneling,” which is the most stimulating part of this procedure. Extubation is performed at the end of the procedure. Prophylactic antiemetics should be administered 30 minutes before the conclusion of anesthesia.
Assess the patient’s neurologic status. Pain can usually be managed with oral preparations.