Chapter 74 Sympathetic Ophthalmia
Introduction
Sympathetic ophthalmia, also known as sympathetic ophthalmitis and sympathetic uveitis, is a rare bilateral diffuse granulomatous uveitis that occurs a few days to several decades after penetrating accidental or surgical trauma to an eye. Both the traumatized eye, commonly referred to as the “exciting” eye, and the fellow eye, referred to as the “sympathizing” eye, are affected. Injury to and/or incarceration of uveal tissue has been a feature of nearly all cases of sympathetic ophthalmia. The clinical signs and symptoms are usually detected in the sympathizing eye within the first 3 months after trauma to the fellow eye.1,2
The notion that injury to one eye may have repercussion in the contralateral eye probably dates back to ancient times, with Hippocrates (460–370 bc), but has also been reported by Agathias, in a compilation from Constantius Cephalis (1000 ad).1,3 However it was William Mackenzie, in 1830, who provided the first comprehensive clinical description and pioneered the use of the term “sympathetic ophthalmia” to describe this entity.1,3 Ernst Fuchs, in 1905, thoroughly detailed the characteristic histopathologic features.4 The pathogenesis of sympathetic ophthalmia has, however, remained an enigma despite years of study, although the two most commonly held views were that it represented an autoimmune response or was infectious. There is now some experimental evidence implicating the development of an autoimmune delayed hypersensitivity reaction to melanocytes or tyrosinase peptide antigen as a possible pathogenetic mechanism.
Epidemiology
Sympathetic ophthalmia is a relatively rare disease, although exact figures are difficult to determine because the onset or the diagnosis, or both, are often delayed for months to years after the initial injury. In addition, definite histopathologic confirmation of the diagnosis is made in only about one-third of suspected cases, but may be established histopathologically in others not suspected clinically.2 In 1972, Liddy and Stuart5 reported an incidence of sympathetic ophthalmia of 0.19% following penetrating injuries and 0.007% following intraocular surgery. Among the general population, sympathetic ophthalmia has been estimated to affect 0.03 per 100 000 persons per year6 and possibly corresponds to 1–2% of all uveitis cases.7 Surgical procedures that may lead to sympathetic ophthalmia include cataract extraction, iridectomy, paracentesis, cyclodialysis, synechialysis, retinal detachment repair, keratectomy, vitrectomy, evisceration, laser cyclophotocoagulation, and others.2,3
Although advances in modern surgical techniques may be contributing to a lower incidence of sympathetic ophthalmia, this is probably being partially offset by more aggressive surgical management of severely traumatized eyes, which in the past would have been promptly enucleated. In fact, the epidemiology of sympathetic ophthalmia has been changing, and more cases are now being associated with intraocular surgery,6–8 in contrast to accidental penetrating trauma.3 Pars plana vitrectomy is one of the leading surgical procedures associated with sympathetic ophthalmia,9 with an estimated risk of around 0.01%;10 cases have been recently reported even after the advent of small-gauge sutureless vitrectomy surgery.11,12 For these reasons, sympathetic ophthalmia should not be considered a disappearing disease and thus should not be neglected.
Some studies have shown a male preponderance, but this is believed to be a reflection of the higher incidence of accidental trauma in males. Indeed, when only cases of surgical trauma are considered, the ratio is similar. In Winter’s series of 257 cases of sympathetic ophthalmia, there was no difference in age incidence.13 Other authors have reported relative peaks in childhood and early adult years, thought to reflect a higher incidence of accidental trauma in these ages, and an additional peak in the sixth and seventh decades, thought to represent an increased incidence of surgical procedures among persons in this age group.14
Pathogenesis
The exact cause of sympathetic ophthalmia is unknown. Clinical studies have shown that the predominant predisposing factors are accidental penetrating trauma, accounting for approximately 60–70% of cases, and penetrating surgical trauma, accounting for nearly 30%. Recent studies have pointed to an inversion of this proportion, with many cases now being associated with surgical rather than accidental trauma.6,7,9 A small percentage of cases are the result of contusion injuries with occult scleral rupture and perforating corneal ulcers. The common denominator in the overwhelming majority of cases is the presence of a penetrating injury in which wound healing is complicated by incarceration of the iris, ciliary body, or choroid.2
Historically, the pathogenesis of sympathetic ophthalmia has been suspected to be infectious. However no organisms have ever been isolated from cases of sympathetic ophthalmia, and infective agents have not induced the disease in laboratory animals. Cases associated with severe infectious keratitis in the absence of a corneal perforation are probably associated with exposure of the uveal antigens to the immune system, through a disrupted hemato-ocular barrier.15,16 Sympathetic ophthalmia may also develop in the setting of posttraumatic or postoperative infectious endophthalmitis and it is possible that such infection may potentiate the development of sympathetic ophthalmia in these cases17 and not prevent it, in contrast to what had previously been suggested.1,18,19 Most cases of sympathetic ophthalmia however develop in the absence of intraocular infection.14
Several investigators have proposed an immunologic basis for sympathetic ophthalmia, in which a T-cell-mediated autoimmune response against an antigenic protein from the uvea, particularly a uveal tyrosinase peptide, may be involved.20–24 Marak25,26 and Wong and colleagues20 demonstrated enhanced transformation of peripheral lymphocytes from patients with histologically confirmed sympathetic ophthalmia when exposed to homologous uveal-retinal extracts in tissue culture, suggesting that these patients have lymphocytes that are sensitized to some component(s) of uveal-retinal antigen. When antigens extracted from the retina are injected into guinea pigs, intraocular inflammation similar to sympathetic ophthalmia develops in these animals.26 Some studies indeed suggest that sympathetic ophthalmia may result from altered T-cell response to one of the soluble proteins associated with the retinal photoreceptor membranes, particularly the retinal S antigen, or to other retinal or choroidal melanocyte antigens.21–23,26,27 However, recent studies reveal that T-cell immune response to tyrosinase peptide may play a role in the development of sympathetic ophthalmia, as in Vogt–Koyanagi–Harada disease.22,28–30
There may be a genetic predisposition to the development of sympathetic ophthalmia. Human leukocyte antigen (HLA) association has been reported in sympathetic ophthalmia, including HLA-A11, HLA-B40, HLA-DR4/DRw53, and HLA-DR4/DQw3 haplotypes.31,32 Studies from Asia and Europe found a significant correlation between HLA-DRB1*4 and HLA-DQB1*04 and the development of sympathetic ophthalmia.33–35 A similar association was also seen in patients with Vogt–Koyanagi–Harada disease. Finally, genetic background may also correlate to disease severity, either through some of those predisposing HLA haplotypes such as HLA-DRB133 or through cytokine polymorphisms such as in interleukin-10, modifying the immune response and the risk of disease recurrence.36
Immunopathology
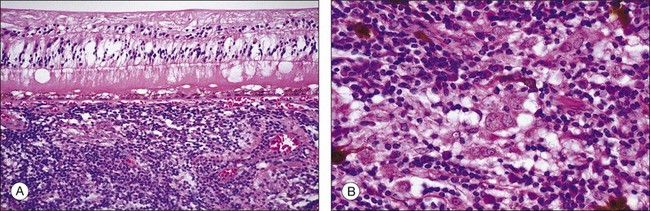
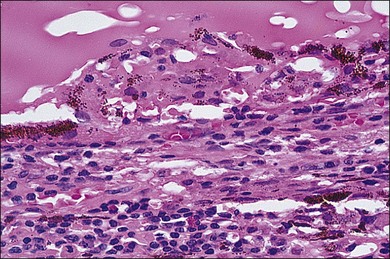
In sympathetic ophthalmia the immunopathologic alterations are similar in both the exciting and sympathizing eyes and typically consist of a diffuse granulomatous inflammation of the uveal tract, made up of lymphocytes, plasma cells, and nests of epithelioid histiocytes; pigment is often present within these epithelioid cells and also within giant cells (Fig. 74.1). In the majority of cases, the inflammatory process does not involve the choriocapillaris or the retina. Absence of necrosis is another characteristic feature. The choroid is diffusely involved and thickened by an infiltration of predominantly lymphocytes, collections of epithelioid cells, and a few giant cells; neutrophils are rarely seen. Plasma cells may be present, particularly in patients treated with corticosteroids. Eosinophils can also be found and are frequently concentrated in the inner choroid, particularly in heavily pigmented individuals. Nodular clusters of epithelioid cells containing pigment are often seen lying between the retinal pigment epithelium (RPE) and Bruch’s membrane; these appear clinically as the drusen-like, yellow-white dots known as Dalen–Fuchs nodules (Fig. 74.2).1,2,4,13,14,37,38 Infiltration of the pars plana of the ciliary body occurs early in the course of disease, and the inflammatory cells from this site may spill over into the vitreous cavity. Similar inflammatory cell infiltration in the iris may result in the clinical appearance of a thickened iris.
The retina is usually free of inflammatory infiltrates. However, few enucleated eyes of sympathetic ophthalmia show collections of mononuclear cells around the blood vessels, and occasional involvement in the areas overlying the Dalen–Fuchs nodules, and in the pars plana region. Other pathologic changes include scleral involvement with inflammatory infiltrates around the emissary veins and extension of the granulomatous process into the optic nerve and surrounding meningeal sheaths, the sites where melanocytes are also present.1,4,14,39 Some of the eyes with characteristic histologic features of sympathetic ophthalmia but also with breaks in the lens capsule may additionally reveal features of phacoanaphylaxis, with zonal granulomatous inflammation around the lens material.39 Even though typical features of sympathetic ophthalmia include nonnecrotizing granulomatous uveitis, there are cases exhibiting atypical features such as nongranulomatous choroiditis or chorioretinal adhesions with the inflammatory process involving the choriocapillaris, as seen in chronic Vogt–Koyanagi–Harada disease.13,39
Immunohistochemical studies have revealed infiltration of predominantly T lymphocytes in the uveal tract.37,40 B lymphocytes may also be present, especially in longstanding disease.41,42 Among the T lymphocytes, both helper (CD4+) and suppressor/cytotoxic (CD8+) cells have been observed,37,40 driving a Th1 response with secretion of proinflammatory cytokines such as interferon-γ and interleukin-2.43 These cells are probably recruited to the eye by the selective expression of intercellular adhesion proteins in the uveal tract (particularly some integrins),44 as well as some other molecules in macrophages and RPE cells, including chemokines, such as monocyte chemotactic protein-1 (CCL2/MCP-1) and stromal cell-derived factor-1 (CXCL12/SDF-1), and metalloproteinases, such as gelatinase-B.42 Relative preservation of the choriocapillaries in the acute phase of the disease process may be associated with secretion of anti-inflammatory cytokines by the RPE.27 A recent study has interestingly shown CD4+ T lymphocytes and melanin-laden macrophages expressing HLA-DR underneath the conjunctiva in the exciting eye, suggesting that antigen processing and presentation may initially take place at that site, further leading to activation of lymphocytes and to the granulomatous response.45
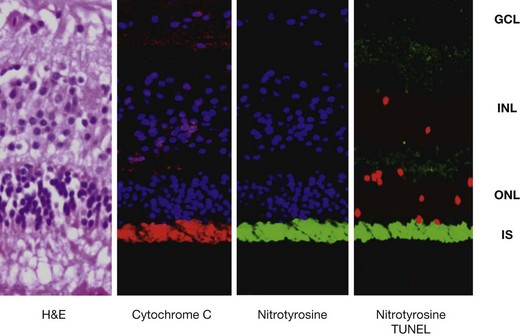
Even though the retina seems to be relatively spared in the pathologic process of sympathetic ophthalmia, tumor necrosis factor (TNF)-α-mediated mitochondrial oxidative stress has recently been localized in the outer retina of enucleated human globes with the disease (Fig. 74.3). This was associated with apoptosis of photoreceptors and probably such photoreceptor damage could be a mechanism leading to vision loss in sympathetic ophthalmia.46
Clinical findings
The clinical onset of sympathetic ophthalmia is typically heralded by the development of an apparently mild intraocular inflammation in the sympathizing eye and worsening of inflammation in the exciting eye. The interval between the time of injury and the onset of inflammation in the sympathizing eye has been reported to be as short as 5 days and as long as 66 years after trauma.1,3,47 In general, however, sympathetic ophthalmia rarely develops sooner than 2 weeks after trauma, with 80% of cases occurring within 3 months and 90% within 1 year of the penetrating injury.2,7,9 The peak incidence occurs between 4 and 8 weeks after accidental trauma, while cases following surgical trauma may have a more delayed onset.48
Symptoms in the sympathizing eye include mild pain, photophobia, and increased lacrimation, blurring of vision, visual fatigue, or even paresis of accommodation. The exciting eye may have a decrease in vision and an increase in photophobia. Moreover, both eyes may show ciliary injection and a partially dilated and poorly responsive pupil.1,2,8,9
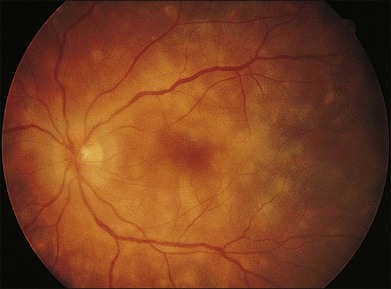
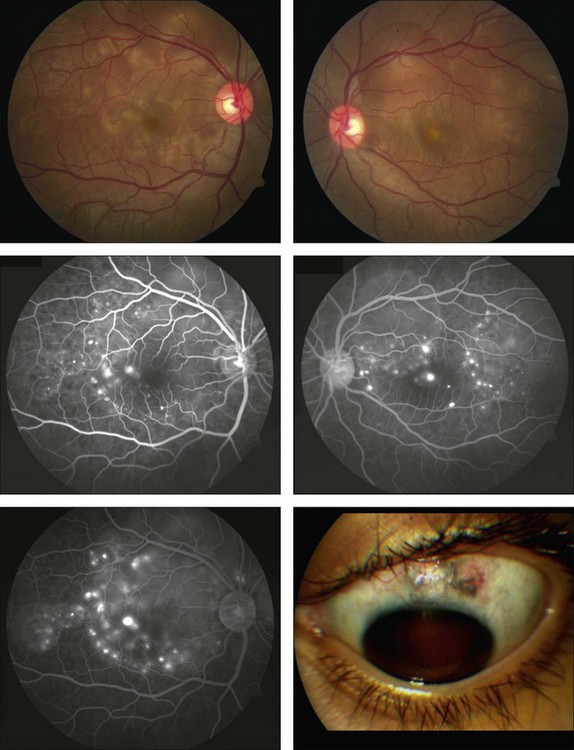
The clinical signs are variable and can be either insidious or fairly rapid in onset. Anterior-segment changes are those of an anterior uveitis, with ciliary injection, keratic precipitates, flare, and inflammatory cells in the anterior chamber. Thickening of the iris and even iris nodules may also be seen and posterior synechiae are common. Posterior-segment findings in sympathetic ophthalmia include inflammatory cell infiltration of the vitreous, hyperemia and edema of the optic disc, diffuse edema and exudative detachment of the retina, as well as small yellow-white deposits beneath the RPE, so-called Dalen–Fuchs nodules.1,2,8,9,13,14 Bullous serous detachments may also be seen in the peripheral retina. Occasionally, multiple deep ill-defined yellowish lesions may be present, corresponding to choroidal granulomas (Fig. 74.4). Inflammatory scleral involvement is rarely seen clinically, but is a common finding on microscopic examination of enucleated eyes. With time, patients may develop depigmentation of the choroid leading to the so-called sunset glow fundus, as well as RPE changes, as seen in individuals with chronic Vogt–Koyanagi–Harada disease.49,50 Although less commonly than in Vogt–Koyanagi–Harada disease, extraocular involvement – including meningismus, dysacusis, vitiligo, poliosis, and alopecia – may also be present in patients with sympathetic ophthalmia.2,49,51
Diagnosis
The diagnosis of sympathetic ophthalmia is essentially clinical.1,2,9 No serologic or immunologic tests are available to aid in the diagnosis. Even though there are no systematized diagnostic criteria, the presence of penetrating ocular trauma (either accidental or surgical) is a fundamental feature. Bilateral intraocular inflammation should also be present, and may be accompanied by exudative retinal detachments and/or optic disc edema early in the disease process, or by choroidal depigmentation (sunset glow fundus) and RPE changes in chronic cases, similarly to Vogt–Koyanagi–Harada disease.52 However, variations in this clinical presentation can challenge or delay the diagnosis. Some imaging studies, such as fluorescein angiography, indocyanine green angiography, B-scan ultrasound, as well as optical coherence tomography (OCT), may be helpful to disclose or delineate better some supportive features of sympathetic ophthalmia.
Fluorescein angiography may typically show multiple progressively fluorescent dots at the level of the RPE (pinpoint leakage; Fig. 74.5), as well as disc leakage, as also seen in Vogt–Koyanagi–Harada disease. Coalescence of the dye from these foci occurs in the areas of exudative detachment.2,53,54 Less frequently there may also be early focal blockage of the background choroidal fluorescence, a finding also noted in acute posterior multifocal placoid pigment epitheliopathy.55 On indocyanine green angiography, numerous hypofluorescent patches may be visible during the intermediate phase, presumably corresponding to the choroidal granulomas. Some of these may become isofluorescent at the later phases.56–58
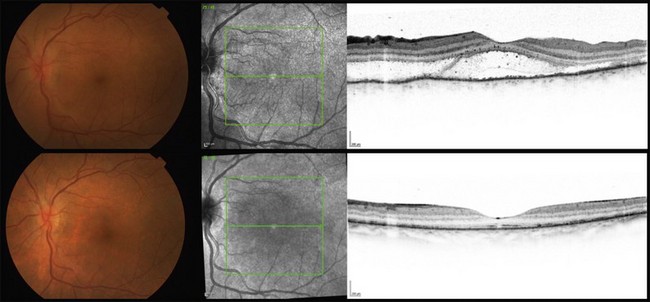
Ultrasound examination may reveal choroidal thickening and also areas of exudative retinal detachment, being particularly useful in the exciting eyes with opaque media.9,17 Usually the choroidal thickening is more prominent around the optic disc and less in the anterior choroid. OCT can nicely delineate the foci of exudative retinal detachment, their resolution following appropriate therapy (Fig. 74.6), as well as other progressive changes in the neurosensory retina and in the RPE.55,59,60
Especially in those severely traumatized eyes that later require enucleation, histopathologic examination may help to make or confirm the diagnosis.2,14,17,39
Differential diagnosis
Sympathetic ophthalmia must be differentiated from several infectious and also other noninfectious uveitides, including syphilis, tuberculosis, sarcoidosis, multifocal choroiditis, and panuveitis. Other bacterial and fungal infections can also produce a granulomatous anterior and/or posterior uveitis, being usually differentiated by history and associated clinical findings. Infectious endophthalmitis must always be considered following any penetrating trauma to the eye. In particular, less virulent microorganisms such as Propionibacterium acnes and some fungi may lead to a picture of chronic endophthalmitis, which should be distinguished from sympathetic ophthalmia. Reactivation of a preexisting uveitis after injury or the development of a posttraumatic iritis or iridocyclitis can also occur.2,9
Phacoanaphylatic endophthalmitis can also closely simulate the clinical picture of sympathetic ophthalmia and these two entities may even coexist in the same eye. Although unilaterality can be a clue, phacoanaphylaxis may also be bilateral but such bilaterality is rare. The incidence of phacoanaphylaxis in cases of sympathetic ophthalmia has been estimated to range between 4 and 25%. Unlike sympathetic ophthalmia, in bilateral phacoanaphylaxis the eye first involved is usually quiet by the time inflammation begins in the fellow eye. Moreover ultrasound examination usually reveals predominant thickening of the anterior uveal tract in phacoanaphylactic endophthalmitis, in contrast to sympathetic ophthalmia, in which such thickening is more pronounced in the posterior uveal tract. A careful slit-lamp examination should always be carried out to search for ruptured lens capsule and fragments of lens cortex in the anterior chamber. In phacoanaphylactic endophthalmitis, lens extraction can be curative, thereby avoiding unnecessary enucleation.17,61
Vogt–Koyanagi–Harada syndrome is a bilateral granulomatous panuveitis, often associated with a prodrome of meningeal and auditory symptoms. The disease may have clinical and histopathologic features identical to sympathetic ophthalmia in both the acute and in the chronic phase. However, findings such as vitiligo and alopecia are more common in Vogt–Koyanagi–Harada syndrome than in sympathetic ophthalmia. A history of penetrating trauma is helpful in this differential diagnosis.2,49,50
Course and complications
Untreated sympathetic ophthalmia runs a long, variable, and complicated course, marked initially by episodes of active intraocular inflammation followed by quiescent periods that can last months to several years. With time, the disease may become chronically active, eventually producing irreversible ocular damage and even phthisis bulbi. Long-term complications of sympathetic ophthalmia include cataract, secondary ocular hypertension or hypotony, glaucoma, persistent cystoid macular edema or retinal detachment, chorioretinal scarring (including epiretinal membrane formation), choroidal neovascularization, subretinal fibrosis, and optic atrophy.2,14,62 In spite of treatment, the overall risk of developing any of these ocular complications reaches 40% per patient per year, with around half of the patients losing vision to <20/40 and roughly one-fourth eventually becoming legally blind.48
Therapy
Although corticosteroids have not been shown to be effective in the prevention of sympathetic ophthalmia, they do constitute the mainstay of its therapy.2,6,8 Large doses of corticosteroids should be given early in the course of the disease and continued for at least 6 months. For the first week, 1.5–2.0 mg/kg of body weight of oral prednisone (or equivalent) is given daily and then gradually tapered over several months, following clinical response of the uveitis. Alternatively, pulse therapy with intravenous methylprednisolone (up to 1 g daily for 3 days), as well as supplementation with sub-Tenon’s injection of triamcinolone acetonide (20–40 mg), may be considered. Topical corticosteroids and mydriatic/cycloplegic agents are used adjunctively as needed. Special care should be taken to monitor side-effects of the systemic corticosteroids, including periodic measurement of blood pressure, body weight, lipids, blood glucose, as well as gastroduodenal protection and prophylaxis of osteoporosis (with calcium and vitamin D supplementation).63
In a number of patients, medical problems or systemic or ophthalmologic complications may prevent the long-term use of high doses of steroids. In these patients, supplemental treatment with immunosuppressive agents (azathioprine, 2–4 mg/kg/day; cyclosporine, 2.5–5 mg/kg/day; mycophenolate mofetil, 1–1.5 g bid; methotrexate, 15–25 mg/week) has been shown to suppress inflammation effectively, allow reduction of corticosteroid therapy to nontoxic levels (<10 mg/day), and, in some cases, induce disease remission. These drugs are usually started as monotherapy, but can be combined or switched in case of lack of adequate response.6,8 Careful monitoring of their side-effects every 4–6 weeks, with the supervision of an internist, is recommended. Bone marrow suppression, renal and/or hepatic toxicity can occur with prolonged use of these agents.63 The alkylating agents cyclophosphamide (2–3 mg/kg/day) and chlorambucil (0.1–0.2 mg/kg/day) are reserved for more severe and refractory cases.63,64 These agents require careful monitoring for side-effects, including hemorrhagic urinary bladder inflammation and development of malignancies.
Biologicals, particularly anti-TNF agents such as infliximab and adalimumab, may also be used in cases of sympathetic ophthalmia that are unresponsive to conventional immunomodulatory agents and, in this setting, favorable results have been anecdotally reported in the recent literature.65,66 However the long-term safety of these agents is still uncertain.67
Intraocular corticosteroids, either administered as intravitreal injections (triamcinolone acetonide 4 mg) or as slow-release devices, such as fluocinolone or dexamethasone intravitreal implants, may also be used, especially in individuals who cannot tolerate the systemic medications.68–70 Special concern should be given to the high risk of cataract and secondary glaucoma associated with these intravitreal devices.
Prevention
The prevention of sympathetic ophthalmia entails careful microsurgical wound toilet and prompt closure of all penetrating injuries. Every attempt should be made to save any eye with a reasonable prognosis for useful vision, but in those eyes with barely discernible or no visual function, and with demonstrable disorganization of the ocular contents, enucleation within 2 weeks after injury is possibly the only way to definitely prevent the development of sympathetic ophthalmia.3 At one time it was believed that the use of steroids following penetrating injury would in some instances prevent the development of sympathetic ophthalmia; this has not been proved to be the case.
Enucleation of the exciting eye once sympathetic ophthalmia has commenced has been a topic of considerable controversy. Some studies suggest that early enucleation of the exciting eye may improve the prognosis for the sympathizing eye14,71; however careful review of the data presented in these studies does not support this conclusion.72 A review by Winter of 257 cases of histologically proven sympathetic ophthalmia indicated no benefit to the sympathizing eye from enucleation of the exciting eye, whether performed briefly before, concomitant with, or subsequent to the development of sympathetic ophthalmia at various elapsed intervals following injury.13 This was also supported by the results of a prospective study.6 Indeed, it is possible that the exciting eye may eventually provide the better visual acuity, and its enucleation would therefore deprive the patient of that visual potential.72
Another controversy is the possible role of evisceration versus enucleation. Even though evisceration may be technically easier and provides a faster recovery,73 it seems not to protect against the development of sympathetic ophthalmia, probably because of retention of uveal remnants in the scleral shell.74 Many cases of sympathetic ophthalmia following evisceration have been described in the older literature,74,75 but it is not clear whether those larger rates were biased by the more limited resources for primary repair of those globes at the time of injury. Sympathetic ophthalmia after evisceration is probably rarer nowadays, but recent cases have been anecdotally reported.76–79 Because this is far less common than in the past,80,81 and management of sympathetic ophthalmia has significantly improved,6,8,9 an issue raises whether evisceration should indeed be preferred to enucleation, particularly in blind eyes, after severe trauma and/or infection. This issue is not yet resolved,73 especially considering the very low incidence of sympathetic ophthalmia and the changing trend from posttraumatic to postsurgical cases.7,8 An additional complication to this controversy is the risk of inadvertently eviscerating occult tumors,82 which may even outweigh the risk of sympathetic ophthalmia per se. It is advisable that patients with penetrating ocular injuries, as well as those undergoing intraocular surgeries with an increased risk of sympathetic ophthalmia (such as pars plana vitrectomy), are counseled about the possibility of developing the disease, early or even long after the traumatic or surgical insult.
Prognosis
Before the use of corticosteroids, the visual prognosis of sympathetic ophthalmia was generally poor. However, after the advent of the corticosteroids and more recently of immunosuppressive agents, this prognosis dramatically improved.8,9 Makley and Azar found that, in patients treated solely with systemic corticosteroids, a visual acuity of 20/60 or better was achieved in most of them, but relapses occurred in 60%, sometimes long after initial disease remission.62 Chan et al. reported visual acuity of 20/40 or better in 50% of patients treated with steroids and immunosuppressive agents.83 With prompt and aggressive corticosteroid therapy, and immunosuppressive agents, as needed, many eyes with sympathetic ophthalmia should retain reasonable vision.
1 Duke-Elder S. Sympathetic ophthalmitis. System of ophthalmology. Duke-Elder S, ed. System of ophthalmology. St Louis: Mosby; 1966;vol 9:558–593.
2 Goto H, Rao NA. Sympathetic ophthalmia and Vogt–Koyanagi–Harada syndrome. Int Ophthalmol Clin. 1990;30:279–285.
3 Albert DM, Diaz-Rohena R. A historical review of sympathetic ophthalmia and its epidemiology. Surv Ophthalmol. 1989;34:1–14.
4 Fuchs E. Über sympathisierende Entzündung (nebst Bemerkungen über seröse traumatische Iritis). Graefes Arch Ophthalmol. 1905;61:365–456.
5 Liddy L, Stuart J. Sympathetic ophthalmia in Canada. Can J Ophthalmol. 1972;7:157–159.
6 Kilmartin DJ, Dick AD, Forrester JV. Prospective surveillance of sympathetic ophthalmia in the UK and Republic of Ireland. Br J Ophthalmol. 2000;84:259–263.
7 Sen HN, Nussenblatt RB. Sympathetic ophthalmia: what have we learned? Am J Ophthalmol. 2009;148:632–633.
8 Vote BJ, Hall A, Cairns J, et al. Changing trends in sympathetic ophthalmia. Clin Experiment Ophthalmol. 2004;32:542–545.
9 Castiblanco CP, Adelman RA. Sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2009;247:289–302.
10 Gass JD. Sympathetic ophthalmia following vitrectomy. Am J Ophthalmol. 1982;93:552–558.
11 Cha DM, Woo SJ, Ahn J, et al. A case of sympathetic ophthalmia presenting with extraocular symptoms and conjunctival pigmentation after repeated 23-gauge vitrectomy. Ocul Immunol Inflamm. 2010;18:265–267.
12 Haruta M, Mukuno H, Nishijima K, et al. Sympathetic ophthalmia after 23-gauge transconjunctival sutureless vitrectomy. Clin Ophthalmol. 2010;4:1347–1349.
13 Winter FC. Sympathetic uveitis; a clinical and pathologic study of the visual result. Am J Ophthalmol. 1955;39:340–347.
14 Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia. A clinicopathologic review of 105 cases (1913–1978). Ophthalmology. 1980;87:109–121.
15 Buller AJ, Doris JP, Bonshek R, et al. Sympathetic ophthalmia following severe fungal keratitis. Eye. 2006;20:1306–1307.
16 Guerriero S, Montepara A, Ciraci L, et al. A case of sympathetic ophthalmia after a severe Acanthamoeba keratitis. Eye Contact Lens. 2011;37:374–376.
17 Rathinam SR, Rao NA. Sympathetic ophthalmia following postoperative bacterial endophthalmitis: a clinicopathologic study. Am J Ophthalmol. 2006;141:498–507.
18 Woods AC. Sympathetic ophthalmia: part 2. Am J Ophthalmol. 1936;19:100–109.
19 Samuels B. Panophthalmitis and sympathetic ophthalmia. Arch Ophthalmol. 1938;20:804–811.
20 Wong VG, Anderson R, O’Brien PJ. Sympathetic ophthalmia and lymphocyte transformation. Am J Ophthalmol. 1971;72:960–966.
21 Rao NA, Robin J, Hartmann D, et al. The role of the penetrating wound in the development of sympathetic ophthalmia: experimental observations. Arch Ophthalmol. 1983;101:102–104.
22 Sugita S, Sagawa K, Mochizuki M, et al. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with Vogt–Koyanagi–Harada disease. Int Immunol. 1996;8:799–803.
23 Hammer H. Cellular hypersensitivity to uveal pigment confirmed by leucocyte migration tests in sympathetic ophthalmitis and the Vogt–Koyanagi–Harada syndrome. Br J Ophthalmol. 1974;58:773–776.
24 Rao NA, Wong VG. Aetiology of sympathetic ophthalmitis. Trans Ophthalmol Soc U K. 1981;101:357–360.
25 Marak GE, Jr. Recent advances in sympathetic ophthalmia. Surv Ophthalmol. 1979;24:141–156.
26 Rao NA, Wacker WB, Marak GE, Jr. Experimental allergic uveitis: clinicopathologic features associated with varying doses of S antigen. Arch Ophthalmol. 1979;97:1954–1958.
27 Rao NA. Mechanisms of inflammatory response in sympathetic ophthalmia and VKH syndrome. Eye. 1997;11:213–216.
28 Yamaki K, Ohono S. Animal models of Vogt–Koyanagi–Harada disease (sympathetic ophthalmia). Ophthalmic Res. 2008;40:129–135.
29 Yamaki K, Gocho K, Hayakawa K, et al. Tyrosinase family proteins are antigens specific to Vogt–Koyanagi–Harada disease. J Immunol. 2000;165:7323–7329.
30 Kawakami Y, Suzuki Y, Shofuda T, et al. T cell immune responses against melanoma and melanocytes in cancer and autoimmunity. Pigment Cell Res. 2000;13(Suppl 8):163–169.
31 Reynard M, Shulman IA, Azen SP, et al. Histocompatibility antigens in sympathetic ophthalmia. Am J Ophthalmol. 1983;95:216–221.
32 Azen SP, Marak GE, Jr., Minckler DS, et al. Histocompatibility antigens in sympathetic ophthalmia. Am J Ophthalmol. 1984;98:117–119.
33 Kilmartin DJ, Wilson D, Liversidge J, et al. Immunogenetics and clinical phenotype of sympathetic ophthalmia in British and Irish patients. Br J Ophthalmol. 2001;85:281–286.
34 Shindo Y, Ohno S, Usui M, et al. Immunogenetic study of sympathetic ophthalmia. Tissue Antigens. 1997;49:111–115.
35 Tiercy JM, Rathinam SR, Gex-Fabry M, et al. A shared HLA-DRB1 epitope in the DR beta first domain is associated with Vogt–Koyanagi–Harada syndrome in Indian patients. Mol Vis. 2010;16:353–358.
36 Atan D, Turner SJ, Kilmartin DJ, et al. Cytokine gene polymorphism in sympathetic ophthalmia. Invest Ophthalmol Vis Sci. 2005;46:4245–4250.
37 Chan CC, Nussenblatt RB, Fujikawa LS, et al. Sympathetic ophthalmia. Immunopathological findings. Ophthalmology. 1986;93:690–695.
38 Kuo PK, Lubin JR, Ni C, et al. Sympathetic ophthalmia: a comparison of the histopathological features from a Chinese and American series. Int Ophthalmol Clin. 1982;22:125–139.
39 Croxatto JO, Rao NA, McLean IW, et al. Atypical histopathologic features in sympathetic ophthalmia. A study of a hundred cases. Int Ophthalmol. 1982;4:129–135.
40 Jakobiec FA, Marboe CC, Knowles DM, 2nd., et al. Human sympathetic ophthalmia. An analysis of the inflammatory infiltrate by hybridoma-monoclonal antibodies, immunochemistry, and correlative electron microscopy. Ophthalmology. 1983;90:76–95.
41 Shah DN, Piacentini MA, Burnier MN, et al. Inflammatory cellular kinetics in sympathetic ophthalmia a study of 29 traumatized (exciting) eyes. Ocular Immunol Inflamm. 1993;1:255–262.
42 Abu El-Asrar AM, Struyf S, Van den Broeck C, et al. Expression of chemokines and gelatinase B in sympathetic ophthalmia. Eye (Lond). 2007;21:649–657.
43 Hooks JJ, Chan CC, Detrick B. Identification of the lymphokines, interferon-gamma and interleukin-2, in inflammatory eye diseases. Invest Ophthalmol Vis Sci. 1988;29:1444–1451.
44 Kuppner MC, Liversidge J, McKillop-Smith S, et al. Adhesion molecule expression in acute and fibrotic sympathetic ophthalmia. Curr Eye Res. 1993;12:923–934.
45 Jayaprakash Patil A, Edward DP, Wong M, et al. The role of perivascular melanophage infiltrates in the conjunctiva in sympathetic ophthalmia. Ocul Immunol Inflamm. 2011;19:186–191.
46 Parikh JG, Saraswathy S, Rao NA. Photoreceptor oxidative damage in sympathetic ophthalmia. Am J Ophthalmol. 2008;146:866–875. e2
47 Zaharia MA, Lamarche J, Laurin M. Sympathetic uveitis 66 years after injury. Can J Ophthalmol. 1984;19:240–243.
48 Galor A, Davis JL, Flynn HW, Jr., et al. Sympathetic ophthalmia: incidence of ocular complications and vision loss in the sympathizing eye. Am J Ophthalmol. 2009;148:704–710. e2
49 Rao NA, Marak GE. Sympathetic ophthalmia simulating Vogt–Koyanagi–Harada’s disease: a clinico-pathologic study of four cases. Jpn J Ophthalmol. 1983;27:506–511.
50 Rao NA. Pathology of Vogt–Koyanagi–Harada disease. Int Ophthalmol. 2007;27:81–85.
51 Comer M, Taylor C, Chen S, et al. Sympathetic ophthalmia associated with high frequent deafness. Br J Ophthalmol. 2001;85:496.
52 Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt–Koyanagi–Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–652.
53 Dreyer WB, Jr., Zegarra H, Zakov ZN, et al. Sympathetic ophthalmia. Am J Ophthalmol. 1981;92:816–823.
54 Sharp DC, Bell RA, Patterson E, et al. Sympathetic ophthalmia. Histopathologic and fluorescein angiographic correlation. Arch Ophthalmol. 1984;102:232–235.
55 Correnti AJ, Read RW, Kimble JA, et al. Imaging of Dalen–Fuchs nodules in a likely case of sympathetic ophthalmia by fluorescein angiography and OCT. Ophthalmic Surg Lasers Imaging. 2010:1–3.
56 Bernasconi O, Auer C, Zografos L, et al. Indocyanine green angiographic findings in sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 1998;236:635–638.
57 Saatci AO, Pasa E, Soylev MF, et al. Sympathetic ophthalmia and indocyanine green angiography. Arch Ophthalmol. 2004;122:1568–1569.
58 Moshfeghi AA, Harrison SA, Ferrone PJ. Indocyanine green angiography findings in sympathetic ophthalmia. Ophthalmic Surg Lasers Imaging. 2005;36:163–166.
59 Gallagher MJ, Yilmaz T, Cervantes-Castaneda RA, et al. The characteristic features of optical coherence tomography in posterior uveitis. Br J Ophthalmol. 2007;91:1680–1685.
60 Gupta V, Gupta A, Dogra MR, et al. Reversible retinal changes in the acute stage of sympathetic ophthalmia seen on spectral domain optical coherence tomography. Int Ophthalmol. 2011;31:105–110.
61 Marak GE, Jr. Phacoanaphylactic endophthalmitis. Surv Ophthalmol. 1992;36:325–339.
62 Makley TA, Jr., Azar A. Sympathetic ophthalmia. A long-term follow-up. Arch Ophthalmol. 1978;96:257–262.
63 Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513.
64 Tessler HH, Jennings T. High-dose short-term chlorambucil for intractable sympathetic ophthalmia and Behçet’s disease. Br J Ophthalmol. 1990;74:353–357.
65 Gupta SR, Phan IT, Suhler EB. Successful treatment of refractory sympathetic ophthalmia in a child with infliximab. Arch Ophthalmol. 2011;129:250–252.
66 Menghini M, Frimmel SA, Windisch R, et al. Efficacy of infliximab therapy in two patients with sympathetic ophthalmia. Klin Monbl Augenheilkd. 2011;228:362–363.
67 Keystone EC. Does anti-tumor necrosis factor-α therapy affect risk of serious infection and cancer in patients with rheumatoid arthritis? A review of longterm data. J Rheumatol. 2011;38:1552–1562.
68 Ozdemir H, Karacorlu M, Karacorlu S. Intravitreal triamcinolone acetonide in sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2005;243:734–736.
69 Chan RV, Seiff BD, Lincoff HA, et al. Rapid recovery of sympathetic ophthalmia with treatment augmented by intravitreal steroids. Retina. 2006;26:243–247.
70 Mahajan VB, Gehrs KM, Goldstein DA, et al. Management of sympathetic ophthalmia with the fluocinolone acetonide implant. Ophthalmology. 2009;116:552–557. e1
71 Reynard M, Riffenburgh RS, Maes EF. Effect of corticosteroid treatment and enucleation on the visual prognosis of sympathetic ophthalmia. Am J Ophthalmol. 1983;96:290–294.
72 Marak GE, Jr. Sympathetic ophthalmia. Ophthalmology. 1982;89:1291–1292.
73 O’Donnell BA, Kersten R, McNab A, et al. Enucleation versus evisceration. Clin Exp Ophthalmol. 2005;33:5–9.
74 Ruedemann AD. Sympathetic ophthalmia after evisceration. Trans Am Ophthalmol Soc. 1963;61:274–314.
75 Green WR, Maumenee AE, Sanders TE, et al. Sympathetic uveitis following evisceration. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:625–644.
76 Griepentrog GJ, Lucarelli MJ, Albert DM, et al. Sympathetic ophthalmia following evisceration: a rare case. Ophthal Plast Reconstr Surg. 2005;21:316–318.
77 Androudi S, Theodoridou A, Praidou A, et al. Sympathetic ophthalmia following postoperative endophthalmitis and evisceration. Hippokratia. 2010;14:131–132.
78 Zhang Y, Zhang MN, Jiang CH, et al. Development of sympathetic ophthalmia following globe injury. Chin Med J. 2009;122:2961–2966.
79 Freidlin J, Pak J, Tessler HH, et al. Sympathetic ophthalmia after injury in the Iraq war. Ophthal Plast Reconstr Surg. 2006;22:133–134.
80 du Toit N, Motala MI, Richards J, et al. The risk of sympathetic ophthalmia following evisceration for penetrating eye injuries at Groote Schuur Hospital. Br J Ophthalmol. 2008;92:61–63.
81 Savar A, Andreoli MT, Kloek CE, et al. Enucleation for open globe injury. Am J Ophthalmol. 2009;147:595–600. e1
82 Eagle RC, Jr., Grossniklaus HE, Syed N, et al. Inadvertent evisceration of eyes containing uveal melanoma. Arch Ophthalmol. 2009;127:141–145.
83 Chan CC, Roberge RG, Whitcup SM, et al. 32 cases of sympathetic ophthalmia. A retrospective study at the National Eye Institute, Bethesda, Md., from 1982 to 1992. Arch Ophthalmol. 1995;113:597–600.