Chapter 15 Alimentary system
COMMON CLINICAL PROBLEMS FROM ALIMENTARY SYSTEM DISEASE
Pathological basis of gastrointestinal signs and symptoms—cont’d
| Sign or symptom | Pathological basis |
|---|---|
| Dysphagia (diffi culty swallowing) |
Heartburn (indigestion)Oesophageal/gastric mucosal irritation, often with infl ammation and ulcerationAbdominal pain
DiarrhoeaExcessive secretion or impaired absorption of fl uid within lumen of gastrointestinal tractSteatorrhoea (fatty stools)Impaired absorption of fat due to reduced lipase secretion or reduced mucosal surface area for absorptionBlood loss
Weight loss
AnaemiaBlood loss (e.g. tumour, ulcer) or impaired absorption of iron, folate or B12 due to mucosal disease
The alimentary system is constantly in contact with dietary contaminants, especially infective agents and environmental toxins, so it is not surprising that it is affected by many diseases. This chapter examines these diseases and, in those of major importance, attempts to relate them to the potentially pathogenic factors present in the human diet.
MOUTH, TEETH, PHARYNX AND SALIVARY GLANDS
NORMAL STRUCTURE AND FUNCTION
The mouth and teeth masticate the food prior to swallowing and digestion. At the same time digestion is initiated by the addition of salivary amylases and lipases.
The mouth is lined by stratified squamous epithelium overlying richly vascular connective tissue. The epithelium is of variable thickness, being thickest over the tongue where there are also papillary projections which account for its rougher texture. The epithelium is mostly non-keratinised, except over the lips, gums and hard palate where slight keratinisation occurs. Elsewhere pathological keratinisation (keratosis) results in the formation of white plaques on the mucosa; this is termed leukoplakia.
The teeth consist principally of dentine, which is similar to bone; it is composed of a collagen matrix mineralised by calcium phosphate (apatite) crystals. It differs from bone, however, in that its cellular constituents (odontoblasts) form a layer over the surface of the dentine, from which long tubular processes ramify through the tissue. The dentine is covered over the exposed part of the tooth (crown) by enamel, which is composed almost entirely of inorganic material arranged in stacked crystalline rods. The dentine of the root is covered by a thin layer of cementum which, as its name implies, attaches the tooth to the periodontal ‘ligament’ lining the socket. Centrally, the tooth has a connective tissue core, the pulp, which links with the narrow root canal.
The salivary glands are usually categorised as either major or minor. The major glands are the parotid, submandibular and sublingual glands; minor glands are scattered throughout the oral cavity. The parotids enclose branches of the facial nerve and a few lymph nodes. The glandular tissue comprises multiple small secretory acini lined by plump cells containing zymogen granules and surrounded by supporting myoepithelial cells. The secretion has a low protein content, hence these glandular units are referred to as serous acini. Small ducts lined by cuboidal epithelium drain the glandular lobules and unite to form the main secretory (Stensen’s) duct. The submandibular glands contain both serous and mucus-secreting cells in mixed acini; the sublingual and minor salivary glands are predominantly or entirely mucus-secreting. The main ducts of the submandibular glands (Wharton’s ducts) are lined by partly ciliated epithelium to facilitate drainage of the more viscid mucous secretion.
CONGENITAL DISORDERS OF THE MOUTH
Hare-lip and cleft palate
Hare-lip may appear as a sporadic defect of development but may also occur as an inherited condition exhibiting male sex linkage. The inherited form occurs both with and without a cleft palate. Where a cleft palate exists alone, a proportion of the cases are due to a dominant gene of low penetrance. Other cases are not genetically determined, as for example in the rubella syndrome (Ch. 5).
Hare-lip may be unilateral or bilateral; it may involve the lip only, or extend upwards and backwards to include the floor of the nose and the alveolar ridge. Cleft palate may vary considerably, from a small defect in the soft palate, which causes little disability, to a complete separation of the hard palate combined with hare-lip. With extensive lesions, there may be considerable difficulty with feeding as the child is unable to suck.
DISEASES OF THE TEETH AND GUMS
While of paramount importance to the dental student, diseases of the mouth, teeth and gums are soon evident to both patient and doctor, and frequently reflect generalised disorders. Their recognition and an understanding of the processes involved are therefore also of wider importance in clinical medicine.
Dental caries
Caries (‘tooth decay’) is the result of acid destruction of the calcified components of the teeth (Fig. 15.1). These structures are in a dynamic equilibrium between de- and re-mineralisation. When the pH falls below 5.5, de-mineralisation outstrips re-mineralisation; erosion of enamel is followed by loss of dentine. The acid is produced by bacteria, usually specific strains of Streptococcus mutans, acting mainly on refined sugar which is trapped in contact with the enamel by ‘plaque’—a mixture of adhesive sugar residues and bacteria. Thus, a lack of oral hygiene, excessive consumption of sugars and under-development of dentine contribute to the development of caries. Penetration of the dentine is followed by bacterial invasion; this can infect the pulp, causing pulpitis.
Gingivitis
Acute gingivitis (inflammation of the gums) is an uncommon infection caused by the anaerobic Borrelia vincentii and fusiform bacilli. It is a severe ulcerative disease, formerly referred to as Vincent’s infection, which can spread widely along the gum margins and deeply to destroy bone.
Chronic gingivitis, by contrast, is a very common condition which represents the response of the gum to adjacent bacterial plaque. Proliferation of anaerobic bacteria, and possibly their production of proteolytic enzymes, leads to chronic periodontitis and gradual destruction of the supporting tissues of the teeth. This results in loosening and eventual loss of teeth.
DISEASES OF THE ORAL MUCOSA
Inflammatory disorders
The oral mucous membrane is affected in a wide variety of mucocutaneous inflammatory disorders such as acute erythema multiforme, lichen planus, Behçet’s syndrome and many others. However, some conditions (discussed below) are restricted to the oral mucosa.
Herpetic stomatitis
Herpetic stomatitis is a very common manifestation of infection by herpes simplex virus. It is characterised by vesiculation and ulceration of the oral mucosa and is usually acquired during childhood. Many patients develop recurrences in later life, appearing as similar lesions on the lips (herpes labialis).
Oral candidiasis
Oral candidiasis (thrush) is caused by the yeast-like fungus Candida albicans. It appears as white plaques on the oral mucosa consisting of enmeshed fungal hyphae, which invade the epithelium, together with polymorphs and fibrin. The infection is more common in neonates, in patients receiving broad-spectrum antibiotics and in immunocompromised individuals.
Aphthous stomatitis
Aphthous stomatitis is a very common disorder in which single or, more usually, multiple small ulcers appear in the oral mucosa. They are shallow, with a grey, necrotic base and a haemorrhagic rim. Many patients suffer from recurrent crops of ulcers which heal spontaneously after several days. The aetiology is unknown but assumed to be immunological; some patients have an associated gastrointestinal disorder, such as coeliac disease or inflammatory bowel disease.
Reparative lesions
The oral mucosa is frequently subjected to minor trauma. In some individuals the reparative processes that follow prove excessive, and the surplus fibrovascular tissue appears as a polyp. Such a reparative lesion in the mouth is termed an epulis, of which ‘congenital’ and giant cell forms are recognised. There is also a similar angiomatous ‘tumour’ of pregnancy, and many of the so-called haemangiomas and fibromas of the mouth have the same histogenesis.
Oral leukoplakia and epithelial dysplasia
Leukoplakia (‘white plaque’) is a clinical term for patches of keratosis on the squamous epithelium that cannot be categorised as any other definable lesion, e.g. lichen planus or frictional keratosis. These lesions may be pre-cancerous: up to 18% of leukoplakias become oral cancer. Thus, in leukoplakia there is hyperkeratosis and hyperplasia of the squamous epithelium with, in some cases, dysplastic changes that herald the onset of malignant change. The finding of dysplasia on histological assessment is currently the best predictor of progression.
In the UK and USA, leukoplakia is associated with heavy cigarette smoking, excessive alcohol consumption and poor dental hygiene. The high incidence in India and Sri Lanka is attributed to the habit of chewing ‘quids’ made up of tobacco dust, areca nut and lime wrapped in a betel leaf.
Tumours
Cancer of the lip is more common than intra-oral cancers and occurs mainly in elderly people. It has a definite relationship to sunlight exposure; therefore, it is much more common on the lower than the upper lip. Lip cancers are usually well-differentiated squamous carcinomas which spread directly into surrounding tissues, and through lymphatics to the regional nodes.
Intra-oral cancers most frequently affect the tongue and commonly develop in areas of leukoplakia (Fig. 15.2); many oral carcinomas are associated with leukoplakia when diagnosed. The predisposing causes are therefore the same as those for leukoplakia but with the possibility that human papillomavirus (HPV) could also be implicated. ‘High-risk’ HPVs (mainly type 16 or 18) are more frequently associated with oral squamous carcinoma than low-risk HPVs. Like lip cancers, oral cancers are usually squamous carcinomas. Initially they are painless and can remain undetected, especially if situated on the posterior third of the tongue, until fixation and swelling interfere with swallowing and speech. Late presentation with nodal metastases and direct spread to vital structures explains the much poorer prognosis of cancer of the tongue compared to that of cancer of the lip.
DISEASES OF THE PHARYNX
Pharyngitis
Viral pharyngitis
The commonest cause of pharyngitis is viral infection, but the causative virus is rarely identified. Most cases are thought to be caused by adeno- and rhinoviruses, but other viral infections, notably those directed at the respiratory tract, can be responsible. Patients with these infections either start with a pharyngitis or develop it during the illness. Thus pharyngitis is a common feature of the common cold, influenza, measles and infectious mononucleosis (glandular fever).
Streptococcal pharyngitis
Although less common than viral infections, streptococcal pharyngitis is important for its complications. In non-immune individuals a widespread skin rash (scarlet fever) develops and occasional patients will develop acute proliferative glomerulonephritis, rheumatic fever or Henoch–Schönlein purpura.
Ulcerative pharyngitis
An ulcerative pharyngitis and tonsillitis is a common complication of agranulocytosis (deficiency of polymorphs) due to a leukaemia or marrow failure. Diphtheria was formerly an important cause of an ulcerative pharyngitis, but has now been largely eradicated in many countries by immunisation.
Tonsillitis
The faucial tonsils are collections of lymphoid tissue covered by non-keratinising squamous epithelium thrown into a series of clefts; these can harbour debris and act as a nidus for infection. The tonsils are thus a frequent site for bacterial infection, producing either an acute inflammation or, more frequently, recurring chronic inflammation leading to tonsillar enlargement (through lymphoid hyperplasia) and general debility.
Tumours
The pharynx can be the site of squamous carcinoma and of intermediate or ‘transitional’ cell carcinomas, the latter exhibiting features of epithelium transitional between squamous and columnar, respiratory-type epithelium. However, most carcinomas in this site are anaplastic (undifferentiated). In addition, the tonsils may be involved by lymphomas.
Nasopharyngeal carcinoma is of interest because of the wide geographical variation in its incidence. It is an uncommon carcinoma in Caucasians, but in some parts of China (particularly Canton) the frequency is 100-fold higher than in European populations. Males are more frequently affected than females. There appears to be a link with prior infection with Epstein–Barr (EB) virus, and the susceptible Chinese show a relatively higher frequency of the histocompatibility haplotypes HLA-A2 and -Bw46.
DISEASES OF THE SALIVARY GLANDS
Sialadenitis
Acute bacterial sialadenitis (inflammation of the salivary glands) is uncommon. It arises by ascending infection from the mouth and occurs in patients with abnormal dryness of the mouth (xerostomia), either as part of a generalised dehydration or as a result of an autoimmune-induced atrophy of the salivary glands (Sjögren’s syndrome). Acute enlargement of the salivary glands is usually due to mumps virus infection.
Recurrent sialadenitis is seen in patients who have some degree of duct obstruction, hyposecretion of saliva and ascending infection. Duct obstruction can be due to a stone (calculus) or to fibrosis. Hyposecretion may be a direct consequence of duct obstruction but may also be due to the acinar atrophy resulting from sialadenitis itself. Bacterial infection leads to recurrent acute inflammation and also acts as a nidus for stone formation.
Tumours
Pleomorphic adenoma
At least two-thirds of all salivary tumours are accounted for by the pleomorphic adenoma or ‘mixed tumour’, and over 80% occur in the parotid gland. As the name implies, this has a varied histological appearance and is composed of a mixture of stromal and epithelial elements (Fig. 15.3). The myxoid stroma, which is rich in proteoglycans, is thought to be produced by myoepithelial cells; thus, despite its biphasic appearance, it is a purely epithelial neoplasm. Occasionally the stroma has a cartilaginous appearance. Pleomorphic adenomas are essentially benign tumours but are prone to local recurrence if surgical removal is incomplete. The facial nerve is vulnerable during attempts at surgical removal. A very small proportion undergo malignant change and are capable of metastasising; these are termed malignant mixed tumours.
Warthin’s tumour
Warthin’s tumour or adenolymphoma is a relatively common salivary gland tumour (5–10% of total). It has a very characteristic appearance: tall columnar epithelial cells line convoluted cystic spaces separated by a dense lymphoid stroma. The term adenolymphoma, with its connotations of lymphoid malignancy, is a misnomer; this is an entirely benign tumour.
Muco-epidermoid tumour
Muco-epidermoid tumour consists of mucus-secreting cells—cells showing squamous differentiation and intermediate cells (small cells that are probable precursors of the mucus-secreting and squamous cells). All should be considered as at least low-grade malignancy but those tumours with a greater proportion of intermediate and squamous cells compared to mucous cells have a more aggressive behaviour. Muco-epidermoid tumours comprise about 10% of all parotid tumours.
Adenoid cystic carcinoma
Adenoid cystic carcinoma is a distinctive malignancy that is relatively more common in the minor salivary glands. It is composed of small epithelial cells arranged in islands showing microcystic change. This tumour has a propensity for perineural spread and is particularly difficult to eradicate surgically.
OESOPHAGUS
NORMAL STRUCTURE AND FUNCTION
The oesophagus is a muscular tube lined mostly by squamous epithelium. It extends from the pharynx to the cardia of the stomach and is about 25cm long in the adult. At the upper end there is the cricopharyngeal sphincter; close to the lower end there is a functional sphincter whose position can be determined only by manometry. The upper sphincter contains striated muscle fibres enabling voluntary control over the initiation of swallowing, whereas the remainder of the muscular tube is composed of smooth muscle which propels the food bolus by peristalsis and is under autonomic control. Entry of food into the stomach is facilitated by relaxation of the distal sphincter. Protection of the lower oesophagus against regurgitation of gastric contents is achieved by the distal sphincter assisted by constricting muscle bands in the diaphragm, and an acute valve-like angle of entry into the stomach. The distal 1.5–2cm of the oesophagus is situated below the diaphragm and is lined by columnar mucosa of cardiac type. The squamo-columnar junction is clearly visible on endoscopy and is usually found at about 40cm (measured from the incisor teeth). Proximal extension of this junction is found in hiatus hernia or when there is columnar metaplasia.
The squamous lining of the oesophagus consists of a layer of non-keratinising squamous epithelium overlying connective tissue papillae containing blood vessels and lymphatics. A narrow layer one to two cells thick at the base of the epithelium forms the proliferative compartment from where cells migrate upwards, mature and desquamate at the surface (Fig. 15.4). These cells acquire an increasing glycogen content as they mature. Scattered argyrophil (neuroendocrine) cells and melanoblasts can also be found in the basal layer.
CONGENITAL AND MECHANICAL DISORDERS
Heterotopic tissue
Patches of fundic-type gastric mucosa are occasionally found above the distal sphincter and separated from the columnar lining of the distal oesophagus. These are assumed to be congenitally misplaced (heterotopic) gastric tissue rather than an acquired change; they can lead to ulceration and stricturing due to local acid/pepsin secretion.
Atresia
Atresia is a failure of embryological canalisation. It is more frequent than agenesis of the oesophagus, which is extremely rare. Atresia is usually associated with an abnormal connection (fistula) between the patent part of the oesophagus and the trachea. The affected child cannot swallow and develops an aspiration bronchopneumonia. Urgent surgical correction is required.
Diverticula
Diverticula are outpouchings of the wall of a hollow viscus. Some represent a saccular dilatation of the full thickness of the wall; others are formed by herniation of mucosa through a defect in the muscle coat. Diverticula are more common in the pharynx but can develop in the oesophagus by either traction (external forces pulling on the wall) or pulsion (forcible distension). These diverticula differ from congenital forms in lacking a muscle coat in their wall. They frequently become permanently distended with retained food and cause difficulties in swallowing (dysphagia).
Hiatus hernia
Defined as the presence of part of the stomach above the diaphragmatic orifice, hiatus hernia is the commonest mechanical disorder of the oesophagus and is found in c. 25% of people undergoing investigation for dyspepsia. The herniation of the stomach with subsequent retraction of the oesophagus is largely due to increased intra-abdominal pressure and loss of diaphragmatic muscular tone with ageing. Predisposing factors include obesity, lifting heavy loads, frequent coughing fits, tight-fitting clothes and frequent bending. The consequent incompetence of the oesophageal sphincter results in regurgitation of gastric contents leading to gastro-oesophageal reflux disease (GORD). Hiatus hernia of itself is usually asymptomatic; the condition presents with the symptoms and complications of GORD.
Achalasia
Achalasia is an uncommon condition in which the contractility of the lower oesophagus is lost and there is a failure of relaxation at the sphincter (cardiospasm).
Normal functioning of the oesophagus is dependent upon the integrity of its co-ordinated muscular activity, which in turn relies on normal neuronal transmission of peristaltic signals. Thus dysphagia may arise from fibrosis and atrophy of the smooth muscle, as occurs in progressive systemic sclerosis, or by destruction or degeneration of the intrinsic nerves. The latter can occur in neurotropic infections such as Chagas’ disease (South American trypanosomiasis), or by unknown mechanisms as in the condition of achalasia.
Achalasia results in slowing or retention of the food bolus with increasing obstruction and dilatation of the oesophagus. The cause of this condition is unknown, but there are reduced numbers of ganglion cells in the myenteric plexus, and both myelinated and unmyelinated axons of the extra-oesophageal vagus nerves show Wallerian degeneration (Ch. 6).
Oesophageal varices
Varices are localised dilatations of veins. The veins of the lower oesophagus are a potential site for porto-systemic shunting of blood when portal venous flow through the liver is impaired. Therefore, in portal hypertension (most commonly resulting from cirrhosis of the liver) the submucosal veins of the oesophagus become congested and dilate (Ch. 16). These enlarged veins elevate the mucosa and protrude into the oesophageal lumen where they are easily traumatised by the passage of food. Haemorrhage is thus a frequent complication and, because of the relatively high pressure within the vascular bed, can be torrential and life-threatening.
Mallory–Weiss tears
Two American physicians’ names are associated with rupture of the oesophageal mucosa resulting from repeated retching or vomiting. The mucosa tears in the distal oesophagus close to the gastro-oesophageal junction. This is an important cause of upper gastrointestinal haemorrhage. Bleeding usually stops after 24–48 hours and the tears heal within 10 days or so.
INFLAMMATORY DISORDERS
Oesophagitis
Acute oesophagitis
Acute oesophagitis is clinically of only minor importance. Spread of bacterial infection from the nasopharynx to involve the oesophagus is a rare occurrence. More important are viral and fungal infections in immunocompromised individuals; for example, herpes simplex and cytomegalovirus infections are occasionally encountered in patients with leukaemias, lymphomas or AIDS. Candidiasis is a more common infection which may give rise to difficulties in swallowing; it is endoscopically recognisable as white plaques with haemorrhagic margins. Candidiasis is also opportunistic in immunodeficiency states and in diabetes mellitus, but can sometimes be found in otherwise healthy individuals. A further cause of acute inflammation and ulceration is the ingestion of corrosive substances; this may be either accidental (as when children swallow chemicals from unlabelled bottles) or taken with suicidal intent.
Chronic oesophagitis
As with chronic inflammation in any site, chronic oesophagitis may be either specific or non-specific. Specific causes are rare, but involvement by tuberculosis and Crohn’s disease are recognised. Non-specific oesophagitis is very common and usually results from regurgitation of gastric contents into the lower oesophagus; this is reflux oesophagitis.
Reflux oesophagitis
Gastro-oesophageal reflux disease (GORD) is very common and is diagnosed when regurgitation causes symptoms or damages the mucosa. There is poor correlation between symptoms and oesophagitis; some patients with severe symptoms have little or no damage to the oesophageal lining whereas others with obvious inflammation on endoscopy may be asymptomatic. Clinically, GORD should be diagnosed on the basis of symptoms alone; the characteristic complaint is an awareness of acid regurgitation with central chest pain or discomfort—‘heartburn’. A defective sphincter mechanism at the cardia predisposes to this gastro-oesophageal reflux, which is therefore an invariable accompaniment of hiatus hernia. Smokers are more likely to have GORD, as are obese people.
Morphology
Exposure of the squamous mucosa to refluxed acid leads to cell injury and accelerated desquamation. The increased cell loss is compensated for by increased proliferation of the germinative cells of the epithelium (basal cell hyperplasia; see Fig. 15.4); this results in fewer mature cells occupying most of the epithelial thickness and is accompanied by elongation of the connective tissue papillae. Such elongation permits extension of the basal layer and possibly reflects an interaction between the proliferating epithelial cells and underlying mesenchyme. The epithelial injury is accompanied by a low-grade inflammatory cell response so that, in general, relatively small numbers of polymorphs (including eosinophils) and lymphocytes are seen within the epithelium and in the underlying connective tissue. Thus the response to reflux embraces both:
Where reflux is severe, cell proliferation cannot keep pace with cell desquamation and ulceration occurs. These areas of ulceration can be the source of haemorrhage, and may even perforate in the most severe cases. Healing is achieved by epithelial regeneration and underlying fibrosis; subsequent shrinkage of fibrous tissue can produce a segmental narrowing (benign oesophageal stricture) in the area of healed ulceration.
Restoration of epithelial continuity is usually achieved by proliferation of squamous cells, but in some patients the lost squamous epithelium is replaced by columnar, intestinal-type epithelium, giving rise to a condition known as ‘Barrett’s oesophagus’.
BARRETT’S OESOPHAGUS
As a result of longstanding reflux, the lower oesophagus comes to be lined by columnar, intestinalised mucosa, an appearance referred to as Barrett’s oesophagus. Opinions vary as to whether this is due to epithelial ‘substitution’—migration of columnar epithelium from the distal 2cm or from the ducts of submucosal mucous glands—or to an effect on the differentiation of progeny cells from a common stem cell (metaplasia). The latter is held to be the main mechanism at work.
In a patient with Barrett’s oesophagus, the endoscopist sees proximal extension of pink columnar mucosa replacing the pearly white squamous epithelium. This extension is seen first as ‘tongues’ extending up from the cardia, and later as a complete ‘cylinder’ of columnar epithelium that can occupy much of the distal half of the oesophagus. Histologically, the epithelium may resemble that of the gastric cardia, but the characteristic ‘specialised’ Barrett’s metaplasia consists of columnar epithelium, with goblet cells and tall intervening mucus-producing cells both secreting intestinal-type mucins—a form of intestinal metaplasia. Metaplasias arise in response to an adverse micro-environment and can be regarded as a defensive response in which the new cell lineage has a survival advantage over the ‘native’ epithelium it has replaced. Thus, an initial change from squamous epithelium to a columnar, gastric-type mucosa is readily understood as a response to acid reflux in that it provides a more resistant mucosa. However, the change to epithelium with intestinal features cannot be explained solely as a defence response to acid. Other factors, such as bile reflux, may play a part, but it may be that patients who develop Barrett’s metaplasia have a different phenotypic response to acid injury from the outset.
Barrett’s oesophagus has become increasingly important following its recognition as a premalignant condition. Although the risk of malignancy is about 100 times higher among patients with Barrett’s oesophagus than in the general population, the absolute risk of developing adenocarcinoma remains small. Only 2–3% of affected patients die from adenocarcinoma and overall life expectancy is unchanged. Nevertheless, it is considered best practice to undertake regular surveillance once Barrett’s oesophagus has been diagnosed. If dysplasia, particularly high-grade dysplasia, is found on biopsy, then the risk of malignancy is greatly increased.
TUMOURS
Benign tumours
Benign tumours are uncommon and comprise about 5% of all neoplasms of the oesophagus; the type most frequently encountered is a leiomyoma. The behaviour of smooth muscle tumours in the alimentary tract is generally difficult to predict (see below) but those arising in the oesophagus are almost invariably benign. Other benign non-epithelial tumours—lipomas, haemangiomas and fibromas—are rare. The only benign epithelial tumour of note is squamous papilloma. Compared with squamous carcinoma (see below) these are rare lesions, certainly in terms of clinical presentation, but are of interest because they are likely to share a common pathogenesis with squamous papillomas at other sites being linked to human papillomavirus (HPV) infection.
Carcinoma
There are two main types of oesophageal carcinoma—squamous and adenocarcinoma. These differ markedly in their aetiology and epidemiology.
Squamous carcinoma
Squamous carcinoma is much more common in males than in females and shows marked geographical variation in incidence. In European countries, the age-standardised annual incidence is around 5 per 100000 population in males and 1 per 100000 in females. However, there are some well-defined high-risk areas, such as north-west France and northern Italy, where the incidence rises to 30 per 100000 in males and 2 per 100000 in females. Globally there are more striking differences. Regions with very high incidence have been identified in Iran, South Africa, Brazil and Central China. In Henan Province in China the mortality rate from carcinoma of the oesophagus exceeds 100 per 100 000 in males and 50 per 100 000 in females.
Epidemiological studies in high-incidence areas have indicated that a high dietary intake of tannic acid, in the form of strong tea or sorghum wheat, or dietary deficiencies of riboflavin, vitamin A and possibly zinc may be important, but other factors such as fungal contamination of foodstuffs, opium usage and thermal injury may also be involved. In Western countries, cigarette smoking and the drinking of alcoholic spirits are associated with a higher incidence.
A factor of current interest is the possible involvement of HPV. Some oesophageal squamous cancers contain HPV in their cells, and viruses of similar subtype can be found in intact and apparently normal oesophageal mucosa. It is therefore possible that virus integrated into the host genome can bring about oncogene activation and carcinogenesis. The involvement of papillomaviruses in the development of bovine oesophageal carcinoma is well established.
Non-specific chronic oesophagitis is common among the general population in high-incidence areas, and biopsies will frequently reveal dysplasia. The squamous epithelium shows cellular pleomorphism: there is disordered maturation with immature cells and mitotic activity appearing close to the surface. The degree of atypia can be categorised as low- or high-grade dysplasia; the latter condition will proceed to invasive carcinoma if surgical resection is not performed. As in the oropharynx, dysplasia is sometimes associated with abnormal keratosis—leukoplakia.
Adenocarcinoma
In the lower third of the oesophagus, adenocarcinomas are the predominant type. They usually develop on the basis of a Barrett’s oesophagus. The incidence has risen dramatically in recent years among white, middle-aged men in European countries and the USA; the reported annual increase in the white male population of the USA is close to 10% which exceeds that of any other malignancy in that population. Although the incidence of adenocarcinoma has increased, it is still a rare disease; in the year 2000 the numbers of new cases in Western countries varied between 1 and 5 per 100000 white males. Nevertheless this incidence approaches, and in some areas exceeds, that of squamous carcinoma of the oesophagus.
Carcinoma of the oesophagus, either squamous or adenocarcinoma, usually commences as an ulcer, but spreads to become annular and constricting so that the patient develops dysphagia (difficulty in swallowing) (Fig. 15.5). However, by the time most patients present, direct spread outside the oesophagus has occurred and the surgical resection rate is only about 40%. Resectability and ultimate survival can be improved by pre-operative chemo-irradiation. Those patients who cannot be surgically treated may undergo chemo- or radiotherapy alone, or receive palliative laser therapy. Unfortunately, a substantial proportion of patients are simply intubated to facilitate adequate nutrition. The long-term outlook is therefore very poor; only 5% survive for 5 years. Most patients die from local disease and bronchopneumonia exacerbated by malnutrition. Unlike many forms of cancer, metastases are uncommon at autopsy.
STOMACH
NORMAL STRUCTURE AND FUNCTION
The stomach acts essentially as a ‘mixing’ reservoir for food during acid–pepsin digestion. Hydrochloric acid and pepsin are, however, only two of many products of the gastric mucosa.
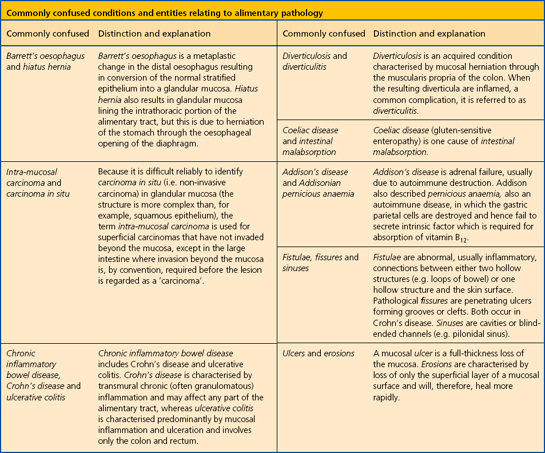
Histologically, the stomach can be divided into three regions—the cardia, body and antrum. The surface of the gastric mucosa and its pits (foveolae) are lined throughout by columnar mucus-secreting epithelium. The mucus secreted by these cells, together with contributions from the antral mucous glands, forms a viscid gel covering the mucosa—the gastric mucus barrier (Fig. 15.6). Bicarbonate and sodium ions, also secreted by surface epithelial cells, diffuse into the unstirred gel and buffer the hydrogen ions entering from the luminal aspect. A pH gradient is thus established, ranging from 1 or 2 at the luminal surface of the barrier, to neutrality at the plasma membrane of the epithelium. The glandular component varies from region to region.
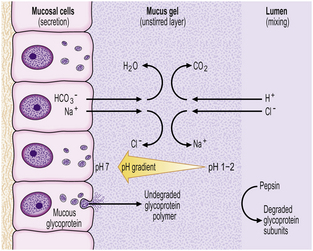
Fig. 15.6 The gastric mucus barrier. The surface epithelial cells (supplemented by foveolar and glandular mucous cells) secrete viscid mucus which forms an unstirred layer between the epithelium and the gastric lumen. The surface cells also secrete sodium and bicarbonate ions into the mucus gel and a pH gradient is established. This constitutes the major defence against acid attack.
The cardiac (or junctional) mucosa is a narrow zone immediately below the termination of the squamous-lined oesophagus; it comprises simple tubular or cystic glands, lined by mucus-secreting cells, in which endocrine cells are scattered.
Body mucosa lines the proximal two-thirds of the stomach and consists of tightly packed tubular glands, the upper parts of which are lined by parietal cells (acid producing) and the lower parts by chief cells (pepsinogen) (Fig. 15.7A). In addition to acid, the parietal cells secrete intrinsic factor, essential for vitamin B12 absorption. Other cells present in body mucosa are mucous neck cells and endocrine cells. The neck cells are found at the bases of the gastric pits, i.e. at the junction between foveolar lining cells and glandular cells, and contain the stem cells of the mucosa together with some immature foveolar cells. The majority of the endocrine cells are so-called enterochromaffin-like (ECL) cells which are readily identifiable by silver staining (argyrophil) techniques. These cells modulate parietal cell activity by releasing histamine in response to stimulatory hormones such as gastrin.
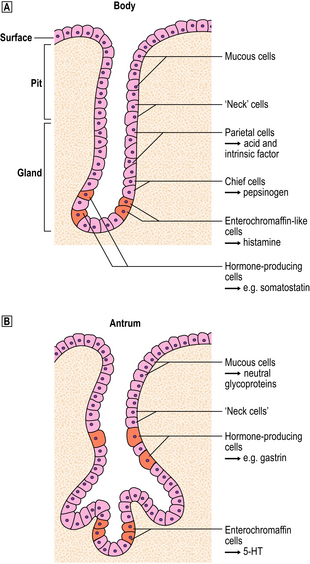
Fig. 15.7 Structure of the gastric mucosa.  Body (corpus) mucosa where the tubular glands contain specialised secretory cells. The ‘neck’ cells represent the proliferative compartment of the gastric pit from where the majority of cells migrate upwards to replenish exfoliated surface cells, and a minority move downwards to replace glandular cells.
Body (corpus) mucosa where the tubular glands contain specialised secretory cells. The ‘neck’ cells represent the proliferative compartment of the gastric pit from where the majority of cells migrate upwards to replenish exfoliated surface cells, and a minority move downwards to replace glandular cells.  Antral mucosa, predominantly composed of mucus-secreting cells but with scattered endocrine cells.
Antral mucosa, predominantly composed of mucus-secreting cells but with scattered endocrine cells.
Antral (or pyloric) mucosa occupies a roughly triangular region proximal to the pylorus, with its base about one-third of the distance along the lesser curvature and its apex a few centimetres from the pylorus on the greater curve. The antral glands are more branched, tortuous and less tightly packed than those in the body (Fig. 15.7B). The glands are lined by mucus-secreting cells with faintly granular cytoplasm and basal nuclei, together with endocrine cells. There may be occasional parietal cells. The endocrine cells of the antrum produce several hormones: G cells secreting gastrin are the most numerous, but others include D cells (which secrete somatostatin), EC cells (5-hydroxytryptamine, 5-HT), P cells (bombesin) and S cells (secretin).
CONGENITAL DISORDERS
Congenital abnormalities, apart from hypertrophic pyloric stenosis, are rare. They include accessory structures lined by gastric mucosa, which are referred to as ‘cysts’ when saccular and not communicating with the gastric lumen, ‘duplications’ if tubular and non-communicating, and ‘diverticula’ if they communicate.
Diaphragmatic hernia
Maldevelopment of the diaphragm can lead to defects though which the stomach, together with parts of the intestine and the spleen, herniate into the left thoracic cavity. Usually only part of the stomach is dislocated into the thorax, but after birth it may become expanded by swallowed air and can rapidly compress the lungs and, very rarely, cause death from respiratory failure.
Pyloric stenosis
An abnormal hypertrophy of the circular muscle coat at the pylorus can cause outflow obstruction from the stomach. The condition, found in approximately 4 per 1000 live births, usually presents 2–3 weeks after birth with projectile vomiting. It is four to five times more common in males than in females. The primary abnormality appears to lie in the enteric nervous system at this site; the interstitial cells of Cajal (see p. 378) are either absent or abnormal.
INFLAMMATORY DISORDERS
Inflammation of the stomach, as with other organs, is usually considered as either acute (often described as ‘haemorrhagic’ or ‘erosive’) or chronic gastritis. One form of chronic gastritis, formerly designated type A, has long been recognised as an autoimmune disorder, but this type is uncommon. The major form of chronic gastritis (type B) is the result of infection with the bacterium Helicobacter pylori. Finally, longstanding bile reflux gives rise to a third major category—a ‘chemical’ type gastritis—which could therefore be termed ‘type C’.
Acute gastritis
Acute gastritis is almost invariably an acute response to an irritant ‘chemical’ injury by drugs or alcohol. The principal drugs involved are non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin, but many others have been implicated. These agents cause a prompt exfoliation of surface epithelial cells and diminished secretion of mucus such that the protective barrier against acid attack (see below) may be compromised. Many of their effects are probably mediated by an inhibition of prostaglandin synthesis.
Depending on the severity of the injury, the mucosal response varies from vasodilatation and oedema of the lamina propria, to erosion and haemorrhage. An erosion is an area of partial loss of the mucosa, as opposed to an ulcer where the full thickness, i.e. below the muscularis mucosae, is lost. The erosions in acute gastritis are frequently multiple and the resultant haemorrhage can be severe and life-threatening. Fortunately, the lesions are transient and heal rapidly by regeneration, so that erosions may well have disappeared 24–48 hours after the bleeding episode.
An acute neutrophilic gastritis (i.e. one in which polymorph infiltration is a dominant feature) is characteristic of the initial response to Helicobacter pylori infection. Acute Helicobacter gastritis is a transient phase which in the majority of individuals is subclinical and over the course of 3–4 weeks gives way to chronic gastritis. In a minority of individuals the infection is spontaneously eradicated and the inflammatory response resolves. The pathological features of acute bacterial gastritis are summarised in Table 15.1.
Chronic gastritis
Autoimmune chronic gastritis
A few patients with chronic gastritis are found to have antibodies in their serum directed against gastric parietal cells and intrinsic factor binding sites. These patients exhibit varying degrees of hypochlorhydria (they are often achlorhydric), and have a macrocytic anaemia resulting from vitamin B12 deficiency; this association of autoimmune gastritis with macrocytic anaemia is called pernicious anaemia.
Histologically, the body of the stomach is maximally affected: there is marked loss of parietal cells (glandular atrophy) and replacement fibrosis of the lamina propria, together with an infiltrate of lymphocytes and plasma cells. In addition, the surface and pit-lining epithelium may show intestinal metaplasia (IM), a change common to all forms of longstanding chronic gastritis. In this form of metaplasia, the neutral, mucin-secreting cells characteristic of the stomach are replaced by goblet cells containing acidic glycoproteins typical of the intestine. In well-developed cases there may also be absorptive cells and Paneth cells. Intestinal metaplasia is generally regarded as a premalignant condition; however, the risk is low and cancer develops in only a very small proportion of people with IM. Another type of metaplasia in the stomach could be more important in cancer development: parietal gland atrophy is accompanied by a multifocal proliferation of mucous glands—usually referred to as pyloric gland metaplasia—and this is more strongly associated with gastric cancer than IM. However, neither IM nor pyloric gland metaplasia is sufficiently closely related to cancer development to be useful for identifying ‘high-risk’ patients.
Helicobacter-associated chronic gastritis
The commonest cause of chronic gastritis is bacterial infection by Helicobacter pylori. This is a Gram-negative organism that inhabits a peculiarly protected niche closely applied to the surface epithelium beneath the mucous barrier where the pH approaches neutrality. Besides taking advantage of this acid-protected niche, the organism has its own intrinsic acid buffering mechanism using its urease and ammonia production to neutralise hydrogen ions gaining access to its periplasmic space. The organism binds to the surface cells and, depending on its virulence, exerts cytopathic effects that lead to accelerated cell exfoliation and a polymorph and chronic inflammatory cell response (Fig. 15.8). H. pylori is found in over 90% of biopsies showing active chronic (type B) gastritis but is very uncommon in the autoimmune type. The gastritis resolves after successful eradication of infection with antibiotics. Interestingly, the organism is found only on gastric epithelium and does not colonise duodenal (or any other intestinal) epithelium.
Fig. 15.8 Gastritis resulting from Helicobacter pylori infection.  At low magnification, the biopsy reveals an influx of chronic inflammatory cells into the mucosa and lymphoid follicle formation.
At low magnification, the biopsy reveals an influx of chronic inflammatory cells into the mucosa and lymphoid follicle formation.  A section specially stained to reveal numerous minute curved Helicobacter adherent to the mucosal surface.
A section specially stained to reveal numerous minute curved Helicobacter adherent to the mucosal surface.
The neutrophil polymorph response provoked by H. pylori is mediated partly by leukotactic complement components liberated through activation of the alternative pathway (Ch. 9), but principally by bacteria-induced production of interleukin-8 by epithelial cells, macrophages and endothelial cells. Polymorphs subsequently release proteases, reactive oxygen metabolites (ROMs) and reactive nitrogen species into the mucosa. ROM production by leukocytes is enhanced by cytokines, such as tumour necrosis factor-alpha (TNF-alpha), and their cytopathic effects may be responsible for the glandular loss (atrophy) that characterises longstanding chronic gastritis. Anti-H. pylori IgA, IgG and IgM antibodies are produced locally by plasma cells in the lamina propria as part of a Th2-mediated response (Ch. 9); these antibodies have a role in the prevention of bacterial adhesion and in opsonisation, but fail to eliminate the infection.
Histologically, Helicobacter-associated gastritis affects the entire stomach mucosa but to a variable degree. The majority of patients have diffuse involvement of the antrum and body with gradual glandular atrophy, replacement fibrosis and intestinal metaplasia. The loss of parietal cells leads to hypochlorhydria and a reduction in the secreted signals that modulate the growth and differentiation of progenitor cells in the gastric mucosa; this could explain the link between atrophy and metaplasia. Patients with widespread gastritis have an increased risk of gastric ulcer and carcinoma compared with uninfected individuals. A second main pattern is where the antrum is markedly inflamed but with little involvement of body mucosa. These individuals have increased acid output rendering the body mucosa more hostile to H. pylori colonisation. Patients with this antrum-predominant gastritis have a greater risk of duodenal ulcer. Overall, however, only 10–15% of individuals infected with H. pylori develop peptic ulcer disease, and the risk of gastric cancer is about 1–3%. The histological features of acute and chronic gastritis are summarised in Table 15.1.
Chemical (reflux) gastritis
The presence of regurgitated bile and alkaline duodenal juice in the stomach provokes epithelial cell loss, compensatory hyperplasia of the proliferative compartment in the gastric foveolae, and vasodilatation and oedema of the lamina propria; this is reflux gastritis. In ‘normal’ people there is little or no regurgitation of duodenal contents into the stomach. Reflux gastritis is seen in the post-operative stomach following operations that destroy or bypass the pylorus, as a result of secondary motility disturbances in patients with gallstones and after cholecystectomy, and in some patients who appear to have a disturbance of antro-duodenal motility or co-ordination. Unoperated patients with bile reflux appear to have a failure in pyloric competence resulting from a disturbance in pyloro-antral motor function; this may be either a primary disturbance, or a defective response to hormones, such as cholecystokinin and secretin, which normally increase pyloric tone during duodenal acidification. The ensuing reflux gastritis stimulates production of gastrin by the antral mucosa; this may also block the effects of cholecystokinin and secretin on the pyloric muscles.
Reflux gastritis may present with bilious vomiting or less severe dyspeptic symptoms; repeated damage to the mucosa may lead to the development of a gastric ulcer.
A similar histological picture to that found with bile reflux can result from long-term usage of NSAIDs; the common denominator is repeated chemical injury. The various types of chronic gastritis are compared in Table 15.2.
Other forms of gastritis
Less common forms of chronic gastritis have been distinguished from the three major types discussed above.
In lymphocytic gastritis the main histological feature is the presence of numerous mature lymphocytes within the surface epithelium. This form is occasionally seen in patients who have peculiarly heaped-up erosions running along prominent rugal folds. Most patients are histologically H. pylori-negative at the time of diagnosis but most show serological evidence of infection. Some cases are related to villous atrophy and altered small intestinal function.
Eosinophilic gastritis is characterised by oedema and a large number of eosinophils in the inflammatory cell infiltrate. It is thought to be an allergic response to a dietary antigen to which the patient has become sensitised, or in some countries to parasitic infestation.
Granulomatous gastritis is a rare form of gastritis in which epithelioid cell granulomas are found. Such granulomas can be part of Crohn’s disease (p. 385) or sarcoidosis, but after exclusion of these causes there remains an isolated granulomatous gastritis of unknown aetiology.
PEPTIC ULCERATION
Peptic ulceration is a breach in the mucosa lining the alimentary tract as a result of acid and pepsin attack. Gastric and duodenal ulcers differ in their epidemiology, incidence and pathogenesis (Table 15.3). They arise as either acute or chronic ulcers.
Table 15.3 Comparison of the epidemiology, incidence and aetiology of gastric and duodenal ulcers
| Feature | Gastric ulcer | Duodenal ulcer |
|---|---|---|
| Incidence (relative) | 1 | 3 |
| Age distribution | Increases with age | Increases up to 35 years of age |
| Social class | More common in low socio-economic classes | Even distribution |
| Blood group | A | O |
| Acid levels | Normal or low | Elevated or normal |
| Helicobacter gastritis | About 70% | 95–100% |
 Major sites: first part of duodenum, junction of antral and body mucosa in stomach, distal oesophagus and gastro-enterostomy stoma
Major sites: first part of duodenum, junction of antral and body mucosa in stomach, distal oesophagus and gastro-enterostomy stomaAcute ulcers
Deeper extension of the erosions in acute gastritis resulting from NSAIDs or acute alcohol overdosage can produce frank ulcers. Acute ulcers occur also in a heterogeneous group of conditions where stress seems to be the common denominator. For example, ulcers may be found following severe burns (Curling’s ulcer), major trauma or cerebrovascular accidents. Such ulcers probably arise as a consequence of mucosal ischaemia, which lowers the mucosal resistance to acid. Extreme hyperacidity, as seen for example in patients with gastrin-secreting tumours (Zollinger–Ellison syndrome), can lead to multiple acute ulcers in the antrum, the duodenum and even the jejunum.
Chronic ulcers
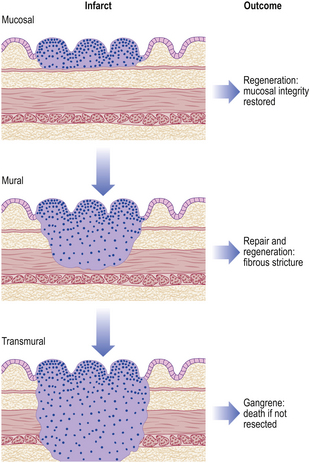
Chronic peptic ulcers (Fig. 15.9) seem to occur most frequently at mucosal junctions. Thus gastric ulcers are often found where antral meets body-type mucosa on the lesser curvature; duodenal ulcers are found in the proximal duodenum close to the pylorus; oesophageal peptic ulcers are found in the squamous epithelium just above the cardio-oesophageal junction; and stromal ulcers—those occurring following construction of a gastro-enterostomy linking stomach and jejunum—are found in the jejunal mucosa immediately adjacent to the gastric mucosa of the stromal margin. This suggests that ulceration is most likely to occur where acid and pepsin first come into contact with a susceptible mucosa.
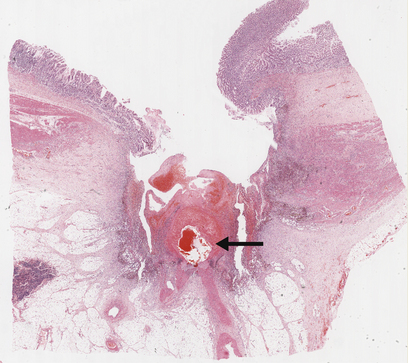
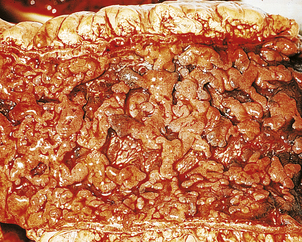
Fig. 15.9 Chronic gastric ulcer. Histological section through the ulcer revealing a deep breach of the main muscle layers and haemorrhage around an artery (arrowed) in the ulcer base. The patient presented with a profuse haematemesis (vomiting blood) and underwent emergency partial gastrectomy.
Pathogenesis
For many years peptic ulceration has been attributed to excessive acid production. However, there are many problems with this hypothesis. People with gastric ulcers frequently have normal or even subnormal acid production, and over one-half of duodenal ulcer patients do not have hyperacidity. Conversely, many people who are hypersecretors of acid do not get ulcers. Furthermore, while most ulcers respond initially to anti-acid treatment there are frequent relapses. It has therefore become increasingly apparent that mucosal defence against acid attack is of considerable importance (Fig. 15.10). Failure of the mucosal defence mechanisms means that ulcers can result from normal or even decreased quantities of acid.
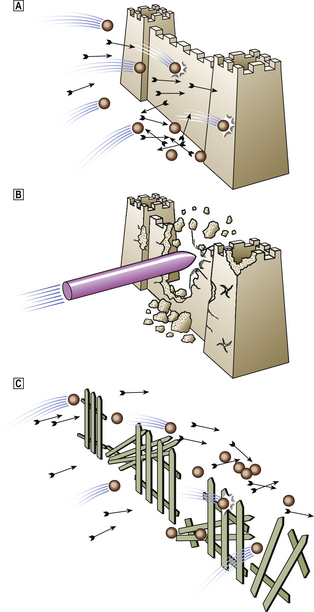
Fig. 15.10 Peptic ulceration. ‘An ulcer represents the adverse outcome of a conflict between aggressive forces in the stomach or duodenum and the defence mechanisms.’ (Sir Francis Avery-Jones)  Normal. Acid/pepsin attack is balanced by the mucus barrier and other defence mechanisms.
Normal. Acid/pepsin attack is balanced by the mucus barrier and other defence mechanisms.  Increased attack. Hyperacidity (as in the Zollinger–Ellison syndrome) or NSAIDs may bring about the ulceration of ‘normal’ mucosa in the stomach and duodenum.
Increased attack. Hyperacidity (as in the Zollinger–Ellison syndrome) or NSAIDs may bring about the ulceration of ‘normal’ mucosa in the stomach and duodenum.  Weakened mucosal defence. This is the major factor in peptic ulceration. Chronic inflammation in the gastric and duodenal mucosa resulting from Helicobacter pylori infection can lead to ulceration in the presence of normal or even reduced levels of acid. Duodenal inflammation results from infection of patches of acid-induced gastric metaplasia in the first part of the duodenum.
Weakened mucosal defence. This is the major factor in peptic ulceration. Chronic inflammation in the gastric and duodenal mucosa resulting from Helicobacter pylori infection can lead to ulceration in the presence of normal or even reduced levels of acid. Duodenal inflammation results from infection of patches of acid-induced gastric metaplasia in the first part of the duodenum.
Gastric ulcers
The pH of the gastric juice under fasting conditions is extremely acidic (between 1 and 2) so that any unprotected gastric mucosa would rapidly undergo auto-digestion.
The mucosal defences against acid attack consist of:
The mucus barrier is the more important of the two lines of defence. The pit-lining and surface epithelial cells of the stomach secrete viscid neutral glycoproteins which form a layer of unstirred mucus on the surface. The mucus itself has acid-resistant properties, but its protective power is greatly enhanced by the establishment of a buffering gradient within the layer brought about by bicarbonate ions.
The surface epithelium constitutes a second line of defence; for its proper functioning it requires integrity of both the apical plasma membrane as a barrier to ion transfer, and cellular metabolic functions, including the production of bicarbonate. These functions are dependent upon an adequate mucosal blood supply.
Ulceration can follow either destruction or removal of the mucus barrier, or a loss of integrity of the surface epithelium. Dissolution of the mucus layer can occur as a primary event as a consequence of duodeno-gastric reflux. The regurgitated bile from the duodenum strips off the mucus barrier and paves the way for acid attack. Acid and bile in combination damage the surface epithelial cells, increasing the permeability of the mucosa. This causes the congestion and oedema of the lamina propria seen in reflux gastritis.
The epithelial barrier may be damaged by the effect of NSAIDs blocking the synthesis of the prostaglandins that normally protect the epithelium. Epithelial injury is also a consequence of H. pylori infection, produced either directly by cytotoxins and ammonia or indirectly as a result of the inflammatory reaction. Thus, in peptic ulcers in the stomach, breakdown of mucosal defence is much more important than excessive acid production.
Duodenal ulcers
Increased production of acid assumes more importance in the pathogenesis of duodenal ulceration; about one-half of such patients have an elevated maximal acid secretion, and even those with a normal maximal acid output may have inappropriately sustained acid secretion without the normal sharp fall-off of acid production during sleep. It has been shown that H. pylori-infected individuals secrete two to six times as much acid as non-infected controls when stimulated by gastrin-releasing peptide. Nevertheless, excess acidity is not the entire explanation and mucosal defence is also important.
The factors causing lowered resistance in the stomach do not usually apply in the duodenum: Helicobacter does not colonise normal duodenal epithelium; the duodenal mucosa is tolerant of bile and pancreatic alkaline secretions; and drugs are generally diluted or absorbed before reaching the duodenum. Nevertheless, Helicobacter is involved in duodenal ulceration because there is gastric metaplasia in response to excess acid. Gastric metaplasia (i.e. a change from intestinal absorptive cells to gastric surface mucous cells) paves the way for colonisation by Helicobacter, which in turn sets up chronic inflammation in the duodenum (duodenitis) and predisposes to ulceration. Furthermore, NSAID-induced ulcers can arise in the duodenum with, or without, pre-existing duodenitis.
Morphology
Grossly, chronic peptic ulcers are usually less than 20mm in diameter but they may be larger and can exceed 100mm in diameter. The edges are clear-cut and overhang the base. Microscopically, the base consists of necrotic tissue and polymorph exudate overlying inflamed granulation tissue which merges with mature fibrous (scar) tissue. The latter frequently occupies the remainder of the wall, with the muscularis propria completely replaced by fibrous tissue. Arteries within this fibrous base often show extreme narrowing of their lumina by intimal proliferation (endarteritis obliterans).
Ulcers heal by a combination of epithelial regeneration, which reconstitutes the mucosa, and progressive fibrosis. Later, shrinkage of the fibrous tissue (cicatrisation) may lead to pyloric stenosis or a central narrowing of the stomach, the so-called hour-glass deformity.
More immediate complications of peptic ulcers include:
Although malignant change is claimed to occur in gastric peptic ulcers, this is a very uncommon event: as far as duodenal ulcers are concerned it can be assumed that they never become malignant.
BENIGN TUMOURS AND POLYPS
A polyp is simply a protuberant mass of tissue; it can either be neoplastic or form as a result of an excessive reparative or regenerative process. The commonest form of polyp involves simple elongation of the gastric pits separated by fibrous tissue or mildly inflamed lamina propria. These are hyperplastic or regenerative polyps and are generally found against a background of Helicobacter-associated gastritis in the gastric antrum. A similar variety is seen in body-type mucosa, but in this instance the main feature is enlargement by cystic dilatation of the specialised fundic glands. These were originally thought to be hamartomatous, but this is questionable and they are best termed simple fundic polyps. Much more rarely, true hamartomas occur, either as adenomyomas, which, as the term implies, are overgrowths of glandular and smooth muscle elements, or as part of the Peutz–Jeghers syndrome, an autosomal dominant disorder where the patient has multiple gastrointestinal hamartomatous polyps and circumoral, macular pigmentation. A further rare cause of a polypoid mass in the stomach is heterotopic pancreas, i.e. the presence of pancreatic tissue separate from the main gland.
Adenomas (i.e. benign epithelial neoplasms) are uncommon in most Western countries but are a relatively frequent finding in Japan and other countries with a high incidence of gastric cancer. When polypoid, these tumours have a strong potential for malignant change and, if subjected to multiple sectioning, around 40% will be found to contain carcinoma on microscopic examination. However, ‘flat adenomas’ with lower malignant potential are increasingly recognised and have to be distinguished from the higher-risk multifocal epithelial dysplasias (see below).
There are two main benign mesenchymal tumours, the leiomyoma and the Schwannoma or nerve sheath tumour. Both are rare. The majority of ‘connective tissue tumours’ in the stomach are stromal tumours. Gastric stromal tumours have a largely unpredictable behaviour and from a management point of view are best considered as ‘low-grade’ malignancy (see below).
MALIGNANT TUMOURS OF THE STOMACH
Carcinoma of the stomach
The incidence of gastric carcinoma, like that of carcinoma of the oesophagus, varies widely both between and within countries. There is a notably high incidence in Japan, China, Colombia and Finland, but even in these countries, as elsewhere in the world, the incidence of carcinoma of the stomach is declining. Despite this fall, gastric cancer is still the second most common fatal malignancy (after lung cancer) in the world, accounting for around 10% of all cancer deaths, with an estimated 750 000 new cases diagnosed annually.
Aetiology
For many years, a sequence of events, starting with chronic gastritis and passing through atrophy and intestinal metaplasia to premalignant dysplasia, has been recognised as the precursor to cancer of the stomach. Given that H. pylori is now known to be the major cause of chronic gastritis, it is logical to implicate this infection in the causation of gastric cancer. The prevalence of H. pylori infection frequently runs parallel with the incidence of gastric cancer in the same populations, and epidemiological studies have shown that patients with antibodies to H. pylori have a higher risk of gastric cancer. The strength of the epidemiological links is such that the International Agency for Research into Cancer has declared that H. pylori is a gastric carcinogen, that is, the infection initiates the events leading to cancer. Given the high prevalence of infection and the comparative rarity of cancer it is highly unlikely that the organism or its products are direct-acting mutagens.
There are several possible indirect mechanisms linking H. pylori infection to gastric cancer. Long-term infection leads to glandular atrophy, which leads to a gradual decline in acid secretion. Hypochlorhydria allows other bacteria to proliferate in the gastric juice; these bacteria are capable of reducing nitrate ions to nitrite and can catalyse nitrosation of amines and amides present in the diet to give rise to potentially carcinogenic N-nitroso compounds. A more likely source of genomic DNA damage in H. pylori gastritis, however, are reactive oxygen metabolites produced by activated polymorphs and macrophages. Interestingly, nitrosation and oxidative damage is minimised by anti-oxidant vitamins, among which ascorbic acid is the most important, and diets rich in fresh fruit and vegetables have long been recognised as protective against gastric cancer. Ascorbic acid secretion into gastric juice is severely compromised in H. pylori gastritis.
When considering the role of H. pylori, several paradoxes have to be explained. First, there is a male preponderance of gastric cancer yet infection rates are similar in females; second, there is a low risk of cancer associated with the longstanding antral gastritis found in duodenal ulcer subjects; and third, some countries have a high rate of infection but a low incidence of gastric cancer (‘the African enigma’). Possible explanations lie in the interplay between an individual’s inflammatory response and the genotype of the infecting bacterium. Regarding the former, interleukin-1 beta (IL1-beta) is a powerful pro-inflammatory cytokine with acid-suppressive actions that governs many aspects of the response to H. pylori. Polymorphisms in the IL1-beta gene cluster allied to infection by more virulent strains of H. pylori could explain widely different risks of cancer development, even within populations. The balance between Th1- and Th2-mediated responses could also play a part (Ch. 9). Th1 cell immune responses are known to encourage the progression of pre-neoplastic changes like atrophy and metaplasia, relative to Th2 responses. Concurrent intestinal helminth infection alters the immune response to H. pylori infection away from a Th1- towards a Th2-mediated reaction and this may be protective against gastric cancer. Thus, in some developing countries, co-infection with intestinal parasites could explain the disparity between H. pylori infection and gastric cancer rates.
Several molecular genetic changes occur in gastric cancer. These include loss of expression of cell adhesion molecules including E-cadherin and beta-catenin, mutations and deletions of tumour suppressor genes, notably TP53, K-ras and the APC gene, and over-expression of oncogenes like c-myc and erbB-2. However, while some of these mutations are consistent with exogenous chemical carcinogens or exposure to endogenous free-radical injury, the nature of the mutational agent cannot be deduced from the genetic lesions with any certainty.
Overall, the evidence points to an unequivocal link between H. pylori infection and gastric cancer. In the year 2000, it was estimated that about 340 000 gastric adenocarcinoma deaths were attributable to H. pylori. The implications for the prevention of this major cancer are clear. Eradication of this infection or vaccination in childhood (when a vaccine becomes available) will have a profound effect on the incidence of gastric cancer.
However, H. pylori is not the only infectious agent to have a role in gastric cancer; over the past 10 years it has become clear that Epstein–Barr virus (EBV) infection is also linked to gastric adenocarcinoma. Worldwide about 10% of gastric cancers are associated with EBV but there is considerable geographical variation; the highest frequency (up to 17%) is found in Latin America. Gastric cancer has been associated with a distinctive strain of EBV, and global DNA methylation with gene ‘silencing’ occurs in EBV-related cancers.
About 8–10% of gastric cancers appear to have an inherited familial component, and in 1–3% of cases germline mutations inactivating the gene (CDH1) encoding the cell-adhesion protein E-cadherin lead to an autosomal dominant predisposition.
Dysplasia and early gastric cancer
The dysplasia–carcinoma sequence is thought to characterise the development of most gastric cancers, but the finding of dysplasia is relatively uncommon in low-incidence countries such as the UK and USA. Most cancers are advanced at the time of initial diagnosis; in the USA 65% of cases present at an advanced stage (pT3/T4) with nearly 85% of tumours accompanied by lymph node metastases at diagnosis. This accounts for the poor prognosis of gastric cancer, which generally has only a 10–15% survival rate at 5 years after diagnosis. Much better results, however, are obtained when patients undergo radical operations with extensive lymph node clearance. Patients who have such ‘potentially curative resections’ with removal of all regional nodes have a 60% chance of surviving 5 years.
Gastric cancers are classified as either ‘early’ or ‘advanced’ on their depth of invasion into the stomach wall (Fig. 15.11). Early gastric cancer (pT1) is confined to either the mucosa (intra-mucosal carcinoma) or submucosa; advanced gastric cancer extends into or beyond the main muscle coats (pT2–4). Cancers can still be ‘early’ even if lymphatic spread has occurred to regional lymph nodes. The importance of this categorisation lies in their differing prognosis: patients with early gastric cancer have a 5-year survival in excess of 90%. The prognosis of advanced cases rests largely on whether surgery has been truly ‘curative’ in removing the entire tumour. Thus, involvement of the resection margins by carcinoma carries a dire prognosis, as does the presence of covert hepatic or distant lymph node metastases. The best guide to prognosis in potentially curative cases appears to be the number of involved lymph nodes and, to some extent, the histological type of carcinoma.
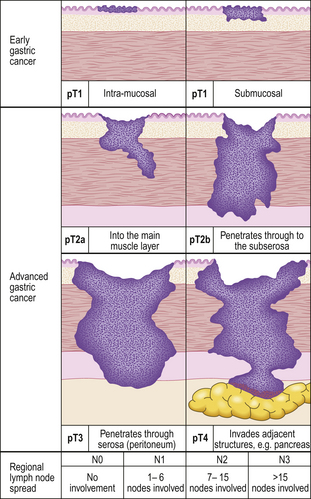
Fig. 15.11 The spread of gastric cancer. Direct spread and lymph node metastases categorised according to the TNM scheme. Spread confined the mucosa or submucosa is classified as ‘early’, whereas any degree of spread into, or beyond, the main muscle layer is ‘advanced’. The prognosis is highly dependent upon the extent of direct spread.
Morphology
Foci of high-grade dysplasia and intra-mucosal carcinoma may be endoscopically visible as slightly elevated plaques or shallow depressions. Histologically, they may be distinguished according to whether invasion of the lamina propria has occurred, but this can be excluded in high-grade dysplasia only by examination of multiple sections from the entire area of involvement. From a practical point of view the distinction is academic; if either of these lesions is diagnosed in a gastric biopsy, resection is essential. With increasing size, the elevated lesions develop into polypoid and later into fungating carcinomas, while the depressed areas present an excavated ulcerated appearance mimicking that seen in chronic peptic ulcer. The distinction between carcinoma and chronic peptic ulcer cannot be made with certainty on clinical, endoscopic or radiological grounds, so that all gastric ulcers should be subjected to cytology or multiple biopsies both before and after therapy.
Carcinomas of the stomach are almost exclusively adenocarcinomas derived from mucus-secreting epithelial cells. Like other carcinomas, they can be graded according to their degree of differentiation; poorly differentiated carcinomas behave more aggressively than well-differentiated types. However, a better guide to histogenesis results from division into either ‘intestinal’ or ‘diffuse’ types according to the scheme devised by Lauren (Fig. 15.12).
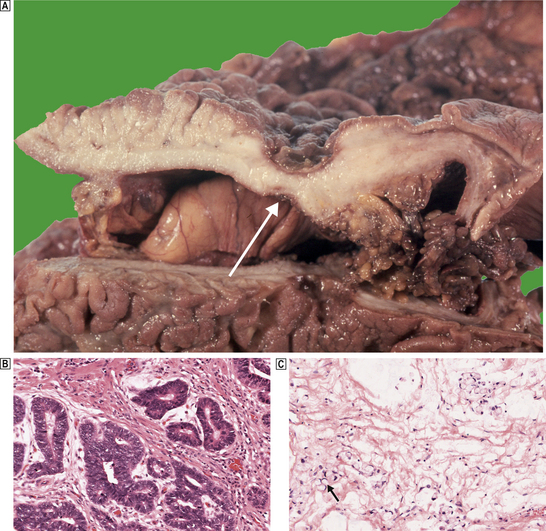
Fig. 15.12 Gastric cancer.  An ulcerated cancer of the stomach, initially thought to be a chronic peptic ulcer but biopsies revealed adenocarcinoma. The carcinoma has spread through the wall and breached the peritoneal surface (pT3; arrowed). The two main histological types of gastric adenocarcinoma are:
An ulcerated cancer of the stomach, initially thought to be a chronic peptic ulcer but biopsies revealed adenocarcinoma. The carcinoma has spread through the wall and breached the peritoneal surface (pT3; arrowed). The two main histological types of gastric adenocarcinoma are:  intestinal-type comprising tubular or glandular formations of cohesive cells, and
intestinal-type comprising tubular or glandular formations of cohesive cells, and  diffuse-type composed of scattered clusters of non-cohesive cells which, in this example, contain a large clear mucin vacuole with compressed nuclei, so-called signet-ring cells (arrowed).
diffuse-type composed of scattered clusters of non-cohesive cells which, in this example, contain a large clear mucin vacuole with compressed nuclei, so-called signet-ring cells (arrowed).
Intestinal-type gastric carcinomas carry a better prognosis than the diffuse type, but this is largely explained by the greater extent of spread of diffuse carcinomas at the time of diagnosis. Interestingly, the intestinal form predominates in high-incidence countries and has a strong correlation with pre-existing Helicobacter-associated chronic gastritis. Diffuse carcinomas predominate in low-incidence countries; this may reflect the increased contribution of genetic factors or EBV infection to cancer development among these populations. Certainly, both autosomal dominant hereditary gastric cancer and EBV-associated cancers are of diffuse type.
Carcinomas spread directly to involve the serosa (pT3), which can lead to peritoneal dissemination. This can result in the formation of a malignant effusion (ascites) or involvement of other organs by transcoelomic spread, of which metastases in the ovaries (Krukenberg tumours) are a classic example. Depending upon the site of the tumour, direct spread can also occur into the pancreas, transverse colon (when fistulation can occur), liver and spleen (pT4). Lymphatic spread is initially to perigastric nodes along both curvatures of the stomach, then to nodes along the right and left gastric, coeliac and splenic arteries (pN1–3). Spread to non-regional nodes such as retro-pancreatic, mesenteric and para-aortic groups is considered to be distant metastasis (M1). Rarely, spread to even more distant nodes is encountered, like the classical involvement of left supraclavicular nodes (Troisier’s sign). Blood-stream spread occurs via the portal vein; liver metastases are frequently evident at the time of presentation.
Other malignant tumours
Adenocarcinomas comprise over 90% of all gastric malignancies. Other malignant tumours include neuroendocrine (carcinoid) tumours (p. 396), malignant stromal tumours and lymphomas.
Stromal tumours
The stomach is the commonest site for gastrointestinal stromal tumours (GIST); approximately 45% of these are malignant and can metastasise. The component spindle cells originate from the interstitial cells of Cajal (see p. 378), and can be identified immunohistochemically by their CD117 (receptor tyrosine kinase) positivity. Affected patients present with symptoms referable to secondary ulceration—haemorrhage, anaemia, anorexia and weight loss. Endoscopically, the tumour protrudes into the lumen and often has a central deep ulcer crater. Stromal tumours behave unpredictably: it is difficult to distinguish between benign and malignant tumours on histological criteria. Features indicating a benign course are small size, encapsulation, very low mitotic activity and absence of necrosis. Malignancy is recognised by the presence of metastases at the time of surgery and can be predicted, to some extent, by an invasive margin and high mitotic activity.
Lymphomas
The stomach is the commonest site for primary lymphomas to arise in the gastrointestinal tract, accounting for around 40% of all cases, and the incidence is steadily increasing. Lymphomas of the stomach represent about 5% of all gastric malignancies and are most frequently of the non-Hodgkin B-cell type; they are closely related to preceding H. pylori infection.
The normal gastric mucosa is virtually devoid of lymphocytes. H. pylori infection provokes an influx of lymphocytes and plasma cells in an active chronic inflammatory reaction. In keeping with a Th2-mediated response, lymphoid follicles with germinal centres appear in the gastric mucosa together with an increase in intra-epithelial lymphocytes in the immediately overlying epithelium. These features recapitulate those of a mucosa-associated lymphoid tissue (MALT); this acquired MALT provides the tissue of origin for gastric B-cell lymphomas. As with gastric carcinoma, epidemiological studies reveal a much increased risk for the subsequent development of gastric lymphoma when H. pylori-infected individuals are compared with uninfected controls. Indeed, patients with these low-grade B-cell lymphomas (marginal zone lymphomas) are almost always H. pylori-positive. A few cases will result from longstanding H. heilmannii gastritis, a much less common infection contracted from pet cats. In these cross-species Helicobacter infections, the predominant Th2 response favours lymphoma development over adenocarcinoma.
The emergence of a monoclonal proliferation of B-lymphocytes associated with aggressive features, evinced by invasion and destruction of epithelium (lympho-epithelial lesions) and replacement of germinal centres by atypical centrocyte-like B-cells, is characteristic of a low-grade malignant MALT lymphoma (Fig. 15.13). Monoclonality is detected using the polymerase chain reaction to show that a significant proportion of the lymphoid cells contain identically rearranged immunoglobulin heavy chain genes.
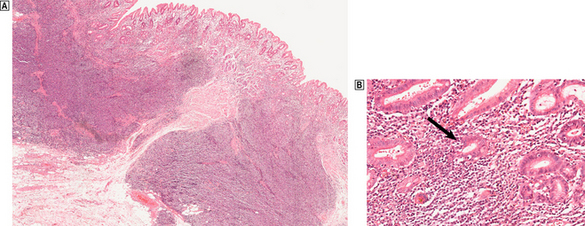
Fig. 15.13 H. pylori-related low-grade (marginal zone) lymphoma in the stomach.  A dense infiltrate of lymphocytes occupies the mucosa and extends deeply into the submucosa.
A dense infiltrate of lymphocytes occupies the mucosa and extends deeply into the submucosa.  At high magnification, glands and the deep parts of the gastric pits are surrounded and infiltrated by lymphocytes including atypical forms. These lympho-epithelial lesions (arrowed) are a precursor to destruction of glands by the malignant infiltrate.
At high magnification, glands and the deep parts of the gastric pits are surrounded and infiltrated by lymphocytes including atypical forms. These lympho-epithelial lesions (arrowed) are a precursor to destruction of glands by the malignant infiltrate.
High-grade (large-cell) lymphomas consist of dense sheets of large ‘blast’ cells and are almost invariably of B-cell lineage. The transition from chronic gastritis to low-grade and then to high-grade lymphoma is associated with specific, reproducible genetic changes, but the cause remains unknown. Interestingly, the low-grade B-cell lymphomas appear to require the continuing antigenic stimulation of Th2 cells to maintain B-cell proliferation. As a consequence, these lymphomas can show complete regression following successful elimination of H. pylori infection. Deeply infiltrating (pT2–4) low-grade tumours and all high-grade lymphomas require treatment with chemo- and/or radiotherapy. Even so, high-grade lymphomas have a relatively good prognosis (compared to adenocarcinoma) when confined to the stomach (50% survival at 5 years), but the outlook worsens considerably when penetration of the serosa (pT3–4) or involvement of regional lymph nodes has occurred. The stomach may also be involved by lymphomas that have arisen elsewhere; the outlook in these systematised cases depends upon the overall extent, histological type and grade of the lymphoma.
INTESTINE
NORMAL STRUCTURE AND FUNCTION
Small intestine
The main functions of the small intestine are:
By providing a vast surface area of specialised epithelium, the villous structure of the mucosa optimises absorption; this can be either passive or under active control. The villi are covered by tightly packed absorptive cells (enterocytes), which themselves have microvilli on the luminal surface along their plasma membranes. This microvillous or ‘brush’ border further increases surface area and, together with the adherent glycoproteins of the glycocalyx, is also the site of hydrolytic enzyme activity, for example disaccharidases and peptidases.
Endocrine cells
Scattered among the absorptive cells are mucus-secreting goblet cells and endocrine cells; the latter are part of the diffuse neuroendocrine system and there are more than 14 different types producing a wide variety of gut hormones, including enteroglucagon, cholecystokinin, gastrin, motilin, secretin and vasoactive intestinal polypeptide (VIP). Neuroendocrine cells are also found among the proliferating cells (enteroblasts) of the intestinal crypts and these produce serotonin (5-HT), which has an important role in the control of gut motility and blood supply. Tumours originating from neuroendocrine cells are discussed further on page 396.
Paneth cells
These are distinctive granulated cells also found at the bases of the small intestinal crypts. Paneth cells are a rich source of defensins, peptides that are important in protecting the intestinal epithelium against microbes. Paneth cells store defensins as inactive propeptides within granules containing inactive forms of the processing enzymes, trypsin and trypsinogen. Upon granule release into the lumen, activated trypsinogen cleaves prodefensin to release the biologically active peptide alpha-defensin 5. Defensins act like broad-spectrum antibiotics with activity against Gram-positive and -negative bacteria, fungi, viruses and parasites.
Brunner’s glands
The duodenal submucosa contains Brunner’s glands, collections of mucus-secreting acini most plentiful proximally. They are much less frequent in the jejunum. Brunner’s glands produce an alkaline mucous secretion that is also rich in epidermal growth factor (EGF). The secretion not only neutralises acidic gastric juice entering the duodenum but, through its high content of luminally active EGF, promotes mucosal regeneration after injury.
Mucosa-associated lymphoid tissue
The connective tissue of the mucosa (lamina propria) contains prominent lymphatics (lacteals), blood capillaries, and a cellular infiltrate comprising lymphocytes, plasma cells, eosinophils and mast cells. The lymphoid cells form an important arm of mucosal immunity, and most of those in the lamina propria are T-helper cells, whereas the intra-epithelial lymphocytes are predominantly T-suppressor cells thought to be important in maintaining tolerance to food antigens. Lymphoid aggregates or follicles with germinal centres are found throughout the intestinal mucosa; they frequently straddle the muscularis mucosae and extend into the superficial submucosa. Dense aggregates are found in the terminal ileum where they form Peyer’s patches. The flattened epithelium over these aggregates contains M-cells, specialised cells capable of antigen binding and processing; they pass antigenic material to underlying helper T-lymphocytes.
Large intestine
The large intestine has several functions:
The large intestine can be divided into six parts—caecum, ascending colon, transverse colon, descending colon, sigmoid and rectum. These divisions are imprecise, but are useful for describing the sites and extent of disease.
Mucosa
The mucosa of the large bowel is devoid of villi. Instead, it comprises perpendicular crypts extending from the flat surface down to the muscularis mucosae, separated by a little lamina propria. Numerically, the predominant cell is of columnar absorptive type, but in tissue sections such cells often appear less numerous than the intervening goblet cells. As in the small intestine, several types of neuroendocrine cell are present, but in health Paneth cells are confined to the right side of the colon and then only sparsely. Also, in contrast to the small intestine, large bowel mucosa has only scanty lymphatics which are concentrated towards the muscularis mucosae. This restricts the metastatic potential of intra-mucosal malignant cells.
Vascular supply
The vascular supply to the colon derives from the superior and inferior mesenteric arteries:
These patterns of blood supply are important in determining the sites and consequences of ischaemia (for example, the ‘watershed’ territory around the splenic flexure is especially vulnerable) and, because lymphatic drainage follows similar patterns, in predicting the likely distribution of lymph node metastases from the site of the tumour.
Nerve supply
The intestine has a complex nerve network comprising autonomic motor and sensory neurones and a separate enteric nervous system. The sympathetic supply originates from ganglia outside the gut in the coeliac and mesenteric plexuses. The parasympathetic ganglia are found within the gut wall, and these, together with the associated neurones, form two nerve networks: the submucosal (Meissner’s) plexus and the myenteric (Auerbach’s) plexus. The nerve plexuses create and conduct the basic electrical rhythm of the gut. Stimulation of parasympathetic nerves increases muscular contraction (particularly in the inner circular layer), blood supply and secretory activity; stimulation of the sympathetic supply has the opposite effects.
The enteric nervous system has sensory receptors in the mucosa and bowel wall that respond to changes in volume and composition of the bowel contents, and through neuronal connections elicits the appropriate response in the effector system. These activities are mediated by a wide variety of neurotransmitters, such as vasoactive intestinal polypeptide, cholecystokinin and somatostatin, some of which were formerly thought to be gut hormones. The interstitial cells of Cajal (ICC) were until recently obscure and neglected partners in the neural control of the gut. These cells are of mesenchymal, non-neural origin and are found intercalated between elements of the enteric nervous system and smooth muscle cells. Some ICC act as a source of spontaneous electrical slow-waves responsible for paced contractions of the musculature (‘intestinal pacemakers’) while others appear to modulate neurotransmission in the gastrointestinal tract.
Appendix
The appendix arises from the caecum. It is a blind-ended structure lined internally by colonic-type mucosa, surrounded by submucosa and muscle coats. In children and young adults the mucosa contains numerous prominent lymphoid follicles. In the elderly, the lumen often shows fibrous obliteration.
CONGENITAL DISORDERS
The duodenum derives from the distal end of the primitive foregut; the jejunum, ileum and proximal colon from the midgut; and the distal colon and rectum from the hindgut. Proper development involves canalisation (development of a lumen), temporary herniation into the extra-embryonic coelom, rotation, and eventual retraction back into the abdominal cavity. Defects arising in the course of this complex process are relatively common.
Atresia and stenosis
Atresia represents either a failure of the gut to canalise or a failure of a segment to develop during fetal growth. A congenital stenosis is a constriction of the bowel arising during fetal development. These lesions are most commonly found in the duodenum or small intestine, and are rare in the colon. Duodenal atresia seems to be a failure of organ development, and around 30% of affected children also have Down’s syndrome; jejuno-ileal atresia commonly appears to be the result of an intra-uterine accident, such as incarceration of the midgut in the physiological umbilical hernia or some other form of vascular occlusion.
Malrotation
The commonest type of malrotation occurs when the large bowel fails to descend into the right iliac fossa after emerging from the physiological umbilical hernia. This means that the caecum remains high in the abdomen and the bands that should fix it in the right iliac fossa (Ladd’s bands) cross the duodenum and compress it, causing extrinsic obstruction.
Duplication and diverticula
Duplication of the bowel may either present as a tubular double-barrelled appearance, or form a cyst in the mesentery. These anomalies can produce an abdominal mass, cause intestinal obstruction, or initiate a volvulus (p. 392). Congenital diverticula are outpouchings of the full thickness of the bowel wall and are found mainly in the duodenum and jejunum. These rarely have clinical consequences, but some patients develop bacterial overgrowth, steatorrhoea and vitamin B12 malabsorption. The diverticula can also undergo perforation and haemorrhage.
Meckel’s diverticulum
Meckel’s diverticulum arises as a result of incomplete regression of the vitello-intestinal duct, such that a tubular diverticulum is present in the ileum. The diverticulum is usually lined by normal small-intestinal mucosa, but occasionally it may contain heterotopic gastric or pancreatic elements. If gastric elements are present, acid and peptic secretion may lead to ulceration at the mouth of the diverticulum and give rise to haemorrhage and perforation. The diverticulum may also become inflamed and present as an acute abdomen which can mimic appendicitis.
Meconium ileus
The term meconium ileus refers to small-intestinal obstruction resulting from thickening and desiccation (inspissation) of the viscid meconium produced by children with cystic fibrosis (Ch. 7). It is seen in about 15% of affected babies and may be complicated by perforation, secondary atresia or volvulus.
Hirschsprung’s disease
Hirschsprung’s disease, or aganglionosis of the intestine, results from a failure of migration of neuroblasts from the vagus into the developing gut, such that the intramural parasympathetic nerve plexuses fail to develop. The distal colon and rectum have an additional parasympathetic supply from extramural nerves derived from the sacral plexus. Under normal circumstances the parasympathetic tone, which controls the contraction of the circular muscle coat, is modulated at the ganglia by the sympathetic innervation. However, in the absence of the myenteric ganglia, the intact extramural parasympathetic supply is unchecked by sympathetic modulation and results in spasm of the circular muscle leading to intestinal obstruction. There is a proliferation of cholinergic nerves derived from this extramural supply throughout the affected segment, and their high content of acetylcholinesterase can be utilised to diagnose Hirschsprung’s disease in frozen sections of rectal mucosa.
Hirschsprung’s disease affects the distal large intestine, extending proximally from the anorectal junction for a variable distance. The rectum and distal colon are usually involved, but the extent varies from 1–2cm to total colonic aganglionosis, or even extension into the small intestine. The effects of the aganglionosis vary from life-threatening total obstruction to mild cases causing chronic constipation. The main cause of death in Hirschsprung’s disease is the development of an acute enterocolitis with endotoxaemia.
Anorectal anomalies
A large variety of malformations have been described that affect the termination of the large bowel. These include:
Anorectal anomalies occur in approximately 1:5000 live births. Most are amenable to surgical correction.
MALABSORPTION
Malabsorption can result from pancreatic disease or various biochemical disorders such as lactase and sucrase–isomaltase deficiency, as well as from small-intestinal diseases. Small-intestinal causes include:
Coeliac disease
Coeliac disease is due to an abnormal reaction to gluten, a constituent of wheat, rye and barley, which results in damage to the surface enterocytes of the small intestine and severely reduces their absorptive capacity.
Incidence and clinical manifestations
Overt coeliac disease affects about 1 in 2000 individuals in the UK, but in the west of Ireland this rises to 1 in 300. Surveys in Europe, South America, Australasia and the USA have shown that approximately 0.5–1% of these populations have undetected coeliac disease. Although coeliac disease can be diagnosed at any age, it presents most frequently in either early childhood or in the third or fourth decades. In contrast to the equal sex distribution in children, there are twice as many females diagnosed as adults. Although the classical features of ‘malabsorption syndrome’—diarrhoea, steatorrhoea, weight loss and fatigue—are seen in severe cases, most patients have milder, less easily recognised, symptoms such as bloating and indigestion, or are largely asymptomatic and present with an unexplained iron-deficiency anaemia. In the UK, the diagnosis is made increasingly in patients with atypical symptoms or anaemia.
Aetiology and pathogenesis
The toxic components of gluten are gliadin and glutenin, but the mechanism by which they induce tissue damage remains uncertain. Gluten is partially digested in the small intestine, but many gluten peptides are resistant to intestinal peptidases like pepsin and chymotrypsin. Although these toxic peptides have direct effects on intestinal permeability through the disruption of tight junctions between enterocytes, it seems increasingly likely that tissue injury is more a consequence of the immune response than a direct effect. A long-established factor in the immune reaction to gluten components is the possession of particular HLA (human leukocyte antigen) genes. Nearly all patients with coeliac disease possess either the HLA-DQ2 or the HLA-DQ8 heterodimer. However, HLA-DQ2 is present in approximately one-third of non-affected Caucasian populations, demonstrating that it contributes to, but is not sufficient for, disease development. The potential importance of HLA-DQ2 lies in its ability to bind the toxic gluten peptides and present them to T-cells.
Evidence of the importance of perturbed T-cell function comes from the recent identification of a disease-related region at 4q27; this harbours the genes for interleukin (IL)-2 and IL-21. IL-2, secreted in an autocrine manner by antigen-stimulated T-cells, is a key cytokine for T-cell activation and proliferation, while IL-21 enhances T-, B- and NK-cell proliferation and interferon-gamma production. One of the gluten peptides (part of alpha gliadin) appears to induce IL-15 production by enterocytes and dendritic cells. IL-15 is also important in T-cell activation and may induce expression of a major histocompatability complex chain-related molecule (MICA) on enterocytes as well as upregulating NKG2D, an activating receptor on intra-epithelial lymphocytes (IELs). The interaction between activated IELs and enterocytes expressing MICA results in direct enterocyte killing.
Morphology
Under normal circumstances, enterocytes are constantly shed from the tips of the villi and replenished by migration of cells up the villi from the proliferative compartment in the crypts (Fig. 15.14). The entire cycle from cell birth through functional maturation to extrusion takes about 72 hours. Moderately accelerated cell loss can be compensated for by increased cell proliferation. With higher rates of cell loss, a stage is soon reached when the increased proliferative compartment cannot maintain a normal number of maturing and functioning ‘end cells’, the size of this compartment diminishes, and villous atrophy results. Shrinkage of villi and reduction in epithelial surface area are thus inevitable consequences of any injury causing a high rate of cell loss in the small intestine. In coeliac disease the ultimate stage of this process is seen; despite a marked increase in size of the proliferative compartment, evidenced by elongation, hypercellularity and high mitotic activity of the crypts (crypt hyperplasia), there is a flat surface (total villous atrophy; Fig. 15.15) and even this is populated by immature cells incapable of proper absorptive activity. Fully developed coeliac disease is therefore characterised by a total malabsorption, affecting sugars, fatty acids, monoglycerides, amino acids, water and electrolytes; the failure to absorb fat is the dominant abnormality in most cases. The loss of mature surface epithelial cells also causes disaccharidase deficiency, so that patients become intolerant of lactose and other sugars. The degenerate surface epithelium is infiltrated by large numbers of T-lymphocytes (IELs). Damaged epithelial cells may release TNF-alpha; this enhances the proliferation and migration of IELs (Fig. 15.16).
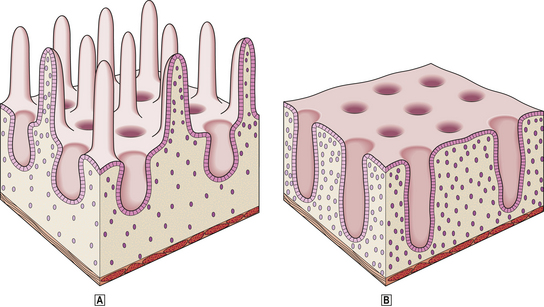
Fig. 15.15 Coeliac disease.  Normal jejunal mucosa.
Normal jejunal mucosa.  Total villous atrophy in coeliac disease. See text for details.
Total villous atrophy in coeliac disease. See text for details.
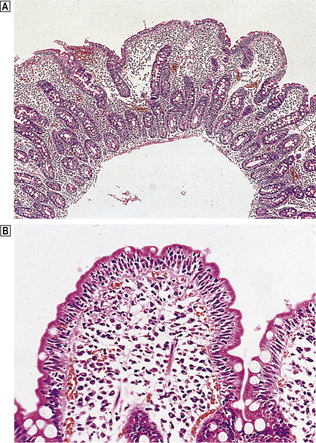
Fig. 15.16 Coeliac disease.  Jejunal biopsy from a patient with coeliac disease showing severe villous atrophy and crypt elongation (hyperplasia).
Jejunal biopsy from a patient with coeliac disease showing severe villous atrophy and crypt elongation (hyperplasia).  Higher power view of shortened villus showing increased numbers of lymphocytes in the surface epithelium and lamina propria.
Higher power view of shortened villus showing increased numbers of lymphocytes in the surface epithelium and lamina propria.
The lesion is more severe in the proximal small intestine—the duodenum and proximal jejunum—and may spare the ileum, although the latter is susceptible to injury if exposed to gluten. In addition to malabsorption, intestinal hormone production from the proximal small bowel is impaired; there may be secondary reduction in pancreatic secretion and bile flow as a result of reduced production or release of pancreozymin, secretin and cholecystokinin. Thus gallstones are commonly present in older patients.
Lesser degrees of gluten intolerance short of classical coeliac disease are increasingly recognised. Investigation of older patients with unexplained weight loss or anaemia sometimes brings to light an increase in IELs with or without mild villous atrophy in duodenal biopsies. Such patients also respond to gluten withdrawal.
Complications
Now that the primary lesion and clinical consequences of coeliac disease can be managed by gluten-free diets, the later effects of the disease are becoming of greater concern. Osteoporosis and sub-fertility have been attributed to coeliac disease but the increase in frequency is small. Of more importance, there is a low but definitely increased risk of malignant lymphoma in the small intestine, and a modest increase in other gastrointestinal cancers. Outside of coeliac disease, the majority of small bowel lymphomas are of B-cell lineage, but in coeliacs they are frequently of a large cell type derived from T-cells (enteropathy-associated T-cell lymphoma). The patient presents with haemorrhage, perforation, small-bowel obstruction or systemic symptoms. A few patients with coeliac disease develop ulceration of the small intestine which is non-lymphomatous; microscopic examination simply reveals non-specific chronic inflammation (chronic ulcerative enteritis).
Tropical sprue
Pathological changes identical to those found in coeliac disease (but usually less severe) are evident in tropical sprue, a form of malabsorption found, as the name indicates, in the tropics and sub-tropics but not apparently in Africa. It occurs most frequently in South-East Asia and the Caribbean. Tropical sprue is characterised by chronic diarrhoea, weight loss and a macrocytic anaemia due to folate or vitamin B12 deficiency. A gluten-free diet has little or no benefit, but the condition may be relieved by broad-spectrum antibiotics. The cause of the disease remains uncertain, but abnormal bacterial colonisation of the upper small bowel is probably involved. Plasma levels of the gut hormones enteroglucagon and motilin are increased, the former slowing intestinal transit.
Repeated bacterial and viral infections are virtually the norm in tropical countries and these may lead to mucosal changes falling short of those seen in tropical sprue. In tropical Africa, for instance, jejunal biopsies from apparently healthy subjects will reveal reduced villous height and increased inflammatory cells when compared to normal Europeans. These minor morphological abnormalities found in asymptomatic residents of tropical countries have been called ‘tropical enteropathy’ but from a local perspective could be considered ‘normal’.
BACTERIAL INFECTIONS
Bacterial infections of the intestinal tract are a major cause of morbidity and mortality throughout the world. Bacterial contamination of water supplies and the consequent diarrhoeal diseases are the major cause of infant mortality in developing countries. From a pathological viewpoint, diarrhoeal pathogens can be separated into those causing an inflammatory diarrhoea with a polymorph exudate, and those causing a secretory diarrhoea with no faecal leukocytes. Bacterial pathogens associated with secretory diarrhoea (e.g. Vibrio cholerae, enterotoxigenic and enterohaemorrhagic Escherichia coli) are generally non-invasive whereas those causing inflammatory diarrhoea (enteroinvasive E. coli, Shigella, Campylobacter or non-typhoidal Salmonella species) readily invade the intestinal epithelium and provoke an intense polymorph reaction.
Salmonella
Food poisoning by Salmonella organisms is a common and increasing problem in many countries. Salmonella infection of food poisoning type (salmonellosis) is generally confined to the gastrointestinal tract. In some patients this results in vomiting and profuse watery diarrhoea, usually with colicky, peri-umbilical pain suggesting predominantly gastric and small-intestinal involvement. However, in others the features relate to the large intestine, with frequent, small-volume, bloody motions, tenesmus and tenderness over the sigmoid colon. In the latter cases, sigmoidoscopy discloses a range of abnormalities varying from mucosal oedema and hyperaemia, to mucosal friability with slough formation and contact or spontaneous haemorrhage. The histological appearances are similarly varied. Some biopsies show oedema, focal interstitial haemorrhage and a mild increase in neutrophil polymorphs; more severe cases show a marked increase in polymorphs, with occasional crypts distended by polymorphs and mucus in the lumen (‘mucoid crypt abscesses’). The crypt pattern, however, remains normal. The disease usually resolves within 10 days as the immune response clears the infection.
Typhoid fever is caused by Salmonella enterica serotype Typhi (S. typhi), and remains a major public health problem in many regions of the world; over 16 million cases are reported each year worldwide. In contrast to the gastroenteritis caused by most Salmonella species, typhoid fever is not a typical diarrhoeal disease and the intestinal pathology is characterised by a macrophage, not a polymorph, infiltrate. Patients usually present with prolonged fever, headache, abdominal discomfort and general debility. Around 10% of these develop severe complicated disease and without specific treatment 5–30% of all patients may die. S. typhi is ingested in contaminated food or water, passes through the stomach and then invades the gut epithelium, possibly in the distal ileum. After penetration through the epithelium, Salmonella are ingested by macrophages and several pro-inflammatory reactions are initiated (e.g. production of cytokines, increased expression of adhesion molecules and upregulation of genes involved in apoptosis). There is further recruitment of macrophages and these may facilitate systemic spread of the bacteria as Salmonella-infected macrophages can survive for several hours. In this way, infected cells pass into the liver and spleen and can be found also in bone marrow and blood. Some find their way back to the intestine. Shedding of S. typhi in the faeces of an infected individual is an essential step in the transmission of typhoid fever.
Bacillary dysentery
Bacillary dysentery is an acute infection of the large intestine characterised by painful diarrhoea, often with blood and mucus in the stools. Shigella sonnei is the commonest cause; it produces relatively minor lesions and seldom causes ulceration. However, Sh. flexneri and Sh. dysenteriae can produce necrosis, sloughing and haemorrhage, giving rise to a picture closely resembling ulcerative colitis.
Campylobacter colitis
Since the early 1900s Campylobacter organisms have been known to cause dysentery and abortion in cattle and domestic animals, but recognition of their role in human disease is relatively recent. Contamination of milk and water supplies with C. jejuni and C. coli is now recognised as a frequent cause of severe gastroenteritis and colitis, particularly in debilitated and malnourished individuals. The histological changes seen in rectal biopsies are non-specific, and are similar to those seen in other forms of infective colitis.
Cholera
Cholera is a form of secretory diarrhoea resulting from infection with Vibrio cholerae. The cholera toxin binds to a specific receptor on epithelial cells which leads to increased adenylate cyclase activity; this in turn results in high cyclic AMP levels in the intestinal mucosa. The affected enterocytes secrete fluid and sodium ions, and the ensuing watery diarrhoea can be extreme, with overwhelming fluid loss and a rapidly fatal outcome. Because the effects are mediated by an exotoxin and there is no bacterial invasion of host tissues, the histological changes are remarkably slight; the mucosa shows mild oedema and goblet cell depletion.
Neonatal diarrhoea
In some of the diarrhoeas of neonates and infants, various strains of Escherichia coli can be isolated. Such infections are more common in bottle-fed infants, and epidemics may occur in children’s wards. Certain defined enteropathogenic serotypes are involved, and these differ from non-pathogenic types in their powers of adhesion to colonocytes and their ability to invade the mucosa. Diarrhoea may be severe, leading to dehydration and death. At autopsy, the small- and large-intestinal mucosa shows mucosal congestion and oedema with focal ulceration.
Staphylococcal enterocolitis
Enterocolitis due to staphylococcal infection is rare, but potentially fatal. The injudicious use of broad-spectrum antibiotics may alter the normal ecology of the intestinal bacterial flora so that the way is open for invasion by organisms that are either completely foreign to the bowel or normally present only in small numbers. The most dangerous of these is Staphylococcus aureus, which, when present in large numbers, can produce sufficient endotoxin to cause a severe enterocolitis. Staphylococcal enterocolitis is usually the result of cross-infection, and typically affects the hospital inpatient who has had contact with an antibiotic-resistant staphylococcus, in particular meticillin-resistant S. aureus (MRSA).
Patients present with sudden onset of severe diarrhoea, accompanied by shock and dehydration. The course can be relatively mild and respond to treatment, but with MRSA is often severe and carries a high mortality. There is widespread superficial ulceration predominantly affecting the small intestine. Microscopically there is acute inflammation of the mucosa with intense congestion and widespread necrosis. The surface of the mucosa is covered by an exudate containing numerous staphylococci.
Gonococcal proctitis
Gonococcal proctitis (inflammation of the rectum) is an acute exudative inflammatory condition which develops by genito-anal spread in females, and results from anal intercourse in males. The histological changes are non-specific, but the demonstration of numerous Gram-negative diplococci in the exudate leads to a presumptive diagnosis. As with other forms of infective colitis, definitive diagnosis depends on culture of the organisms.
Tuberculosis
Tuberculosis is almost entirely confined to the small intestine. In primary infection, an inconspicuous intestinal lesion is accompanied by gross enlargement of mesenteric nodes. This was the form of infection characteristic of bovine tuberculosis, a variety now virtually eliminated from the UK through the introduction of tubercle-free herds of cattle and the pasteurisation of milk.
Secondary tuberculous enteritis is a complication of extensive pulmonary tuberculosis which results from the swallowing of infected sputum. The typical alimentary lesion is ulceration of the ileum, the ulcer having formed by coalescence of caseous foci in the mucosa and submucosa. As the ulcers enlarge they follow the path of the lymphatics around the circumference of the intestine and eventually encircle the bowel. Healing is by fibrosis, and strictures may result from subsequent cicatrisation. The inflammatory exudate on the serosal aspect of the bowel may organise and form fibrous adhesions.
Ileo-caecal tuberculosis is a distinctive form of infection consisting of an ulcerative, granulomatous and fibrotic process occurring around the ileo-caecal valve, with variable extension into both ileum and caecum. The thickening and stenosis present a picture that is frequently indistinguishable from Crohn’s disease, although, in tuberculosis, distinct pale tubercles can be seen in the serosa. Patients recognised as having active intra-abdominal tuberculosis are treated by chemotherapy, but surgery may be required for the treatment of complications or for diagnosis. The major complications are intestinal obstruction by strictures and adhesions, perforation of ulcers (although this is uncommon because of the marked fibrous reaction), and malabsorption resulting from widespread mucosal involvement or blockage to lymphatic drainage.
Actinomycosis
Actinomycosis usually presents as a localised chronic inflammatory process most commonly related to the appendix or caecal area. The organism, Actinomyces israelii, is a normal commensal of the mouth, and when swallowed may resist acid digestion and infect the bowel. The infection is protracted and characterised by chronic suppuration and the formation of sinuses (openings on to the skin) and fistulae (abnormal connections with other hollow viscera). Histology reveals inflamed granulation tissue, and foci of suppuration containing the characteristic colonies of organisms visible to the naked eye as ‘sulphur granules’ in the watery pus.
Whipple’s disease
Whipple’s disease is a rare bacterial infection usually involving the small intestine. The causative organism has been identified as Tropheryma whippelii, and this infection, in combination with alterations in immune responsiveness, produces multisystem involvement with joint pains, weight loss, pigmentation, lymphadenopathy and malabsorption. The mucosa from affected individuals shows infiltration of the lamina propria by numerous granular macrophages containing abundant glycoprotein. On electron microscopy, the Whipple bacillus and granular material derived from the bacterial cell wall can be found in these macrophages. Patients usually respond to prolonged treatment with tetracyclines.
Clostridium difficile enteritis
Some patients taking a broad-spectrum antibiotic develop diarrhoea resulting from overgrowth of the intestinal commensal bacterium, C. difficile. In most patients this is not severe and responds to withdrawal of the antibiotic; this is generally referred to as antibiotic-associated colitis. However, in a small proportion (generally elderly or post-operative subjects) a fulminant enteritis with profuse diarrhoea and dehydration can lead to death in the more debilitated patients. On biopsy, there is superficial loss of epithelial cells and a volcano-like eruption of mucin, polymorphs and fibrin forming a pseudomembrane on the surface; this is pseudomembranous colitis. C. difficile is a Gram-positive bacillus that is non-invasive but produces a highly cytopathic toxin (toxin A) which is responsible for the enteritis.
Lymphogranuloma venereum
Proctitis due to lymphogranuloma venereum is principally a disease of females. This chlamydial infection begins in the genital tract and is thought to spread to the rectum via lymphatics. The deeper tissues are most heavily involved, and rectal stricture is the likely clinical problem. While non-specific chronic inflammation is usually pronounced, granulomas are a characteristic histological finding and these may show central necrosis when the disease is active.
VIRAL INFECTIONS
In many cases of presumed infective gastroenteritis or colitis no bacteria are isolated, and viral infection is probably responsible. Acute viral gastroenteritis is a major public health problem and as a cause of illness is second only to the common cold. However, the positive identification of viruses in contaminated food is difficult. The minute infecting dose required and the insensitivity of the available tests mean that laboratory identification is not always possible. The principal agents are rotaviruses, parvoviruses and ‘small round structured’ viruses including calicivirus. The most prevalent examples of the latter are the co-called ‘Norwalk-like’ viruses, a common cause of non-bacterial acute gastroenteritis resulting from contamination of food. In the small intestine these viruses produce degenerative changes in absorptive cells, minor shortening of villi and crypt hyperplasia, and inflammatory cell infiltration of the lamina propria.
Rare viral infections of the large bowel include cytomegalovirus colitis. Cytomegalovirus colitis has been described both as a primary infection and as a complication of ulcerative colitis. Infection is readily recognised by the presence of large intranuclear inclusions in cells within the mucosa.
FUNGAL INFECTIONS
Fungal infections of the alimentary tract are rare. Histoplasmosis may produce a striking picture of multiple inflammatory polyps in the small and large intestines, and on microscopy the intracellular Histoplasma capsulatum can be identified.
Mucor and Rhizopus are phycomycetes with non-septate hyphae that are widely distributed in nature. Although these organisms are usually non-pathogenic, gastrointestinal involvement in debilitated or immunosuppressed patients is becoming increasingly common. The oesophagus, stomach and colon are most frequently involved, and in addition to ulceration there is thrombosis of submucosal vessels with intravascular growth of the fungi. Despite this propensity for vascular infection, distant spread is surprisingly rare.
PARASITIC DISEASES
Giardiasis
Infection with the protozoan parasite Giardia lamblia produces a generally mild malabsorption state. It is a cause of ‘traveller’s diarrhoea’ and of diarrhoea in childhood, in people with IgA deficiency, and following gastric surgery. It has been suggested that the malabsorption state is due to heavy infestation blocking access of nutrients to the surface epithelium; however, this is unlikely, as the numbers of organisms are rarely sufficient.
Amoebiasis
Amoebiasis is a disease of the large intestine resulting from infection with the protozoan Entamoeba histolytica. It is worldwide in its distribution, though more prevalent in the tropics than in temperate climates. Vegetative forms are present in the large bowel in infected individuals; these are passed in the stools, encyst into a more resistant form, and may survive in food and fluid and be re-ingested later. The cysts pass unharmed through the stomach; on reaching the intestine the cyst wall is dissolved, liberating the active amoebae (Fig. 15.17). These secrete a cytolytic enzyme which enables them to pass through the intestinal epithelium and, in disrupting the mucosa, release red blood cells which they then ingest. Contamination of food and water is brought about by human carriers, infected rats, or flies. Carriers may either be individuals known to have suffered an attack in the past, or be apparently healthy people, some of whom may have symptomless lesions in the bowel. Direct spread via anal intercourse is another recognised route of infection.
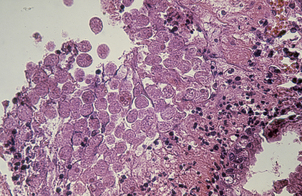
Fig. 15.17 Amoebiasis. High-power view of disintegrating colonic mucosa covered in an exudate in which there are large numbers of trophozoites. Some of these contain ingested red blood cells.
The disease can lead to discrete oval ulcers, which are characteristically ‘flask-shaped’ in section, or to a diffuse colitis. Blood-stream spread can result in liver abscesses, a potentially fatal complication.
Balantidiasis
Balantidiasis is a rare form of colitis caused by the ciliated protozoan Balantidium coli. It may be acute or chronic. Most cases are found in tropical or sub-tropical countries among debilitated, malnourished individuals. Gross and microscopic findings in the tissues are much like those in amoebiasis. The organism is readily detected by microscopy in both the lumen and the mucosa: it is so large as to dwarf the surrounding host cells.
Schistosomiasis
Infestation of the large intestine by Schistosoma occurs most commonly with S. mansoni and S. japonicum but can also be found with S. haematobium. Humans may become infected while wading or bathing in water contaminated with the second larval stage (cercaria) of the fluke. The cercariae penetrate the skin, enter venules, and are carried through the circulation to the portal veins in the liver, where they mature to form the adult flukes (Fig. 15.18). The adults migrate to either the submucosal veins of the gut or the venous plexus in the bladder, where they lay their eggs. The ova pass through the intestinal wall into the faeces or through the bladder wall into the urine. The cycle is completed in water contaminated with egg-containing urine or faeces. The eggs hatch out, liberating miracidia (first larval stage) which infect a snail, the intermediate host within which the second larval stage of cercariae develop, later to emerge in their free-swimming form.
The pathological changes in schistosomiasis are essentially the result of an inflammatory reaction to the eggs in the tissues of the intestinal wall. Lesions are commonest in the rectum and left colon and are then nearly always due to S. mansoni; S. haematobium may be responsible if the lesions are in the right side of the colon and the appendix.
Cryptosporidiosis
Cryptosporidiosis is caused by a coccidial organism of the genus Cryptosporidium. These are common parasites in a variety of reptiles, birds and mammals. They are a frequent cause of diarrhoea in children, and are increasingly encountered in AIDS sufferers. Infection usually results from drinking contaminated water. Severe acute colitis with surface exudation and ulceration may result. Cryptosporidia cannot be recognised in stool specimens, so a biopsy or mucosal scraping is needed to make the diagnosis.
DRUGS AND THE INTESTINE
Many drugs adversely affect the gastrointestinal tract but, given their widespread use, non-steroidal anti-inflammatory drugs (NSAIDs) are the most important. The effects of aspirin and NSAIDs on the stomach and their association with gastro-duodenal ulceration are well known. Less attention has been given to their effects on the intestinal tract. Examination by capsule endoscopy (a pill-sized camera that is swallowed and passes through the intestine naturally) reveals visible small bowel lesions in the majority of people taking NSAIDs. Longer-term use can lead to small bowel ulceration, strictures and peculiar membranous mucosal ‘diaphragms’ that partly occlude the lumen. NSAID-related ulceration occurs occasionally in the caecum and proximal colon, but more subtle effects are usual in the large intestine. These include various forms of microscopic colitis where chronic inflammation is found histologically but there are no endoscopic abnormalities. These patients usually present with watery diarrhoea. Interestingly, the COX-2-selective agents protect both the stomach and intestine from NSAID injury.
Other drugs that affect the intestine include agents causing diarrhoea either by interfering with fluid absorption (secretagogues) or by an osmotic effect, and laxative abuse can lead to diarrhoea. A few drugs, such as neomycin and colchicine, directly damage the mucosa and cause malabsorption, and colitis can be a side-effect of gold salts and penicillamine used in the treatment of rheumatoid arthritis. Drugs used for chemotherapy in cancer treatment can cause ulcers and perforations.
CHRONIC INFLAMMATORY DISORDERS
Although several chronic inflammatory conditions affect the intestinal tract, by convention the term ‘inflammatory bowel disease’ (IBD) is used to cover two diseases that exhibit overlapping clinical and pathological features: Crohn’s disease and ulcerative colitis. Crohn’s disease most commonly affects the small bowel, but, when it involves the colon, the differential diagnosis from ulcerative colitis can be a problem. The main distinguishing features are listed in Table 15.4.
Table 15.4 Chronic (idiopathic) inflammatory bowel disease: distinguishing features of Crohn’s disease and ulcerative colitis
| Feature | Crohn’s disease | Ulcerative colitis |
|---|---|---|
| Distribution | Commonly terminal ileum, but may occur anywhere from mouth to anus | Colon and rectum |
| Skip lesions | Common | Rare |
| Affected bowel | Thickened wall and narrowed lumen | Mucosal ulceration and dilated lumen |
| Extent of inflammation | Transmural | Mainly mucosal |
| Granulomas | Often present (c. 60%) | Absent |
| Fissures and fistulae | Common | Rare |
| Cancer risk | Slightly raised | Significantly raised |
Crohn’s disease
In 1932, Burrill Bernard Crohn and his colleagues established regional enteritis as a distinct entity. Previously, the condition had been confused with intestinal tuberculosis, then a common disease in Western countries. The chronic inflammation and ulceration in Crohn’s disease predominantly affect the terminal ileum, but all parts of the alimentary tract from the mouth to the anus may be involved and more than one site may be affected. ‘Satellite’ lesions can also occur in skin remote from the peri-anal area. However, involvement outside the small and large intestine is uncommon. About two-thirds of patients have only small-intestinal involvement, about one-sixth only large-intestinal involvement, and in one-sixth of patients both small and large bowel are affected.
Crohn’s disease usually presents with either small-intestinal obstruction or abdominal pain which may mimic acute appendicitis; other presentations can relate to its complications (see below). The course of the disease is chronic, with exacerbations and remissions not always linked to therapy. Onset is usually in early adult life, with about half of all cases beginning between the ages of 20 and 30 years and 90% between 10 and 40 years. Slightly more males than females are affected.
Morphology
Intestinal involvement by Crohn’s disease is frequently segmental; that is, lengths of diseased bowel are separated by apparently normal tissue. Such separated segments of disease are referred to as ‘skip lesions’.
The earliest evidence of involvement visible with the naked eye is the presence of small discrete shallow ulcers with a haemorrhagic rim. These ulcers have been likened to the common aphthous ulcers of the mouth and are thus often described as ‘aphthoid’; however, there is no aetiological link between the two conditions. Later, the more characteristic longitudinal ulcers develop which progress into deep fissures (Fig. 15.19). The process comes to involve the full thickness of the wall and subsequent fibrosis leads to considerable narrowing in the diseased segments (Fig. 15.20). This produces a characteristic radiological sign where only a trickle of contrast medium passes through the affected segment (the ‘string sign’). Where longitudinal fissures cross oedematous transverse mucosal folds, a ‘cobblestone’ appearance results. The mesenteric lymph nodes are enlarged by reactive hyperplasia and may also contain granulomas.
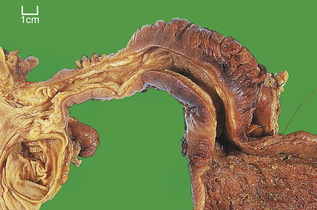
Fig. 15.20 Crohn’s disease. The terminal ileum is severely narrowed due to thickening of the bowel wall by the chronic inflammatory process. On the right, the lumen is passively dilated in response to the presence of the obstructive lesion.
Microscopy reflects the gross appearances. Inflammatory involvement is discontinuous: it is focal or patchy. Collections of lymphocytes and plasma cells are found, mainly in the mucosa and submucosa, but usually affecting all layers (transmural inflammation). The classical microscopic feature of Crohn’s disease is the presence of granulomas. These consist of epithelioid macrophages and giant cells surrounded by a cuff of lymphocytes. The giant cells are usually of the Langhans’ type, but may resemble foreign body giant cells. The granulomas are distinguished from those of tuberculosis by the absence of central caseous necrosis. While they are virtually diagnostic of the condition, granulomas are found in only 60% of cases of Crohn’s disease; in their absence the diagnosis must be based on a summation of the less specific histological changes. In addition to the aggregated transmural pattern of inflammation, these changes include the finding of vertical fissure ulcers and marked submucosal oedema, lymphangiectasia, fibrosis and neuromatoid hyperplasia (enlargement and proliferation of submucosal nerves).
Complications
The complications of Crohn’s disease are summarised in Table 15.5. Widespread involvement of the small intestine can lead to a malabsorption syndrome, but the commonest cause of malabsorption in Crohn’s disease is iatrogenic. Repeated resections of small intestine can lead to a ‘short bowel syndrome’ in which adequate nutrition can be maintained only by intravenous or intraperitoneal alimentation. Fistula formation is a frequent complication; deep penetration by ulcers produces fistulae between adherent loops of bowel and, particularly after surgical intervention, leads to entero-cutaneous fistulae.
Table 15.5 Complications of Crohn’s disease
| Complication | Comment/example |
|---|---|
| Malabsorption syndrome | Often iatrogenic (‘short bowelsyndrome’) |
| Fistula formation | Causes malabsorption when loops of bowel are bypassed |
| Anal lesions | Skin tags, fissures, fistulae |
| Acute complications | Perforation (haemorrhage, toxic dilatation—rare) |
| Malignancy | Increased risk—adenocarcinoma |
| Systemic amyloidosis | Rare |
Approximately 60% of patients have anal lesions. These include simple skin ‘tags’, fissures, and fistulae into the anal canal or peri-anal skin. Acute complications such as perforation, haemorrhage and toxic dilatation do occur but are seen much less frequently seen in Crohn’s disease than in ulcerative colitis. In the long term, there is an increased risk of malignancy, particularly in the small intestine, but the overall risk is less than in patients with ulcerative colitis because more people with Crohn’s disease have the affected areas resected. Interestingly, there are reports of an increase in malignancy outside the digestive tract in patients with longstanding Crohn’s disease. Systemic amyloidosis is a rare, long-term complication that results from excessive production of serum amyloid A protein (Ch. 7).
Ulcerative colitis
In temperate climates ulcerative colitis is the commonest cause of diarrhoea associated with the passage of blood, mucus and pus. It is a non-specific inflammatory disorder of the large intestine, usually commencing in the rectum and extending proximally to a varying extent. Unlike Crohn’s disease, ulcerative colitis is confined to the large intestine. Involvement of the terminal ileum in a so-called ‘backwash ileitis’ is occasionally seen, but this is thought to represent chronic inflammation provoked by incompetence of the ileo-caecal valve, rather than an actual part of the disease.
Morphology
Ulcerative colitis is continuous in its distribution. Thus the disease, which is typically maximal in the rectum, extends proximally and continuously to involve the colon. Some cases are confined to the rectum (proctitis), others to the rectum and sigmoid (distal colitis), while others may exhibit a total colitis extending into the caecum. The disease does not involve the mucosa of the anal transitional zone or the anal canal, but a small proportion of patients do have anal tags and fissures.
The ulcers are irregular in outline and orientation and become confluent (Fig. 15.21); they extend horizontally to undermine adjacent, less involved, mucosa which remains as discrete islands. Usually the ulceration remains superficial (see Fig. 15.19), involving mucosa and submucosa, but in severe cases there is extension into the main muscle coats and perforation is likely. There is intense hyperaemia of the intact mucosa and haemorrhage from the ulcers.
Microscopically, there is diffuse infiltration of the mucosa by mixed acute and chronic inflammatory cells. Polymorphs are seen in the interstitium, but are particularly evident as aggregates within distended crypts (crypt abscesses). There are widespread degenerative changes in surface and crypt lining epithelium, with marked depletion of their mucin content. Crypts undergo destruction during the acute phase, and when regeneration occurs they are frequently distorted by branching or dilatation. This disturbance of the crypt pattern is a useful diagnostic pointer in quiescent cases, when the inflammatory features may have totally subsided. Thus, in longstanding disease, rectal biopsy will reveal crypt atrophy and distortion, and there may be metaplastic features such as the acquisition of Paneth cells. Ulcerative colitis is a recognised premalignant condition, and a few cases will reveal epithelial dysplasia.
Complications
The complications of ulcerative colitis are summarised in Table 15.6.
Table 15.6 Complications of ulcerative colitis
| Complication | Comment/example |
|---|---|
| Blood loss | May be acute (haemorrhage) or chronic, leading to anaemia |
| Electrolyte disturbances | Due to severe diarrhoea in acute phase |
| Toxic dilatation | May develop insidiously |
| Colorectal cancer | Overall incidence 2% |
| Skin involvement | Pigmentation, erythema nodosum, pyoderma gangrenosum |
| Liver involvement | Fatty change, chronic pericholangitis, sclerosing cholangitis, cirrhosis, hepatitis |
| Eye involvement | Iritis, uveitis, episcleritis |
| Joint involvement | Ankylosing spondylitis, arthritis |
Local complications
Haemorrhage is occasionally massive and life-threatening, but more often occurs as chronic blood loss leading to iron-deficiency anaemia. In the acute phase, severe diarrhoea with a markedly increased loss of water and mucus can lead to serious electrolyte disturbances. A further hazard of the acute phase is so-called toxic dilatation. Toxic dilatation occurs when ulceration affects large areas of the muscle coats and their viability and contractile strength are impaired. The resultant adynamic segment—commonly the transverse colon—becomes progressively distended, and the consequent thinning of the wall predisposes to perforation. Since there are few adhesions to localise its spread, perforation into the peritoneal cavity results in generalised faecal peritonitis and a fatal outcome is likely. Frequent radiographs should be taken in the seriously ill patient, as toxic dilatation may develop insidiously.
Systemic complications
Patients with ulcerative colitis are at risk of developing systemic problems (Table 15.6). These include:
Malignancy
The overall incidence of colorectal cancer in ulcerative colitis is low, around 2%, but this rises to about 10% in patients who have had the disease for 25 years. The increased risk over that for the general population warrants colonoscopic surveillance of longstanding cases. The clinical factors apparently associated with a higher cancer risk are:
In practice, patients with extensive colitis of longer than 8–10 years’ duration are usually admitted into surveillance programmes and undergo regular (usually annual) colonoscopy and multiple biopsy. If high-grade (severe) dysplasia is seen, then the development of carcinoma is considered imminent and total resection is warranted.
Pathogenesis of inflammatory bowel disease
Epidemiological factors
The geographical incidence of IBD (Crohn’s disease and ulcerative colitis) varies considerably. These diseases are much more common in northern Europe and the USA than in countries of southern Europe, Africa, South America and Asia, although increasing urbanisation and prosperity is leading to a higher incidence in parts of southern Europe and Japan. Even within Europe and the USA the incidence of Crohn’s disease varies widely from around 4 up to 65 affected persons per 100000 population. In northern Europe and the USA the incidence of ulcerative colitis varies between 12 and 140 per 100000 population, but lower rates occur in underdeveloped countries with warmer climates. In low-incidence countries there may be diagnostic confusion with chronic infective colitis. The epidemiological data combined with twin studies and patterns within families indicate that IBD combines both genetic and environmental influences in its pathogenesis.
Environmental factors
The most clearly determined environmental influence on the susceptibility to IBD is that of cigarette smoking. Smokers have an increased risk of developing Crohn’s disease, whereas they are apparently protected against ulcerative colitis. While this is an intriguing finding, it gives little clue to the aetiology of IBD. More interest has focused on infective agents.
Ever since a Scottish surgeon and farmer, Dr T Kennedy Dalziel, recognised the similarity between a mycobacterial infection—Johne’s disease—affecting his pedigree cattle and (presumed) Crohn’s disease affecting some of his patients, there has been varying interest in the role of Mycobacterium paratuberculosis. These mycobacteria can be frequently identified in milk samples destined for human consumption so infection is entirely possible, but tests for mycobacterial DNA by the polymerase chain reaction in affected tissues have produced conflicting results. Likewise, trials of anti-mycobacterial treatment in Crohn’s disease have been equivocal. The case for M. paratuberculosis as an aetiological agent in Crohn’s disease remains unproven. Even more controversial is the putative role of measles virus and the MMR (measles, mumps and rubella) vaccine in IBD; the majority view disputes this possibility.
The most compelling evidence for the involvement of microbial agents implicates the gut flora. Diversion of the faecal stream brings about improvement in segments affected by Crohn’s disease; likewise antibiotic and probiotic treatment can lead to remissions in IBD, and animal models of colitis require the presence of gut flora for the disease to become manifest. Of itself, however, the composition of the flora cannot be the critical factor. Current interest is centred on the underlying genetic changes that determine how an individual reacts to the ingress of bacteria (or bacterial antigens) into the intestinal mucosa.
Genetic factors
The first breakthrough came in 1996: a gene conferring susceptibility to Crohn’s disease (designated IBD1) was identified on chromosome 16 in families with multiple affected members. Five years later, the IBD1 gene was identified as nucleotide oligomerisation domain (NOD)-2, and more recent studies have confirmed that NOD2 mutations are associated with susceptibility to Crohn’s disease but may protect against ulcerative colitis.
NOD2 is expressed in dendritic cells, macrophages and intestinal epithelial cells, but the greatest concentration is found in Paneth cells, where NOD2 is also an inducer of alpha-defensins. Thus, a mutation resulting in defective NOD2 activity could lead to an increase in bacterial density or a qualitative change in the gut flora consequent upon reduced alpha-defensin production. Furthermore, the NOD2 gene has a leucine-rich domain at its C-terminal that is important for binding a dipeptide derived from the breakdown of bacterial peptidoglycans by mucosal dendritic cells. This dipeptide binding activates NOD2 and initiates a mechanism leading to inhibition of nuclear factor kappa B, a key transcription factor in pro-inflammatory responses. In normal dendritic cells, therefore, NOD2 activation down-modulates cytokine production resulting from exposure to bacterial components.
More recently, studies in patients with Crohn’s disease have revealed further relevant loci related to genes that control IL-23 production and genes involved in autophagy that could influence antigen delivery to immune cells, and in the generation of free radicals essential for effective bacterial killing.
Immunopathology
Inappropriate and persistent T-cell activation may be the basis of both ulcerative colitis and Crohn’s disease. Under normal circumstances the mucosal immune system is tolerant of luminal bacterial antigens, and this tolerance is dependent upon the relationship between intestinal epithelium and suppressor T-cells. Classically, antigen handling is attributed to M-cells over the mucosal lymphoid follicles, but direct luminal antigen sampling by dendritic cell processes extending through intact surface epithelium is another possibility. Increased mucosal permeability to antigens in IBD is more likely to be a consequence of the inflammatory response rather than the initiating event, and the cytokine TNF-alpha has been implicated through its action on epithelial tight junctions. In ulcerative colitis, increased permeability and ingress of bacterial antigens may lead to immune complex phenomena and some of the extra-intestinal complications.
In IBD, one or more genetically determined defects result in a mucosal immune system that overreacts to normal constituents of the gut flora. Despite this common basis, Crohn’s disease and ulcerative colitis are immunologically distinct entities. Crohn’s disease is associated with a Th1 cell-mediated response characterised by elevated production of interferon-gamma and TNF-alpha. The Th1 response is governed by the interleukins (IL)-12 and, to a lesser degree, by IL-23. The prominence of IL-12 in the induction of a Th1 response has an important bearing on the role of NOD2 gene mutations in Crohn’s disease. IL-12 production is a consequence of the activation of nuclear factor kappa B that follows stimulation of so-called Toll-like receptors on the surface of dendritic cells by bacterial peptidoglycans. We have seen (above) that NOD2 activation is necessary for the negative regulation of nuclear factor kappa B. Thus, enhanced IL-12 production and Th1 cell activation in Crohn’s disease may be due to a failure of mutated NOD2 to downregulate Toll-like receptor signalling.
Although helper T-cells isolated from the colonic mucosa of ulcerative colitis patients produce interferon-gamma, they produce much more IL-5 than patients with Crohn’s disease, suggesting that a Th2 response is of more importance in ulcerative colitis. Other cytokines characteristic of the Th2 response are increased in ulcerative colitis, including IL-13. Indeed, IL-13 produced by natural killer T-cells is thought to be central to the immunopathology of ulcerative colitis; IL-13 impairs intestinal barrier function by affecting tight junctions, increasing epithelial apoptosis and slowing epithelial restitution.
Despite these two distinctive patterns of response, it is acknowledged that the Th1 and Th2 routes converge into a common pathway of tissue injury. Mucosal damage in IBD is now accepted to occur as a result of sustained interactions between immune and non-immune cells. For example, in both diseases, IL-1-beta and TNF-alpha enhance the expression of adhesion molecules on vascular endothelium and increase chemokine production, thereby attracting blood-borne inflammatory cells into the tissues. These cytokines also stimulate mucosal myofibroblasts to synthesise matrix-degrading enzymes that contribute to mucosal damage. Pro-inflammatory responses in myofibroblasts are also induced by IL-22, a cytokine produced in excess by helper T-cells in active IBD. The similarity of the consequent events in Crohn’s disease and ulcerative colitis explains the response of both diseases to certain therapies—e.g. corticosteroids and immunosuppressants—but more success is likely to be achieved by agents aimed at specific immunological targets. Already, blockade of IL-12 has been shown to be effective in Crohn’s disease, while strategies to block IL-13 in ulcerative colitis are a challenge for the future.
VASCULAR DISORDERS
Ischaemic injury to the intestine occurs either as a consequence of obstruction to the mesenteric arterial supply (occlusive ischaemia) or in circumstances where, despite patency of the vessels, the blood supply falls to a level at which the nutrition of mucosa cannot be maintained (non-occlusive ischaemia). Thus, in the territory supplied by the superior mesenteric artery, occlusive ischaemia results either from embolism originating from atrial or ventricular mural thrombosis (about 50% of cases) or from thrombosis, usually on the basis of atherosclerosis (in about 25%). Non-occlusive ischaemia is a consequence of systemic hypotension, vasoconstriction, viscosity disturbances, arterial narrowing and certain drugs, such as digitalis and cocaine (in about 20%).
An uncommon cause of acute intestinal ischaemia (less than 5%) is mesenteric venous thrombosis which, by preventing outflow of blood, causes infarction by intense congestion. Predisposing factors include hypercoagulability states, portal hypertension, intra-abdominal inflammation and previous surgery or abdominal trauma.
Pathogenesis
Total vascular occlusion results in segmental anoxic or hypoxic injury, the extent depending on the adequacy of the collateral supply; cell death appears to ensue from a lethal ingress of calcium ions through the damaged plasma membrane. However, much of the mucosal injury in non-occlusive ischaemia develops after the period of hypoperfusion, i.e. when normal perfusion and oxygenation have been restored. This is an example of a ‘reperfusion injury’ of the kind seen after myocardial and cerebral ischaemia and following iatrogenic ischaemia, as occurs during aortic aneurysm repair or in organ transplantation. Reperfusion injuries are mediated by free radical formation (Ch. 6), TNF-alpha and arachidonic acid metabolites; these are responsible for the membrane injuries that bring about mucosal disintegration in the reperfusion phase.
Acute ischaemia
Acute mesenteric ischaemia is a life-threatening emergency with mortality rates between 60% and 100%. Sudden ischaemia results in varying degrees of infarction of the bowel wall. Such infarcts can be classified, according to the depth of involvement, as mucosal, mural or transmural (Fig. 15.22).
Mucosal infarction
Mucosal infarction is usually considered transient or reversible because the lesion can be followed by complete regeneration. However, mucosal damage leads to release of chemokines that cause an influx of polymorphs and their adhesion to vascular endothelium coupled with platelet aggregation further compromise the microcirculation. Furthermore, the ‘leaky’ mucosa allows potentially toxic agents to enter the circulation; this can bring about further systemic cardiovascular deterioration (‘shock’) and gradual progression of the intestinal lesion to transmural infarction.
Mural infarction
Mural infarction reaches into the submucosa or into, but not through, the muscularis propria. The mucosa is variably ulcerated and, where intact, is haemorrhagic and elevated by marked submucosal oedema. As with mucosal infarction, this stage can progress to full-thickness infarction. The deeper extent of necrosis with involvement of connective tissues necessitates healing by granulation tissue formation and a more prolonged process of repair. If the patient recovers, this is likely to lead to fibrous stricture formation.
Transmural infarction
Transmural infarction of the intestine is the most common consequence of acute ischaemia. The infarct extends through the muscularis propria to the serosa, and is synonymous with gangrene (death of tissue together with bacterial infection). The bowel becomes flaccid and dilates, and the serosal aspect is deeply congested and coated in a thin layer of fibrin. The wall becomes friable and liable to perforation. The infarct is usually widespread, affecting several loops of small intestine, but can be segmental. Segmental infarction results either from occlusion of distal mesenteric vessels, or by mechanical obstruction of the supply to a loop of intestine. This type of involvement is amenable to surgical treatment, but many patients already have peritonitis, endotoxaemia and severe circulatory problems at the time of diagnosis, so operative results remain poor. Massive infarction, most commonly seen in the small intestine following complete occlusion of the superior mesenteric artery, has a hopeless prognosis.
Chronic ischaemia
Chronic ischaemia leads to two main problems:
Strictures are encountered most often in the large intestine, particularly in the ‘watershed’ area around the splenic flexure of the colon. The patients generally present with the consequences of large bowel obstruction. Chronic mesenteric insufficiency is used to describe a condition in which there is insufficient blood flow to the small intestine to satisfy the demands of increased motility, secretion and absorption that develop after meals. The insufficiency is usually manifest as pain (so-called ‘mesenteric angina’), but patients may also have diarrhoea and malabsorption.
Necrotising enterocolitis
Necrotising enterocolitis (NEC) is an uncommon condition arising from a combination of ischaemia and infection. NEC is the most serious acquired gastrointestinal disease in the newborn, accounting for 1–3% of neonatal intensive care unit admissions. It carries a high risk of death with mortality rates between 20% and 50%. The disease manifests as abdominal distension and bloody stools with respiratory and circulatory disturbances. No single bacterial pathogen has been consistently identified, but organisms are frequently isolated and include E. coli, Klebsiella, C. difficile and Clostridium perfrigens. Prematurity is the only consistent risk factor; an immature mucosal barrier coupled with an impaired humoral (secretory IgA) and cellular immune response to bacteria are the suggested mechanisms. A substantially higher incidence of NEC has been found in formula-fed infants compared with exclusively breast-fed babies, suggesting that the former lack a degree of immuno-protection.
The disease can also (rarely) affect adults where it is caused by infection with Clostridium perfringens Type C, which produces a powerful beta-toxin. A peculiar form of NEC was that found among natives of Papua New Guinea after ceremonial feasting on huge quantities of sweet potato and inadequately cooked pork, including pig intestines. This condition was referred to as ‘pigbel’, pidgin English for abdominal pain after eating pig!
Adults with NEC present with severe abdominal pain and diarrhoea. Paralytic ileus develops and progresses to intestinal infarction, sepsis and shock. The appearances are typically those of gas gangrene, with either segmental or total involvement of the small and large intestines by coagulative necrosis and intramural gas bubble formation.
Vascular anomalies
Vascular anomalies in the gut are uncommon but enter into the differential diagnosis of gastrointestinal haemorrhage. Their classification is confused; some are congenital malformations that form part of recognised syndromes, while other, possibly identical, lesions are claimed to be acquired. Congenital types include arteriovenous malformations and telangiectasias; acquired forms are usually termed angiodysplasias. Angiodysplasia of the colon is an occasional cause of blood loss from the large bowel. This condition is more common in the elderly and can be diagnosed by mesenteric angiography.
DISORDERS RESULTING FROM ABNORMAL GUT MOTILITY
Diverticular disease
Diverticula are herniations of mucosa into the intestinal wall. The herniations are of the pulsion type and form at sites of potential weakness, notably where lymphoid aggregates breach the muscularis mucosae. They extend through the muscularis propria at the point of entry or exit of blood vessels and bulge into the subserosa.
Diverticula can be found anywhere in the intestinal tract, but the colon, and particularly the sigmoid, is by far the commonest site (Fig. 15.23). Most diverticula occur between the mesenteric and anti-mesenteric longitudinal muscle bands—the taenia coli. The affected segment of colon shows thickening of the muscularis propria, and prominence of the mucosal folds so that they almost occlude the lumen and have a ‘concertina-like’ appearance. The disease is generally acknowledged to result from a deficiency of fibre in the diet. Sigmoid motility is peculiarly sensitive to the bulk of the colonic contents and when this is low, as with a low-fibre diet, abnormally high intra-luminal pressures are generated which push the mucosa into the wall.
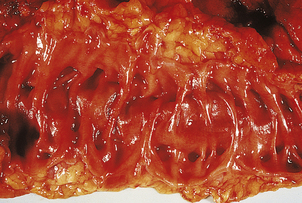
Fig. 15.23 Diverticulosis of the sigmoid colon. The mucosal surface is ridged due to hypertrophy of the underlying muscle. The openings of the diverticula can be seen between the mucosal ridges.
Complications
Diverticular disease presents as abdominal pain and altered bowel habit, but it is also prone to develop some serious complications, the most common being diverticulitis (Fig. 15.24) The faecal contents can lead to abrasion of the herniated mucosa, or a microscopic perforation in the apex of a diverticulum can occur, which allows infection by faecal organisms and the development of a suppurative diverticulitis. This in turn can cause a peri-colic abscess and a fistula may form into the bladder, vagina or small intestine; more seriously, a peri-diverticular abscess may perforate and produce a generalised faecal peritonitis.
Diverticula can be the source of haemorrhage from the colon. This usually arises from areas of granulation tissue in an inflamed diverticulum, but the precise source is sometimes difficult to identify.
Intussusception
An intussusception is an invagination of one segment of bowel into another, thus causing intestinal obstruction. A lesion in the wall of the bowel disturbs normal peristaltic contractions, forcing the lesion and a segment of proximal bowel into a distal segment. Several lesions can act as the apex of an intussusception, including polyps, ingested foreign bodies, a Meckel’s diverticulum, an area of intramural haemorrhage (e.g. in Henoch–Schönlein purpura; Ch. 23) and lymphoid hyperplasia. Such hyperplasia close to the ileo-caecal valve is the cause of the ileo-colic intussusception, the most common form of this disorder.
Volvulus and strangulation
Intestinal obstruction can result from a twist in the bowel that occludes its lumen (volvulus) or when a segment of bowel becomes trapped in a defect in either the posterior peritoneum or mesentery (internal herniation), or herniates into an inguinal or para-umbilical peritoneal sac. The neck of the sac may then constrict the bowel and compromise its blood supply (strangulation). Volvulus occurs around a ‘fulcrum’ such as a Meckel’s diverticulum or a congenital band of fibrous tissue, or around an abnormally long mesentery. About two-thirds of cases affect the small intestine; most of the remaining one-third affect the sigmoid colon.
TUMOURS OF THE INTESTINE
Paradoxically, the small intestine, with its vast surface area and a cell turnover rate higher than that of any other tissue in the body, is an uncommon site for primary neoplasms. Adenomas and adenocarcinomas are distinctly rare in the small intestine, yet the much shorter large bowel is a very common site of neoplasia. The low incidence of carcinoma means that other neoplasms, such as neuroendocrine cell tumours and lymphomas, assume more importance in the small intestine where they are relatively more common than in the large bowel.
Polyps
A polyp is simply a protuberant growth and there is thus a wide variety of histological types. These can be broadly divided into epithelial and mesenchymal polyps (of which the latter are distinctly uncommon), and into benign and malignant categories (Table 15.7). Even epithelial polyps are rare in the small intestine and some, such as metaplastic polyps, are confined to the large bowel. Thus, the following account is confined to large-intestinal polyps.
Table 15.7 Polyps of the large intestine
| Type of polyp | Benign | Malignant |
|---|---|---|
| Epithelial |
Mesenchymal
Benign epithelial polyps
Benign epithelial polyps fall into four categories: adenomas, and inflammatory, hamartomatous and metaplastic polyps.
Adenomas
Adenomas are very common; there is an increased incidence with age so that at 60 years they are found in about 20% of the population. There are two main histological types—tubular (75%) and villous (10%); the remaining 15% are intermediate in pattern and are designated tubulo-villous.
Tubular adenomas are generally small (usually less than 10mm in diameter), and macroscopically resemble a raspberry. Most have a stalk (pedunculated) and a minority have a broad base (sessile). Microscopically, they consist of numerous cross-sectioned crypt profiles lined by mucus-secreting epithelium showing varying degrees of dysplasia.
Villous adenomas are usually sessile; they are often over 20mm in diameter and some extend over a wide area as a thick, carpet-like growth. Microscopically, they consist of elongated villi in a papillary growth pattern; the villi are again lined by columnar epithelium showing dysplasia. Large adenomas may secrete copious electrolyte-rich mucus, leading in rare instances to hypokalaemia and acute renal failure, but their real importance lies in their propensity for malignant change.
However, not all adenomas are polypoid. Non-polypoid (‘flat’) adenomas are defined as adenomas whose height is less than twice the thickness of the adjacent normal mucosa, and are increasingly recognised as an alternative source of colorectal carcinomas.
Inflammatory polyps
These usually arise in the context of inflammatory bowel disease, and represent excessive reparative and regenerative tissue formed in the aftermath of mucosal ulceration. In most cases there is a preponderance of granulation or mature fibrovascular tissue, so their categorisation as epithelial is somewhat debatable.
Hamartomatous polyps
These rare polyps may be solitary, like the majority of so-called ‘juvenile’ polyps, or be multiple and occur throughout the gastrointestinal tract, as in Peutz–Jeghers syndrome. These multiple polyp syndromes carry a substantial risk of malignancy.
Metaplastic (or hyperplastic) polyps
These polyps are of unknown histogenesis, but the surface cells are hypermature compared to normal epithelium. They are common lesions, being found with increasing age, and are most frequently situated in the rectum. Microscopically they are sessile with elongated crypts, but the majority show no dysplasia. Their characteristic feature is the ‘serrated’ appearance of the cells lining the upper crypt and at the surface. In contrast to adenomas, these polyps have little or no malignant potential.
There is a category of polyp, however, that has appearances intermediate between metaplastic polyp and adenoma and is termed a ‘serrated adenoma’. Although of low malignant potential, such polyps exhibit a distinct pathway of genetic changes leading to adenocarcinoma.
Malignant epithelial polyps
Examples of malignant epithelial polyps are polypoid carcinomas and ‘carcinoid’ polyps. Some adenocarcinomas develop as protuberant growths and appear endoscopically as polyps, hence the term ‘polypoid carcinoma’. The vast majority of adenocarcinomas, however, arise within pre-existing adenomas (polypoid or ‘flat’) and these constitute the bulk of ‘malignant polyps’. A very small minority of polyps are neoplasms derived from neuroendocrine cells (see p. 396); such carcinoid polyps have a low malignant potential and only give rise to metastases late in their course. Thus, complete local removal is usually curative.
The adenoma–carcinoma sequence
Adenomas are probably the precursors of most, if not all, colorectal cancers. Evidence in favour of a link comes from a number of sources, but the strongest is the hereditary condition of familial adenomatous polyposis (FAP). FAP is a rare autosomal disease carried by either parent and transmitted as a Mendelian dominant. Both sexes are equally affected. Adenomas, mainly in the large intestine (Fig. 15.25) but also in the small bowel, develop during the second and third decades and subsequently undergo malignant change, with an almost inevitable progression to adenocarcinoma by the age of 35 years. The gene responsible for FAP is on the long arm of chromosome 5; interestingly, a somatic mutation has been identified on chromosome 5 in cases of sporadic (non-inherited) colorectal cancer (see below).

Fig. 15.25 Familial adenomatous polyposis. The colonic mucosa is studded with numerous adenomatous polyps. These are premalignant.
Histologically, the finding of residual adenomatous tissue in some cancers, and the observation of early invasive malignancy developing in adenomas, is further evidence supporting the adenoma–carcinoma sequence. Examination of adenomas showing early malignancy has demonstrated an association with increasing size, villous growth pattern and more severe dysplasia, although non-polypoid (flat) adenomas frequently show high-grade dysplasia in spite of their small size. Adenomas and carcinomas are frequently found together in a resected segment of bowel. Such patients have an increased risk of developing a second cancer in the remaining large intestine, compared with patients having carcinoma alone.
Recently, genome-wide scans have revealed a colorectal cancer susceptibility locus on chromosome 8q24 that is common to some sporadic adenomas, providing genetic evidence of the link and indicating that the locus is involved in tumour initiation rather than progression.
Molecular pathology of the adenoma–carcinoma sequence
In no other tumour system are the genetic events underlying the development of carcinoma as clearly understood as they are in colorectal cancer. In recent years, however, it has become increasingly clear that acquired epigenetic changes are of major importance. The genetic/epigenetic abnormalities are:
Activation of oncogenes
The oncogenes most frequently altered in colorectal cancer are c-Ki-ras and c-myc. Point mutations in Ki-ras mean that the protein can no longer hydrolyse bound GTP to GDP. Persistence of GTP-ras, the active form of the protein, results in continual signalling of cell division. Overexpression of c-myc is a feature of most colorectal cancers; c-myc encodes a nuclear phosphoprotein that is required for DNA synthesis, and increased expression may well be followed by increased cellular proliferation. While mutations are a proven cause of activation of oncogenes, they are not necessarily the main mechanism. Epigenetic change in DNA methylation is likely to be the key event; in particular, age-related DNA hypomethylation may contribute to oncogene activation.
Loss or mutations of tumour suppressor genes
Tumour suppressor genes appear to be very important in colorectal carcinoma. FAP results from point mutations in a tumour suppressor gene, the APC (adenomatous polyposis coli) gene on chromosome 5q, and subsequent deletion of the accompanying normal allele results in the loss of tumour suppressor function that leads to colorectal cancer. Mutations and deletions of the APC gene, and of other tumour suppressor genes, have also been identified in sporadic (i.e. non-hereditary) colorectal cancer. Other genes implicated are MCC (mutated in colorectal cancer) whose gene product is involved in cell cycle control, DCC (deleted in colorectal cancer) involved in the control of apoptosis, and TP53. The TP53 gene product (p53) is a nuclear protein that can hold the cell cycle at the G1/S checkpoint and allow time for successful DNA repair, or initiate apoptosis if the DNA damage is irreparable. Finally, deletions affecting the nm23 gene may facilitate metastasis.
Just as with oncogene activation, mutations are not the only mechanism leading to loss of function of tumour suppressor genes. Hypermethylation of CpG dinucleotides (cytosine and guanine linked by a phosphodiester bond) has been incriminated. CpG islands—DNA segments with increased concentration of this dinucleotide—are found at transcriptional start sites and close to promoter regions, and hypermethylation has been linked to the transcriptional silencing of tumour suppressor genes. Interestingly, CpG island hypermethylation is found in about a third of metaplastic polyps; it is likely that these are the polyps with malignant potential.
Defects in DNA repair
Mutation or loss of function of the TP53 gene is not the only way in which DNA repair can be compromised. Highly conserved genes have been discovered that recognise mismatched nucleotides in complementary DNA strands and orchestrate the enzymes that effect repairs. Defects in these genes and the ensuing replication errors are manifest as microsatellite instability. Alterations in two mismatch repair genes, hMLH1 and hMSH2, have been identified in most kindreds with hereditary non-polyposis colorectal cancer (HNPCC, Lynch syndrome). Similar alterations in these and other ‘housekeeper’ genes are found in sporadic cancers, with around 15% of cases showing high microsatellite instability.
The sequence of these genetic events in causing colorectal cancer is not as critical as the overall accumulation of changes, but the different prevalences of mutations and deletions between adenomas and invasive carcinoma do suggest that there is a preferred order (Fig. 15.26).
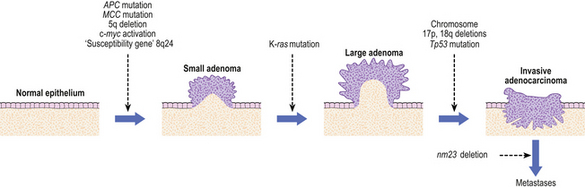
Fig. 15.26 Molecular genetics of adenoma–carcinoma sequence. Analysis of adenomas, small and large, and of invasive carcinomas and their metastases reveals a cascade of gene mutations, deletions and activations corresponding to the altered behaviour of the tumour cells. However, epigenetic changes are also of critical importance.
Colorectal cancer
Cancer of the colon and rectum is one of the commonest forms of malignancy in developed countries. For example, it accounts for about 10% of all cancer registrations in the UK, where the death rate is second only to that of lung cancer, with gastric cancer a close third. The incidence appears to be rising.
Aetiology
Apart from the role played by inherited genetic factors, and a few cases developing in the unstable mucosa of ulcerative colitis, the most important factor in the aetiology of colorectal cancer appears to be environmental. Epidemiological evidence indicates that this is dietary. Diet affects the bacterial flora of the large bowel, the bowel transit time, and the amount of cellulose, amino acids and bile acids in the bowel contents. Some types of bacteria, the nuclear dehydrogenating clostridia (NDC), can act on bile acids to produce carcinogens. Similarly, bacterial transformation of amino acids may result in carcinogen (or co-carcinogen) production. On the other hand, a high content of fermentable cellulose leads to high levels of volatile fatty acids which appear to be ‘protective’ in that they provide nutrition and aid maturation of the epithelial cells. Thus, the type of diet that has been linked to colorectal cancer is a high-fat, high-protein, low-fibre diet. High fat leads to an increase in bile salt production and higher load of faecal bile acids to react with NDC; high protein favours the transformation of amino acids by bacteria; low fibre reduces volatile fatty acids and prolongs intestinal transit so that there is more time for bacterial action on the contents and more prolonged contact between any carcinogen generated and the mucosa. These factors, more than anything else, account for the high incidence of colorectal cancer in developed countries.
Clinicopathological features
Approximately 50% of cancers occur in the rectum, where they are equally divided between the upper, middle and lower thirds; about 30% occur in the sigmoid colon and the rest are equally distributed in the ascending, transverse and descending colon. This anatomical distribution is of practical importance, as about 50% of large bowel cancers can be reached with the examining finger and 80% with the sigmoidoscope.
In the rectum, the majority of cancers are of the ulcerating type (Fig. 15.27) and usually present with rectal bleeding. The stenosing type is more common in the descending colon and sigmoid, where it usually produces obstruction relatively early because of the narrowing of the lumen and the solid consistency of the faeces at this site. Polypoid and larger fungating cancers are more common in the right colon, where they tend to give rise to recurrent occult bleeding and the patient develops iron-deficiency anaemia. By virtue of the fluid bowel contents and the greater distensibility of the caecum and ascending colon, these tumours are more likely to be advanced at the time of presentation.
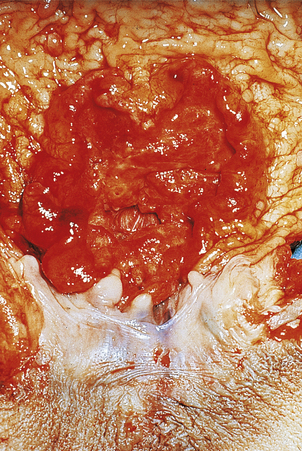
Fig. 15.27 Carcinoma of the rectum. Ulcerated carcinoma arising in the lower rectum close to and invading the anal canal.
Microscopically the cancers are adenocarcinomas, showing varying degrees of mucin production and glandular differentiation. These histological features are of little or no value in patient management, however, and the role of the pathologist after cancer surgery is to determine the completeness of excision and the extent of spread. If microscopic examination of the resection margins (especially the circumferential margin) establishes that all the tumour has been removed and the operation has been potentially curative, then the extent of spread through the bowel wall and the presence of lymph node metastases are the major prognostic determinants. The extent of spread is given by the Dukes’ and/or TNM stage (Fig. 15.28). Unfortunately, only about 70% of patients with colorectal cancer undergo a potentially curative operation; in about 15–25% of patients only a palliative operation is possible because they have widespread peritoneal deposits or liver secondaries, and the remainder are totally inoperable. However, with pre-operative radiotherapy the proportion of potentially curative operations is set to increase, and patients formerly considered inoperable because of liver metastases are now undergoing partial liver resections. Overall, the outlook for patients with colorectal cancer has improved remarkably in recent years.
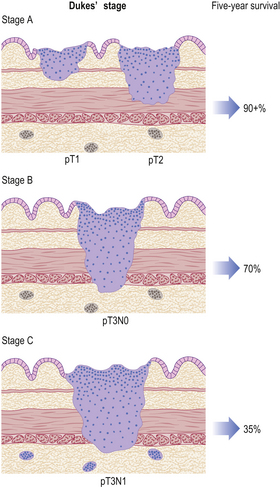
Fig. 15.28 The spread of colorectal cancer; Dukes’ stage and equivalent TNM stage, together with average 5-year survival. Dukes’ A: The tumour is confined to the submucosa (pT1) or muscle layer (pT2). Dukes’ B: The tumour has spread through the muscle layer, but does not yet involve the lymph nodes (pT3 N0). Dukes’ C: Any tumour involving lymph nodes irrespective of extent of direct spread. Here, spread has progressed through the main muscle coats into the subserosa and involves local lymph nodes (pT3 N1) but has not breached the peritoneal surface or invaded an adjacent organ (pT4).
Neuroendocrine tumours
Neuroendocrine tumours of the gastrointestinal tract are widely known as carcinoid tumours but, as they originate from the diffuse neuroendocrine system, they are better termed neuroendocrine tumours. Basically, there are two microscopic types. Some are composed of solid nests of regular cells with lightly staining nuclei and express immunochemical neuroendocrine markers; this is the typical carcinoid pattern. The second type, rare in the gastrointestinal tract, is an undifferentiated small- or large-cell tumour indistinguishable from their counterparts in the lung (Ch. 14) and more explicitly termed a neuroendocrine carcinoma. Both types occur as rare neuroendocrine tumours of the oesophagus where the undifferentiated types have to be distinguished from direct involvement by a primary lung tumour.
Gastric neuroendocrine tumours are relatively frequent, comprising up to 30% of all gastrointestinal neuroendocrine tumours. Two forms are encountered. More common are multiple small polypoid tumours found in association with autoimmune chronic gastritis. A second form is a solitary and, usually, larger polyp which is a sporadic finding with no associated disease. In the former, the glandular atrophy leads to achlorhydria which stimulates antral G-cells to produce gastrin. Persistent hypergastrinaemia leads to hyperplasia of the ECL cells (see p. 366) in the body of the stomach from which, after a latent period of many years, multiple tumours arise. This clinicopathological distinction is important in terms of behaviour. Neuroendocrine tumours associated with autoimmune gastritis do not metastasise and can be considered benign; thus, only the larger polyps need to be removed. In contrast, the sporadic tumours carry a definite risk of metastasis and require complete removal whatever their size.
Most duodenal neuroendocrine tumours are gastrinomas, i.e. gastrin-producing tumours, and are associated with the Zollinger–Ellison syndrome, but they can also be somatostatinomas, calcitonin-producing tumour, non-functioning or poorly differentiated neuroendocrine carcinomas.
Neuroendocrine tumours of the distal jejunum and ileum account for about 25% of these lesions. They are often over 20mm in diameter and have frequently spread to the mesenteric lymph nodes at the time of diagnosis. Indeed, about 20% of patients will have liver metastases at presentation. These tumours contain serotonin (5-hydoxytrytamine; 5-HT), substance P, kallikrein and catecholamines. 5-HT released by the tumour exerts local effects on the intestine but is inactivated in the liver by monoamine oxidases to form 5-hydroxyindole acetic acid (5-HIAA); this is excreted in the urine. The local effects are diarrhoea and borborygmi (excessive bowel sounds) because 5-HT stimulates intestinal contractility. Once metastases have formed in the liver, the tumour products (5-HT and kinins) are released directly into the hepatic veins and can affect the right side of the heart and the lungs before oxidation takes place in the pulmonary vasculature; this results in the carcinoid syndrome. The patient develops cyanotic flushing of the face, and there can be stenosis or incompetence of the pulmonary and tricuspid valves. The heart shows smooth muscle proliferation within the endocardium; this may result from bradykinin stimulation of mesenchymal cells which undergo differentiation to muscle cells. The spread of mid-gut neuroendocrine tumours and their effects are summarised in Figure 15.29.
Neuroendocrine tumours in the appendix are generally small (less than 20 mm in diameter), situated at or near the tip, and are discovered incidentally in specimens removed for abdominal pain. Such small tumours can be considered benign, and no further treatment is necessary. Larger tumours do occasionally metastasise and post-appendicectomy investigation and follow-up is advisable for tumours over 20 mm in diameter.
Colonic neuroendocrine tumours are rare but those in the rectum account for up to 20% of these gastrointestinal lesions. Histologically, colonic neuroendocrine tumours are usually poorly differentiated neuroendocrine carcinomas that have often metastasised at the time of diagnosis. The more common rectal neuroendocrine tumours are usually small and found incidentally at endoscopy. Histologically, they have a typical carcinoid appearance and a generally benign nature; metastases occur only when they are over 20 mm in diameter or have extended into the main muscle coat.
Lymphomas
Lymphomas are the commonest malignant tumours in the small intestine but are rare in the large bowel. Mention has already been made of the development of malignant lymphoma in coeliac disease—enteropathy-associated T-cell lymphoma—but this accounts for only a small proportion of the total; the majority of cases in developed countries have no predisposing cause. In the Middle East and South Africa, however, lymphoma of the small intestine frequently follows alpha heavy chain disease, a condition in which there is initially a benign proliferation of plasma cells secreting incomplete immunoglobulins.
In Western countries, non-enteropathy-associated lymphomas account for 60–80% of intestinal lymphomas and are most commonly of diffuse large B-cell type. They appear as plaques or polypoid masses that may be multiple, and give rise to abdominal pain, obstruction (either directly or by intussusception) and anaemia through intestinal blood loss. Other specific types are Burkitt’s lymphoma, mantle-cell lymphoma and follicle-centre lymphoma but these are all rare.
APPENDIX
The appendix can be the site for neuroendocrine (carcinoid) tumours, adenocarcinomas and lymphomas, but these are rare compared with the frequency of non-specific suppurative inflammation.
APPENDICITIS
Aetiology
Several factors are claimed to predispose to acute inflammation of the appendix, including faecoliths (hard pellets of faeces arising from dehydration and compaction) and food residues, lymphoid hyperplasia (as occurs in childhood and with some viral infections), diverticulosis of the appendix, and the presence of a carcinoid tumour.
Specific inflammations can also affect the appendix, and very occasional cases are due to Yersinia pseudotuberculosis, typhoid, tuberculosis and actinomycosis. The appendix is also involved by ulcerative colitis and Crohn’s disease.
Pathogenesis
Acute inflammation commences in the mucosa following a breach in the epithelium that permits infection by bowel flora. Infection leads to mucosal ulceration and a polymorph response, with exudation of cells and fibrin into the lumen. Further spread involves all the layers of the appendix and eventually causes a peritonitis over the serosal aspect. The build-up of fluid exudate within the wall increases tissue pressure and this, together with toxic damage to blood vessels and subsequent thrombosis, can lead to superimposed ischaemia. In this way the distal part of the appendix can become gangrenous and perforate.
Complications
Complications of acute appendicitis include those arising from perforation, such as generalised peritonitis, abscess and fistula formation, and the consequences of blood spread, suppurative pyelophlebitis (inflammation and thrombosis of the portal vein), liver abscess and septicaemia. The inflammation may become chronic, or obstruction to the neck of the appendix may lead to mucus retention in its lumen causing a mucocele. This does not often give rise to clinical problems but, on rare occasions, may rupture and disseminate mucus-secreting epithelial cells into the peritoneal cavity—pseudomyxoma peritonei.
ANUS AND ANAL CANAL
NORMAL STRUCTURE AND FUNCTION
The anal canal begins at the upper border of the internal sphincter at the level of the insertion of the puborectalis portion of levator ani (the so-called anorectal ring), and extends down to the groove between the terminal ends of the internal and external sphincters. It is 30–40 mm long.
Histologically, the upper part of the canal is lined by rectal-type glandular mucosa, the lower part by non-keratinising squamous epithelium. The upper end of the squamous portion is clearly delineated by the pectinate (or dentate) line. Proximal to this is a narrow zone of ‘transitional’ mucosa, consisting of columnar epithelium with multilayered small basal cells, which merges with the rectal-type mucosa of the upper segment.
The sensory nerves of the anal canal and the muscle sphincters are of vital importance in the control of defecation.
DISEASES OF THE ANUS AND ANAL CANAL
Fissures, fistulae and abscesses are common anal conditions that arise either in isolation or as part of Crohn’s disease. Anorectal tuberculosis is very rare in the UK, but is common in countries with a high incidence of pulmonary tuberculosis. Lesions of syphilis and other sexually transmitted diseases may occur at the anus.
Haemorrhoids (‘piles’)
Haemorrhoids are varicosities resulting from dilatation of the internal haemorrhoidal venous plexus. The mechanisms involved in their formation are not clearly understood, although chronic constipation with straining at stool is most commonly invoked. As such they are largely a consequence of the low-residue ‘Western’ diet and are relatively uncommon in developing countries.
Haemorrhoids present with rectal bleeding as streaks of blood on the outside of the stool. They may prolapse through the anal verge and can undergo secondary thrombosis and inflammation, whereupon they become acutely painful.
Tumours
Warts
Warts (condyloma acuminata) are the commonest benign tumours of the anus. They are often multiple and are almost always attributable to human papillomavirus (HPV) infection. Approximately 90% are related to HPV types 6 or 11. Their high incidence in homosexual males suggests venereal transmission through anal intercourse. There is also an increased risk of anal squamous carcinoma; those infected with HPV 16 and 18 have the highest risk of malignant change.
Carcinoma
There are three categories of carcinoma corresponding to the three kinds of epithelium found in the anal canal:
Squamous carcinomas at the anal verge and arising in peri-anal skin tend to be well differentiated. They appear as ulcerated lesions with rolled margins, and cause pain or bleeding. Those in the anal canal tend to be poorly differentiated squamous carcinomas and spread upwards into the lower rectum, outwards to involve the sphincters, and along lymphatics to involve the lateral pelvic and inguinal nodes. Anal carcinomas have a higher incidence in homosexual males, particularly those infected with HIV. Some are known to have developed in pre-existing anal intra-epithelial neoplasia and viral warts, and in particular in so-called giant condyloma acuminata.
Basaloid carcinomas are now considered to be a subtype of squamous carcinoma and, stage for stage, have the same prognosis. Irradiation and chemotherapy alone or in combination achieve at least 70% survival at 5 years. Surgery is usually reserved for large tumours that fail to respond to irradiation. Adenocarcinomas at the upper end of the anal canal are treated as for low rectal tumours.
Correa P., Schneider B.G.. Etiology of gastric cancer: what is new? Cancer Epidemiology Biomarkers and Prevention. 2005;14:1865-1868.
Day D.W., Jass J.R., Price A.B., et al. Morson and Dawson’s gastrointestinal pathology, 4th edn. Oxford: Blackwell, 2003.
Fortun P.J., Hawkey C.J.. Nonsteroidal anti-inflammatory drugs and the small intestine. Current Opinion in Gastroenterology. 2007;23:134-141.
Fox J.G., Wang T.C.. Inflammation, atrophy and gastric cancer. Journal of Clinical Investigation. 2007;117:60-69.
Fox M., Forgacs I.. Gastro-oesophageal reflux disease. British Medical Journal. 2006;332:88-93.
Gordon P.H., Nivatvongs S.. Neoplasms of the colon, rectum and anus, 2nd edn. New York: Informa Healthcare, 2007.
Iacobuzio-Donahue C.A., Montgomery E.A.. Gastrointestinal and liver pathology. Foundations in diagnostic pathology. Edinburgh: Churchill-Livingstone, 2006.
Kemp Z., Thirwell C., Sieber O., Silver A., Tomlinson I.. An update on the genetics of colorectal cancer. Human Molecular Genetics. 2004;13:R177-185.
Klöppel G., Anlauf M.. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Practice and Research. Clinical Gastroenterology. 2005;19:507-517.
Kucharzik T., Maaser C., Lügering A ., et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflammatory Bowel Disease. 2006;12:1068-1083.
Lagergren J.. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54(suppl 1):i1-i5.
Lock G.. Acute intestinal ischaemia. Best Practice and Research. Clinical Gastroenterology. 2001;15:83-98.
Monteleone G., Fina D., Caruso R., Pallone F.. New mediators of immunity and inflammation in inflammatory bowel disease. Current Opinion in Gastroenterology. 2006;22:361-364.
Raffatellu M., Chessa D., Wilson R.P., Tükel C., Akçelik M., Bäumler A.J.. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infection and Immunity. 2006;74:19-27.
Reibel J.. Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Critical Reviews in Oral Biology and Medicine. 2003;14:47-62.
Russell R.K., Wilson D.C., Satsangi J.. Unravelling the complex genetics of inflammatory bowel disease. Archives of Disease in Childhood. 2004;89:598-603.
Ryan D.P., Mayer R.J.. Anal carcinoma: histology, staging, epidemiology, treatment. Current Opinion in Oncology. 2000;12:345-352.
Strober W., Fuss I., Mannon P.. The fundamental basis of inflammatory bowel disease. Journal of Clinical Investigation. 2007;117:514-521.
Tamura G.. Genetic and epigenetic alterations in tumor suppressor and tumor-related genes in gastric cancer. Histology and Histopathology. 2002;17:323-329.
van Heel D.A., West J.. Recent advances in coeliac disease. Gut. 2006;55:1037-1046.

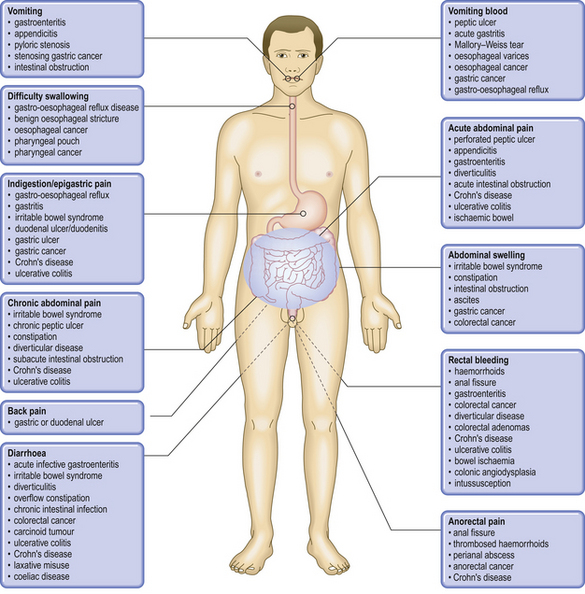




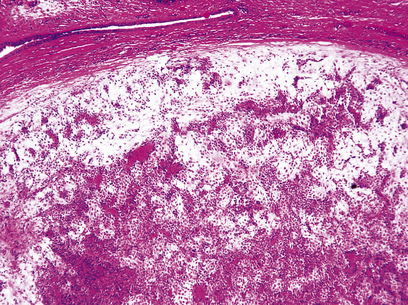
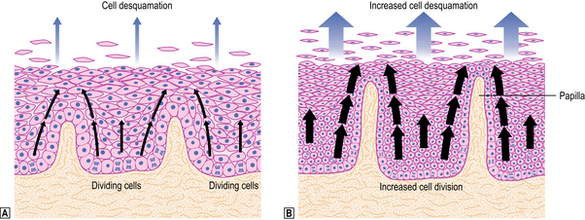
 Normal cell proliferation and migration in the squamous epithelium.
Normal cell proliferation and migration in the squamous epithelium.  In reflux oesophagitis, increased proliferation to compensate for increased cell desquamation results in basal zone hyperplasia and elongation of connective tissue papillae.
In reflux oesophagitis, increased proliferation to compensate for increased cell desquamation results in basal zone hyperplasia and elongation of connective tissue papillae.








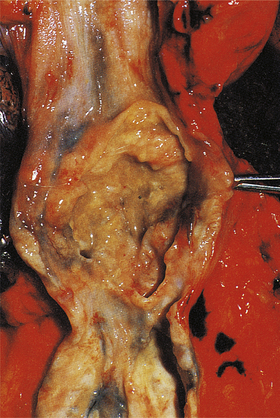




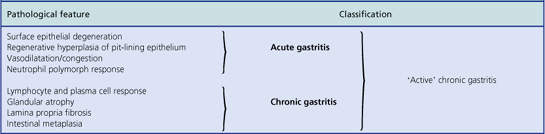
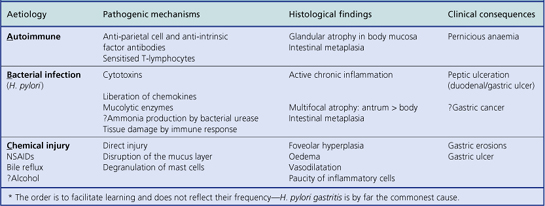













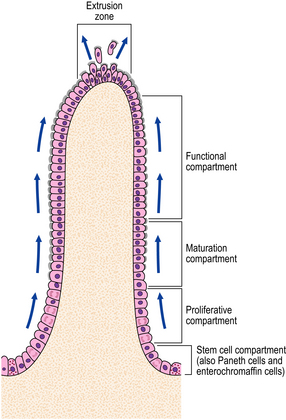




 and those of ulcerative colitis
and those of ulcerative colitis .
.