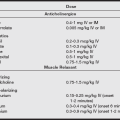
Renal system
A Acute renal failure
Definition
Acute renal failure (acute tubular necrosis, vasomotor neuropathy, lower nephron necrosis) is defined as the sudden inability of the kidneys to vary urine volume and content appropriately in response to homeostatic needs.
Pathophysiology
Acute renal failure is classified according to its predominant cause or on the basis of urine flow rates. The cause of acute renal failure has prerenal, renal, or postrenal origins. Prerenal failure results from hemodynamic or endocrine factors that impair renal perfusion, renal failure results from tissue damage, and postrenal failure results from urinary tract obstruction. Prerenal or postrenal failure is reversed with attention to hemodynamics or relief of obstruction. Acute renal failure caused by parenchymal disease or damage is more serious and often requires hemodialysis. Common causes are listed below.
Primary renal disease
Failure classified according to urine flow rates is known as oliguric, nonoliguric, or polyuric failure. Oliguria is defined as a urinary flow rate less than 0.5 mL/kg/hr in a patient subjected to acute stress. This rate is higher than that seen in unstressed patients because acutely stressed patients cannot maximally concentrate urine. Polyuric failure is associated with elevations of blood urea, nitrogen, and serum creatinine levels and is characterized by urine flow rates that exceed 2.5 L/day.
Conditions that lead to prerenal oliguria include acute reductions in glomerular filtration rate (GFR), excessive reabsorption of salt or water, or both. Increases in circulating levels of catecholamines, antidiuretic hormone (ADH), or aldosterone are physiologic factors that can decrease urinary output. Hypotension may or may not be present in the initiation of acute renal failure. If not reversed, prerenal oliguria may progress to parenchymal damage and tubular necrosis.
Acute tubular necrosis
Acute tubular necrosis may be produced by a variety of factors that interfere with glomerular filtration or tubular reabsorption. The pathogenesis of acute tubular necrosis may be divided into an initiation period, a maintenance period, and a recovery period. Renal hypoperfusion or a nephrotoxic insult may initiate renal failure. Surgical patients with external and internal fluid losses or sepsis may have renal hypoperfusion. The initiating insult culminates in the development of one or more maintenance factors, such as decreased tubular function, tubular obstruction, and sustained reductions in renal blood flow and glomerular filtration. Urine flow and solute excretion are reduced. When the maintenance period has begun, pharmacologic interventions to improve renal blood flow do not reverse the failure.
Prerenal oliguria
Prerenal oliguria is associated with physiologic mechanisms that conserve salt and water. In this case, urine has low sodium levels and high osmolality. Urine sodium levels are high, and osmolality is low. Renal damage is also associated with a progressive rise in serum urea, creatinine, uric acid, and polypeptide levels. Serum potassium levels may increase by 0.3 to 3 mEq/L/day, and a decrease occurs in the serum levels of sodium, calcium, and proteins such as albumin. The creatinine clearance remains the single most helpful test in defining renal status and predicting the prognosis in cases of severe renal dysfunction.
A number of conditions may place patients at high risk for acute renal failure and are listed in the following section. Renal reserve decreases progressively with age. For each year after 50 years of age, creatinine clearance decreases by 1.5 mL and renal plasma flow by 8 mL. Older patients are less able to cope with fluid and electrolyte imbalance and are more prone to renal damage. Overall mortality rates associated with acute renal failure increase from 50% for those younger than age 40 years to 80% for those older than age 60 years.
Prevention and management
Prevention of renal failure can be based on the following generalizations:
• The most common cause of failure is prolonged renal hypoperfusion.
• Prophylaxis reduces mortality more effectively than dialytic therapy.
• The duration and magnitude of the initiating renal insult are critical in determining the severity of failure.
A key strategy in reducing the incidence of renal failure is limiting the magnitude and duration of renal ischemia. Although a number of preventive strategies have been described, none apart from maintenance of normovolemia appears to be effective.
Anesthetic considerations
In the preoperative preparation of surgical patients, high-risk patients and procedures should be identified. Reversible renal dysfunction should be sought, and fluid losses and hypovolemia should be corrected by intravenous fluids. Perioperative ADH and renin–angiotensin–aldosterone secretion can be minimized with adequate hydration before anesthetic induction. Administration of saline rather than solutions low in sodium is helpful in prevention of aldosterone secretion, hyponatremia, and oliguria.
Oliguria often signals inadequate systemic perfusion, and prevention of acute renal failure requires its rapid recognition through adequate monitoring. In addition to standard monitors and a urinary catheter, monitors for patients with questionable cardiac and pulmonary function should include a direct arterial line for blood pressure monitoring and a central venous pressure (CVP) or Swan-Ganz catheter, when appropriate, for assessment of cardiac function and volume status.
Perioperative
Use of a urinary catheter is the only means of monitoring renal function in the operating room. A fluid challenge is necessary if hourly urinary output decreases to below acceptable levels.
The use of diuretics in the face of inadequate urinary output must be carefully evaluated. Although diuretics may not be effective during the initiation of failure, large doses of furosemide may convert oliguric renal failure into nonoliguric failure, which is easier to manage. In the maintenance phase, doses of furosemide in excess of 1 g may be required to convert oliguric failure to nonoliguric failure. Diuretic therapy must be associated with aggressive monitoring and intravascular volume expansion.
Although the mechanism is unknown, prophylactic administration of mannitol in well-hydrated patients protects renal function. Loop diuretics may also prevent acute renal failure. Mechanisms for protection include the inhibition of sodium reabsorption and the prevention of tubular obstruction through the maintenance of high flow and pressure within the tubules and the reversal of intrinsic renal vasoconstriction. Prophylactic use of diuretics may be of benefit in the case of jaundice in surgical patients, excessive exposure to contrast media, hyperuricemia, or the presence of pigment in the urine. Fenoldopam, a dopamine receptor agonist, also may be helpful.
Fenoldopam mesylate (Corlopam) is a selective DA1 receptor agonist. It causes both systemic and renal arteriolar vasodilation and has no impact on DA2, α-adrenergic, or β-adrenergic receptors. Unlike dopamine, which causes renal vasoconstriction at higher doses, fenoldopam at high dose produces even greater renal vasodilation. Fenoldopam is more than six times as potent as dopamine in increasing renal blood flow.
Management of acute renal failure
If acute renal failure develops, it progresses through four distinct phases: onset, the oliguric phase, the diuretic phase, and the recovery phase. Onset, or the initiation phase, precedes actual necrotic injury and correlates with a major alteration in renal hemodynamics. The oliguric phase reflects four pathophysiologic processes:
1. Obstruction of tubules by cellular debris, tubular casts, or tissue swelling
2. Total reabsorption or backleak of urine filtrate through damaged tubular epithelium and into the circulation
3. Tubular cell damage with leakage of adenosine triphosphate (ATP) and potassium and edema
The diuretic phase signifies that tubular function is returning. It is marked by a large daily urinary output (more than 3 L) secondary to the osmotic diuretic effect produced by an elevated blood urea nitrogen (BUN) and impaired ability of tubules to conserve sodium and water. The recovery phase is characterized by gradual improvement of renal function over 3 months to 1 year.
After renal failure is established, the primary consideration in management is the maintenance of fluid and electrolyte balance. The early use of hemodialysis for the prevention of severe fluid and electrolyte imbalance is necessary during the oliguric and diuretic phases. The clinical management of oliguria is discussed in the following section.
Algorithm for clinical management of perioperative oliguria
• Oliguria is less than 0.5 mL/kg but may be 1 to 2 mL/kg in a patient who has received mannitol.
• Assume oliguria is prerenal until proven otherwise.
• Do not give a diuretic to “make urine” in the face of intravascular hypovolemia or hypotension.
• Do not give diuretics if there are signs of fluid overload or if oliguria persists despite fluid challenges and stabilized hemodynamics or if there is pigment nephropathy.
• If improvement is not noted with fluid challenge or diuretics, institute invasive hemodynamic monitoring.
• Maximize renal blood flow by enhancing cardiac function: normalize preload, heart rate, rhythm, consider afterload reduction with vasodilators or inodilator agents.
• Prophylactic pharmacologic agents may be used when renal risk is high, but there is little evidence to suggest that they are better at maintaining GFR than volume.
B Chronic renal failure
Definition
Chronic renal failure is a slow, progressive, irreversible condition characterized by diminished functioning of nephrons and a decrease in renal blood flow, GFR, tubular function, and reabsorptive capacity.
Pathophysiology
Although many conditions may lead to renal failure, primary causes include glomerulonephritis, pyelonephritis, diabetes mellitus, vascular or hypertensive insults, and congenital defects. The systemic effects of chronic renal failure are shown in the following box.
The general course of progressive renal failure may be divided into three stages: decreased renal reserve, renal insufficiency, and end-stage renal failure or uremia. As the number of functioning nephrons declines, the signs, symptoms, and biochemical abnormalities become more severe.
Clinical signs or laboratory evidence of renal disease are absent until fewer than 40% of normal-functioning nephrons remain. Loss of nephron function without symptoms is known as a decrease in renal reserve. Renal insufficiency occurs when only 10% to 40% of nephrons are functioning adequately. Nocturia occurs secondary to a decrease in concentrating ability. Although affected patients seem well compensated when excretory capacity is unstressed, little renal reserve is present. Elimination of a large protein load or excretion of certain drugs is impaired, and preservation of remaining nephron function is a major goal. Toxic substances such as aminoglycosides potentiate existing damage, and aminoglycoside toxicity is enhanced in the presence of either volume depletion or arterial hypotension. Radiocontrast exposure in patients with chronic renal insufficiency often causes further reversible decreases in renal function in those with either myocardial failure or diabetes mellitus.
As renal function deteriorates further, end-stage renal disease (ESRD) develops. During this stage, concentrating and diluting properties of the kidney are severely compromised, and electrolyte, hematologic, and acid–base disturbances are common. The loss of 95% of functioning nephrons culminates in uremia, which is associated with volume overload and congestive heart failure. Uremia, which can be viewed as urine in the blood, adversely affects almost every organ system. Death occurs unless dialysis is performed.
Treatment
Dialytic therapy
Approximately 250,000 people in the United States today require chronic dialysis with an estimated 8% increase each year.
Dialysis techniques
Dialysis is a general term used to describe therapy in which solute moves from blood through a semipermeable membrane into a chemically prescribed solution. The movement of solute, which is called diffusive transport, depends on differences in molecular concentration between the blood compartment and the dialysate. Ultrafiltration is a technique in which a hydraulic pressure difference across a semipermeable membrane causes the bulk removal of fluid and solute by convective transport.
Major types of dialysis include hemodialysis and peritoneal dialysis. In hemodialysis, blood moves through a device that exposes it to an individually prescribed dialysate solution across a semipermeable membrane. Hemodialysis requires systemic or regional anticoagulation. In peritoneal dialysis, the blood compartment is the peritoneal microvasculature, and the semipermeable membrane is the peritoneal lining. The dialysate is infused into and withdrawn from the abdominal cavity. Movement of water occurs down an osmotic gradient from blood to dialysate and may be increased with an increase in the glucose concentration of the dialysate. Diffusive transport is influenced by the solute concentration within the dialysate.
Anesthetic considerations
Preoperative
Preoperative preparation of patients with advanced renal disease should include an evaluation of recent laboratory measurements, coexisting diseases, and current medications. Patients with ESRD should undergo determination of BUN and serum creatinine levels, complete blood count, bleeding time measurement, and electrolyte studies preoperatively. Special attention should be given to serum potassium, the type of and schedule for dialysis, and volume status.
Discussions regarding premedication should take into consideration unexpected sensitivity to central nervous system depressants and delayed gastric emptying. Benzodiazepines are useful as premedicants because of their oral route of administration and hepatic metabolism. Midazolam has virtually no active metabolites, and its half-life is only slightly prolonged in renal failure. Although this drug is useful when it is carefully titrated, patients with renal disease may be more susceptible to the sedative-hypnotic effects of this benzodiazepine than those without renal dysfunction. Reduced protein binding may be responsible for increased sensitivity to these drugs in these patients.
Gastric hyperacidity and gastrointestinal bleeding are common in patients with renal failure. H2 blockers and magnesium-free antacids should be considered. Cimetidine has been used, but renal elimination accounts for 80% of total elimination, and elimination is impaired with reduced renal function. Although newer H2 antagonists are now available, all H2-receptor blockers are very dependent on renal excretion. Metoclopramide is partly excreted unchanged in the urine and accumulates in patients with renal failure.
Intraoperative monitoring
The selection of monitors for a patient with diminished or absent renal function is based on the physiologic status of the patient and the proposed surgical procedure. Frequent measurements of blood pressure and continuous recording of temperature and heart rate and rhythm are essential. Electrocardiography may allow early detection of hyperkalemia.
The decision to use invasive monitors depends on the patient’s functional cardiac reserve and the severity and control of hypertension. Continuous monitoring of intraarterial blood pressure is helpful when major surgical procedures are performed. A femoral or dorsalis pedis artery is sometimes used for cannulation because vessels in the upper extremities may be needed later for vascular shunts. Vascular volume and fluid replacement can be guided by CVP or pulmonary artery catheter monitoring. A pulmonary artery catheter is useful if interpretation of the CVP is questionable or cardiac disease is present.
Vascular shunts and fistulas must be protected. Patency is easily monitored with Doppler imaging. Because of the immunocompromised state of these patients, strict aseptic technique is required during the placement of vascular catheters.
Regional anesthesia
Regional anesthesia is tolerated by patients with advanced renal disease, provided no significant coagulation disorder is present and the mean arterial pressure is maintained. Regional techniques avoid most of the pharmacokinetic and pharmacodynamic problems associated with general anesthetics and sedative drugs. Major concerns regarding this type of anesthesia include psychological intolerance, coagulation abnormalities, the presence of peripheral neuropathies, difficulty in making intravascular volume adjustments, and risk of infection.
Arteriovenous shunts or fistulas may be surgically created with the use of local infiltration or brachial plexus block. In addition to providing analgesia, brachial blocks improve surgical conditions by providing maximum vascular vasodilation and abolishing vasospasm. The duration of brachial plexus block has been reported to be shortened by 40% in patients with chronic renal failure. A shortened duration of action would support the use of a longer acting local anesthetic, such as bupivacaine, especially if prolonged surgery is anticipated. However, data suggest a similar duration of anesthesia with brachial plexus blocks in patients with renal failure and normal renal function.
With regard to spinal or epidural anesthesia, patients with long-standing renal disease often have undergone multiple procedures and prefer general anesthetic techniques. In addition to the history, the bleeding time, platelet count, prothrombin time, partial thromboplastin time, and fibrinogen level should be evaluated before subarachnoid or epidural catheters are placed in uremic patients. An opioid or opioid and local epidural solution rather than local solution alone allows continuous monitoring of neurologic function.
Peripheral neuropathies should be discussed with the patient and documented before regional anesthesia is undertaken. The incidence of hypotension with subarachnoid or epidural blockade may be increased because of effects of chronic hypertension or hypovolemia related to recent dialysis. Correction of hypovolemia postoperatively is hazardous. Recession of the sympathetic block in patients who cannot undergo diuresis may lead to pulmonary edema. One must weigh the advantages of fluid infusion against the effects of pressor drugs with these factors in mind.
Patients with ESRD are often acidotic, and local anesthetic toxicity may be increased with acidosis. The onset and duration of blocks also have been shown to vary in these patients.
General anesthesia
Intravenous drugs: Intravenous anesthetics can be used in patients with advanced renal disease, but the response of these patients may be more variable than normal. Variability arises from a complex interplay among changes in volume of distribution (which is often increased), protein binding (which may be low), low pH, and dependence on renal excretion for the parent drug or metabolites.
The action of many drugs is potentiated by metabolic abnormalities associated with renal failure. Highly protein-bound drugs have more target organ effect in the presence of hypoalbuminemia. The acidemic state associated with renal failure increases the proportion of the agent that is unionized and unbound and therefore more available to target tissue. Anemia associated with renal failure increases cardiac output and enhances delivery to the brain. Uremia alters the blood–brain barrier; this also increases the sensitivity to intravenous drugs.
Uremic patients are generally anemic and may require high inspired oxygen concentrations. Because intravenous anesthesia is often supplemented with nitrous oxide, the inspired concentration of oxygen is reduced. Volatile agents are more reliable in controlling hypertension, and their action is more easily reversed. For these reasons, inhalation agents may be preferable for general anesthesia.
Volatile anesthetic agents
Inhalation agents offer some advantage in patients with renal failure. Although biotransformation of some agents may produce metabolites excreted by the kidneys, elimination of volatile agents does not rely on renal function. Inhalation agents potentiate neuromuscular blocking drugs, allowing administration of reduced doses. Although the potency of these agents allows them to be administered without nitrous oxide, excessive depth of anesthesia may lead to a depression of cardiac output. Reductions in cardiac output and tissue blood flow must be avoided in these patients with anemia if tissue oxygen delivery is to be maintained. A disadvantage of sevoflurane relates to its biotransformation and potential nephrotoxic effects.
Both regional and general anesthesia have been used successfully in patients with advanced renal disease. Advantages and disadvantages of both techniques are shown in the table below.
Regional versus General Anesthesia
| Technique | Advantages | Disadvantages |
| Regional | Patient responsiveness Minimal changes in renal hemodynamics |
Presence of peripheral neuropathy Tendency for bleeding Patient anxiety Prolonged procedures Hypotension with sympathetic block; may cause reluctance to expand volume |
| Inhalation anesthetics | Good airway control Blood pressure control Duration not dependent on urinary excretion Less neuromuscular blocking with drugs required Fio2 can be increased because N2O not necessary |
Alterations in renal hemodynamics Decreased cardiac output Hypotension Biodegradation and potential nephrotoxicity; halothane, 15%–20%; sevoflurane, 5%; isoflurane, 0.2%; desflurane, 0.02% |
| Intravenous anesthetics | Hemodynamic stability | Unpredictable response Hypertension Greater need for N2O and neuromuscular blockers |
Intravenous fluid management
Perioperative management of fluids and electrolytes in patients with renal disease is critical. The state of hydration affects renin, aldosterone, and antidiuretic levels. Dehydration and hypovolemia lead to elevations in these hormones and to a decline in urinary output.
Perioperative renal function
Surgical patients at high risk for acute renal failure and those with advanced disease who do not require hemodialysis present unique challenges. Preservation of renal function intraoperatively is a major goal. Preservation of renal function is dependent on the maintenance of intravascular volume and cardiovascular stability and on the avoidance of events that cause renal vasoconstriction. Preoperative hydration with 10 to 20 mL/kg of balanced salt solution may be helpful. Intraoperatively, urinary output is the only time monitor for renal function. A urinary output of 0.5 to 1 mL/kg/hr intraoperatively and postoperatively is recommended in these patients.
Decreased renal reserve
The goal of fluid management in patients with decreased renal reserve is the maximization of renal perfusion. Basal fluids should be replaced; 5% dextrose in water (D5W) with 50 to 70 mEq/L of Na+ is appropriate. Potassium should be administered as needed to sustain a normal plasma level. Deficit and intraoperative losses should be replaced as for a normal patient. Third-space losses can be replaced with balanced salt solution. Although it is better to err on the side of excess with respect to volume replacement in these patients, if the ratio of replacement crystalloid solution to lost blood exceeds 3:1, consideration should be given to the use of a colloid solution. The administration of large volumes of crystalloid solution may be associated with pulmonary edema as fluids are mobilized. This generally occurs on the second to the fourth postoperative day.
Renal insufficiency
In patients with renal insufficiency, volume deficits should be replaced preoperatively, as in normal patients. Basal fluids must be carefully regulated because these patients cannot tolerate much deviation. Overall basal fluid requirements must be related to metabolic rate and be designed to provide an overall fluid balance that allows an isotonic urine to carry excreted electrolytes and waste products. Intraoperative losses greater than 10% to 15% of the blood volume should be replaced with colloid solution on a 1:1 basis after red blood cell losses are corrected. Smaller losses can be replaced with the usual 3:1 ratio of crystalloid infusion to blood loss. Third-space losses are ideally replaced initially with crystalloid solution without potassium or excess chloride. Initial third-space losses should be replaced with crystalloid solution at a rate of 2 to 3 mL/kg/hr. The critical goal in patients with renal insufficiency is sustaining blood volume. Monitoring of colloid osmotic pressure and hemoglobin can guide the choice between crystalloid and colloid infusions. If hemoglobin and colloid osmotic pressure are increasing, crystalloid solution is clearly indicated. If they are decreasing, crystalloid solution should be withheld in favor of colloid solution. Close monitoring of blood pressure, heart rate, CVP, pulmonary artery occlusion pressure, and cardiac output also guides fluid titration. This is especially true in patients with cardiac or respiratory compromise.
End-stage renal disease
With regard to perioperative fluid management, patients with ESRD who are hemodialysis dependent require special attention. Although these patients are similar to normal patients in terms of fluid deficit, basal, and third-space requirements, they have a narrow margin of safety. The patient’s ability to compensate for either fluid excess or fluid deficiency progressively declines as renal function is lost.
Fluid deficits must be replaced preoperatively in patients with ESRD. If deficits exceed 10% to 15% of the blood volume, invasive monitoring is justified. Dialysis is recommended on the day before anesthesia to allow time for equilibration of fluid and electrolyte shifts that are common with dialysis. Electrolyte levels must be checked before anesthesia.
Basal fluids in patients with ESRD should be replaced in a manner similar to that for patients with renal insufficiency. Volume restriction is recommended for intraoperative losses. Third-space losses should be replaced with a balanced salt solution that contains no potassium and small amounts of chloride. Close monitoring of hemoglobin and cardiac filling pressures is indicated for all major procedures. Patients with ESRD generally require dialysis within 24 to 36 hours after major surgery.
Uremia
Deficit replacement in patients with uremia must be guided by hemodynamic monitoring. Basal fluids should be replaced with red blood cells, fresh-frozen plasma, or colloid solutions. Third-space losses are best replaced with crystalloid solutions in association with frequent monitoring of hemoglobin and cardiac filling pressures. A moderate degree of volume overload is not a grave problem. Many uremic patients require dialysis within 24 to 36 hours for the removal of mobilized fluid and the control of hypertension.
Although volume overload is most often emphasized in patients with ESRD, complications of hypovolemia are also serious. Hypotension associated with hypovolemia increases the risk of thrombosis of the arteriovenous fistula and predisposes to cardiac and cerebral ischemia. Hemodynamic goals include the avoidance of hypotension and gross fluid overload. This can be accomplished only through careful titration with the patient well monitored.
C Urolithiasis
Definition
Urolithiasis, or “kidney stones,” refers to the presence of calculi, typically composed of calcium oxalate, in the urinary system. These calculi result from hypercalcemia or hyperoxaluria. Other types of stones include magnesium ammonium phosphate, calcium phosphate, and uric acid.
Pathophysiology
Causes of hypercalcemia and hypercalciuria are primarily hyperparathyroidism, vitamin D intoxication malignancies, and sarcoidosis. Small bowel bypass is associated with hyperoxaluria. Alterations in urine pH and the presence of metabolic disturbances can also result in formation of renal stones, which differ in composition from the typical oxalate variety.
Treatment
Extracorporeal shock wave lithotripsy is a noninvasive treatment of renal stones. It transmits shock waves through water and focuses them on the stone by biplanar fluoroscopy. For patients with arrhythmias, shock waves can be delivered during the heart’s refractory period (<20 msec after R wave) to avoid initiating further cardiac arrhythmias. Patients with artificial cardiac pacemakers risk pacemaker dysfunction with this form of therapy. Percutaneous nephrostomy can also be performed for removal of stone, obstruction, or biopsy. A needle is passed into the renal collecting duct, a catheter can be placed over the needle, and the kidney is drained. Anesthetic options include local anesthesia with sedation, regional anesthesia, and general anesthesia.
Anesthetic considerations
General anesthesia, intravenous sedation, or regional anesthesia, including epidural and intercostal nerve blocks with local infiltration, may be used to provide analgesia during lithotripsy. Regardless of anesthetic regimen, the patient must remain still because movement (including excessive diaphragmatic excursion) can move the stone from the wave focus. The sitting position may be associated with peripheral pooling of blood, especially with the use of regional anesthesia and resultant vasodilation. Immersion in water increases hydrostatic pressures on the abdomen and thorax, which can displace blood into the central circulation. This increase in CVP may result in acute congestive heart failure in patients with limited cardiac reserve. The hydrostatic forces on the thorax likewise result in decreases in chest wall compliance and functional residual capacity. This may produce ventilation/perfusion mismatches. Water in the immersion tub should be kept warm to avoid hypothermia. An alternative option to an immersion tank is a lithotripsy table. Extracorporeal shock wave lithotripsy is contraindicated in patients with abdominal aortic aneurysms, spinal cord tumors, or orthopedic implants in the lumbar region. Parturients, obese patients, and patients with coagulopathies also are not good candidates for this procedure.