Respiratory system
A Adult respiratory distress syndrome
Definition
The term acute respiratory failure is often used synonymously with acute (formerly adult) respiratory distress syndrome (ARDS). Although ARDS may be caused by or associated with a variety of clinical conditions, most patients with this disease demonstrate similar clinical and pathologic features regardless of the cause of lung injury. Common features include: (1) a history of a preceding noxious event that served as a trigger for the subsequent development of ARDS; (2) an interval from hours to days of relatively normal lung function after the insult; and (3) the rapid onset and progression over several hours of dyspnea, severe hypoxia, diffuse bilateral pulmonary infiltration, and stiffening and noncompliance of the lungs.
Incidence and prevalence
Risk factors for the development of ARDS appear to be additive. The incidence of occurrence is 25% with the presence of one risk factor, 42% with the presence of two, and 85% with the presence of three. The mortality rate for ARDS remains high, ranging from 50% to 70% and often exceeds 90% when gram-negative septic shock precedes ARDS development. Events and risk factors associated with the development of ARDS include: (1) shock (septic, cardiogenic, or hypovolemic), (2) trauma, (3) pulmonary infection (e.g., with Pneumocystis carinii [jiroveci] or Escherichia coli), (4) disease states that result in the release of inflammatory mediators (e.g., extrapulmonary infections, disseminated intravascular coagulation, anaphylaxis, coronary bypass grafting, and transfusion reactions), (5) exposure to various agents (e.g., narcotics, barbiturates, and O2), (6) diseases of the central nervous system (CNS), (7) aspiration (e.g., of gastric contents or as in drowning), and (8) metabolic events (e.g., pancreatitis and uremia).
Pathophysiology
The pathophysiology of ARDS is centered around severe damage and inflammation to the alveolocapillary membrane. Irrespective of the cause of acute respiratory failure, the lung’s structural response to injury and subsequent repair occurs in a similar fashion. Although the exact mechanisms of this response and repair remain unclear, research has focused on the release of cytokines and membrane-bound phospholipids from the capillary endothelium and the activation of leukocytes and macrophages (via the complement system) within the lungs.
Clinical manifestations
The clinical presentation includes patients who are dyspneic, hypoxic, and hypovolemic and require intubation and mechanical ventilation. Recovery of lung function is unpredictable. Milder cases resolve quickly, but others progress to fibrosis and death.
Treatment
Because lung infections (e.g., P. carinii [jiroveci] pneumonia) mimic ARDS, antibiotic therapy often is initiated before the cause of respiratory failure is known. Maintenance of tissue oxygenation and replacement of lost intravascular fluids are the main goals of therapy. Preservation of end-organ perfusion is of utmost importance. Treatment is supportive and includes correction of hypoxia, preload and afterload reduction, and inotropic support as indicated.
Anesthetic considerations
Anesthetic preparation includes evaluation of the patient’s respiratory, cardiac, and renal status. Ventilator settings should be noted and special attention devoted to peak inspiratory pressures and positive end-expiratory pressure (PEEP) levels. If the anesthesia ventilator cannot accommodate these settings, then arrangements must be made to bring the patient’s ventilator into the operating room. The nature of lung sounds and amount of secretions should be noted. The presence of excess secretions should alert the anesthesia provider to the potential risk of airway obstruction. The degree of barotrauma from prolonged mechanical ventilation with high levels of PEEP can be assessed by the presence of chest tubes and subcutaneous emphysema secondary to pneumothorax. The effectiveness of therapy with bronchodilators should be assessed because the use of these drugs may be initiated preoperatively and continued intraoperatively if effective. An arterial line should be placed preoperatively and arterial blood gas (ABG) analysis performed. If possible, lactic acid values should be determined.
Volume status should be evaluated closely because patients with ARDS often are hypovolemic. Invasive monitoring via central venous lines and pulmonary artery catheters often is available, and cardiac filling pressures along with CO values should be assessed. Patients requiring inotropic support may arrive for surgery with infusions of dopamine or dobutamine. For all procedures, renal function should be monitored with a bladder catheter. Antibiotic therapy should be continued intraoperatively, and continuation of steroid preparations should be considered if patients were receiving these medications preoperatively.
Because patients with ARDS often are hemodynamically unstable, careful titration of anesthetic agents and adjunct agents is necessary. Owing to the multisystemic involvement characteristic of ARDS, drug metabolism and elimination should be carefully considered.
Transport should be carefully planned so that complications are minimized and safe arrival in the intensive care unit is ensured. Patients should undergo pulse oximetry, electrocardiography (ECG), and blood pressure transport monitoring (by arterial line or noninvasively) before departure from the operating room.
Breath sounds should be continually assessed with a precordial stethoscope. A full tank of O2 and PEEP adapter valves should be available for transport. The potential need for emergency medications and a defibrillator should be considered. If the patient’s ventilator needs to be returned to the intensive care unit, plans should be made so that it arrives there before the patient does. Finally, if possible, another member of the anesthesia team should accompany the patient during transport. Pulmonary dysfunction is the most common cause of postoperative complications after the administration of general anesthesia. To minimize pulmonary derangement, the anesthesia provider must identify patients who are at risk for the development of pulmonary impairment and must have a thorough understanding of the preexisting lung dysfunction.
B Aspiration pneumonia
Definition
Two entirely separate clinical aspiration disorders exist. One occurs after the aspiration of solid food and produces a picture of laryngeal or bronchial obstruction, an the other results from direct acid injury to the lung and produces an “asthma-like” syndrome. Pulmonary aspiration occurs when the gastric contents escape from the stomach into the pharynx and then enter the lungs. This results from preexisting disease, airway manipulation, and the inevitable compromise in protective reflexes accompanying the anesthetized state. Aspirates are commonly categorized as contaminated, acidic, alkaline, particulate, and nonparticulate. Fewer than half of all aspirations lead to pneumonia. Pneumonia occurs most often in patients with aspiration of infected material or who are immunocompromised.
Incidence and prevalence
Although the incidence of regurgitation is estimated to be frequent (as high as 15%), pulmonary aspiration complicates only about one in 3000 anesthetics. This incidence is roughly doubled for cesarean section surgery and emergency surgery. Fortunately, the majority of aspiration incidents require little or no treatment.
Pathophysiology
Although vomiting and gastroesophageal reflux are common clinical events, aspiration usually occurs only when normal protective reflexes (swallowing, coughing, gagging) fail. Three broad categories of failure are (1) depression of reflex protection, (2) alteration in anatomic structures, and (3) iatrogenic disorder. Reflex responses to aspiration are automatically blunted with depression of consciousness. The most common setting for depression of reflex protection occurs during anesthesia induction and emergence.
Three aspiration syndromes have been identified: (1) chemical pneumonitis (Mendelson syndrome), (2) mechanical obstruction, and (3) bacterial infection. Because acute chemical pneumonitis poses the greatest difficulty to anesthesia providers, the pathophysiology, presentation, and anesthetic implications of Mendelson syndrome are discussed. The triphasic sequence of (1) immediate respiratory distress combined with bronchospasm, cyanosis, tachycardia, and dyspnea followed by (2) partial recovery and (3) a final phase of gradual return of function is characteristic of Mendelson syndrome. This acute chemical pneumonitis is caused by the irritative action of hydrochloric acid, alkaline aspirates, or particulate materials, which are damaging to the lungs.
The pathophysiology of aspiration pneumonitis is typically characterized by four stages: (1) The aspirated substance causes immediate damage to the lung parenchyma, resulting in tissue necrosis. (2) Atelectasis results within minutes caused by a parasympathetic response that leads to airway closure and a decrease in lung compliance. (3) One to two hours after the injury, there is an intense inflammatory reaction characterized by pulmonary edema and hemorrhage. Inflammatory cytokines play a central role in this, including interleukin-8 and tumor necrosis factor alpha (TNF-α) released by alveolar macrophages. Neutrophils also play a key role in this phase by releasing oxygen radicals and proteases. Fluid fills the alveolar capillary membrane, causing hypoxia and hypercarbia. (4) Later, secondary injuries result from fibrin deposits and necrosis of alveolar cells by 24 hours after the insult.
Clinical manifestations and diagnosis
Arterial hypoxemia, the hallmark sign of aspiration pneumonitis, is frequently the first sign of aspiration. Because the majority of aspiration incidents are asymptomatic or mildly symptomatic, unexplained hypoxemia occurring in otherwise healthy patients postoperatively may frequently be a vague sign of silent aspiration. Other signs to alert the anesthetist to the possibility of aspiration include tachypnea, dyspnea, tachycardia, hypertension, and cyanosis.
Anesthetic considerations
Preoperative management
When dealing with aspiration, “an ounce of prevention is worth a pound of cure.” Avoiding the use of general anesthesia is the most effective means of preventing aspiration. However, regional and local sedation anesthesia is unrealistic for many procedures and in certain patient populations. When the use of general anesthesia is unavoidable, taking the following steps may help minimize the risk of aspiration or at least limit its consequences.
• Adhere to nil per os (NPO) policy.
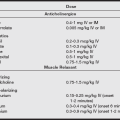
• Use pharmacologic prophylaxis for aspiration. Agents such as gastrokinetics, histamine blockers, anticholinergics, antacids, proton pump inhibitors, and antiemetics are all used alone or in various combinations to raise gastric pH and lower volume (see the table below).
• Consider rapid sequence induction and the application of cricoid pressure.
• Other nonpharmacologic mechanisms such as elevating the patient’s head may offer limited benefit.
Drug Prophylaxis for Anesthesia
| Medication Type | Common Examples | Recommendation |
| Gastrointestinal stimulants | Metoclopramide | No routine use* |
| Gastric acid secretion blockers | Cimetidine | No routine use* |
| Famotidine | No routine use* | |
| Ranitidine | No routine use* | |
| Omeprazole | No routine use* | |
| Lansoprazole | ||
| Antacids | Sodium citrate | No routine use* |
| Sodium bicarbonate | ||
| Magnesium trisilicate | ||
| Antiemetics | Droperidol | No routine use* |
| Ondansetron | ||
| Anticholinergics | Atropine | No use† |
| Scopolamine | ||
| Glycopyrrolate | ||
| Combinations of the medications above | No routine use* |
*The routine preoperative use of these medications to decrease aspiration risk in patients with no apparent increased risk is not recommended.
†The use of anticholinergics to decrease aspiration risk is not recommended.
Data from American Society of Anesthesiologists. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology 2011;114(3):495-511.
Intraoperative management
If intubation is not expected to be difficult, a rapid-sequence induction (rather than awake endotracheal intubation) is indicated in the patient with aspiration risk. There is little evidence that “modified” rapid sequence technique (which allows for gentle mask ventilation) would worsen aspiration incidence, and this approach may be preferable in patients at risk for rapid oxygen desaturation. Because difficult intubation itself is a risk factor for aspiration, there should be a low threshold for performing awake intubation in a patient with aspiration risk who may also pose airway challenges. Endotracheal intubation is considered the optimal approach for airway isolation; however, regurgitated material can seep around the endotracheal tube (ETT) cuff, particularly if it is not lubricated. Preventive measures in the anesthetic plan include ensuring that the patient is fully awake before extubation, that the patient is manifesting protective reflexes, minimizing any residual neuromuscular blockade, avoiding narcosis, and emptying the stomach.
If vomiting or aspiration occurs during induction, immediate treatment includes tilting of the patient’s head downward or to the side, rapid suctioning of the mouth and pharynx, and intubation. There is little benefit in performing tracheal or bronchial suctioning in most cases, and bronchoscopy should be reserved for patients who are suspected of having aspirated solid material. If aspiration is severe, surgery may be postponed. ABG analysis should be performed for determination of the extent of hypoxemia. Early application of PEEP is recommended for improving pulmonary function and combating atelectasis. Oxygenation should be supported with supplemental oxygen only to the minimum extent necessary.
Treatment of aspiration pneumonitis
• Suction the mouth and pharynx.
• Use deeper suctioning only for particulate material.
• Administer oxygen only to the extent needed.
• Administer lidocaine to inhibit neutrophil response.
• Steroids can lead to superinfection and are generally not indicated
• Intubate as needed to support oxygenation.
• Use a bronchodilator and PEEP to support ventilation.
• Administer antibiotics only if indicated or for fever or an elevated white blood cell count lasting longer than 48 hours.
C Asthma
Definition
Asthma is a chronic inflammatory disorder of the airways characterized by increased responsiveness of the tracheobronchial tree to a variety of stimuli. Many cells and cellular elements play a role, particularly mast cells, eosinophils, T lymphocytes, neutrophils, and epithelial cells. In susceptible individuals, this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and cough, particularly at night and in the early morning. These episodes are usually associated with widespread but variable airflow obstruction that is often reversible either spontaneously or with treatment.
Various subtypes of asthma exist. The most important consideration is the identification of exacerbating factors whenever possible. A well-known system classifies asthmas as either extrinsic or intrinsic. Although this system is conceptually helpful, its two groups are not mutually exclusive. Whereas extrinsic asthma (or allergic asthma) most commonly affects children and young adults and involves infectious, environmental, psychological, or physical factors, intrinsic asthma (or idiosyncratic asthma) usually develops in middle age without specifically identifiable attack-provoking stimuli. The term atopy, which refers to a hereditary, immunoglobulin E (IgE)–mediated, clinical hypersensitive state, is often used when extrinsic asthma is described.
Incidence and prevalence
Up to 15 million persons in the United States have asthma. It is the most common chronic disease of childhood, affecting an estimated 4.8 million children. People with asthma collectively have more than 100 million days of restricted activity and 470,000 hospitalizations annually. More than 5000 people die of asthma each year.
Pathophysiology
Asthma is a heterogeneous clinical syndrome characterized by episodes in which airways are hyperresponsive, interspersed with symptom-free periods. Bronchoconstriction is a factor long associated with the asthmatic symptom complex, but asthma is much more than bronchoconstriction. Airway inflammation and a nonspecific hyperirritability of the tracheobronchial tree are now recognized as being central to the pathogenesis of even mild cases of asthma. Permanent changes in airway anatomy, referred to as airway remodeling, magnify the inflammatory response.
Allergic asthma (atopic or immunologic disease) is triggered by antigens that provoke a T-lymphocyte–generated, IgE-mediated immune response. It is often associated with a personal or familial history of allergic disease. Potent biochemical mediators released from proinflammatory and airway epithelial cells promote vasoconstriction, increased smooth muscle tone, enhanced mucous secretion, submucosal edema, increased vascular permeability, and inflammatory cell chemotaxis. Leukotrienes have been identified as especially potent spasmogenic and proinflammatory substances. Released molecules that are toxic to the airway epithelium cause patchy desquamation, which exposes cholinergic nerve endings and compounds the bronchoconstrictive and hyperresponsive response.
The asthmatic diathesis creates airways that are inflamed, edematous, and hypersensitive to irritant stimuli, and the degree of airway hyperresponsiveness and bronchoconstriction appears to parallel the extent of inflammation. When airway reactivity is high, asthmatic symptoms are generally more severe and unrelenting, and the amount of therapy required to control the episode is greater.
The mechanisms underlying idiosyncratic asthma (nonimmunologic disease) are less clearly defined. Nonimmunologic asthma occurs in patients with no history of allergy and normal serum IgE. These patients typically develop asthmatic symptoms in response to some provocative or noxious stimulus such as cold air, airway instrumentation or irritation, climate changes, or an upper respiratory illness. Recent upper respiratory infection may precipitate bronchospasm in any patient, but the risk is higher in patients with a history of asthma.
The mechanism of exercise-induced asthma is unknown. Regardless of the mechanism, most symptoms last less than 1 hour and are usually quickly reversed with administration of β2-adrenergic receptor agonists. Occupational asthma develops when irritants directly stimulate vagal nerve endings in the airway epithelium. Infection-induced asthma with acute inflammation of the bronchi may be caused by viral, bacterial, or mycoplasmal infections. Aspirin-induced asthma occurs when, in some predisposed persons, cyclooxygenase promotes an increase in leukotriene levels via the arachidonic acid pathway, thereby triggering the asthma attack. This peculiar response can occur with the use of other nonsteroidal anti-inflammatory agents. The aspirin-induced asthma variant is not IgE mediated or allergic in nature; furthermore, it is clinically associated with nasal polyps. Patients with aspirin-induced asthma may be at increased risk of bronchospasm after ketorolac (Toradol) administration.
Clinical manifestations
Key hallmarks of asthma in the awake patient include the following:
• Dyspnea (may parallel the severity of expiratory airflow obstruction)
• Cough (productive or nonproductive; frequently at night or early morning)
• Labored respirations with accessory muscle use
• Tachypnea (a respiratory rate >30 breaths/min and a heart rate of 120 beats/min suggests severe bronchospasm)
Typically, attacks are short lived, lasting minutes to hours. Between attacks, the patient with asthma may be entirely symptom free; however, underlying airway remodeling is still evident. Severe obstruction persisting for days or weeks is known as status asthmaticus. Use of accessory muscles of respiration and the increased work of breathing associated with a protracted asthmatic episode can result in respiratory muscle fatigue and respiratory failure. During exacerbations, pulmonary function tests may reflect acute expiratory airflow obstruction (decreased forced expiratory flow [FEF]25%–75% and decreased ratio of forced expiratory volume in one second to forced vital capacity [FEV1/FVC]). Viscid mucous secretion may compound the airway narrowing and produce airway collapse.
The asthmatic episode produces not only airflow obstruction but also gas exchange abnormalities. The resulting low ventilation/perfusion (V/Q) state produces arterial O2 desaturation. Hypoxemia is common, but in most patients with acute bronchospasm, CO2 elimination is relatively well preserved until V/Q abnormalities are severe. An increased arterial CO2 tension may indicate impending respiratory failure in an acutely ill patient with asthma. Chronic asthma may eventually lead to irreversible lung destruction, loss of lung elasticity, pulmonary hypertension (PH), and lung hyperinflation.
Anesthetized patients
In anesthetized patients, prominent manifestations of the asthmatic episode are wheezing, mucous hypersecretion, high inspiratory pressures, a blunted expiratory CO2 waveform, and hypoxemia. Mechanical ventilation and positive airway pressure (PAP) are associated with a higher incidence of air trapping and lung hyperinflation, and the associated barotrauma can result in a pneumothorax. Additionally, alveolar overdistention may lead to decreased venous return and diminution of CO. The combination of impaired ventilation and hypoxia can precipitate increased pulmonary vascular resistance, enhanced right ventricular afterload, and finally hemodynamic collapse.
The onset of an asthmatic episode may occur abruptly in surgical patients. Airway manipulation, acute exposure to allergens, or the stress of surgery can provoke wheezing in a patient who was previously asymptomatic. Wheezing often suggests potentially reversible bronchoconstriction, but the extent or degree of wheezing is a notoriously poor indicator of the degree of airway obstruction. Care must be taken to differentiate wheezing of asthmatic origin from other causes of wheezing such as pneumothorax, ETT obstruction, endobronchial intubation, anaphylaxis, pulmonary edema, and pulmonary aspiration.
D Chronic obstructive pulmonary disease
Definition
Chronic obstructive pulmonary disease (COPD) is a “disorder characterized by abnormal tests of expiratory flow that does not change markedly over periods of several months of observation.” Asthma, chronic bronchitis, and emphysema are all common obstructive diseases characterized by decreased air flow through the tracheobronchial tree and small airways.
The terms chronic obstructive pulmonary disease and chronic obstructive lung disease are widely used as synonyms for the combination of chronic bronchitis and emphysema. Because of the prevalence of cigarette smoking, the combination of these two entities is encountered much more commonly than either of the two in its “pure” form. As a rule, the combination of chronic bronchitis and emphysema is seen in those who smoke heavily, and the disease process takes 30 years or longer to manifest. Differential diagnosis of COPD compared with other common lung disorders is described in the following table.
Differential Diagnosis of Chronic Obstructive Pulmonary Disease
| Diagnosis | Suggestive Features* |
| COPD | Onset in midlife; symptoms slowly progressive; long-term smoking history; dyspnea during exercise; largely irreversible airflow limitation |
| Asthma | Onset early in life (often childhood); symptoms vary from day to day; symptoms occur at night or in early morning; allergy, rhinitis, or eczema also present; family history of asthma; largely reversible airflow limitation |
| Congestive heart failure | Fine basilar crackles on auscultation; chest radiograph shows dilated heart, pulmonary edema; pulmonary function tests indicate volume restriction, not airflow limitation |
| Bronchiectasis | Large volumes of purulent sputum; commonly associated with bacterial infection; coarse crackles or clubbing on auscultation; chest radiograph or CT scan shows bronchial dilation, bronchial wall thickening |
| Tuberculosis | Onset at all ages; chest radiograph shows lung infiltrate or nodular lesions; microbiologic confirmation; high local prevalence of tuberculosis |
| Obliterative bronchiolitis | Onset at younger age, in nonsmokers; may have history of rheumatoid arthritis or fume exposure; CT scan taken on expiration shows hypodense areas |
| Diffuse panbronchiolitis | Most patients are male and nonsmokers; almost all have chronic sinusitis; chest radiograph and HRCT scan show diffuse small centrilobular nodular opacities and hyperinflation |
COPD, Chronic obstructive pulmonary disease; CT, computed tomography; HRCT, high-resolution computed tomography.
*These features tend to be characteristic of the respective diseases but do not occur in every case. For example, a person who has never smoked can develop COPD (especially in developing countries, where other risk factors may be more important than cigarette smoking); asthma can develop in adult and even elderly patients.
From Rable KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:532-555.
Incidence and prevalence
COPD affects an estimated 15 to 20 million Americans and is the fifth leading cause of death in the United States, accounting for approximately 60,000 deaths each year. Chronic bronchitis and emphysema are the most common causes of COPD.
Pathophysiology
The dominant feature of the natural history of COPD is progressive air flow obstruction, as reflected by a decrease in FEV1. Three causes of decreases in FEV1 are as follows:
Clinical manifestations
The clinical presentation of COPD varies markedly, and crippling changes for one person may be a minor incapacity for another. Chronic productive cough and progressive exercise limitation are the hallmarks of COPD. The clinical and functional changes are noted in the following tables.
Clinical Hallmarks: Predominant Bronchitis versus Predominant Emphysema
| Blue Bloater, Predominant Bronchitis | Pink Puffer, Predominant Emphysema | |
| General appearance | Overweight; dusky; warm extremities | Thin, often emaciated; pursed-lip breathing; anxious; prominent use of accessory muscles; normal-to-cool extremities |
| Age (years) | 40–55 | 50–75 |
| Onset | Cough | Dyspnea |
| Cyanosis | Marked | Slight to none |
| Cough | More evident than dyspnea | Less evident than dyspnea |
| Sputum | Copious | Scanty |
| Upper respiratory infections | Common | Occasional |
| Breath sounds | Moderately diminished | Markedly diminished |
| Cor pulmonale and right-sided heart failure | Common | Only during bouts of respiratory infection |
| Radiographic features | Normal diaphragm position; cardiomegaly; lungs normal or with increased bronchovascular markings | Small pendulous heart; low, flat diaphragm; areas of increased radiolucency |
| Course | Ambulatory but constantly on verge of right-sided heart failure and coma | Incapacitation of breathlessness punctuated by life-threatening bouts of upper respiratory infections; prolonged course culminating in right-sided heart failure and coma |
Modified from Fishman AP, et al, ed. Fishman’s Pulmonary Diseases and Disorders. 4th ed. New York: McGraw-Hill; 2008.
Functional Hallmarks: Predominant Bronchitis versus Predominant Emphysema
| Blue Bloater, Predominant Bronchitis | Pink Puffer, Predominant Emphysema | |
| FEV1/FC | Reduced | Reduced |
| FRC | Mildly increased | Markedly increased |
| TLC | Normal to slight increase | Considerably increased |
| RV | Moderately increased | Markedly increased |
| Lung compliance | Normal or high | Normal or low |
| Recoil pressure | Normal or high | Low |
| MVV | Moderately decreased | Markedly decreased |
| Airway resistance | Increased | Normal to slightly increased |
| Dlco | Normal or low | Low |
| Arterial Pao2 | Moderate to severe decrease | Mildly to moderately reduced |
| Arterial hypercapnia | Often present | Present during an acute respiratory infection |
| Hematocrit | Generally high; may reach 70% | Normal or slightly high; uncommonly exceeds 55% |
| Pulmonary arterial pressure | Generally increased | Normal or slightly increased |
Dlco, Diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; FRC, functional residual capacity; MVV, maximum voluntary ventilation; Pao2, partial pressure of arterial oxygen; RV, residual volume; TLC, total lung capacity.
From Fishman AP, et al, ed. Fishman’s Pulmonary Diseases and Disorders. 4th ed. New York: McGraw-Hill; 2008.
Anesthetic considerations
Preoperative evaluation
The surgical site and the preoperative status of the patient are critical factors in determining the incidence of postoperative complications. Multiple factors are predictive of postoperative respiratory difficulties, but no preoperative pulmonary function test establishes absolute contraindications to surgery. The preoperative evaluation of patients with COPD should determine the severity of the disease and identify treatments for reducing inflammation, improving secretion clearance, treating underlying infection, and increasing airway caliber that can ensure the best surgical outcome. Supplemental administration of O2 usually is recommended if the Pao2 is less than 60 mmHg, the hematocrit is greater than 55%, or evidence of cor pulmonale is present. Bronchodilators should be used if the patient exhibits some degree of airway obstruction.
The presence of COPD does not dictate the use of specific drugs or techniques for the management of anesthesia. It is crucial to realize that COPD patients are susceptible to the development of acute respiratory failure during the postoperative period. Therefore, continued intubation of the trachea and mechanical ventilation of the lungs may be necessary, particularly after thoracic and upper abdominal surgery. Postoperative ventilation is more likely to be needed in those patients with low Pao2 and dyspnea at rest.
Regional anesthesia
Regional anesthesia is useful if sedation is not needed, and it may be safer than general anesthesia. Anesthesia providers must avoid complacency if they use regional rather than general anesthesia, monitoring for potential adverse side effects (e.g., pneumothorax, impaired muscle function). Regional anesthetic techniques that produce sensory anesthesia above T6 are not recommended because of the potential for decreasing expiratory reserve volume, impairing cough effort, and creating anxiety-provoking weakness.
Postoperative implications
Postoperative care of patients with COPD is directed at minimizing the incidence and severity of pulmonary complications because such patients are at increased risk for the development of acute respiratory failure. Postoperative pulmonary complications most often are characterized by atelectasis followed by pneumonia and decreases in Pao2.
Ambulation should be encouraged in order to increase functional residual capacity (FRC) and improve oxygenation via V/Q matching. Incentive spirometry with maintenance of peak inflation for 3 to 5 seconds reexpands collapsed alveoli. Expiratory maneuvers (e.g., floating of balls on an expiratory spirometer) generate pleural pressures that exceed airway pressures and thereby can cause alveolar collapse.
E Cor pulmonale
Definition
The term cor pulmonale or pulmonary heart disease refers to patients exhibiting pulmonary arterial hypertension (PAH) resulting in progressive right ventricular hypertrophy, dilation, and eventual cardiac decompensation. This arises from disorders that affect ventilatory drive or musculoskeletal respiratory mechanics; pulmonary airway, infiltrative, fibrotic, or vascular diseases; and diseases that are primarily cardiac but affect the pulmonary circulation and the lungs.
Incidence and prevalence
In individuals older than 50 years of age, cor pulmonale is the third most common cardiac disorder (after ischemic heart disease and hypertensive cardiac disease). The male-to-female ratio of incidence of the disease is 5:1; 10% to 30% of patients admitted to the hospital with coronary heart failure exhibit cor pulmonale.
The prognosis is determined by the pulmonary disease responsible for the increased pulmonary vascular resistance (PVR). In patients with COPD in whom Pao2 can be maintained at near-normal levels, the prognosis is favorable. However, cor pulmonale associated with hypoxic lung disease is associated with a 70% rate of mortality within 5 years after onset of associated peripheral edema. The prognosis is poor for patients in whom cor pulmonale is the result of gradual obstruction of pulmonary vessels by intrinsic pulmonary vascular disease or pulmonary fibrosis. These anatomic changes cause irreversible alterations in the pulmonary vasculature, resulting in fixed elevations of PVR.
Pathophysiology
COPD is associated with the functional loss of pulmonary capillaries and the subsequent arterial hypoxemia; these events initiate pulmonary vasoconstriction, which is the leading cause of chronic cor pulmonale. Sustained pulmonary vasoconstriction produces hypertrophy of the smooth muscle in the tunica media and an irreversible increase in the PVR. In the presence of chronically elevated pulmonary capillary pressure, the lungs are increasingly resistant to pulmonary edema because lymph vessels expand and their ability to carry fluid away from the interstitial spaces increases. The lymphatic pumping action creates a suction effect, which results in a negative pleural pressure. The rate at which right ventricular dysfunction develops depends on the magnitude of pressure increase in the pulmonary circulation and on the rapidity with which this increase occurs. For example, PE may result in right ventricular failure in the presence of a mean PAP as low as 30 mmHg. By contrast, when PAH occurs gradually, as it does in COPD, right ventricular compensation occurs; congestive heart failure rarely occurs before mean PAP exceeds 50 mmHg.
Clinical manifestations and diagnosis
Clinical manifestations of cor pulmonale often are nonspecific and obscured by coexisting COPD. Right-sided heart catheterization usually is required for diagnosis. Cardiac catheterization combined with pulmonary angiography provides the most definitive information on the degree of PAH, cardiac reserve, and the effects of pulmonary vasodilator treatment. Symptoms of cor pulmonale are retrosternal pain, cough, dyspnea on exertion, weakness, fatigue, early exhaustion, and hemoptysis. Occasionally, hoarseness secondary to left recurrent laryngeal nerve compression by the enlarged pulmonary artery is present. Syncope on effort may occur, reflecting the inability of the right ventricular stroke volume to increase in the presence of a fixed elevation of PVR.
Physical signs of cor pulmonale include the following:
• Elevation of jugular venous pressure
• Cardiac heave or thrust along the left sternal border and S3 gallop
• Presence of an S4 secondary to significant right ventricular hypertrophy
• Possible murmur of pulmonic and tricuspid insufficiency
• Hepatomegaly, ascites, and lower extremity edema (late signs)
Treatment
The three major drug classes for treatment of PAH are prostanoids, endothelin receptor antagonists, and phosphodiesterase inhibitors. The goals of treatment are decreasing the workload of the right ventricle, reducing PVR, preventing increases in PAP, and avoiding major hemodynamic changes. Improvement of gas exchange is the primary focus of treatment in COPD patients with cor pulmonale. Treatment includes supplemental administration of O2 in order to maintain a Pao2 of greater than 60 mmHg or an arterial O2 saturation of greater than 90%. O2 is the only vasodilator with a selective effect on pulmonary vessels that is not associated with a risk of worsening hypoxemia.
Anesthetic considerations
Regional anesthesia technique may be appropriate as long as a high sensory level of anesthesia is not required because any decrease in systemic vascular resistance in the presence of a fixed PVR may produce undesirable degrees of systemic hypotension.
General anesthesia
Volatile agents decrease PVR. Studies have demonstrated that PAP is decreased by isoflurane. N2O has been shown to increase PVR in patients with primary pulmonary hypertension (PPH). Intravenous agents, with the exception of ketamine, appear to have little effect on PVR. During all stages of anesthesia, manipulations that increase PAP must be avoided. Five key principles should be followed:
F Cystic fibrosis
Incidence and prevalence
Cystic fibrosis affects an estimated 30,000 persons in the United States, with 1000 new cases per year. Most are diagnosed between the ages of 3 months and 6 years, but 8% are not diagnosed until after 18 years. One in 31 Americans is an asymptomatic carrier of the defective gene. More than 80% of those born with cystic fibrosis are born to parents with no history of the disease.
Pathophysiology
Cystic fibrosis is caused by a mutation of a gene on chromosome 7. The result is defective chloride ion transport in epithelial cells in the lungs, pancreas, liver, gastrointestinal tract, and reproductive organs. This decrease in chloride transport results in a decrease of sodium and water transport. This results in dehydrated, viscous secretions that can cause obstruction, destruction, and scarring of exocrine glands.
Laboratory results
Because cystic fibrosis is an expiratory airflow obstructive disease, most pulmonary function test results will be abnormal. The white blood cell count may be elevated because of frequent pulmonary infections.
Clinical manifestations
Pancreatic insufficiency, meconium ileus at birth, diabetes mellitus, azoospermia, and obstructive hepatobiliary tract disease (cirrhosis and portal hypertension) are often present. The primary causes of morbidity and mortality are bronchiectasis and COPD. Typically, a cough, chronic purulent sputum production, and exertional dyspnea will be seen.
Treatment
Treatment is similar to that for bronchiectasis and focuses on alleviation of symptoms (mobilization and clearance of lower airway secretions and treatment of pulmonary infections) and correcting organ dysfunction (pancreatic enzyme replacement). Treatment of secretions is usually done by chest physical therapy with postural drainage. Bronchodilator therapy may also be instituted. Antibiotics are often given to relieve the increased secretions from pulmonary infections. If no pathogens are seen, bronchoscopy to remove lower airway secretions may be indicated if there is no response to common antibiotics.
Anesthetic considerations
Management is based on the same principles as in patients with COPD and bronchiectasis. Elective procedures are delayed until optimal pulmonary function is ensured by controlling infections and removing secretions from the airways. Vitamin K may be given if liver function is poor or if absorption of fat-soluble vitamins is poor in the gastrointestinal tract. Preoperative sedation is probably unnecessary because of possible respiratory depression. Maintenance of anesthesia with a volatile agent and oxygen can decrease airway resistance by decreasing the bronchial smooth muscle tone. Volatile agents are also helpful in decreasing the responsiveness of hyperreactive airways. Humidified gases are also important to decrease the viscosity of secretions. Frequent suctioning is also necessary.
G Pneumothorax and hemothorax
Definition
Pneumothorax is the presence of air or gas in the pleural space. Hemothorax is the presence of blood in the pleural space.
Incidence and prevalence
Spontaneous pneumothorax occurs unexpectedly in healthy persons, most often in men 20 to 40 years of age. Secondary pneumothorax and hemopneumothorax are generally the result of trauma.
Pathophysiology and treatment
Pneumothorax
Pneumothorax can be subdivided into three categories, depending on whether air has direct access to the pleural cavity. In simple pneumothorax, no communication exists with the atmosphere. Additionally, no shift of the mediastinum or hemidiaphragm results from the accumulation of air in the intrapleural space. The severity of pneumothoraces is graded on the basis of the degree of collapse: collapse of 15% or less is small, collapse of 15% to 60% is moderate, and collapse of greater than 60% is large. Treatment of simple pneumothorax is determined by the size and cause of injury and may include catheter aspiration or tube thoracostomy; close observation of the patient with simple pneumothorax is essential.
In communicating pneumothorax, air in the pleural cavity exchanges with atmospheric air through a defect in the chest wall. Because the exchange of air through the site of injury may often be heard, this entity is commonly known as a “sucking chest wound.” Treatment measures include administration of supplemental oxygen, tube thoracostomy, and intubation; mechanical ventilation may be indicated.
Tension pneumothorax develops when air progressively accumulates under pressure within the pleural cavity. If the pressure becomes too great, the mediastinum shifts to the opposite hemithorax, and this causes compression of the contralateral lung and great vessels. Tension pneumothorax is potentially lethal; therefore, immediate treatment is essential. Decompression of the chest can be performed with the insertion of a 16- or 18-gauge angiocatheter into the second or third interspace anteriorly or the fourth or fifth interspace laterally. A rush of air is heard when decompression occurs. The angiocatheter must be covered if the sucking of more air into the pleura is to be prevented.
Hemothorax
Hemothorax is the accumulation of blood in the pleural cavity. It usually is a result of trauma, but other causes include the rupture of small blood vessels in the presence of inflammation, pneumonia, tuberculosis, or erosion by tumors. The treatment of hemothorax consists of airway management as necessary, restoration of circulating blood volume, and evacuation of the accumulated blood. Thoracostomy may be indicated if the initial bleeding rate is greater than 20 mL/kg/hr. If bleeding subsides but its rate remains greater than 7 mL/kg/hr, chest radiographic findings worsen, or hypotension persists after initial blood replacement and decompression, thoracostomy is indicated.
Pathogenesis
Different presentations may be distinguished, according to the mechanism of injury, as described in the following sections.
Spontaneous
Pneumothorax usually is caused by rupture of alveoli near the pleural surface of the lung after a forceful sneeze or cough. This mechanism is most common in persons with a long, narrow chest and in those with emphysema.
Traumatic
Hemothorax, pneumothorax, and flail chest may occur after blunt chest trauma; however, they most frequently occur after rib fracture. Hemopneumothorax also may occur with penetrating injury.
Iatrogenic
Hemothorax and pneumothorax may occur after any of the following:
• Subclavian central line insertion (incidence is 2% to 16%)
• Supraclavicular block to the brachial plexus (incidence is 1%; hemothorax and pneumothorax can be complications of interscalene block but are rare with intercostal block)
• Barotrauma (from overdistention of the alveoli by PEEP; an abrupt deterioration of alveolar oxygen tension and cardiovascular function during PEEP administration should arouse suspicion of pulmonary barotrauma, especially pneumothorax)
• Exposure to high airway pressures (e.g., during mechanical ventilation)
• Other surgical procedures (e.g., mediastinoscopy, radical neck dissection, mastectomy, or nephrectomy)
Laboratory results
The chest radiograph frequently shows subcutaneous emphysema, pneumomediastinum, and pneumopericardium. Free fluid, as with hemothorax, may be best seen on a cross-table lateral radiograph.
Anesthetic considerations
Pneumothorax or hemothorax can significantly interfere with oxygenation and ventilation. Decreased cardiac output, as with tension pneumothorax, can exacerbate intrapulmonary shunting. Cyanosis indicates both cardiac and pulmonary involvement. Nitrous oxide can quickly expand the size of a pneumothorax if a chest tube is not in place. Nitrous oxide is acceptable for use if the chest tube is patent and functioning. A closed pneumothorax is a contraindication to the administration of nitrous oxide. Decreased pulmonary compliance (increased pulmonary inspiratory pressure) during administration of anesthesia to patients with a history of chest trauma may reflect the expansion of an unrecognized pneumothorax.
In the case of ventilator-related pneumothorax, the mortality rate is significantly reduced if a chest tube is placed in less than 30 minutes. Development of tension pneumothorax during general anesthesia is manifested by sudden hypotension, loss of pulmonary compliance, or decreased ventilating volume. Patients with significant trauma may require fluid resuscitation and cardiovascular support. Agents chosen should have minimal cardiac depressant effects.
H Pulmonary arterial hypertension
Definition
Pulmonary arterial hypertension usually represents an advanced stage of a large number of cardiovascular diseases. The mortality rate associated with PAH is high. PAH exists if the mean level of PAP increases by 5 to 10 mmHg or if pulmonary artery systolic pressure exceeds 30 mmHg and mean PAP exceeds 20 mmHg.
Incidence
Pulmonary arterial hypertension may be (1) primary or idiopathic (unexplained) or (2) secondary to an associated condition. In young adults, the female-to-male incidence of primary pulmonary arterial hypertension (PPAH) is 4:1; this incidence is similar to that in older groups of men and women.
Pathophysiology
Pulmonary arterial hypertension may be caused by many associated conditions, including pulmonary venous hypertension caused by left atrial outflow obstruction or pulmonary venous occlusive disease and PAH caused by hyperdynamic circulation (e.g., secondary to burns or sepsis), vasoconstriction, viscosity, obstruction, and reactive vascular disease.
Pulmonary arterial hypertension is characterized by a rapidly progressive course with a 79% mortality rate within 5 years of clinical diagnosis. The degree of increase in pressure in the pulmonary circulation has an important influence on the patient’s life expectancy. Resistant PAH has long been identified as a major cause of early death. The prognosis is largely determined by right ventricular integrity.
Pulmonary arterial hypertension is characterized by an increase in vascular tone and the growth and proliferation of pulmonary vascular smooth muscle. Initial reversible vasoconstriction may progress to muscle hypertrophy and irreversible degeneration.
Clinical manifestations and diagnosis
Pulmonary arterial hypertension may be either acute or chronic. In almost all patients with PAH, dyspnea and exercise intolerance usually are the first complaints. Patients also may have angina. Right atrial hypertrophy or right ventricular hypertrophy (or both) may be evident on ECG. Chest radiography may demonstrate an enlarged pulmonary artery. Cardiac catheterization combined with pulmonary angiography is most informative in assessment of PAH, cardiac reserve, and the effects of pulmonary vasodilator therapy. Vasodilator therapy is attempted when a vasoconstrictor component is identified. Vasodilator challenge may be performed with cardiac catheterization using a rapid and effective pulmonary vasodilator such as nitroglycerin, isoproterenol, nifedipine, prostaglandin E1, prostacyclin, prostaglandin E2, hydralazine, nitroprusside, or adenosine for evaluation of the reversibility of PAH. Frequently, open-lung biopsy is performed for assessment of the histopathologic composition of small pulmonary arteries. Noninvasive evaluation includes Doppler echocardiography for measurement of the velocity of tricuspid regurgitation (which correlates well with invasive PAP measurements) and pulmonic peak flow velocity.
Anesthetic considerations
Attempts to alleviate PH disease states have had varied success. Vasodilator agents are used most commonly and may be helpful in patients with reversible vasoconstriction. A list of vasodilators used in PAH is provided in the table below. Possible beneficial effects of pulmonary arterial dilation are preservation of lung function; prevention of right ventricle deterioration; and, it is hoped, improved survival. The principal objectives during anesthesia in patients with PAH are prevention of increases in PAH and avoidance of major hemodynamic changes. Considerations that apply to the care of patients with cor pulmonale also apply to those with PAH. Information regarding PAH and intravenous induction agents is lacking; however, most agents either have little effect on PVR or decrease it. Ketamine, which causes an increase in PVR, may be the exception.
Key points
• PH has been classified by the World Health Organization into five groups: (1) PAH, (2) PH with heart disease, (3) PH with lung disease, (4) PH with pulmonary thromboembolism, and (5) miscellaneous.
• PAH has further been classified into (1) idiopathic IPAH, (2) heritable, (3) related to other conditions, (4) PPH of the newborn, and (5) pulmonary veno-occulusive disease (PVOD).
• Heritable PH has been associated with bone morphogenic protein receptor 2 (BMPR2), but mutations are not always associated with PH, indicating other defects or insults are required. Evidence also supports involvement of a voltage-gated potassium ion channel and serotonin transporter.
• The basis for treatment of IPAH stems from deficiencies in prostacyclin and nitric oxide release and excess endothelin-1 found in patients with PH.
• PH can be diagnosed to some extent by physical examination, chest radiography, ECG, and echocardiography, but establishing the diagnosis with certainty requires a right-sided cardiac catheterization, which can check for left heart failure and response to a pulmonary vasodilator.
• Therapy includes moderate exercise, warfarin, oxygen, and calcium channel blockers.
• Targeted therapy with the prostacyclins, phosphodiesterase inhibitors, and endothelin receptor antagonists has made major improvements in the lives of patients with PAH.
The natural history of patients with groups 2 and 3 PH are influenced by their left heart and lung disease. In most cases, the presence of PH in addition to the underlying disease portends a poor prognosis.
Pulmonary Arterial Hypertension: Determinants of Prognosis*
| Determinants of Risk | Lower Risk (Good Prognosis) | Higher Risk (Poor Prognosis) |
| Clinical evidence of RV failure | No | Yes |
| Progression of symptoms | Gradual | Rapid |
| WHO class† | II, III | IV |
| 6-minute walk test‡ | Longer (>400 m) | Shorter (<300 m) |
| CPET | Peak Vo2 >10.4 mL/kg/min | Peak Vo2 <10.4 mL/kg/min |
| Echocardiography | Minimal RV dysfunction | Pericardial effusion, significant RV enlargement or dysfunction, RA enlargement |
| Hemodynamics | RAP <10 mmHg, CI >2.5 L/min/m2 | RAP >20 mmHg, CI <2.0 L/min/m2 |
| BNP§ | Minimally elevated | Significantly elevated |
BNP, Brain natriuretic peptide; CI, cardiac index; CPET, cardiopulmonary exercise testing; peak Vo2, average peak oxygen uptake during exercise; RA, right atrium; RAP, right atrial pressure; RV, right ventricle; WHO, World Health Organization.
*Most data available pertain to idiopathic pulmonary arterial hypertension (PAH). Few data are available for other forms of PAH. One should not rely on any single factor to make risk predictions.
†WHO class is the functional classification for PAH and is a modification of the New York Heart Association functional class.
‡The 6-minute walk test is also influenced by age, gender, and height.
§Because there are currently limited data regarding the influence of BNP on prognosis and many factors including renal function, weight, age, and gender may influence BNP, absolute numbers are not given for this variable.
From McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-1619.
Nonspecific treatments of PH include loop diuretics, digoxin, and anticoagulant therapy with warfarin when indicated. Traditional therapies include dihydropyridine calcium channel blockers such as nifedipine or amlodipine, which can modestly decrease pulmonary arterial pressures in vasoresponsive patients but can also cause sudden death in nonvasoresponsive patients.
Drug Treatment Options for Patients with Pulmonary Hypertension
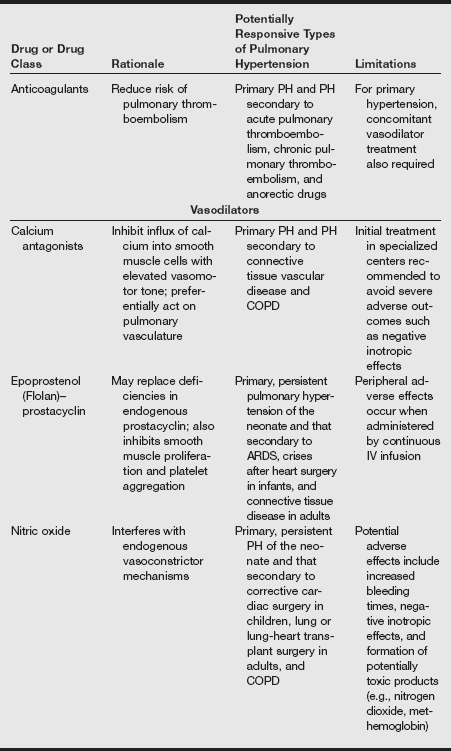
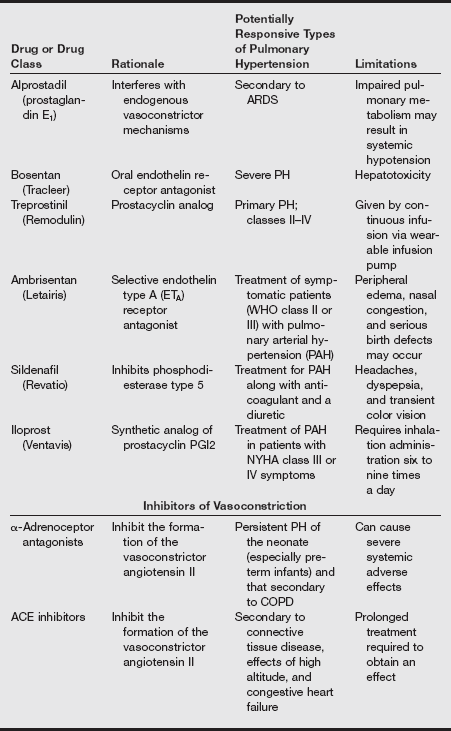
ACE, Angiotensin-converting enzyme; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; IV, intravenous; PH, pulmonary hypertension.
Modified from Treprostinil (Remodulin) for pulmonary arterial hypertension. Med Lett. 2002;44:80-82; Sildenafil (Revatio) for pulmonary arterial hypertension. Med Lett 2005;47:165-167; Ambrisentan (Letairis) for pulmonary arterial hypertension. Med Lett 2007;49:87-90.
Other therapies include prostacyclins such as epoprostenol and treprostinil; both are pulmonary and systemic vasodilators but must be given by continuous intravenous infusion through an indwelling catheter or by subcutaneous injection (treprostinil). Iloprost is an inhaled form of prostacyclin apparently with fewer side effects that can improve exercise capacity and symptom scores, but it requires frequent dosing. Side effects and economic costs are significant obstacles to the use of all these agents. Bosentan is a nonselective endothelin receptor antagonist, and sitaxsentan is a selective endothelin receptor antagonist. Both improve functional status and physiologic measures in PH.
Sildenafil, which inhibits phosphodiesterase type 5 in the pulmonary vasculature, has also been approved for this indication based on results of a controlled trial that showed it improved 6-minute walk distance, New York Heart Association (NYHA) functional class, pulmonary artery pressure, cardiac index, and oxygenation. Sildenafil (2005) is also less expensive than the prostacyclins and endothelin receptor antagonists. Dipyridamole also has some phosphodiesterase type 5 activity.
Inhaled nitric oxide is a selective pulmonary vasodilator that is often used to treat persistent PH of the newborn. In adults with PH, a 2-year trial of inhaled nitric oxide therapy combined with dipyridamole demonstrated improvements in exercise capacity, symptoms, and hemodynamic measures. A European consensus panel has released guidelines for the use of nitric oxide in this condition.
Surgical therapies may be general (lung transplant) or specific, as in the repair of congenital shunting lesions, mitral stenosis, or atrial defects. Pulmonary thromboendarterectomy is effective in some patients with PH associated with saddle pulmonary embolus.
I Pulmonary edema
Definition
Pulmonary edema is the accumulation of excess fluid in the interstitial and air-filled spaces of the lung. The mechanisms responsible for its development include an increase in hydrostatic pressure within the pulmonary capillary system, an increase in the permeability of the alveolocapillary membrane, and a decrease in intravascular colloid oncotic pressure.
Pulmonary edema is classified as being either cardiogenic (high pressure, hydrostatic) or noncardiogenic (increased permeability).
Cardiogenic pulmonary edema
Cardiogenic pulmonary edema is initiated by some type of left-sided heart incompetence or failure. Left ventricular failure implies that there is a decrease in left ventricular contractility, which ultimately leads to a reduction in both stroke volume and cardiac output. Incomplete left ventricular emptying elevates left ventricular end-diastolic volume, which, in turn, elevates left ventricular end-diastolic pressure. Increased left ventricular end-diastolic pressure is “reflected back,” causing elevation of the left atrial, pulmonary venous, and pulmonary capillary pressures. When pulmonary capillary pressure reaches levels of 20 to 25 mmHg (normal range, 10–16 mmHg), the rate of fluid transudation often exceeds lymphatic drainage capacity, and alveolar flooding occurs.
Noncardiogenic pulmonary edema
Noncardiogenic pulmonary edema is associated with an increase in endothelial permeability caused by an insult that disrupts the barrier function of the blood–tissue interface. Noncardiogenic pulmonary edema is associated with the leakage of both fluid and protein from the vascular space. Because this respiratory membrane disruption cannot be easily or directly measured, noncardiogenic pulmonary edema is said to exist when suspicious chest radiographic evidence coexists with insufficient hemodynamic basis. The presence of pulmonary wedge pressure less than 12 mmHg and the absence of a significant history of cardiac disease generally suffice for exclusion of a hemodynamic mechanism.
Neurogenic pulmonary edema
Neurogenic pulmonary edema begins with a massive outpouring of sympathetic nervous system stimulation triggered by CNS insult. This centrally mediated CNS overactivity typically occurs in the hypothalamic area. Excessive sympathetic activation induces remarkable hemodynamic alterations, primarily systemic and pulmonary vasoconstriction. The left ventricle fails because of the inordinate pressure work imposed by the systemic hypertension, and pulmonary blood volume increases because of the functional imbalance between the failing left ventricle and the normal right ventricle. Although this sequence seems to parallel that of hemodynamic pulmonary edema, a permeability component exists, as evidenced by the high protein concentration found in the pulmonary secretions of these patients.
Uremic pulmonary edema
Uremic pulmonary edema is seen in those patients with renal insufficiency or failure. Overhydration and expansion of the circulating blood volume lead to increases in pulmonary capillary pressures. Again, a “leaky” component exists because of the metabolic abnormalities associated with uremia. Reducing the circulating blood volume of these patients by hemodialysis promotes the resolution of this type of pulmonary edema.
High altitude–related pulmonary edema
High altitude–related pulmonary edema may occur in the absence of left ventricular failure whenever someone overexerts himself or herself before acclimating to a high altitude. The pathogenesis of this form of pulmonary edema is unclear but may be the result of intense hypoxic pulmonary arterial vasoconstriction or massive sympathetic discharge triggered by cerebral hypoxia.
Pulmonary edema caused by upper airway obstruction
Pulmonary edema resulting from upper airway obstruction is caused by the prolonged, forced inspiratory effort against an obstructed upper airway. The most common cause of this type of pulmonary edema in adults is laryngospasm after extubation and general anesthesia. In children, pulmonary edema after obstruction caused by croup, epiglottitis, and laryngospasm also is well documented. Vigorous inspiration against obstruction creates high negative intrathoracic, transpleural, and alveolar pressures that enlarge the pulmonary vascular volume and subsequently the interstitial fluid volume. The capacity of the lymphatics becomes overwhelmed, and interstitial fluid transudes into the pulmonary alveoli. Hypoxia causes a massive sympathetic discharge that results in systemic vasoconstriction and a translocation of fluid from the systemic circulation to the already expanding pulmonary vascular and interstitial spaces. Hypoxia also increases pulmonary capillary pressures. Because hypoxia alters myocardial activity, left atrial function and left ventricular function are reduced. During obstruction, vigorous inspiratory efforts are unsuccessful because of the airway obstruction. Unsuccessful expiration produces an increase in intrathoracic and alveolar pressures. Intrinsic PEEP also is produced during this stage. Relief of the obstruction results in cessation of intrinsic PEEP.
The consequence of these events is the sudden massive transudation of fluid from the pulmonary interstitium into the alveoli, which results in pulmonary edema. The severity of pulmonary edema is determined by the extent of prior alveolar and capillary damage and the immensity of hemodynamic and cardiovascular alterations.
Clinical manifestations
Physical examination reveals an increased work of breathing. As water accumulates, the lungs become heavy and noncompliant, and a decrease in FRC occurs. This increase in the volume of extravascular lung fluid provides a potent stimulus for surrounding interstitial stretch receptors (j-receptors) whose activation results in tachypnea. Tachypnea is not relieved by the administration of oxygen and the return of arterial oxygen tension (Pao2) to normal. Intercostal retractions and use of accessory muscles are apparent on physical examination. Signs of sympathetic stress stimulation such as hypertension, diaphoresis, and tachycardia often are noted. The expectoration of pink, frothy sputum signals that alveoli have been flooded.
The detection of basilar crackles on auscultation is the traditional hallmark of early pulmonary edema. In reality, by the time these crackles become audible, excess water has already flooded the alveoli and has overflowed into the terminal bronchioles. It is in the bronchioles, not in the alveoli, that the crackles of pulmonary edema are generated. The earliest and most often disregarded clinical sign is rapid, shallow breathing.
In cardiogenic pulmonary edema, heart size may be increased. High central venous pressures (CVPs), an S3 or S4 gallop, and jugular venous distention often are observed. Chest radiography is still the most reliable and expedient tool for early detection of pulmonary edema. In cardiogenic pulmonary edema, the cardiac silhouette may appear abnormal or enlarged; in noncardiogenic pulmonary edema, it can be enlarged or remain normal. Interstitial edema can be observed before the alveoli flood and the onset of clinical signs occurs. Pleural effusions are common, and a “whited-out” or “butterfly” appearance may be noted.
Arterial blood gas analysis reveals hypoxemia secondary to V/Q abnormalities. When right-to-left shunting is great, the Pao2 can be affected by any change in the central venous oxygen content. Increases in oxygen consumption or decreases in cardiac output further reduce the Pao2. The arterial carbon dioxide tension (Paco2) may be low, normal, or elevated. The initial hypocarbia is related to tachypnea and high minute volumes; at later stages, hypercarbia is frequently secondary to muscle fatigue and exhaustion. Changes in pH usually reflect changes in Paco2, but metabolic and V/Q or lactic acidosis may occur from tissue oxygen deficiency, low cardiac output, or sepsis.
Treatment and anesthetic considerations
Treatment includes prompt recognition of the condition, securing a patent airway, supportive therapy with oxygenation, and the administration of diuretics. Although the onset of pulmonary edema after laryngospasm usually is immediate, cases have been reported of the occurrence of pulmonary edema several hours after laryngospasm. Therefore, it is recommended that patients who develop laryngospasm be observed postoperatively longer than the typical 60 to 90 minutes. The diagnosis of pulmonary edema and its differentiation into cardiac and noncardiac categories require the taking of a detailed medical history, physical examination, chest radiography, and ABG analysis.
Pulmonary edema is considered a medical emergency, and immediate intervention is required for treating the underlying disease, supporting other failing organ systems, and optimizing oxygen delivery. Oxygen should be administered by nasal cannula, face mask, or ETT. If oxygenation does not improve with the administration of high fractions of inspired oxygen, positive-pressure ventilation with either PEEP or continuous PAP must be initiated. Institution of positive-pressure mechanical ventilation in patients with acute pulmonary edema usually results in a prompt increase in oxygenation and, in some cases, in cardiac output. Improvement occurs because of superior inflation and V./Q. matching.
Pharmacologic therapy includes the use of vasodilators, inotropes, steroids, and diuretics. Morphine sulfate has been used in the treatment of cardiogenic pulmonary edema because of its venodilatory properties. Nitroprusside is very effective at decreasing preload, afterload and left ventricular afterload. This may result in better cardiac function, with a subsequent lowering of left atrial pressures. Inotropic agents such as dopamine or dobutamine improve myocardial contractility and lower cardiac filling pressures. In patients with chronic congestive heart failure and pulmonary congestion, digitalis augments contractility and promotes decreases in left atrial and ventricular filling pressures.
Fluid balance is managed with both fluid restriction and diuresis. This therapy helps achieve a “negative” fluid balance in hydrostatic pulmonary edema. Potent diuretics such as furosemide not only lower left atrial filling pressure by decreasing systemic venous tone but also induce diuresis of the expanded extravascular volume. The type of fluid, whether crystalloid or colloid, that should be used in the presence of pulmonary edema remains controversial. Regardless of type used, it is generally agreed that fluid administration proceeds slowly.
J Pulmonary embolism
Definition
Pulmonary embolism (PE) is considered by some to be a clinical manifestation of deep venous thrombosis (DVT) rather than a separate entity. Most emboli (90%) arise in the proximal deep veins of the lower extremities, with the remainder originating from pelvic veins. DVT at proximal sites is more likely to cause symptoms. Three major factors promote the formation of venous thrombi: stasis of blood flow, venous injury, and hypercoagulation states. Other less common causes of PE include air, tumor, bone, fat, catheter fragments, and amniotic fluid. Fillers used in illicit drug preparations by intravenous drug abusers also may cause PE. Of particular concern to anesthesia providers are air emboli caused by the opening of venous structures during surgery or by disconnected intravenous lines.
Risk Factors for Venous Thrombosis Based on Virchow Triad
| Stasis | Congestive heart failure or cor pulmonale |
| General anesthesia | |
| Immobility | |
| Obesity | |
| Prior venous thrombosis | |
| Varicose veins | |
| Hypercoagulability | Disseminated intravascular coagulation |
| Estrogen therapy or oral contraceptive use | |
| Infection | |
| Malignancy | |
| Nephrotic syndrome | |
| Pregnancy | |
| Thrombophilias: Anticardiolipin antibody, factor V Leiden mutation, protein C and S deficiencies, antithrombin III deficiency | |
| Vascular injury | Trauma |
| Surgery |
Modified from Ozsu S, Oztuna F, Bulbul Y, et al. The role of risk factors in delayed diagnosis of pulmonary embolism. Am J Emerg Med 2011;29(1):26-32; Epley D: Pulmonary emboli risk reduction. J Vasc Nurs 2000;18(2):61-68, 69-70; Dijk FN, et al. Pulmonary embolism in children. Paediatr Respir Rev 2012;13(2):112-122.
Most pulmonary emboli resolve within 8 to 21 days of the initial presentation. Chronically unresolved emboli that lodge in major pulmonary arteries may become incorporated into the vascular walls and obstruct blood flow. Patients with such emboli are surgical candidates, representing approximately 1000 cases in the United States each year.
Pathophysiology
When a thrombus has formed, it rarely remains static. It can be dissolved through fibrinolysis, become “organized” into a vessel wall, or be released into the circulation. Because thrombi are most friable early in their development, it is then that the greatest risk for embolism exists. Emboli are most often seen in the lower lung lobes, which receive the greatest amount of blood flow. Fortunately, these lower lung lobes also tend to receive the least ventilation, so much of the V./Q. ratio is preserved in patients with small- to moderate-sized emboli. The three components of the Virchow triad—stasis, hypercoagulability, and vessel wall injury—lead to venous thrombosis.
Clinical manifestations and diagnosis
The patient’s clinical presentation depends largely on the size of the embolus. Signs and symptoms of PE vary, and the differential diagnosis according to size of emboli may be difficult. Dyspnea of sudden onset appears to be the only common historical complaint. Hypoxia is a constant feature of PE, possibly owing to intrapulmonary shunting.
Associated Factors in Patients with Pulmonary Embolism*
| Finding | Incidence (%) |
| Dyspnea | 96 |
| Tachycardia | 71 |
| Acute onset of symptoms (<48 hr) | 70 |
| Syncope | 35 |
| Arterial hypotension (SBP <90 mmHg) | 34 |
| Congestive heart failure | 32 |
| History of venous thrombosis | 29 |
| Recent major operations (within 10 days) | 27 |
| Cancer | 12 |
| Major trauma or fracture within 10 days | 11 |
| Chronic pulmonary disease | 11 |
| Stroke | 2 |
*Data from 1001 patients with pulmonary embolism.
From Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997;30(5):1165-1171.
Massive emboli can produce sudden cardiac collapse. Preceding symptoms range from pallor, shock, and central chest pain to sudden loss of consciousness. In patients with cardiac collapse, the pulse becomes rapid and weak, blood pressure decreases, neck veins become engorged, and cardiogenic shock may be present or impending. Also, a decrease in Petco2 and an increase in Paco2 occur, with the difference between the values for these two indexes increasing as conditions worsen. If a pulmonary artery catheter is in place, PAPs are observed to increase rapidly; also, the ECG may begin to show right ventricular strain. The prognosis for these patients is very poor.
Diagnostic testing
Few of the common preoperative tests indicate the presence of PE. A number of imaging and laboratory tests are now available for diagnosis as listed below. In patients with PE, ABG analysis generally reveals hypoxemia and increased differences between Paco2 and Paco2, which result from ventilation of unperfused alveoli.
Diagnostic Tests for Suspected Pulmonary Embolism
| Test | Comments |
| Oxygen saturation | Nonspecific but suspect PE if there is a sudden otherwise unexplained decrement |
| Electrocardiogram | May be normal, especially in younger, previously healthy individuals; may provide alternative diagnosis, such as MI or pericarditis |
| Echocardiography | Best used as a prognostic test in patients with established PE rather than as a diagnostic test; many patients with larger PE will have normal echocardiography findings |
| Lung scanning | Usually provides ambiguous results; used in lieu of chest CT for patients with anaphylaxis to contrast agent, renal insufficiency, or pregnancy |
| Chest CT | Most accurate diagnostic imaging test for PE; beware if CT results and clinical likelihood are discordant |
| Pulmonary angiography | Invasive, costly, and uncomfortable; used primarily when local catheter intervention is planned |
| D-dimer | An excellent “rule out” test if normal, especially is accompanied by non-high clinical suspicion |
| Venous ultrasonography | Excellent for diagnosing acute symptomatic proximal DVT but a negative test result does not rule out PE because a recent leg DVT may have embolized completely; calf vein imaging is operator dependent |
| MRI | Reliable only for imaging proximal segmental pulmonary arteries; requires gadolinium but does not require iodinated contrast agents. |
CT, Computed tomography; DVT, deep venous thrombosis; MI, myocardial infarction; MRI, magnetic resonance imaging; PE, pulmonary embolism.
From Goldhaber SZ. Pulmonary embolism. In Bonow RO, Mann DL, Zipes DP, et al, eds. Braunwald’s Heart Disease. 9th ed. Philadelphia: Saunders; 2012:1686.
Advantages and Disadvantages of Diagnostic Tests for Suspected Pulmonary Embolism
| Diagnostic Test | Advantages | Disadvantages |
| Plasma D-dimer ELISA | A normal result makes PE exceedingly unlikely | Level is elevated in patients with many systemic illnesses that mimic PE, such as pneumonia and MI |
| Level is elevated in patients with sepsis, cancer, postoperative state, and pregnancy | ||
| ECG | Universally available; may indicate ominous acute cor pulmonale or benign pericarditis | Acute cor pulmonale on ECG is not specific for PE; not a sensitive test |
| Chest radiography | Usually has minor abnormalities but occasionally pathognomonic; may suggest alternative diagnoses such as pneumothorax | Not specific |
| Venous ultrasonography | Excellent for detecting symptomatic proximal DVT; surrogate for PE | Cannot image iliac vein thrombosis; imaging of calf is operator dependent; DVT may have embolized completely, resulting in a normal result |
| Contrast venography | Used to be gold standard; excellent for calf veins; necessary for catheter-based interventions. | Can cause chemical phlebitis; uncomfortable; costly; may fail to result in diagnosis of massive DVT because veins are filled with thrombus and cannot be opacified |
| Lung scanning | High-probability scans are reliable for detecting PE; normal or near-normal scans are reliable for excluding PE | Most scans are neither high probability nor normal or near normal; ventilation scans are falling out of favor; most test results are equivocal |
| Chest CT | New-generation scanners constitute the new gold standard for diagnosis | Older-generation scanners are insensitive for important but distal PE |
| MRI | Excellent for anatomy and cardiac function; contrast agent does not cause renal failure | In preliminary use; not widely available; experience very limited |
| Echocardiography | Excellent for identifying right ventricular dilatation and dysfunction that is not obvious clinically, thus providing an early warning of potentially adverse outcome | Not specific; many patients with PE have normal echocardiograms; the test cannot reliably differentiate causes of right ventricular dysfunction |
| Pulmonary angiography | Necessary for catheter-based interventions | Invasive, costly, uncomfortable |
CT, Computed tomography; DVT, deep venous thrombosis; ECG, electrocardiogram; ELISA, enzyme-linked immunosorbent assay; MI, myocardial infarction; MRI, magnetic resonance imaging; PE, pulmonary embolism.
Massive PE is associated with severe hypoxemia and hypocapnia. An initial difference between Paco2 and Petco2 is common early during the embolic event. Some common conditions associated with an increased risk for DVT are listed as follows.
Treatment
Aggressive efforts at prevention have been successful in reducing the incidence of DVT in surgical patients. Treatment is mainly aimed at prevention of further embolism and at provision of ventilatory support as shown in the table below. Use of graded compression stockings, intermittent pneumatic compression, administration of various anticoagulants and thrombolytics, and ambulation are typical measures for preventing embolus formation. It must be remembered that PE is a mechanical disease caused by acute pulmonary obstruction in a previously healthy patient.
Prevention of Venous Thromboembolism
| Condition | Strategy |
| Total hip or knee replacement; hip or pelvis fracture | Warfarin (Coumadin) (target INR, 2.5) for 4–6 wk |
| LMWH/subcut (e.g., fondaparinux 2.5 mg subcut except for total knee replacement) or rivaroxaban 10 mg daily or dalteparin 2500–5000 units daily subcut where available | |
| IPC ± warfarin | |
| Gynecologic cancer surgery | LMWH: consider 1 month of prophylaxis |
| Thoracic surgery | IPC or GCS plus UFH 5000 units bid or tid |
| High-risk general surgery (e.g., prior VTE, current cancer, or obesity) | IPC or GCS plus UFH 5000 units bid or tid LMWH |
| General, gynecologic, or urologic surgery (without prior VTE) for noncancerous conditions | GCS plus unfractionated heparin 5000 units bid or tid Dalteparin 2500 units subcut once daily Enoxaparin 40 mg subcut once daily |
| Neurosurgery, eye surgery, or other surgery when prophylactic anticoagulation is contraindicated | GCS ± IPC |
bid, Twice a day; GCS, graduated compression stockings; INR, international normalized ratio; IPC, intermittent pneumatic compression; subcut, subcutaneous; tid, three times a day; UFH, unfractionated heparin; VTE, venous thromboembolism. *Approved only for total hip replacement prophylaxis.
Modified from Goldhaber SZ. Deep vein thrombosis and pulmonary thromboembolism. In Fauci AS, Kasper DL, Braunwald E, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008:1651-1657; Goldhaber SZ. Pulmonary embolism. In Libby P, Bonow RO, Mann DL, et al, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 8th ed. Philadelphia: Saunders; 2008:1879.
Treatment requires rapid intervention before vital signs are affected by hypoxia and mechanical failure of the heart. Guidelines for treatment of PE are summarized in the following box.
Surgery
Surgical intervention often is indicated for patients who are unresponsive to other measures. Procedures that used to be the mainstay of this intervention, such as ligation of the inferior vena cava, are now rarely performed. Currently, the most common surgical procedure for patients with PE is placement of an umbrella filter, which traps thromboemboli. It is estimated that 30,000 to 40,000 patients receive such filters annually in the United States. The filter is placed in the inferior vena cava under fluoroscopic guidance, usually below the renal veins at the level of L2 to L3. Suprarenal placement is required when a thrombus directly involves the renal veins or has propagated above the level of the renal veins.
Anesthetic considerations
Anesthesia for patients at risk for PE is aimed at supporting vital organ function and minimizing anesthetic-induced myocardial depression. The use of a high Fio2 aids in prevention of pulmonary vasoconstriction, and the monitoring of PAP helps the anesthesia provider optimize right-sided heart function and assess the effects of anesthetic management on PVR. Many anesthesia providers choose not to place pulmonary artery catheters because of concerns about the possibility that these catheters will dislodge clots in the right side of the heart.
Intravenous fluid infusion must be adjusted so that right ventricular stroke volume is optimized in the presence of marked increase in afterload. A continuous catecholamine infusion may be needed to enhance cardiac contractility. Induction is often performed with etomidate or ketamine (for maintenance of hemodynamic stability), but ketamine must be titrated judiciously because it may increase PVR.
The use of N2O is generally believed to be acceptable. However, this may not be possible with the use of a high Fio2. Use of N2O should be discontinued if PVR increases. Obviously the use of N2O is contraindicated in patients with venous air embolism. Patients with moderate to severe PE often are experiencing acute right-sided heart failure. Cardiac function can be optimized by the use of minimally depressing cardiac agents such as narcotics.
Persistent severe hypotension, such as that accompanying a massive PE, may necessitate the use of a cardiotonic agent. The goal is preservation of perfusion to the brain and heart until cardiopulmonary bypass is started and surgical removal of the clot attempted. As always, heparin should be readily available, and when needed, it should be administered into a central line while blood aspiration is verified before and after injection. Reports of operative mortality during pulmonary embolectomy range from 11% to 55%, with much higher rates among patients experiencing cardiac arrest.
Detection of pulmonary embolism during anesthesia
In the intubated patient under general anesthesia, combinations of symptoms may occur. A decreasing Petco2 and tachycardia usually are the first symptoms seen in PE. These can be followed by a decrease in Sao2 and the generation of ABG values that indicate unexplained arterial hypoxemia. Increased PAP and CVP can be seen in combination with a decrease in systolic and diastolic blood pressures. Bronchospasm may occur. Finally, ECG changes that indicate right axis deviation, incomplete or complete right bundle branch block, or peaked T waves may be observed in the presence or absence of an accompanying systolic ejection murmur.
Intraoperative management
Several measures can be taken to support anesthetized patients with suspected PE. First and most important, an airway must be established by intubation if the patient is not already intubated. Second, delivery of the anesthetic agent must be discontinued, and administration of a 100% Fio2 initiated. Next, the circulatory system should be supported with the infusion of intravenous fluids or blood (or both) as needed and the use of sympathomimetics (e.g., dobutamine or dopamine) initiated if necessary. Dysrhythmias should be treated with intravenous administration of lidocaine, and the patient should receive PEEP for optimization of O2 transport across the alveolar membrane as blood pressure is adequate.
Patients with PE present particular management challenges in their postoperative courses, including reperfusion edema, persistent hypoxemia, pericardial effusion, psychiatric disorders, and pulmonary blood flow steal. The areas of the lung to which pulmonary artery flow has been restored are subject to development of reperfusion pulmonary edema, presumably as a manifestation of oxidant- and protease-mediated acute lung injury. Other possible causes are extracorporeal circulation, anticoagulation, and an increase in perfusion pressure in a previously obstructed pulmonary artery. Complications include immediate pulmonary hemorrhage and respiratory disturbance, and death may occur. This phenomenon has been labeled pulmonary blood flow steal and is believed to be caused by postoperative redistribution of regional PVR and not by rethrombosis or embolism. It may develop 3 to 5 days after surgery.
K Restrictive pulmonary diseases
Definition
Restrictive pulmonary disease is defined as any condition that interferes with normal lung expansion during inspiration. Typically, it includes disorders that increase the inward elastic recoil of the lungs or chest wall. Consequently, the alteration in pulmonary dynamics results in decreases in lung volumes and capacities and in lung or chest wall compliance. Some restrictive diseases produce ventilation abnormalities and V/Q mismatching, and others lead to impairment of diffusion. FEV1 and FVC are both decreased owing to a reduction in TLC or a decrease in chest wall compliance or muscle strength. However, the FEV1/FVC ratio is normal or elevated.
Incidence and prevalence
Statistics vary according to the acute intrinsic, chronic intrinsic, or chronic extrinsic nature of the restrictive disease.
Pathophysiology
Impairment-producing restrictive pulmonary diseases can be classified as (1) acute intrinsic, (2) chronic intrinsic, or (3) chronic extrinsic. Acute intrinsic disorders are primarily caused by the abnormal movement of intravascular fluid into the interstitium of the lung and alveoli secondary to the increase in pulmonary vascular pressures occurring with left ventricular failure, fluid overload, or an increase in pulmonary capillary permeability. Examples of acute intrinsic disorders include pulmonary edema, aspiration pneumonia, and ARDS. Chronic intrinsic diseases are characterized by pulmonary fibrosis. Conditions that produce fibrosis of the lung include idiopathic pulmonary fibrosis, radiation injury, cytotoxic and noncytotoxic drug exposure, O2 toxicity, autoimmune diseases, and sarcoidosis. Chronic extrinsic diseases can be defined as disorders that inhibit the normal lung excursion. They include flail chest, pneumothorax, atelectasis, and pleural effusions. They also include conditions that interfere with chest wall expansion, such as ascites, obesity, pregnancy, and skeletal and neuromuscular disorders.
Clinical manifestations
Clinical manifestations depend on the disorder but may include tachypnea, dyspnea, cough, bronchospasm, pulmonary vascular vasoconstriction, PH, cor pulmonale, and arterial hypoxemia. The pulmonary function tests reveal decreased vital capacity with normal expiratory flow rates.
Treatment
Treatment also depends on the specific restrictive disorder and may include oxygen therapy, bronchodilators, corticosteroids, and mechanical ventilation with PEEP.
Anesthetic considerations
• Restrictive pulmonary disease does not dictate drug choices for induction and maintenance of general anesthesia.
• Drugs should be selected and administered to avoid postoperative ventilatory depression.
• Regional anesthesia may be acceptable, but sensory levels of blockade above T10 may impair respiratory muscle function.
• Controlled ventilation may maximize oxygenation and ventilation.
• Poorly compliant lungs may require high inspiratory pressures.
• Postoperative mechanical ventilation may be necessary.
• Extubation should be done only when patients clearly meet the criteria.
• Decreased lung volumes may impair cough and interfere with postoperative secretion removal.
L Tuberculosis
Definition
Tuberculosis is a chronic granulomatous disease that is spread primarily by aerosol transmission.
Incidence and prevalence
In the past and before specific antimicrobial therapy, tuberculosis was a significant cause of death and disability in North America. It currently affects approximately 28,000 persons in North America. Elderly, debilitated, and malnourished individuals, and people who are immunosuppressed and living in crowded conditions are most often affected.
Pathophysiology
Tuberculosis is caused by the acid-fast bacillus Mycobacterium tuberculosis. After being inspired, the bacilli multiply, causing nonspecific pneumonitis.
Some bacilli migrate to the lymph nodes, encounter lymphocytes, and precipitate the immune response. Phagocytes engulf colonies of bacilli in the lung, isolate them, and form granulomatous tubercles. Infected tissues inside the tubercles create caseation necrosis (a cheeselike material). Isolation of the bacilli is completed by formation of scar tissue around the tubercle. After approximately 10 days, the immune response is complete, and further bacilli multiplication is prevented. After isolation of bacilli and development of immunity, tuberculosis can remain dormant for life. Reactivation may occur if live bacilli escape into bronchi or in states of decreased immunity. Patients with laryngeal tuberculosis or lung cavitation have the highest infectivity rate.
Laboratory results
Diagnosis is made by positive tuberculin skin (purified protein derivative) testing, chest radiography, and positive sputum culture. A positive skin test result alone for tuberculosis may indicate only exposure to the tuberculin bacteria and is not by itself evidence of active disease. Chest radiography findings consistent with tuberculosis in the presence of acquired immune deficiency (AIDS) may be atypical.
Clinical manifestations
Many patients are asymptomatic. Common manifestations include a low-grade fever, fatigue, weight loss, anorexia, lethargy, and a worsening cough (i.e., purulent sputum). Some patients occasionally develop pleural effusions, meningitis, bone or joint disease, genitourinary abscesses, or peritonitis. Chest pain, dyspnea, and hemoptysis are not common.
Anesthetic considerations
Patients are placed in respiratory isolation until such time or therapy that they are no longer transmitters of the disease. Whenever possible, disposable equipment (i.e., filters and anesthesia circuits) should be used. Nondisposable equipment must be thoroughly sterilized. Elective surgery should be postponed in actively infected patients until adequate chemotherapy has been administered (usually 3 weeks of treatment) and verified by a negative sputum sample result. The implications of organ dysfunction that may be secondary to the disease or its treatment must be considered. Although the disease most commonly affects the pulmonary system, chemotherapeutic agents used for treatment may lead to organ toxicity (i.e., liver, kidneys, or peripheral nervous system).