Genitourinary system
A Cystectomy
The bladder is usually removed for cancer but may also be removed for severe hemorrhagic or radiation cystitis. In a radical cystectomy for invasive cancer in women, the uterus, fallopian tubes, ovaries, and a portion of the vaginal wall are removed. In men, the ampulla of the vas deferens, prostate, and seminal vesicles are removed. There is also pelvic lymph node dissection, and a urinary diversion is created (through the intestine).
a) Cardiac, respiratory, neurologic, and endocrine systems: Assessment is routine.
b) Renal: Gross hematuria may be a symptom. Check renal function tests as well as evidence of a urinary tract infection.
c) Gastrointestinal: Patients are at risk for fluid and electrolyte imbalance because of bowel preparation.
a) Laboratory tests: Complete blood count, electrolytes, blood urea nitrogen, creatinine, glucose, prothrombin time, partial thromboplastin time, type and screen for 2 to 4 units of blood, and urinalysis
b) Diagnostic tests: Electrocardiography and chest radiography for most of this patient population
a) Monitors: Standard, arterial line, and central venous pressure; urine output not measurable during this procedure; two large-bore, reliable intravenous (IV) lines available
b) Additional equipment: Epidural insertion and infusion supplies (if using), and warming devices for the patient and fluids
d) Drugs and fluids: 6 to 10 mL/kg/hr of crystalloid for maintenance; 2 to 4 units of blood readily available
Combined general and epidural or general anesthesia with standard induction is used. The patient may be anemic because of hematuria and hypovolemic because of bowel preparation. Attempt to correct these conditions before induction. Maintenance is routine, with special attention paid to fluid calculations and keeping the patient warm. Plan to extubate immediately postoperatively unless the patient is unstable during the procedure or when prior respiratory complications prevent early extubation.
Epidural or patient-controlled analgesia should be planned for preoperative use. Watch the patient for signs of hypovolemia, anemia, or pulmonary edema resulting from fluid shifts intraoperatively.
B Cystoscopy
Cystoscopy is the use of instrumentation to examine the urinary tract. A cystoscope may be used for diagnostic or therapeutic procedures such as for workup of hematuria, stricture, and tumor; removal and manipulation of stones; placement of stents; and follow-up of therapy. Retrograde pyelography and other dye studies may be used. This procedure is usually performed on an outpatient basis.
The technique of choice is regional blockade or general anesthesia.
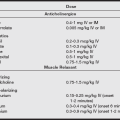
a) IV sedation: Midazolam (Versed), fentanyl, or propofol in sedation doses
b) Regional blockade: Analgesia to J10 required
c) General anesthesia: Laryngeal mask airway or oral endotracheal tube
a) Induction: No specific indications
(1) Diagnostic dyes may be administered. Use indigo carmine dye (α-sympathomimetic effects) cautiously in patients with hypertension or cardiac ischemia. Methylene blue dye may cause hypertension. Oxygen saturation readings may be altered by dye administration.
(2) Persistent erection may occur in younger male patients, thus preventing manipulation of the cystoscope. Use deeper anesthesia.
(3) Water or irrigation solution may be used to distend the bladder. See the discussion of transurethral resection of the prostate later in this section.
(4) Quadriplegic or paraplegic patients may undergo repeated cystoscopies. Autonomic hyperreflexia is possible if the injury is above level T5.
C Extracorporeal shock wave lithotripsy
Extracorporeal shock-wave lithotripsy (ESWL) is a technique that uses high-energy shock waves to fragment renal calculi into small particles. A biplanar fluoroscopy unit is used to focus the shock wave on the target stone. The shock wave is repeated several thousand times and causes the stone to disintegrate. The focused, reflected shock wave passes through the water and enters the body through the flank.
Modern lithotriptors do not require the patient to be submerged in water. Although they do use water for the production of shock waves, a membrane over the shock-wave generator encapsulates the fluid. Transmission of shock waves to the patient is ensured by the use of coupling gel between the patient and the generator membrane.
2. Preoperative assessment and patient preparation
a) Routine preoperative assessment with laboratory tests based on any abnormalities found in the history and physical examination. Consider cardiac status; many hemodynamic changes are associated with this procedure.
b) Absolute contraindications are pregnancy bleeding disorders and abnormal coagulation parameters. Relative contraindications include these active urinary tract infection and a urinary tract obstruction distal to the stone that prevents passage of stone fragments.
c) Relative contraindications include aortic aneurysm, spinal tumors, orthopedic implants in the lumbar region, morbid obesity, AILD (automatic implantable cardioverter-defibrillator), uncontrolled arrhythmias, and coagulation disorders.
d) Ureteral stent placement before ESWL may be used to move the stone upward in the ureter, where it is amenable to therapy.
e) Adequate IV hydration aids in the passage of stone fragments.
f) Prophylactic antibiotics may be given.
g) Electrocardiography, automated cuff measurement of blood pressure, and pulse oximetry are indispensable during lithotripsy.
h) The electrocardiograph must be of good quality because the R wave is used to trigger the shocks. Synchronization of the shock wave to the electrocardiograph has reduced the incidence of cardiac dysrhythmias but has not totally eliminated them. These dysrhythmias are attributed to mechanical stimulation of the heart. Supraventricular premature complexes and premature ventricular complexes are the most common dysrhythmias noted. Atropine or glycopyrrolate may be given to increase the heart rate and thus the shock-wave rate.
a) Patient movement: For lithotripsy to be most effective, the stone must remain at the focal point. Because patient movement and patterns of respiration can change kidney and stone position, movement must be minimized and ventilation carefully controlled. The number and intensity of shock waves can be reduced when stone movement is minimized.
b) Anesthetic techniques: Various anesthetic techniques have been used for ESWL. General anesthesia is advantageous because of its rapid onset and control of patient movement. Other techniques include spinal or epidural anesthesia, patient-controlled analgesia, monitored anesthesia care, and topical anesthesia with eutectic local anesthetics. Continuous infusions of propofol, methohexital, ketamine, and alfentanil have been used alone or with midazolam for ESWL anesthesia.
Spinal anesthesia has the advantage of a rapid onset, and a pure opiate spinal using sufentanil is a common technique. Disadvantages include hypotension, spinal headache, and the inability to reinforce the block.
Although epidural anesthesia is associated with a slower onset, hypotension is less, and the block can be reinforced as needed. A dermatomal level of T4 or T6 must be achieved as renal innervation is derived from T10 to L2.
D Laparoscopic nephrectomy
Indications for nephrectomy include calculus, hemorrhage, hydronephrosis, hypertension, neoplasms, transplantation, trauma, chronic infection, and vascular disease. Partial nephrectomy is performed to preserve as much renal function as possible. Surgery of the kidney is usually accomplished through a flank incision.
2. Preoperative assessment and patient preparation
a) History and physical examination: Individualized for the patient’s condition
(1) IV pyelography with nephrotomography: Identify a renal mass.
(2) Ultrasonography: This differentiates simple cysts from solid tumors.
(3) Arteriography: This determines whether the kidney is suitable for renal transplantation.
(a) Prothrombin time, partial thromboplastin time, complete blood count, electrolytes, and glucose
(b) Glomerular filtration rate: Blood urea nitrogen, plasma creatinine, and creatinine clearance
(c) Renal tubular function: Urine concentration ability, sodium secretion, proteinuria, hematuria urine sediment, and urine volume
c) Preoperative medications and IV therapy
(1) Identify the date of last hemodialysis.
(2) Epidural catheter: Perform a test dose in the preoperative area.
(4) Use small incremental doses of benzodiazepines to facilitate anxiolysis.
(5) Have a minimum of two peripheral IV tubes (16 to 18 gauge) with moderate fluid replacement.
(6) In patients with renal failure, administer hypotonic solutions: 5% dextrose in water or 5% dextrose and 0.45% saline.
(2) An arterial line and central venous pressure monitoring may be necessary to trend volume status, especially if the patient is elderly with coexisting medical disease.
(3) A noninvasive blood pressure cuff should not be placed in an arm with an arteriovenous fistula.
(1) Dopamine: Low dose (2–5 mcg/kg/min) to increase urinary output
(2) Furosemide (Lasix), mannitol, or both for stimulation of urinary output
(3) Indigo carmine (hypertension resulting from an α-agonist) or methylene blue (hypotension and interference with the pulse oximeter) administration (IV to assess urinary flow)
(1) A lateral decubitus position is used, with the kidney bar raised.
(2) With low calcium levels, skin and nerve damage occur easily.
(3) Inadequate support of the head may lead to Horner syndrome (ptosis, enophthalmos, miosis, and anhidrosis) postoperatively.
(4) Evaluate the radial pulse after placement of an axillary roll.
(5) Respiration is impaired secondary to ventilation–perfusion mismatching, decreased functional reserve capacity, decreased vital capacity, and decreased thoracic compliance.
(6) Reassess breath sounds after movement; an endotracheal tube may migrate into the mainstem bronchus during positioning.
a) See the discussion of radical prostatectomy later in this section.
b) Consider rapid-sequence induction because renal patients may be considered to have a full stomach.
(1) Opioids can be used because only a small amount of the drug is excreted unchanged by the kidneys.
(2) Succinylcholine is contraindicated if the potassium is elevated.
(3) Cisatracurium does not require a functional kidney because it is degraded by Hofmann elimination and is a good choice for muscle relaxation. Laudanosine is a metabolite and is associated with seizures. This is not a concern with short-term perioperative use.
(4) Vecuronium and rocuronium may be used for muscle relaxation.
(5) Regardless of blood volume status, renal patients may respond to induction of anesthesia as if they are hypovolemic.
(6) Induction of anesthesia and intubation of the trachea can be safely accomplished with IV drugs plus a nondepolarizing muscle relaxant.
(7) Propofol is highly protein bound, and this may necessitate a reduced dosage. Propofol is exclusively metabolized by the liver, and metabolites are inactive.
(8) Ketamine is less protein bound with less than 3% renal excretion.
(9) Etomidate is 75% protein bound and does not require adjustment.
(1) Maintain normal end-tidal carbon dioxide levels.
(2) If working on a donor nephrectomy, the eleventh rib may be removed; pneumothorax is a complication; therefore, nitrous oxide is best avoided.
(3) Maintain urinary output; use medications if necessary.
(4) Inhalation anesthetic is used to control intraoperative hypertension.
(1) If hypertension occurs on emergence, administer a vasodilator.
(2) Renal patients are considered to have a full stomach; some practitioners require an “awake” patient before extubation.
(3) Initiate regional blockade through the epidural catheter for postoperative analgesia before the end of the case.
a) Continue to assess volume status.
b) Obtain a chest film. Rule out pulmonary edema and pneumothorax, which may occur after administration of large volumes of fluid in the flank position or from removal of the eleventh rib, respectively.
c) For patients with renal failure, normeperidine, the major metabolite of meperidine, may accumulate and result in prolonged depression of ventilation and seizures.
E Laparoscopic urologic surgery
Laparoscopy is the process of inspecting the abdominal cavity through an endoscope. Some examples of surgical procedures that can be done laparoscopically include varicocelectomy, percutaneous stone retrieval, nephrectomy, transplants, and radical prostatectomy.
Carbon dioxide is used to insufflate the abdominal cavity to facilitate view during this procedure. Several pathophysiologic changes can occur after carbon dioxide pneumoperitoneum and extremes of patient positioning required for the procedure.
Unique problems specific to urologic surgery are listed below.
a) The urogenital system is a retroperitoneal system. As such, carbon dioxide insufflated in this space communicates freely with the thorax and subcutaneous tissue. Subcutaneous emphysema can occur and may extend to the head and neck. In severe cases, it may lead to submucous swelling and airway compromise in the unprotected airway.
b) In long cases, carbon dioxide may not be reabsorbed and acidosis may develop. Because carbon dioxide insufflation coupled with steep Trendelenburg position and long procedures may increase intraabdominal and intrathoracic pressure, controlled ventilation is mandatory.
c) Increased pressure exerted by the insufflation may also affect renal and hepatic function. The pneumoperitoneum can cause renal cortical vasoconstriction because of activation of the sympathetic nervous system. Decreased renal perfusion activates the renin–angiotensin–aldosterone system, which causes vasoconstriction. These effects are additive to those seen with surgical stress. Renal and hepatic perfusion may be altered.
d) Some suggestions to minimize the impact of positive pressure pneumoperitoneum are (1) lowering insufflation pressures, (2) operating in a gasless environment, (3) substituting inert gas for carbon dioxide, (4) using drugs to antagonize the neuroendocrine response, (5) volume expansion, and (6) using mechanical devices. It has been reported that the use of intermittent sequential pneumatic compression activated over the lower limbs 15 minutes after the pneumoperitoneum improves splanchnic and renal perfusion. This technique augments cardiac output and lowers systemic vascular resistance.
F Penile procedures
Penile procedures are commonly performed for the following three indications: (1) to repair congenital defects such as hypospadias (typically a pediatric procedure), which is usually a pediatric procedure; (2) for penectomy or penile resection as a result of penile cancer; and (3) for implants to compensate for impotence. Organic impotence is often secondary to diabetes, hypertension or side effects from the systemic treatments or spinal cord trauma.
Assessment is individualized based on the patient’s condition.
5. Anesthetic technique and perioperative management
a) For pediatric patients, an inhalation induction is typical for general anesthesia. Intubation is often desired because hypospadias repair generally takes longer than 2 hours.
b) For penectomy or prosthetic insertion, a regional or general anesthetic may be used, depending on the preferences of the patient, and presence of comorbidities medical condition. Muscle relaxation is not required, and blood loss is minimal.
c) Some practitioners perform a caudal block for pediatric patients just before awakening for postoperative pain control.
Urinary retention is common and may be intensified with the use of a regional anesthetic.
G Percutaneous nephrolithotomy
Removal of kidney stones 25 mm or smaller can also be accomplished through percutaneous nephrolithotomy. This procedure requires general anesthesia and postoperative hospitalization. Stones are removed via a rigid operating scope inserted in the lower calyx of the kidney under fluoroscopy. After being located, calculi are pulverized using laser, electrohydraulic, or ultrasound probes placed directly on the stones. The procedure is performed with the patient in the prone position; therefore, associated anesthetic considerations apply.
H Radical prostatectomy
Open prostatectomy refers to removal of the prostate with or without the prostatic capsule. Several surgical approaches may be used, including suprapubic, transvesical, retropubic, perineal, and transcoccygeal. In suprapubic or transvesical procedures, the prostate is removed through the cavity of the bladder. Retropubic prostatectomy is performed through a low abdominal incision without opening the bladder. The transcoccygeal approach allows maximal surgical access to the posterior lobes of the prostate. Perineal prostatectomy is most often performed for cancer of the prostate when it is confined to the capsule. This procedure may also be done with the assistance of a robot.
2. Preoperative assessment and patient preparation
a) History and physical examination: These are individualized based on the patient’s condition; assess for symptoms of metastatic disease.
(1) Plasma concentration of prostate-specific antigen is increased in prostate cancer.
(2) Blood urea nitrogen, creatinine, electrolytes, complete blood count, coagulation profile, and type and cross-match are obtained.
c) Preoperative medication and IV therapy
(1) Bowel preparation will render the patient in a dehydrated state.
(2) Have a minimum of two peripheral IV lines (18 and 16 gauge) with moderate fluid replacement.
(3) Epidural catheter: Perform a test dose in the preoperative area.
(5) Small incremental doses of benzodiazepines may be given to ease patient preparation.
(2) Arterial line and central venous pressure monitoring may be necessary to trend volume status, especially if the patient is elderly with coexisting medical diseases. Central venous pressure monitoring may also be indicated for potential of venous air embolism related to positioning of the patient.
(1) Although controversial, low-dose dopamine (2 to 5 mcg/kg/min) may be initiated to increase urinary output.
c) Position: Supine; the surgeon may request use of kidney rest and for the patient’s body to be partially flexed. Expect the need to rotate the patient from side to side for optimal surgical viewing.
a) Regional blockade, general anesthesia, or a combination of both
b) Technique of choice: General anesthesia with endotracheal intubation
c) Regional blockade: Epidural catheter placement in preoperative area; analgesia to T6 to T8
d) General anesthesia: Endotracheal intubation
e) Regional blockade with general anesthesia: Smaller doses of each with the combination technique
a) Induction: The patient may be dehydrated and show an exaggerated response to medications.
(1) Initiate warming modality and use fluid warmers.
(a) Radical retropubic: Supine with the operating room table broken in the midline or kidney rest to provide slight hyperextension; a slight Trendelenburg position until the patient’s legs are parallel to the floor
(b) Radical perianal: Exaggerated lithotomy position combined with flexion of the trunk and a Trendelenburg tilt
(3) The Foley catheter is discontinued during the case, and volume status (blood loss) is difficult to quantify.
(4) Muscle relaxation is necessary.
(5) Methylene blue or indigo carmine is administered after reanastomosis of the urethras. A momentary and artificial decrease in the pulse oximetry reading will occur.
a) Obtain hemoglobin and hematocrit.
b) Continue to trend volume status.
c) For postoperative pain management, consider patient-controlled analgesia, epidural techniques, or a combination of ketorolac and opiates.
d) Early postoperative complications include deep venous thrombosis, pulmonary embolus, and wound infection.
e) Late complications include incontinence, impotence, and bladder neck contracture.
f) Rhabdomyolysis is seen with the extreme lithotomy position and may progress to acute renal failure. Monitor urine output and maintain at more than 0.5 mL/kg/hr.
I Renal transplant
Renal transplantation has been performed for nearly a century and is an accepted means of replacing kidney function in patients with end-stage renal disease who are on maintenance dialysis. In this procedure, the donor kidney is placed extraperitoneally in the recipient’s iliac fossa. The renal artery is anastomosed to the internal iliac artery, the renal vein to either the external or the common iliac vein and the ureter to the bladder. The anesthesia provider plays a vital role in management of the viability of the transplanted kidney. Three interrelated variables affect surgical outcomes: management of the donor, preservation of the harvested organ, and perioperative care of the transplant recipient. Additionally, improved surgical and immunosuppressive techniques have contributed to better outcomes in terms of graft survival.
2. Preoperative assessment and patient preparation
a) Harvested organ preservation
(1) Ischemic time, beginning with the clamping of the donor’s renal vessels and ending with the vascular anastomosis in the recipient, is a crucial factor in graft preservation. When renal ischemic time is less than 30 minutes, diuresis begins quickly, but if it is 2 hours or longer, a variable period of oliguria or anuria may occur. The definition of renal ischemic times for warm and cold preservation techniques is noted in the following table.
| Warm | Cold | |
| Begins | Clamping of donor vessels; initial placement in recipient | Perfusion of harvested organ with cold preservation solution; storage at 4° C |
| Ends | Vascular anastomosis in recipient; interrupted with perfusion of cold preservation solution | Perfusion by recipient |
(1) Choice of anesthesia for the living, related donor is not critical.
(2) Adequate amounts of balanced salt solution should be administered to ensure a brisk diuresis from the donor kidney and to offset reduced venous return resulting from use of the flank position.
(3) The greatest risk to the donor is hemorrhage.
(4) Adequate IV access and blood must be available in the event that transfusion becomes necessary.
(5) If the donor kidney is obtained from a brain-dead patient, preservation of graft function is the highest priority. The loss of sympathetic tone after brain death may produce mild hypotension despite adequate volume replacement. Many patients with irreversible cerebral dysfunction are hypovolemic and require vigorous fluid resuscitation.
(6) If pharmacologic support of the cardiovascular system is necessary, a dopamine infusion at a rate of 1 to 3 mcg/kg/min is recommended. Renal vasoconstrictive properties of high-dose vasopressors reduce immediate allograft function and increase the risk of kidney damage. Maintenance of urinary output is paramount and may warrant the use of diuretics and a low-dose dopamine infusion.
(1) Because cadaveric kidneys can be preserved for 36 to 48 hours with cold perfusion, time is sufficient for optimal preparation of the transplant recipient.
(2) The recipient should be free of acute illness and infections because of the likelihood of their spread during immunosuppressive therapy.
(3) Acute alterations in fluid and electrolyte balance should be corrected with dialysis carried out 24 hours before transplantation. Postdialysis laboratory values should be checked, and the serum potassium (K+) level should be below 5.5 mEq/L. Coagulation studies and acid–base status should be normal. Serum creatinine concentration should be below 10 mg/dL and blood urea nitrogen level below 60 mg/dL after dialysis.
(4) Anesthetic considerations are summarized in the following box.
(5) Chronic anemia is common, and transfusion is not required if oxygen delivery is adequate. Because of the danger of volume overload, anemia should be corrected during dialysis with transfusion of packed red blood cells.
(6) Abnormal platelet function, as well as ineffective production of factor VIII and von Willebrand factor, accounts for the syndrome of uremic coagulopathy seen in patients with renal failure. Correction of coagulation abnormalities has been accomplished through dialysis and administration of conjugated estrogen and desmopressin.
(7) Patients should fast for 6 to 8 hours before surgery if possible.
(8) Premedication may include narcotics or benzodiazepines in usual to reduced doses, depending on the status of the patient.
(9) The use of antacids, H2 antagonists, and metoclopramide should be considered if gastric emptying is delayed; however, reduced doses should be considered because these drugs depend on the kidney for excretion, and metoclopramide is partially excreted unchanged in the urine.
(1) Both regional and general anesthesia have been used successfully for renal transplantation.
(2) Spinal and epidural anesthesia are both satisfactory, and because the procedure is extraperitoneal and in the lower half of the abdomen, the block can be kept low.
(3) Advantages of regional anesthesia include a more aseptic technique, avoidance of the use of muscle relaxants and other drugs excreted by the kidney, and the fact that endotracheal intubation is not required. Intubation may increase the risk of nosocomial pneumonia. Pulmonary infection occurs in 10% to 15% of transplant recipients and is associated with a high mortality rate. An additional advantage of regional anesthesia is postoperative analgesia.
(4) Disadvantages of regional anesthesia techniques in these patients include hypotension associated with sympathetic blockade, the length of the procedure, and heparinization of the kidney. Sympathetic blockade can make control of blood pressure difficult in patients who may be hypovolemic.
(5) Because transplantation procedures may last several hours, large amounts of sedation may be needed to supplement regional techniques. Because local heparinization of the kidney is often used, the use of continuous regional techniques may be contraindicated. For these reasons, general anesthesia is now the preferred approach in patients who undergo transplantation.
(1) When general anesthesia is used, nitrous oxide combined with volatile agents, particularly isoflurane, or short-acting opiates is well tolerated.
(2) The skeletal muscle relaxant properties and minimal metabolism make isoflurane an attractive choice.
(3) Reductions in cardiac output secondary to the negative inotropic effects of volatile drugs must be minimized to avoid suboptimal tissue oxygenation in these anemic patients.
(4) The choice of muscle relaxant must take into consideration the unpredictable nature of renal function after transplantation. Relaxants that are independent of renal function for plasma clearance, such as atracurium and cis-atracurium, are excellent for this patient population.
(5) The pharmacokinetics of anticholinesterase drugs used for antagonizing nondepolarizing muscle relaxants is unchanged within 1 hour after renal transplantation.
(6) Succinylcholine can be used to facilitate intubation if serum K+ level is normal.
c) Other drugs and considerations
(1) Mannitol is included in many transplant protocols. It does not depend on renal tubular concentrating mechanisms to promote urinary formation, and it facilitates urinary output and a reduction in tissue and intravascular volume.
(2) The effect of low-dose dopamine administration on cadaver graft function has also been evaluated. An infusion rate of 1 to 3 mcg/kg/min preoperatively does not affect early or late graft function. In normovolemic and hemodynamically stable patients without severe vascular disease and in patients who do not receive kidneys subjected to prolonged hypertension, preservation, or anastomotic times, infusion of dopamine at a rate of 1 to 3 mcg/kg/min perioperatively does not affect early or late graft function. In these circumstances, early graft function is dependent on ischemic changes, and late graft function is dependent on the management of rejection.
(3) Cardiac arrest has been reported after completion of the renal artery anastomosis to the transplanted kidney. Arrest occurred at the time the occlusion clamp was released and was attributed to hyperkalemia from washout of the K+-containing solutions used to preserve the kidney. If clamping of the external iliac artery is necessary during the procedure, K+ can be released from the ischemic limb. Unclamping may also result in hypotension from the release of vasodilating substances from ischemic limbs and the subsequent increase in vascular capacity.
(1) Tacrolimus (Prograf) is indicated for the prophylaxis of organ rejection in patients receiving allogeneic liver or kidney transplants. It is recommended that tacrolimus be used concomitantly with adrenal corticosteroids. Because of the risk of anaphylaxis, tacrolimus injection should be reserved for patients unable to take tacrolimus capsules orally. To avoid excess nephrotoxicity, tacrolimus should not be used simultaneously with cyclosporine. The recommended starting oral dosage of tacrolimus is 0.2 mg/kg/day administered every 12 hours in two divided doses. The initial dose of tacrolimus may be administered within 24 hours of transplantation but should be delayed until renal function has recovered (as indicated, for example, by a serum creatinine <4 mg/dL). In patients unable to take oral tacrolimus capsules, therapy may be initiated with tacrolimus injection. The initial dose of tacrolimus should be administered no sooner than 6 hours after transplantation. The recommended starting dose of tacrolimus injection is 0.03 to 0.05 mg/kg/day as a continuous IV infusion.
(2) Sirolimus (Rapamune) is indicated for the prophylaxis of organ rejection in patients receiving renal transplants. It is recommended that sirolimus be used initially in a regimen with cyclosporine and corticosteroids. In patients at low to moderate immunologic risk, cyclosporine should be withdrawn 2 to 4 months after transplantation and the sirolimus dose should be increased to reach recommended blood concentrations. Sirolimus inhibits T lymphocyte activation and proliferation that occurs in response to antigenic and cytokine (interleukin [IL]-2, IL-4, and IL-15) stimulation by a mechanism that is distinct from that of other immunosuppressants. Sirolimus also inhibits antibody production. Sirolimus should be administered orally once daily at 2 mg after initial loading.
(3) Mycophenolate mofetil (Cellcept) inhibits immunologically mediated inflammatory responses and tumor development. Selective, noncompetitive, and reversible inhibitor of inosine monophosphate dehydrogenase, mycophenolic acid, the active metabolite, inhibits the de novo synthesis pathway of guanosine nucleotides without being incorporated into DNA. Mycophenolate mofetil is indicated for the prophylaxis of organ rejection in patients receiving allogeneic renal, cardiac, or hepatic transplants. It should be used concomitantly with cyclosporine and corticosteroids. Mycophenolate mofetil IV is an alternative dosage form to mycophenolate mofetil capsules, tablets, and oral suspension; it IV should be administered within 24 hours after transplantation. Mycophenolate mofetil IV can be administered for up to 14 days; patients should be switched to oral mycophenolate mofetil as soon as he or she can tolerate oral medication.
(4) Azathioprine (Imuran) is a bone marrow–toxic derivative of 6-mercaptopurine. Although its mechanism of action is unknown, a single dose of 5 mg/kg is administered intravenously at the time of transplantation. The drug is added to an IV drip chamber and administered over 10 to 30 minutes. Maintenance doses of 2 mg/kg/day are used thereafter if leukocyte count is greater than 4000 white blood cells per microliter. Imuran is associated with dose-dependent neutropenia and occasionally with thrombocytopenia.
(5) Orthoclone (OKT3) is a mouse monoclonal antibody to the T3 antigen or human lymphocyte. It is administered daily by slow IV injection. A standard dose in patients who weigh more than 25 kg is 5 mg given by slow IV push. This drug is given only intravenously and is administered for 14 days. Patients receiving OKT3 are given antibiotics to minimize the risk of opportunistic infection. Risks associated with the use of this drug include anaphylaxis, pulmonary capillary leak, and fluid overload.
(6) Cyclosporine is a fungal metabolite that suppresses IL-II production and amplification of cell-mediated immunity. Side effects include nephrotoxicity, hypertension, hirsutism, tremor, and anaphylaxis. Use of the drug early after transplantation delays recovery of allograft function. It is not administered until renal allograft function has reduced serum creatinine level to half of the admission value. The induction dose is 12 mg/kg in two divided doses, and plasma levels are maintained by periodic IV or oral doses. The anesthetic action of drugs may be altered in individuals who receive even a single dose of cyclosporine. Several animal studies have shown that a single dose of this immunosuppressant increases the hypnotic effects of phenobarbital and the analgesic effect of fentanyl. The drug also enhances the neuromuscular blockade produced by vecuronium and atracurium.
(7) Steroid administration is common in patients who undergo renal transplantation. Both prednisone and methylprednisolone sodium succinate (Solu-Medrol) have potent anti-inflammatory and immunosuppressive effects. They are also associated with impaired fibroblast proliferation and function and with impaired wound healing. Prednisone administration is initiated with a dose of 2 mg/kg/day and slowly tapered to maintenance doses. Adjustments in the dosage of prednisone must be made according to the clinical situation. Methylprednisolone is used prophylactically in a dose of 2 mg/kg intravenously. It is also used for treatment of acute allograft rejection at a dose of 0.5 to 1 g/day for 3 days. The maximum dose is 6 g.
(1) In addition to routine monitors, a Foley catheter is inserted for the assessment of graft function.
(2) Central venous pressure lines are not routinely inserted; their use may indirectly improve graft function by improving the assessment of hydration status.
(3) A pulmonary artery catheter is useful if cardiac compromise is suspected or if the kidney is expected to have delayed graft function.
(4) Protection of vascular access and fistula patency is of prime importance with the use of blood pressure cuffs or if arterial cannulation is necessary.
(5) Sterile precautions during insertion of invasive lines are extremely important because transplant patients are immunocompromised.
(6) Strict adherence to aseptic technique is mandatory in the management of these lines, catheters, and endotracheal tubes. Commitment to aseptic technique on the part of the entire team may make the difference between safe transplantation and death for the patient.
(1) Fluid management may be generous or conservative.
(2) Fluid replacement should be with normal saline or with dextrose in saline, generally at a maintenance infusion rate.
(3) Immediate function of the transplanted kidney cannot be guaranteed, and excessive intraoperative fluid replacement can lead to pulmonary edema and swelling of the grafted kidney.
J Robotic urologic surgery
Robotic-assisted surgery is an emerging technique for management of various urologic procedures such as prostatectomy. One of the commercially available systems is the da Vinci surgical system (Intuitive Surgical, Mountain View, CA). It consists of a surgeon’s console for surgical work, a surgical cart that houses the video and lighting equipment, and a robotic tower that supports three or four robotic arms.
The surgeon’s console provides the surgeon with a three-dimensional, 10×–magnified view through a binocular viewpoint. Interaction is through “masters” into which the surgeon’s hands are inserted. Free movement is possible from the masters to robotic instruments. Endoscopic instruments include graspers, hooks, scissors, knives, and surgical energy devices.
Robotic surgery requires a coordinated approach by anesthetist and surgeon because the surgery is performed using a modified laparoscopic technique and can be very long in duration.
a) The Trendelenburg position is used. Major complications of surgery in the Trendelenburg position include: (1) neuropathies; (2) central venous pressure elevation; (3) intraocular or intracranial pressure elevation; (4) increased pulmonary venous pressure; (5) decreased pulmonary compliance; (6) reduced functional residual capacity; and (7) swelling of the face, eyelids, conjunctiva, and tongue.
b) Major anesthetic considerations for robotic procedures are summarized in the following box.
K Scrotal procedures
Scrotal procedures are considered minor operative procedures and can be performed on an outpatient basis. In adults, most elective scrotal procedures can be performed under local anesthesia with sedation. The most common procedures include surgery for infertility, hydrocele, and undescended testicle and orchiectomy for cancer.
The most common emergency scrotal operation is for testicular torsion. These patients will be in acute pain and should be considered to have a full stomach.
a) Most patients who present for infertility concerns, hydrocele, and minor procedures are essentially healthy.
b) Those who present for orchiectomy require careful preoperative evaluation for possible metastasis.
c) No specific laboratory tests or medications are required except those that are suggested from the preoperative evaluation.
b) Position: Supine. The patient’s arms usually are out at the sides. The lithotomy position may be requested.
c) Drugs and tabletop: Sedatives and narcotics. The tabletop should be set up for emergency general anesthesia.
Pain management may be commenced intraoperatively by the use of narcotics, ketorolac, or both. Some patients, especially if being treated for malignancy, may experience nausea and vomiting.
L Transurethral resection of the prostate
Transurethral resection of the prostate (TURP) is one of the most commonly performed surgical procedures in men older than 60 years of age. These patients are often at greater anesthetic risk because they are more likely to have cardiovascular or pulmonary problems. The procedure consists of opening the outlet channel from the bladder with the use of a resectoscope in the urethra for electrically cutting away the obstructing median and lateral lobes of prostate tissue. Bleeding is controlled with a coagulation current. For visualization of the surgical field continuous irrigation is used to wash away blood and dissected prostatic tissue and to distend the bladder.
Various types of irrigating fluid have been used. Although distilled water is associated with the least optical impairment, hemolysis of red blood cells is an unacceptable side effect. Normal saline or lactated Ringer solution is highly ionized and promotes dispersion of high current from the resectoscope. For these reasons, irrigating solutions typically consist of sorbitol (2.70 g) and mannitol (0.54 g) in 100 mL of water (Cytal) or glycine 1.5%. Glycine is slightly hypoosmolar to the blood but is used widely because of its low cost. Average features of a TURP are listed in the following table.
Average Parameters with a TURP
| Parameter | Average |
| Resection time | <77 min |
| Resectate mass | 20-48 g |
| Absorbed volume | 1 L |
| Blood loss | 175-534 mL |
| Speed of TURP syndrome onset | 15 min |
| Serum sodium nadir | 132-135 mmol/L |
Adapted from Gravenstein D, Hahn RG. TURP syndrome. In Lobato EB, Gravenstein N, Kirby, RR, et al, eds. Complications of Anesthesiology. Philadelphia: Lippincott Williams & Wilkins; 2008: 474-491.
a) Spinal anesthesia and general anesthesia have both been used for TURP procedures. Some clinicians believe that spinal anesthesia is ideal because the signs and symptoms of hypervolemia and bladder perforation are more easily detected. A T10 sensory level is necessary for adequate anesthesia. There is no consensus as to the superiority of either technique.
b) Although general anesthesia may mask early complications, it may be desirable in select patients who need pulmonary support or cannot tolerate a fluid load for compensation of a loss of sympathetic tone or when other contraindications to spinal anesthesia exist.
c) Some key points for anesthesia management of TURP are listed in the following box.
b) Fluid absorption: Large amounts of irrigating solution can be absorbed through venous sinuses. The amount absorbed and the rate of absorption depend on the size of the gland to be resected, the congestion of the gland, the duration of resection, the pressure of the irrigating solution, the number of sinuses open at any one time, and the experience of the resectionist.
(1) An average of 10 to 30 mL of fluid can be absorbed per minute of resection time, and 6 to 8 L can be absorbed in cases that last up to 2 hours. In general, limiting resection time to 1 hour is desirable.
(2) TURP syndrome is a general term used to describe complications specifically related to absorption of irrigating fluid include volume overload with pulmonary edema and dilutional hyponatremia.
(3) Central nervous system (CNS) symptoms associated with hyponatremia include restlessness, headache, irritability, confusion, visual disturbances, nausea, coma, and seizures.
(4) Serum sodium (Na+) concentrations of 120 mEq/L appear to be borderline for the development of severe symptoms. Electrocardiographic changes characterized by widening of the QRS complex and ST-segment elevation are seen when the serum level decreases to 115 mEq/L.
(5) At levels less than 100 mEq/L, ventricular tachycardia and fibrillation can occur.
(6) CNS symptoms can be detected more easily in patients receiving regional anesthesia.
(7) Progressive increases in blood pressure, central venous pressure, or pulmonary artery wedge pressure (when monitored) suggest hypervolemia.
(8) Hyponatremia in such cases results from water excess rather than from Na+ loss. Fluid restriction, serial electrolyte screening, and airway and cardiac support are initial treatments for TURP syndrome. Hypertonic saline (3%–5% sodium chloride) and diuretics (furosemide, 0.15–0.5 mg/kg).
(3) These symptoms are better recognized when the patient has regional anesthesia if the regional technique does not produce a high block. With general anesthesia, only the surgeon can appreciate the inability to recover bladder fluid as a sign of perforation. Intraperitoneal fluid will be excreted by the kidney.
(4) If hemodynamic instability occurs, suprapubic drainage is effective for removal of excess intraperitoneal fluid.
d) Glycine, which can also be used as bladder irrigation, can produce toxicity when absorbed.
(1) Glycine, an amino acid normally found in the body, is a major inhibitory transmitter.
(2) Signs and symptoms include nausea, vomiting, fixation and dilation of the pupils, weakness, and muscle incoordination.
(3) Transient blindness after TURP has been attributed to edema of the cortex, atropine, and hyponatremia.
(4) Glycine may also result in CNS toxicity as a consequence of its biotransformation to ammonia. Ammonia toxicity results in encephalopathy and delayed awakening in the postoperative period. NH3 yields glutamine, which is metabolized to the inhibitory neurotransmitter serotonin. Hyperammonemia also decreases the production of dopamine and norepinephrine, which are central excitatory neurotransmitters.
(1) The use of high voltage for cutting and coagulation during TURP may result in skin burns.
(2) Electrocardiography pads may be placed at other sites so that potential burns are avoided.
(1) Blood loss during TURP generally is related to the weight of the resected tissue, operating time, and skill of the surgeon.
(2) Assessment of blood loss is difficult because of the dilution of blood in irrigating fluid.
(3) Hematocrit may be increased, decreased, or unchanged, depending on the amount of fluid in the intravascular space at the time.
(4) Blood transfusion should be based on preoperative hematocrit, the duration and difficulty of resection, and a general assessment of the patient.