Chapter 13 Survivorship and Late Effects
General Considerations
The QUANTEC Initiative
This chapter will summarize and expand upon several reviews published as a special issue in the International Journal of Radiation Oncology Biology Physics (volume 76, issue 3, Supplement), all of which were written as part of the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) initiative. QUANTEC arose from a proposal from the Science Council of the American Association of Physicists in Medicine (AAPM) to revise and update guidelines published by Emami in 1991.1 QUANTEC’s goals are (1) to provide a critical review of the current literature on quantitative dose-response and dose-volume relationships for clinically relevant normal-tissue endpoints; (2) to produce practical guidelines to allow reasonable toxicity risks based on dose-volume parameters; and (3) to identify future research initiatives. Using the QUANTEC reviews as a backbone, this chapter will focus on recently published data relevant to late toxicity from radiation, with an emphasis on dose-volume metrics. Key summary points of the QUANTEC reviews are briefly summarized at the end of each section, and the QUANTEC reviews are referenced at the end of each section. Table 13-1 summarizes the dose-volume metrics that are supported by the literature; this table was modified from the one published in the QUANTEC issue.2
Although the QUANTEC initiative and associated reviews address a broad range of issues relating to normal tissue damage, this chapter will focus on late toxicity after an initial course (i.e., excluding re-irradiation) of conventional radiation (i.e., excluding hypofractionated regimens).3 Studies relating three-dimensional dose/volume metrics to clinical outcomes will be the main subject.
Nervous System: Brain
Dose-Volume Data
Recent prospective studies in adults have shown that partial (and limited) brain irradiation in the dose range of 50 to 60 Gy causes minimal to no discernible effect on memory and cognition.4–9 However, another recent study has suggested that patients undergoing partial brain irradiation for low-grade glioma, versus patients who do not undergo irradiation, are at greater risk for neurocognitive deficits, particularly in attention, executive functioning, and information processing.10 More detailed studies, correlating neurocognition with susceptible regions within the brain, are needed. Certainly, ample data suggest that the volume of brain that is irradiated is a factor in the degree of radiation-associated neurocognitive decline (with whole brain irradiation causing more serious deficits).11 In adult patients who have received whole brain irradiation, neurocognitive decline is well described. However, the degree to which brain irradiation (versus factors such as surgical or chemotherapeutic treatment, the presence of hydrocephalus or comorbid illnesses, and tumor progression) may affect neurocognitive function is not clear.9,12,13
For children, younger age at irradiation and larger irradiated volumes (with higher doses having a greater impact) are associated with objectively worse neurocognitive function.14–17 For example, central nervous system prophylaxis for acute lymphoblastic leukemia with 24-Gy whole-brain irradiation in addition to intrathecal methotrexate (versus methotrexate alone) results in a median 13-point IQ reduction at 5 years after irradiation as well as poorer academic achievement and self-image and greater psychological distress at 15 years.18 Lower risks are associated with lower doses of 14 to 18 Gy.19–21 For medulloblastoma, increasing the brain dose in the 18- to 36-Gy range has been correlated with greater neurocognitive decline.14–16,22,23 Girls are observed to be more vulnerable to injury than boys.24 Reported toxicities are lower (or not detected) when 14 to 18 Gy is used.6–8 Radiation necrosis can occur in any part of the brain. Although there may be regional variations of susceptibility within the brain related to differences in vascularity, glial cell population, and so on, data on this topic are sparse, and it is generally believed that location does not generally affect susceptibility to necrosis. However, damage to specific regions, such as the brainstem, is more likely to cause symptoms. There is a paucity of data correlating dose-volume parameters with the risk of radiation necrosis with conventional fractionated therapy, though many studies have documented an association of fraction size with the risk of necrosis.
In a study from Queen Elizabeth Hospital in China, 1008 patients with nasopharyngeal cancer, treated before 1985, received 45.6 to 53.2 Gy in 3.8-Gy fractions, 50.4 Gy in 4.2-Gy fractions, or 60 Gy in 2.5-Gy fractions.25 The 10-year risk of temporal lobe necrosis was 18.6% in those treated with 4.2-Gy fractions versus less than 5% for the other dose schemes (p <.001). A multiinstitutional Chinese study examined 1032 patients with nasopharyngeal cancer treated after 1990 with one of several fractionation schemes (mostly 2- to 3.5-Gy fractions, though one scheme used a 1.6-Gy twice-daily component).26 The 5-year actuarial incidence of necrosis ranged from 0% (after 66 Gy in 2-Gy fractions) to 14% (after 2.5 Gy × 8 followed by 1.6 Gy twice daily to 71.2 Gy). In both of the above-mentioned studies, the combination of total dose and dose per fraction significantly affected the risk; a shorter overall treatment time and twice-daily fractionation also increased the risk. A Chinese study comparing 71.2 Gy in 1.6-Gy twice-daily fractions versus 60 Gy in 2.5-Gy fractions was terminated early because of excessive neurologic toxicity, including temporal lobe toxicity, in both arms. The risk of toxicity was greater and the interval to developing toxicity was shorter, with the twice-daily regimen.27 In another Chinese study, 27% of patients receiving an accelerated hyperfractionated regimen (64 Gy in 1.6-Gy twice-daily fractions) versus 0% of patients receiving hyperfractionated irradiation (70.8 Gy in 1.2-Gy twice-daily fractions) developed symptomatic radiation necrosis.28
Radiation necrosis has also been studied in patients with primary brain tumors. The risk is dose dependent, with doses of less than 50 Gy rarely causing necrosis.29–31 In patients treated for brain metastases, there was a low (<2%) risk of necrosis developing after 1.6 Gy twice daily to the whole brain (32 Gy), followed by a boost to 54.4 to 74.4 Gy, and no necrosis was seen after a boost to 48 Gy.32,33
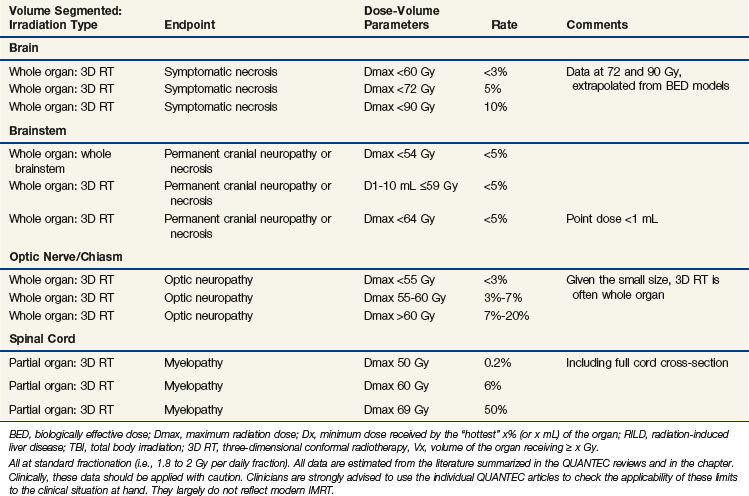
Summary and Other Key Points from the QUANTEC Review34
A high level of evidence to quantify the risks of radiation-induced brain injury is lacking. For brain necrosis, the brain appears to be especially sensitive to fraction size in excess of 2 Gy and to twice-daily fractionated treatment. Symptomatic necrosis is uncommon with doses of less than 60 Gy with conventional (1.8- to 2-Gy) fractionation, though the risk increases with increasing dose (see Table 13-1). More detailed studies correlating neurocognition with susceptible regions within the brain are needed. Long-term (>5 years) follow-up is necessary to best assess neurologic and cognitive decline. For children, younger age and higher whole brain dose strongly correlate with cognitive decline. Future studies should provide a clear definition of toxicity and reported actuarial rates (as opposed to crude rates) that can be correlated with detailed normal brain dose-volume metrics.
Nervous System: Brainstem
Dose-Volume Data
Several institutions have published their dose-volume constraints for the brainstem, most of which have not reported any brainstem toxicity. These constraints for patients undergoing external beam radiation therapy for head and neck cancer include a V60 of less than 5 mL, V65 less than 3 mL,35 V55 less than 0.1 mL,36 D1 less than 54 Gy,37 and maximum less than 50 Gy38; and for patients undergoing proton or combined proton/photon therapy for base of skull lesions include less than 63 to 64 Gy CGE (cobalt Gray equivalent) to the brainstem surface and 53 to 54 CGE to the brainstem center.39–42
Some patients in these studies received a maximum brainstem dose of 66 to 68 CGE (greater than the desired constraints) in order to adequately treat the tumor.39,41,42 In one study, the dose constraint of 63 CGE to the brainstem surface and 54 CGE to the brainstem center was “relaxed” in 38% and 17% of patients, respectively, with no reported neurologic toxicity. In these patients, the volume receiving a dose above the threshold dose was 0.2 mL and 1.2 mL for the brainstem surface and center, respectively.41 In another study, two of four patients developing neurologic toxicity received maximal doses in excess of 64 Gy CGE to the brainstem surface and 53 CGE to the brainstem center.42
In an analysis of 367 patients from Massachusetts General Hospital, an increased risk of late toxicity was associated with the maximal delivered dose (>64 CGE), V50 (>5.9 mL), V55 (>2.7 mL), V60 (>0.9 mL) and a history of diabetes, hypertension, and more than two surgical procedures of the base of the skull, on univariate analysis.43,44 On multivariate analysis, only the V60 and a history of diabetes and more than two surgical procedures remained significant. A V60 of less than 0.9 mL versus more than 0.9 mL resulted in toxicity-free survival rates of 96% versus 79% (p = .0001), and on multivariate analysis resulted in an 11.4 risk ratio (p = .001). In a recent study from St. Jude Children’s Research Hospital on 68 patients with infratentorial ependymoma, several interrelated variables were associated with brainstem injury, including tumor volume (and, therefore, radiation volume), surgical morbidity, male gender, younger patient age, and cerebrospinal fluid shunting. The radiation dose was homogeneous between 54 and 59.4 Gy.45
In a study of 40 patients undergoing IMRT for meningioma, one patient developed fatal brainstem necrosis after receiving a maximum dose to the brainstem of 55.6 Gy, with 4.74 mL exceeding 54 Gy.46 This demonstrates that other poorly understood factors likely increase the risk of brainstem toxicity because this dose constraint would be considered acceptable in most of the studies referenced above.
Nervous System: Spinal Cord
Dose-Volume Data
It is well-accepted that the spinal cord can tolerate 45 to 50 Gy with conventional fractionation, though the TD5 (tolerance dose with 5% injury) is most likely much higher. The spinal cord is generally limited to 45 to 50 Gy because the anticipated risk of cord injury must be very low to be clinically acceptable. In an analysis of several studies, Schultheiss calculated the probability of cervical cord myelopathy after full cross-sectional irradiation as 0.03% after 45 Gy, 0.2% after 50 Gy, and 5% after 59.3 Gy.48 The thoracic spinal cord was calculated to be less sensitive than the cervical spinal cord (although because of the dispersion of data, a good fit could not be obtained). This model does not incorporate spinal cord volume, and this might be acceptable given the “series” nature of the cord. Nevertheless, Schultheiss cautions that long lengths of cord, concomitant chemotherapy, and other factors may increase the risk.
Little data exist exploring the dose-volume tolerance of the spinal cord. No spinal cord toxicity was reported in a study in which the V50 was less than 0.1 mL36 or in another study in which the D1 was less than 45 Gy.37 Massachusetts General Hospital recently studied 85 patients treated to the cervical spinal cord in the range of 45 to 59.4 Gy (1.5-Gy equivalent fractions) equivalent uniform dose (EUD), 42- to 57.5-Gy maximal dose to the cord center, and 57- to 74-Gy maximal dose to the cord surface.49 Fifteen percent of these patients experienced Lhermitte’s syndrome (self-limited transverse myelitis, with symptoms of electric shock-like sensation most notable with neck flexion), and 5% developed objective neurologic findings at or below the cord level treated. Toxicity was not significantly correlated with cord length, cord volume, maximal dose to the cord center, maximal dose to the cord surface, or effective uniform dose. The authors conclude that an EUD to the cervical cord of 60 Gy in 1.5-Gy fractions or 52.5 Gy in 2-Gy fractions is safe.
Nervous System: Optic Nerves and Chiasm
Organ Function and Clinical Significance
It is well-accepted that the entire system of optic nerves and the chiasm may be treated to 54 Gy using conventional fractionation with minimal risk of late visual toxicity, though lower doses can result in other ophthalmologic toxicity.51 In a study from M.D. Anderson Cancer Center (MDACC) in which 219 patients were treated before three-dimensional irradiation was available, 10-year actuarial rates of optic neuropathy were 0%, 3%, and 34% for 43 to 49 Gy at approximately 1.9 Gy per fraction, 50 to 60 Gy at approximately 2.1 Gy per fraction, and 61 to 76 Gy at approximately 2.2 Gy per fraction, respectively. Chiasm damage was similar, with rates of 0%, 8%, and 24% for 15 to 49 Gy at approximately 1.5 Gy per fraction, 50 to 60 Gy at approximately 2 Gy per fraction, and 61 to 76 Gy at approximately 2.1 Gy per fraction, respectively.52 Greater total dose as well as larger fraction size affected risk.53–56 With three-dimensional planning, small volumes of optic nerve and chiasm may tolerate higher doses.
Dose-Volume Data
Several institutions have published their dose-volume constraints for the optic nerves and chiasm, with most not reporting any neurologic visual toxicity. These constraints for patients undergoing external beam radiation therapy for head and neck cancer include V55 of less than 0.1 mL of the optic nerves and chiasm,36 D1 of less than 54 Gy for the optic nerves, and D1 of less than 45 Gy for the optic chiasm37 and a maximum of less than 54 Gy to the optic nerves and 52 Gy for the chiasm. For patients undergoing proton or combined proton/photon therapy for base of skull lesions, doses include less than 55 to 56 GGE40–42 or less than 60 Gy CGE39 to the optic nerves and optic chiasm.
Several studies have reported ophthalmologic toxicity after irradiation. In a study from the University of Florida, the optic nerve dose (defined as the minimum dose delivered to one-third of the optic nerve) in patients who developed optic neuropathy was 50.4 to 79 Gy (median, 68 Gy).56 In a University of Michigan study,57 seven patients developed ophthalmologic toxicity: one patient received a chiasm maximum of 59.5 Gy, and six patients received an optic nerve maximum of 47.5 to 75.5 Gy (average, 63 Gy). Moderate to severe optic nerve complications (four patients) were associated with doses of more than 64 Gy. In one study, four patients developed ophthalmologic toxicity; three had received a maximal dose of 56 to 62 CGE to the optic chiasm/nerves and one had received a maximum dose of more than 62 CGE. In two studies, bilateral visual loss occurred, with no evidence of tumor progression 8 months after conventional radiation; in one study, the prescribed target dose was 49.3 Gy (maximum dose, 56.1 Gy; chiasm maximum dose not reported, but <1 mL received >45 Gy),58 and in the other study, the chiasm maximum dose was less than 58 CGE.40
Some patients tolerate a maximum optic nerve or chiasm dose of more than 60 Gy or more than 63 to 69 CGE.38,39,41,42,57 This demonstrates that other poorly understood factors likely increase the risk of optic nerve and chiasm toxicity. An optic nerve maximum dose of more than 80 Gy and a chiasm maximum dose of more than 70 Gy were tolerated in some patients in the University of Michigan study discussed above.57 In another study, the dose constraint of 56 CGE to the optic nerve and chiasm was “relaxed” in 28% and 48% of patients, respectively, with no reported visual toxicity. In these patients, the volume receiving a dose above the threshold dose was 0.11 mL and 0.12 mL for the optic nerves and chiasm, respectively; the volume receiving a dose more than 105% of the threshold dose was 0.05 mL and 0.01 mL, respectively.41
Nervous System: Hearing
Organ Function and Clinical Significance
The cochlea and acoustic nerve are the essential auditory structures that are susceptible to radiation injury and consequential sensory neural hearing loss. These are small structures and, therefore, dose-volume measures are less determinable and clinically relevant. Other dose-dependent susceptible parts of the auditory system include the external ear and ear canal, tympanic membrane, ossicles, and eustachian tube.60 Platinum-based chemotherapy also is a well-established cause of sensory neural hearing loss. Factors such as baseline function and patient age are also relevant.
Dose-Volume Data
Several studies suggest that the dose to the cochlea correlates with the rate of sensory neural hearing loss. A study from the University of Florida showed that the incidence of sensory neural hearing loss increased consistently with the dose to the cochlea; the 10-year actuarial risk of sensory neural hearing loss was 3% at doses of less than 60.5 Gy versus 37% at doses of more than 60.5 Gy .60
Several other studies have shown hearing loss to be directly related to the inner-ear dose,61–65 with sensory neural hearing loss becoming more apparent at doses of more than 45 to 50 Gy.62,64,65 Cisplatin dose is also relevant to the threshold for auditory impairment. Cisplatin doses as low as 270 mg/m2 can result in hearing loss when combined with cranial radiotherapy dosages of 40 to 50 Gy.66 The sequence of chemoradiotherapy also influences risk. Risk and severity of ototoxicity are greater when cisplatin is administered after cranial irradiation.
Parotid Glands
Dose-Volume Data
The University of Michigan has published several studies investigating radiation dose-volume effects on serially measured stimulated and unstimulated salivary flow from a given parotid gland. With salivary flow measured up to 12 months after irradiation, significant parotid sparing was observed after a mean parotid dose below 24 Gy (for unstimulated flow) to 26 Gy (for stimulated flow).68,69,70 Mean doses correlated with a V15 of 67% or less, a V30 of 45% or less, and a V45 of 24% or less. The TD50 was 28.4 Gy.
Investigators at Washington University also examined whole salivary flow. Their technique differed from that of the University of Michigan researchers in that they measured flow from all glands, though they included only patients whose submandibular glands received more than 50 Gy in an attempt to minimize this confounding variable.71,72 Salivary flow was noted to be exponentially reduced by approximately 0.054/Gy of the mean parotid dose (i.e., e(−0.054* mean dose)), equating to a reduction to 25% of the pretreatment salivary flow with a mean dose of 25.8 Gy, essentially equivalent to the University of Michigan number.73 The mean dose model was more predictive of the risk of late effects than threshold dose levels of V5 to V70 as well as other models studied.
Other groups have corroborated the mean dose as a significant variable affecting salivary function,74–79 with a Belgian study suggesting a lower threshold mean dose of 22.5 Gy.80 A Dutch group demonstrated that the TD50 for stimulated salivary function (risk of <25% of pretreatment rates) improved with time, with a TD50 of 34 Gy at 6 weeks to 40 Gy at 6 months, 42 Gy at 12 months, and 46 Gy at 5 years.81,82 In a recent University of Michigan study, with flow rates measured in 142 patients up to 24 months after irradiation, the salivary flow rate was modeled as a function of time.83 With mean parotid doses of less than 25 Gy, the model predicted salivary function recovery to the pretreatment level of functioning at 12 months. For mean parotid doses of more than 30 Gy, stimulated saliva did not return to pretreatment functioning after 2 years.
The radiation dose to the submandibular glands may also affect salivary function. In a study from Helsinki University, the mean unstimulated salivary flow was 60% of the pretreatment function among patients who had one submandibular gland spared (mean dose of 26 Gy and range from 21 to 34 Gy) and 25% among those who did not (p = .006).84 However, sparing of a submandibular gland did not affect the stimulated saliva flow rates. Investigators from Utrecht have demonstrated that the mean submandibular dose is a significant variable affecting the sensation of sticky saliva.85
Larynx and Pharynx
Organ Function and Clinical Significance
The larynx and pharynx are involved with phonation and swallowing, respectively. Late complications include laryngeal edema and fibrosis, laryngeal dysfunction, dysphagia, and necrosis. A study from the University of Michigan showed that damage to the pharyngeal constrictors and the glottic and supraglottic larynx may cause dysphagia and aspiration after chemoradiation.87
Dose-Volume Data: Larynx
In a study from the University of Texas at Galveston (with most patients receiving irradiation alone), the mean laryngeal dose and laryngeal V30 to V70 were significantly associated with laryngeal edema of grade 2 or higher with a univariate analysis, whereas the mean laryngeal dose and N stage were significant with multivariate analysis.88 The V50, which was highly correlated with the mean laryngeal dose, was significant in a multivariate analysis in which the mean laryngeal dose was replaced by V50. The 1-year rate of grade 2 or higher edema was 20% after a mean laryngeal dose of 43.5 Gy (or V50 <27%), versus 45% after a mean dose of 44 to 57 Gy (or 94% < V50 > 27%) versus more than 80% after a mean dose of more than 57 Gy (or V50 >94%).
There is a paucity of data correlating dose-volume metrics with vocal dysfunction. Generally, after therapeutic doses of 60 to 66 Gy for early-stage glottic cancer, the risks of vocal dysfunction are low. In a study from the University of Iowa, point doses in excess of 66 Gy to the aryepiglottic folds, preepiglottic space, false vocal cords, and lateral pharyngeal walls resulted in a sharp increase in the risk of vocal dysfunction.89
Dose-Volume Data: Swallowing
Several studies have investigated dose-volume metrics predictive of swallowing dysfunction. In a study from the University of Michigan, the mean dose to the pharyngeal constrictors was correlated to the risk of aspiration.91 The risk of aspiration increased with a mean dose of more than 60 Gy to the pharyngeal constrictors, and with a V40 of more than 90%, V50 of more than 80%, V60 of more than 70%, and V65 of more than 50%, and with a V50 of more than 50% for the larynx. Three patients who developed strictures had a V70 of the pharyngeal constrictor of more than 50%.
In a study from Aarhus University, several significant correlations were found between both subjective and objective swallowing problems and DVH parameters of the base of the tongue, pharyngeal constrictors, supraglottic larynx, esophageal sphincter, and glottic larynx.92 Doses of less than 60 Gy to the supraglottic region, larynx, and upper esophageal sphincter resulted in a low risk of swallowing difficulties and aspiration.
In a study from the University of Iowa, point doses to the false vocal cords, lateral pharyngeal walls, and upper esophageal sphincter were correlated with dietary changes.89 As an example, the dose to the left lower pharyngeal wall for patients who had not had oral intake 1 year after treatment was 79.7 Gy versus 65.1 Gy for those with an unrestricted diet. A greater radiation dose to the aryepiglottic folds was associated with greater weight loss, and a greater radiation dose to the false vocal cords was associated with percutaneous endoscopic gastrostomy (PEG) tube use.
In a study from Rotterdam, doses of more than 50 Gy to the pharyngeal constrictors resulted in a 20% probability of dysphagia, with an incremental 19% risk for each additional 10 Gy to the constrictors.93 A study from the Dana-Farber Cancer Institute94 also demonstrated that doses to the pharyngeal constrictors of 50 Gy or more, as well as doses to the larynx of 50 Gy or more, were significantly associated with aspiration and stricture risk.
Lungs
Organ Function and Clinical Significance
Radiation damage to the lung can result in pneumonitis and fibrosis. Symptomatic radiation pneumonitis is characterized by dyspnea, cough, and sometimes a low-grade fever, typically occurring several weeks to months after irradiation. Long-term lung fibrosis can lead to respiratory insufficiency. It is often challenging to distinguish radiation-related pulmonary symptoms from comorbid illnesses (e.g., exacerbation of chronic obstructive pulmonary disease [COPD], infection, or cardiac events).95 Objective reductions in the lung’s ability to move and exchange gas can be measured by formal pulmonary function tests (PFTs).
Dose-Volume Data: Pneumonitis and Fibrosis
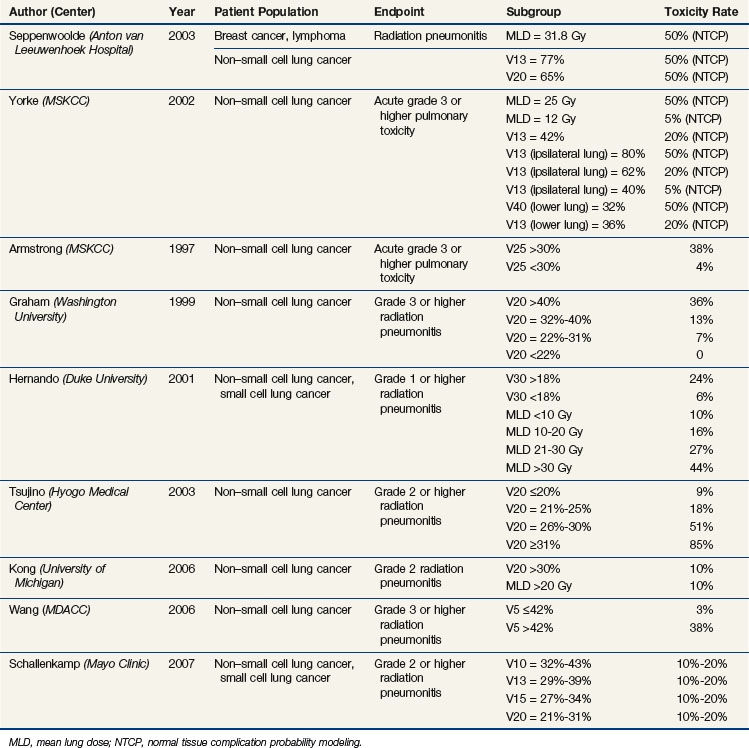
Several parameters have been shown to be associated with the risk of radiation pneumonitis, including the V5 to V70, mean lung dose (MLD), and model-based parameters.96,97 These variables are correlated with each other, accounting for the fact that in most studies examining a range of Vx variables, many or most are significant.* Table 13-2 summarizes many of the studies discussed below.
A normal tissue complication probability (NTCP) analysis from the Netherlands, in collaboration with the University of Michigan, suggests that using the mean lung dose (linear function) is more predictive than using the Vx (step function).101 However, the V13 tended to be more predictive in situations where the mean lung dose exceeded 20 Gy or the V13 exceeded 50%. The TD50 values in this study were a mean lung dose of 30.8 Gy, V13 of more than 77%, and V20 of more than 65%, similar to the mean lung dose of 31.8 Gy reported in an earlier multiinstitutional study.112 From an NTCP analysis from the Memorial Sloan-Kettering Cancer Center (MSKCC),99 a mean lung dose of approximately 26 Gy, a V13 of more than 80% of the ipsilateral lung, or a V40 of more than 32% of the lower lung resulted in a 50% risk of developing late complications. A mean lung dose of approximately 12 Gy or a V13 of more than 40% of the ipsilateral lung resulted in a 5% late complication risk. A V13 of 36% of the lower lung, 42% of the total lung, or 62% of the ipsilateral lung resulted in a 20% risk of developing late grade 3 or higher complications.
In a 1997 study from the MSKCC, of patients treated with irradiation alone, there was a significantly increased risk of grade 3 or higher pulmonary toxicity, 38% with a V25 of more than 30% versus 4% with a V25 of less than 30% (p = .04).113 In subsequent studies from this same group, significant variables for predicting grade 3 or higher pulmonary toxicity included the mean lung dose, the range of V5 to V40 of the total lung, V5 to V40 of the ipsilateral lung, and V5 to V50 of the lower lung.99,104 The range of V5 to V20 for the ipsilateral lung was most predictive.
Washington University investigators also demonstrated that the risk of pneumonitis significantly correlated with the V20; the 2-year incidence of grade 2 or higher radiation injury was 36% versus 13% versus 7% versus 0%, with a V20 of more than 40%, 32% to 40%, 22% to 31%, and less than 22% (p = .0013), respectively.96 In another study from Washington University, radiation pneumonitis was significantly correlated with the V5 to V80, with peak significance in the ranges of V5 to V15 and V70 to V75; radiation pneumonitis was also significantly correlated with the dose delivered to 5% to 100% of the lung (D5 to D100), with peak significance in the D30 to D40 and V90 to V95 ranges.107
In a 2001 study from Duke University (in which 18% of patients received concurrent chemoradiotherapy), a V30 of more than 18% versus less than 18% was associated with a risk of grade 1 or higher radiation pneumonitis of 24% versus 6% (p = .0003).98 Mean lung doses of less than 10 Gy, 10 to 20 Gy, 21 to 30 Gy, and more than 30 Gy were associated with risks of 10%, 16%, 27%, and 44%, respectively. A Japanese study of patients treated with platinum-based chemoradiotherapy found a 6-month risk of grade 2 or higher radiation pneumonitis to be 85%, 51%, 18.3%, and 8.7% (p <.0001), with a V20 of 31% or higher, 26% to 30%, 21% to 25%, and 20% or less, respectively.114 In a University of Michigan study, a 10% risk for grade 2 or higher pneumonitis and fibrosis was associated with a V20 of more than 30% and a mean lung dose of more than 20 Gy. These thresholds provided a positive predictive value of 50% to 71% and a negative predictive value of 85% to 89%.115 In a study from the MDACC, the mean lung dose and V5 to −V65 were highly correlated with the risk of pneumonitis, and the V5 was the most significant factor in a multivariate analysis.106 For a V5 of 42% or less versus more than 42%, the risk of grade 3 or higher pneumonitis at 1 year was 3% versus 38% (p = .001). In a Mayo Clinic study, the V10 to V13 was most predictive of radiation pneumonitis; a V10 of 32% to 43%, V13 of 29% to 39%, V15 of 27% to 34%, and V20 of 21% to 31% resulted in a 10% to 20% risk of pneumonitis.111
Several dose escalation studies have used the V20, Veff, and/or NTCP (normal tissue complication probability) to allocate patients into given dose levels.116–119 In the RTOG 93-11 dose escalation study, patients with a V20 of less than 25% experienced a 7% to 16% 18-month actuarial rate of grade 3 or higher late lung toxicity with prescribed doses of 70.9 to 90.3 Gy; the absolute risk of grade 2 or higher late lung toxicity was 30% to 45%, with one fatal lung complication at the 90.3-Gy dose level. Patients with a V20 of 25% to 36% treated to doses of 70.9 to 77.4 Gy experienced 15% grade 3 or higher late toxicity at 18 months and an absolute risk of grade 2 or higher late lung toxicity of 40% to 60%.116 The D15 was the most predictive variable for radiation pneumonitis.110
In a Dutch study, the effect of regional dose in the lung was investigated, with the lung divided into central and peripheral, ipsilateral and contralateral, caudal and cranial, and anterior and posterior subvolumes.120 The mean regional dose to the posterior, caudal, ipsilateral, central, and peripheral lung subvolumes significantly correlated with the incidence of steroid-requiring radiation pneumonitis. In a similar study from the MSKCC, the risk of radiation pneumonitis was better correlated with the radiation dose to the inferior aspect of the lung rather than the superior aspect.99
Tumor location within the chest may also be a factor affecting risk of pneumonitis. In the above-mentioned study from Washington University,107 inferior tumor location was the most significant predictor of radiation pneumonitis. Tumor location was not a strong correlate to radiation pneumonitis in a study of patients treated in RTOG 93-11, perhaps attributable, in part, to differences in treatment (with RTOG 93-11 designed to treat smaller volumes to higher doses) and differences in tumor size and location (the RTOG 93-11 tumors tended to be smaller and more superiorly located).110 Using a combined data set of patients from RTOG 93-11 and Washington University, tumor location, in addition to mean lung dose, was significant.
IMRT has been demonstrated to provide unique dose distributions for some patients with advanced-stage non–small cell lung cancer.121 The IMRT-based dose distributions appear to reduce the risk of normal tissue injury in patients with non–small cell lung cancer. Investigators at the MDACC compared their rates of lung toxicity in patients treated with IMRT versus three-dimensional planning and noted a reduction in toxicity with the IMRT approach.122 Investigators at the MSKCC noted a similarly low rate of clinical lung injury in patients with non–small cell cancer treated with IMRT.123 In the MDACC study, a V5 of more than 70% was associated with a 21% risk of grade 3 or higher pneumonitis versus a 2% risk with a V5 of 70% or less (p = .017).
IMRT has also been used to treat patients with mesothelioma. In a study from the Dana-Farber Cancer Institute, in which patients received thoracic IMRT after pneumonectomy for mesothelioma, 6 of 13 patients developed fatal pneumonitis.124 The median V20, V5, and mean lung dose for patients who developed pneumonitis was 17.6%, 98.6%, and 15.2 Gy, respectively, versus 10.9%, 90%, and 12.9 Gy for those who did not develop pneumonitis. Although these differences were not significant, the severity of the toxicities merits caution when large volumes are being treated after a pneumonectomy. In a study from Duke University, 1 of 13 patients treated with IMRT for mesothelioma died from pneumonitis, and 2 others developed symptomatic pneumonitis.125 The median V20, V5, and mean lung dose for patients who developed pneumonitis were 2.3%, 92%, and 7.9 Gy, respectively, versus 0.2%, 66%, and 7.5 Gy for those who did not develop pneumonitis, versus 6.9%, 92%, and 11.4 Gy for the patient who developed fatal pneumonitis. In a study of mesothelioma patients from the MDACC, 6 of 63 patients died from pulmonary-related causes (including 2 patients with fatal pneumonitis).126 The V20 was significant on univariate and multivariate analyses (p = .017), with a V20 of more than 7% corresponding to a 42-fold increase in the risk of pulmonary death.
Dose-Volume Data: Pulmonary Function
Changes in pulmonary function tests following thoracic irradiation reflect a combination of improvements due to tumor shrinkage (typically, acute changes) and declines due to radiation-induced injury (both acute and chronic changes).128 Several studies have noted long-term declines in pulmonary function tests following thoracic irradiation.129–131 The association between shortness of breath following irradiation and decline in pulmonary function tests is complex.132 Duke University investigators have shown a correlation between the V30 and NTCP with changes in the forced expiratory volume in 1 second (FEV1) and carbon monoxide diffusion in the lung (DLCO).133 The University of Michigan studies, however, did not find any correlation between the V20, mean lung dose, or Veff with changes in the FEV1 or DLCO.134 Attempts have been made to model the risk of changes in pulmonary function tests,135,136 although this is a complicated area of study.
Similarly, imaging tests provide clear objective metrics. Several studies have noted an association between regional irradiation doses and changes in regional imaging tests (CT-defined density or SPECT-defined perfusion/ventilation).137–140 The extent and severity of these imaging changes is related to changes in global lung function (assessed as either symptoms or changes in pulmonary function tests), but the correlations are relatively weak.128,135,136,140
Heart
Dose-Volume Data
Abundant studies have demonstrated an increased risk of cardiac morbidity following left-sided thoracic irradiation versus right-sided thoracic irradiation in patients treated for breast cancer. Reducing the dose prescribed to the mediastinum and reducing the volume of heart in the radiation field reduces the risk of late toxicity.141–143 Recent studies from Duke University demonstrated that an increased percentage of the left ventricle irradiated correlates with a greater risk of cardiac perfusion defects.144–146 Even over the range of low-dose exposure (~8 to 20 Gy) to small volumes of the cardiac apex, an increased risk of heart disease has been reported.147
A study from Stockholm used NTCP modeling to predict the risk of late heart toxicity in women treated for breast cancer.148 In their models, the TD50 was optimized to a value of 52 Gy to the myocardium. A 5% risk of excess cardiac mortality at 15 years was associated with a myocardial dose of approximately 30 Gy, a V33 of more than 60%, a V38 of more than 33%, or a V42 of more than 20%. Calculations using the whole heart volume (as opposed to the myocardial volume) yielded similar values.
The same group used a similar analysis to assess cardiac risk in Hodgkin’s disease patients.149 Patients were stratified into two risk groups: those with a V38 of more than 35% and those with a V38 of less than 35%. The excess mortality risk at 15 years was 7.9% and 4.7%, respectively. The TD50 was calculated to be 70 Gy. A heart dose of 42 Gy resulted in a 5% NTCP, whereas a heart dose of 53 Gy resulted in a 10% NTCP. The corresponding values in the breast cancer patients were 37 Gy and 44 Gy, respectively (lower threshold doses and a steeper gradient were used). The differences in complication probabilities and the TD50 between the breast and Hodgkin’s disease cohorts suggest that radiation exposure to different portions of the heart result in differences in cardiac risk,149 though there may be other confounding variables not easily identified (e.g., patient age at treatment, similar risk factors between breast cancer and cardiac disease).
There are few data on correlating heart dose-volume metrics with the subsequent ejection fraction. In a study of patients with esophageal cancer treated with chemoradiation at Roswell Park Cancer Institute, the radiation dose to the heart, including the left ventricle and left anterior descending artery (quantified as V20 to V40), was not clinically or statistically associated with changes in the ejection fraction (albeit this was a small study, with limited follow-up).150
A study from the MDACC described the risk of pericardial effusion in patients treated for esophageal cancer.151 A mean dose of more than 26 Gy and relative volumes of the pericardium treated at doses greater than 3 to 50 Gy (rV3 to V50), with the strongest association being rV30. At 18 months following irradiation, for an rV30 of less than 46% versus more than 46%, the rate of pericardial effusion was 73% versus 13% (p = .001); for a mean pericardium dose of less than 26 Gy versus more than 26 Gy, the rate of pericardial effusion was 73% versus 13% (p = .001). A study from the University of Michigan also demonstrated that a mean dose of more than 27 Gy and a maximum dose of 47 Gy correlated with the risk of pericardial effusion; however, only patients treated with 3.5-Gy fractions developed pericardial effusions.152
Radiotherapy has been associated with valvular heart disease.153 The incidence has been related to mediastinal radiation doses of more than 30 Gy and younger age at irradiation. Subclinical valvular disease has been detected at 2 years to more than 20 years following irradiation,153 but it appears to take much longer for clinical symptoms to become apparent (median interval, 22 years from irradiation to symptoms). For patients receiving irradiation for Hodgkin’s lymphoma, at a median of more than 10 years after irradiation, aortic disease usually consists of mixed stenosis and regurgitation and is more common than mitral and right-sided valvular disease.153,154
Anthracyclines and Other Risk Factors
In Hodgkin’s disease patients, radiation exposure, in conjunction with anthracyclines, may impair the ejection fraction and increase the risk of myocardial infarction, congestive heart failure, and valvular disorders. Data on the combined effects of anthracycline and irradiation remain sparse. A recent report of 1474 Hodgkin’s lymphoma survivors younger than 41 years at treatment and followed for a median of 18.7 years has shed some light.155 Risks of myocardial infarction and congestive heart failure were significantly increased, with standard incidence ratios of 3.6 and 4.9, respectively, for Hodgkin’s lymphoma survivors versus the general Dutch population. Mediastinal irradiation alone increases the risks of myocardial infarction, angina pectoris, congestive heart failure, and valvular disorders (twofold to sevenfold). The addition of anthracyclines further elevated the risks of congestive heart failure and valvular disorders from mediastinal irradiation, with hazard ratios of 2.81 and 2.10, respectively. The 25-year cumulative incidence of congestive heart failure following combined irradiation and anthracycline chemotherapy was 7.9%.
Other risk factors for cardiac disease, particularly coronary artery disease, must be considered. For example, investigators from the University of Rochester assessed the risk of coronary artery disease in survivors of Hodgkin’s lymphoma and also the prevalence of cardiac risk factors. The relative risk of cardiac death was 3.1 for males versus 1.8 for females. Other risk factors were more common than in the general population; among patients with Hodgkin’s lymphoma experiencing morbid cardiac events, 72% smoked, 72% were male, 78% had hypercholesterolemia, 61% were obese, 28% had a positive family history, 33% had hypertension, and 6% had diabetes.156
Gastrointestinal System: Esophagus
Organ Function and Clinical Significance
The esophagus is a slightly moveable hollow, distensible organ. On CT images, the cross-sectional esophageal area or volume is highly variable, but these images may not accurately reflect the true anatomy of the organ. In gross esophageal specimens, the cross-sectional area of the esophagus is fairly uniform at all axial levels.158
Dose-Volume Data
In a series from Washington University, grade 3 to 5 esophageal toxicity (acute and late effects) was associated with a maximal dose (Dmax) of more than 58 Gy, a mean dose of more than 34 Gy, and the administration of concurrent chemotherapy.159 The V55 was not significant. A Chinese study reported that a maximal dose of more than 60 Gy and the use of concurrent chemotherapy were significant factors for esophageal toxicity (acute and late toxicity).160 In a series from Duke University, the V50, the surface dose receiving 50 Gy or more (S50), the length of esophagus receiving more than 50 to 60 Gy, and a circumferential Dmax of more than 80 Gy were significant predictors of late esophageal toxicity.161 A V50 of more than 32% or an S50 of more than 32% resulted in a crude rate of approximately 30% for late esophageal toxicity versus 7% below these thresholds (p = .02 and .04, respectively). With more than 3.2 cm of the esophagus receiving more than 50 Gy, late toxicity occurred in approximately 30% of patients versus 4% of those with more than 3.2 cm receiving more than 50 Gy (p = .008). In another series from Duke University, late toxicity of grade 1 or higher was correlated with several dose parameters: the entire circumference receiving 50 Gy or more and 55 Gy or more; 75% of the circumference receiving 70 Gy or more; and a maximal percentage of the circumference receiving 60 to 80 Gy or more.162 The rate of grade 1 or higher late toxicity was approximately 5% in patients with a V50 to V70 of 0% to 30% versus approximately 25% in those with a V70 of 31% to 64% and approximately 10% in those with a V50 of more than 60% (nonsignificant). Acute esophageal toxicity was the greatest predictor of late toxicity. In two studies, most of the patients who developed late grade 3 or higher toxicity had developed acute grade 3 or higher toxicity, though roughly 25% to 40% of patients who developed grade 3 or higher late toxicity had only grade 0 to 2 acute esophageal toxicity.159,160
Gastrointestinal System: Stomach and Small Bowel
Organ Function and Clinical Significance
Symptoms from radiation-related late toxicities of the stomach and small bowel include dyspepsia, gastric ulceration, diarrhea, bowel obstruction, and bowel ulceration, fistula, or perforation. The primary long-term endpoint considered for the small bowel is stricture and diarrhea. For the stomach, perforation and ulceration are commonly considered. Several patient-related variables such as history of diabetes, age, race, body habitus, and prior surgery likely affect the risk of late toxicity.164 Because the stomach and small bowel are mobile and distensible, determining the accurate dose-volume (or dose-surface) constraints is challenging. Factors affecting risk of late toxicity include total dose (with doses in excess of 40 to 50 Gy increasing the risk of late complications), fractional dose, prior abdominal surgery (which increases the risk of bowel obstruction), and concurrent chemotherapy use.
Dose-Volume Data
Dose
Late radiation-induced stomach injury has been reported to occur with increasing frequency with increasing doses. In a study from Walter Reed Army Institute of Research, the rates of gastric ulceration were 4% and 16% after treatment with less than 50 Gy versus more than 50 Gy. Similarly, the rates of perforation were 2% and 14% in the same dose cohorts. Overall, a dose of approximately 50 Gy to the stomach is associated with an incidence of about 2% to 6% of severe late injury. The volume effect for late stomach injury is not well defined. For late small bowel toxicity, doses of approximately 50 Gy are associated with obstruction and perforation rates of approximately 2% to 9%. Prior abdominal surgery appears to increase the risk of late small bowel injury following irradiation. In an EORTC study, the rate of complications was 3% without prior abdominal surgery versus 12% with prior abdominal surgery.165
Dose Volume
There is a paucity of good quantitative data on dose-volume metrics that predict for gastric or bowel late toxicity. Nevertheless, there are data that demonstrate a volume effect. The risk of bowel obstruction among patients with rectal cancer whose fields extended to L1 or L2 was 30% versus 9% in those treated with smaller pelvic fields only.166 The University of Michigan researchers investigated gastric and duodenal bleeding after irradiation of liver tumors.167 Normal tissue complication modeling was consistent with a dose threshold (~60 Gy) for bleeding without a large volume effect.
Summary and Other Key Points from the QUANTEC Review168
The stomach and the intestines are mobile structures with interfraction and intrafraction motion. This makes data regarding doses, volumes, and outcomes less certain. Two different approaches have been considered when defining small bowel volumes. Either the entire potential space of small bowel-containing volume (i.e., incorporating all regions where bowel can be situated)169 or the actual visualized loops of bowel on the planning CT scan170 can be considered as an organ at risk. Using the entire potential small bowel space, it is suggested that the small bowel exposed to 45 to 50 Gy should be less than 195 mL to reduce acute toxicity (not discussed above)169; for the visualized loops of bowel, it is recommended that the V15 should be less than 120 mL.170 Although these dose constraints were derived from acute toxicity data, they provide guidelines that should help minimize the risk of late toxicity as well. For the stomach, the recommendation is to maintain the dose to the whole stomach at less than 45 Gy; a maximum point dose might be an important predictor of toxicity, but more data are needed to confirm this hypothesis.
Gastrointestinal System: Rectum
Organ Function and Clinical Significance
The most common late radiation-related rectal complication is bleeding. Rectal ulceration and fistula are much less common. Other late injuries include stricture and decreased rectal compliance, which can result in frequent small stools. The anus is also at risk of late complication, including stricture and laxity, leading to fecal incontinence. Patients are at a higher risk of late rectal sequelae, including gastrointestinal bleeding, proctitis, diarrhea, and tenesmus, with higher maximal and mean rectal doses. Several patient-related variables, such as history of diabetes and/or vascular disease, inflammatory disease, and age, may affect the risk of late toxicity.164,171,172 Prior abdominal surgery is also relevant.173
Dose-Volume Data
Abundant dosimetric data have shown a correlation of risk with rectal volume and surface and rectal wall doses. Table 13-3 summarizes many of the studies discussed below.
TABLE 13-3 Summary of Selected Studies Analyzing Dose-Volume Parameters Predictive of Rectal Toxicity
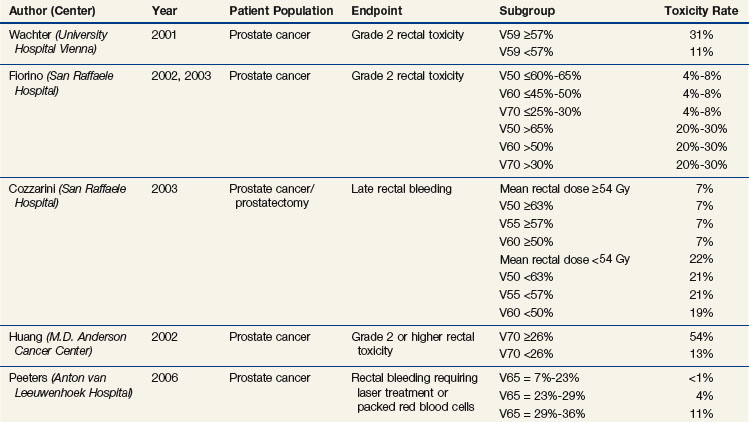
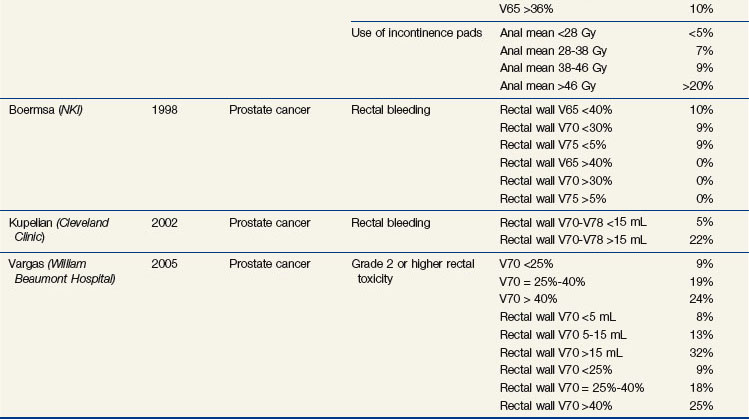
Investigators from the MSKCC have shown a significant difference in the DVHs between patients who developed rectal bleeding versus those who did not after conformal irradiation for prostate cancer.174 The percentage of rectum exposed to 62% and 102% of the prescription dose (70.2 or 75.6 Gy) was significant; the rectal wall being encompassed by the 50% isodose line, higher maximal dose to the rectum and smaller rectal volume were also significantly adverse risk factors.174,175 In a recent study of 1571 patients treated at the MSKCC, the use of IMRT and the lack of acute rectal toxicity predicted for lower risk of late rectal toxicity.176
An Austrian study found that a V59 of 57% or more resulted in increased grade 2 rectal toxicity (31% vs. 11%, p = .003).177 Two Italian multicenter studies found a significant dose volume effect from V50 to V70, with suggested cutoff values for the V50 of 60% to 65% or less, V60 of 45% to 50% or less, and V70 of 25% to 30% or less; the risk of grade 2 or higher rectal complications was approximately 4% to 8% versus approximately 20% to 30% above and below the cutoff values.178,179 A rectal volume of less than 55 mL was also a significant risk factor for late bleeding. In the postprostatectomy setting, this Italian group demonstrated that a mean rectal dose of 54 Gy or more, V50 of 63% or higher, V55 of 57% or higher, V60 of 50% or higher, and a rectal volume of less than 60 mL were predictive of late bleeding.180
In a randomized trial of 70 Gy versus 78 Gy from the MDACC in the treatment of early-risk to intermediate-risk early-stage prostate cancer, the risk of late grade 2 or higher rectal complications was significantly greater with a rectal V70 of 25% or higher versus a V70 of less than 25% (46% vs. 16%; p = .001).181 A retrospective analysis from the MDACC showed that the risk is a continuous function of dose and volume, with suggested cutoff points for lowering the complication risk of a V60 of 41% or less, V70 of 26% or less, V76 of 16% or less (or 3.8 mL), and V78 of 5% or less (or 1.4 mL).182 At 6 years, the risk of late grade 2 or higher rectal complications was 54% for patients with a rectal V70 of 26% or more versus 13% for a V70 of less than 26%.
Among patients treated in the Dutch randomized trial with 68 Gy versus 78 Gy,172 the mean anal dose (as well as the V5 to V60) significantly predicted the rate of grade 2 or higher gastrointestinal toxicity (at 4 years, 16% vs. 31% for a mean dose <19 Gy vs. >52 Gy).183 The mean dose (as well as the V5 to V70) also predicted the risk for use of incontinence pads (at 5 years, <5% vs. >20% for a mean dose <28 Gy vs. >46 Gy). The anorectal V65 (as well as the V55 to V60) was significantly predictive of rectal bleeding (4-year risk <1% and >10% for a V65 <23% vs. >29%).183
The percentage of rectum or rectal wall receiving a given dose can be somewhat subjective (i.e., based on how much of the rectum is segmented); using the absolute volume of rectum184 or rectal wall is less subjective, though defining the rectal wall is not standardized. The rectum extends from the rectosigmoid junction to the anus, with the inferior extent generally defined as the level of the anal verge, above the anus, the ischial tuberosities, or 2 cm below the ischial tuberosities. Studies from William Beaumont Hospital demonstrated that the rectal volume as well as rectal wall V50 to V70 values predict late toxicity, with the rectal wall being more predictive of grade 2 to 3 late effects; acute toxicity is also predictive of late toxicity.185 MDACC investigators have also shown the rectal wall dose to be predictive of late rectal bleeding.186 From a 1998 Dutch study, recommendations for the volume of rectal wall exceeding 65 Gy, 70 Gy, and 75 Gy are less than 40%, less than 30%, and less than 5%, respectively.187 Data from the Cleveland Clinic184 and William Beaumont Hospital185 showed a significantly increased risk of grade 2 or higher rectal toxicity with a rectal or rectal wall V70 to V78 of 15 mL or higher versus less than 15 mL (~20% to >30% vs. ~5% to 10%).185
Gastrointestinal System: Liver
Dose-Volume Data
In the RTOG 84-05 phase I study, in which patients with liver metastases received whole liver irradiation with 1.5-Gy twice-daily fractions, none of the 122 patients who received 27 to 30 Gy developed biochemical evidence of liver toxicity, compared with 5 of 51 who received 33 Gy.189 In a study of 79 patients treated with liver radiotherapy at the University of Michigan, only those patients who received whole liver radiotherapy (1.5 to 1.65 Gy twice daily, with or without a boost) developed late radiation toxicity (crude risk of 9 of 33 patients vs. 0 of 46 patients treated with partial liver irradiation). Those who received a mean dose of more than 37 Gy (delivered twice daily with infusional fluorodeoxyuridine) and less sparing of normal liver had a greater risk of late radiation toxicity (crude risk of 9 of 34 patients vs. 0 of 45 patients).190
Several studies have explored partial liver irradiation in more detail, many of which used the mean liver dose as a dose-volume metric. In a later study from the University of Michigan, no late liver toxicity was observed with a mean liver dose of less than 31 Gy, with NTCP models being optimized with a TD50 of 43 Gy and a TD5 of 31 Gy for whole liver irradiation; the risk of complications was strongly dependent on the volume of liver irradiated.191 The NTCP models predict a TD5 in excess of 80 Gy if less than one-third of the liver is irradiated. With irradiation of two-thirds of the liver, the TD5 is on the order of 50 Gy and the TD50 is on the order of 60 Gy.
In a series from Taipei, patients with irradiated hepatocellular carcinoma who developed late liver toxicity had received a mean hepatic dose of 25 Gy (vs. 20 Gy in patients without toxicity; p = .02).192 The TD50 and TD5 for irradiation to the whole liver, two-thirds of the liver, and one-third of the liver were modeled to be approximately 43 Gy/25 Gy, 50 Gy/28 Gy, and 67 Gy/38 Gy, respectively. The volume effect of liver irradiation was less in this series. In another study from the same group, the mean liver dose and hepatitis B virus positivity were significant predictors of radiation toxicity; with NTCP modeling, the TD50 was approximately 50 Gy.193
In a Korean study of 105 patients with hepatocellular carcinoma, the mean dose and V20 to V40 parameters to the total liver and normal liver (total liver minus GTV) were investigated.194 The total liver V30 was the only significant parameter (p <.001). Grade 2 or higher liver toxicity was observed in only 2.4% of patients with a total liver V30 of 60% or less and 55% of patients with a total liver V30 of more than 60% (p <.001).
Genitourinary System: Kidney
Organ Function and Clinical Significance
Late kidney toxicity may be underreported due to its long latency period and the fact that it may be attributed to more common causes. Late renal complications include decreased kidney function, azotemia, hypertension, and kidney atrophy. Chronic radiation nephropathy in its mildest forms may not be diagnosed until years after therapy. Clinically silent abnormalities may include only proteinuria and azotemia with urinary casts and mild or no hypertension. Certainly, patients with only one functioning kidney, or poor baseline renal function, are more vulnerable to damage (with or without symptoms) for any given volume of renal irradiation. Platinum- and ifosfamide-based chemotherapeutics can also affect renal function. Cisplatin at dosages greater than 200 mg/m2 can result in glomerular or tubular injury and renal insufficiency.196 Ifosfamide can cause glomerular and tubular toxicity, with renal tubular acidosis, or Fanconi’s syndrome. A study examining grade 3 to 4 ifosfamide-induced nephrotoxicity amongst adult and pediatric patients found a prevalence of 17% in both, and neither age nor cumulative ifosfamide dose was a risk factor.197
Dose-Volume Data
Several studies have investigated whole kidney dose tolerance, either after whole abdominal irradiation or total body irradiation (generally delivered with lower fractional doses). Renal toxicity can occur after bilateral kidney doses of 10 Gy or higher, and the risk is quite high (50% to 80%) after 20 Gy. The kidneys have a relatively low threshold for damage. The dose volume effect on the kidneys has been long recognized, even before CT scanning was available for planning, because the kidneys are well visualized on plain simulation films. From these studies, when more than half of the kidney receives doses over 20 to 30 Gy, or more than one third receives over 30 to 40 Gy, patients are at increased risk of developing renal atrophy, decreased kidney function, and hypertension.1,198–200
There is little published on dose-volume parameters to predict late renal toxicity, in part because clinicians make an effort to minimize the volume of kidney exceeding the accepted tolerance dose. Low doses of 10 to 15 Gy to large volumes of kidney increase the risk of nephrotoxicity,201–203 whereas for smaller volumes of kidney, doses exceeding 20 to 25 Gy can result in late renal toxicity.201,203–205 In a series from Heidelberg, normal tissue complication modeling was used to estimate the risk of late complications.204 A median dose of approximately 17.5 to 21.5 Gy and 22 to 26 Gy corresponded to a 5% and 50% late complication risk (anemia, azotemia, hypertension, and edema), respectively. In another German study, reduced kidney function, as measured by scintigraphic changes, was analyzed as a function of dose and volume.201 After irradiation of 10% to 30%, 30% to 60%, and 60% to 100% of the kidney volume to 20 Gy, the incidence of reduced activity was less than 10%, approximately 40%, and more than 70%. After irradiation of 10% to 30%, 30% to 60%, and 60% to 100% of the kidney volume to 30 Gy, the incidence of reduced activity was approximately 35%, more than 90%, and more than 98%. In a Dutch study of patients with gastric cancer (treated with concurrent irradiation and cisplatin or capecitabine), a left kidney V20 of 64% or higher and a mean left kidney dose of 30 Gy or higher were associated with a significant decrease in left kidney function as compared with right kidney function.205
Genitourinary System: Bladder
Genitourinary System: Penile Bulb
Organ Function and Clinical Significance
The penile bulb is located at the base of the penis, caudal to the prostate.208 The irradiation dose to the penile bulb can affect erectile function, either as a direct result of damage to this structure (less likely) or damage to surrounding structures, whose radiation-induced damage is correlated with the dose exposure of the penile bulb. The most common scenario in which the penile bulb is irradiated is in the treatment of prostate cancer. IMRT is often used to minimize the dose to the penile bulb.209,210 Interpretation of erectile dysfunction and the effect of penile bulb dose is complicated by preexisting function, comorbid conditions, and other therapies that may hinder (i.e., hormonal therapy) or help (i.e., drugs used to treat erectile dysfunction) erectile function. Also, determining which patients experience erectile dysfunction—a toxicity with varying severity—also complicates data interpretation.
Dose-Volume Data
Several studies have investigated dose-volume parameters to predict risk of erectile dysfunction. In several studies, no correlation was discerned for penile bulb dose and erectile function.210–212 In one study, attempts were made to reduce the dose to the penile bulb (mean dose of 25 Gy), and, therefore, few patients received a high dose to the penile bulb.210 In another study of 70 patients, no correlation was found for mean dose or maximal dose to the penile bulb, penile crura, or superiormost 1 cm of the penile crura; DVHs were also compared and found to be similar.211
In a small, early study of 21 patients from the University of California, San Francisco, patients receiving a D70 of less than 40%, 40% to 70%, and more than 70% to the penile bulb had a 0%, 80%, and 100% risk, respectively, of experiencing radiation-induced impotence.213 In a study of 29 patients from Thomas Jefferson University, several dose-volume metrics were analyzed; a D30 of more than 67 Gy, D45 of more than 63 Gy, D60 of more than 42 Gy, and D75 of more than 20 Gy to the proximal penis were correlated with increased erectile dysfunction as well as ejaculatory function.214 In a study from Royal Marsden Hospital, a D90 of over 50 Gy to the penile bulb was associated with significantly worse erectile function, whereas values for D15, D30, and D50 showed a similar (albeit not significant) trend toward increased doses in impotent versus intermediately potent versus potent patients.215
The largest study (158 patients) to date to investigate penile bulb dose is an analysis of the RTOG 9406 dose escalation study.216 A median dose of 52.5 Gy or more was associated with a greater risk of impotence (50% vs. 25% at 5 years).
1 Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122.
3 Milano MT, Constine LS, Okunieff P. Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat Oncol. 2008;3:36.
25 Lee AW, Foo W, Chappell R, et al. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;40:35-42.
26 Lee AW, Kwong DL, Leung SF, et al. Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys. 2002;53:75-85.
43 Debus J, Hug EB, Liebsch NJ, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39:967-975.
48 Schultheiss TE. The radiation dose-response of the human spinal cord. Int J Radiat Oncol Biol Phys. 2008;71:1455-1459.
49 Marucci L, Niemierko A, Liebsch NJ, et al. Spinal cord tolerance to high-dose fractionated 3D conformal proton-photon irradiation as evaluated by equivalent uniform dose and dose volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;59:551-555.
57 Martel MK, Sandler HM, Cornblath WT, et al. Dose-volume complication analysis for visual pathway structures of patients with advanced paranasal sinus tumors. Int J Radiat Oncol Biol Phys. 1997;38:273-284.
60 Bhandare N, Antonelli PJ, Morris CG, et al. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67:469-479.
70 Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg. 2003;27:832-837.
73 Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055-1069.
83 Li Y, Taylor JM, Ten Haken RK, et al. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:660-669.
91 Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289-1298.
92 Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiother Oncol. 2007;85:74-82.
96 Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non–small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323-329.
101 Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys. 2003;55:724-735.
107 Hope AJ, Lindsay PE, El Naqa I, et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys. 2006;65:112-124.
110 Bradley JD, Hope A, El Naqa I, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985-992.
112 Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose. An analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1-9.
149 Eriksson F, Gagliardi G, Liedberg A, et al. Long-term cardiac mortality following radiation therapy for Hodgkin’s disease. Analysis with the relative seriality model. Radiother Oncol. 2000;55:153-162.
151 Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707-714.
162 Ahn SJ, Kahn D, Zhou S, et al. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335-347.
174 Jackson A, Skwarchuk MW, Zelefsky MJ, et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys. 2001;49:685-698.
178 Fiorino C, Sanguineti G, Cozzarini C, et al. Rectal dose-volume constraints in high-dose radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57:953-962.
182 Huang EH, Pollack A, Levy L, et al. Late rectal toxicity. Dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:1314-1321.
191 Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810-821.
192 Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma. Dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156-162.
216 Roach M, Winter K, Michalski JM, et al. Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406. Findings from a prospective, multi-institutional, phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys. 2004;60:1351-1356.
1 Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122.
2 Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19.
3 Milano MT, Constine LS, Okunieff P. Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat Oncol. 2008;3:36.
4 Armstrong CL, Hunter JV, Ledakis GE, et al. Late cognitive and radiographic changes related to radiotherapy. Initial prospective findings. Neurology. 2002;59:40-48.
5 Brown PD, Buckner JC, O’Fallon JR, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J Clin Oncol. 2003;21:2519-2524.
6 Laack NN, Brown PD, Ivnik RJ, et al. Cognitive function after radiotherapy for supratentorial low-grade glioma. A North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63:1175-1183.
7 Torres IJ, Mundt AJ, Sweeney PJ, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology. 2003;60:1113-1118.
8 Vigliani MC, Sichez N, Poisson M, et al. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults. 4-year results. Int J Radiat Oncol Biol Phys. 1996;35:527-533.
9 Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas. A comparative study. Lancet. 2002;360:1361-1368.
10 Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma. Long-term follow-up. Lancet Neurol. 2009;8:810-818.
11 Gregor A, Cull A, Traynor E, et al. Neuropsychometric evaluation of long-term survivors of adult brain tumours: relationship with tumour and treatment parameters. Radiother Oncol. 1996;41:55-59.
12 Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388-1395.
13 Kwok Y, Won M, Regine WF, et al. Neurocognitive impact of whole brain radiation on patients with brain metastases: secondary analysis of RTOG BR-0018, ASTRO. Los Angeles, 2007. Available at http://www.oncolink.org/conferences/article.cfm?c=3&s=47&ss=266&id=1706
14 Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma. A Children’s Cancer Group study. J Clin Oncol. 2001;19:3470-3476.
15 Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence. The influence of dose and age on IQ score. J Clin Oncol. 1992;10:1390-1396.
16 Jannoun L, Bloom HJ. Long-term psychological effects in children treated for intracranial tumors. Int J Radiat Oncol Biol Phys. 1990;18:747-753.
17 Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma. Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691-3697.
18 Hill JM, Kornblith AB, Jones D, et al. A comparative study of the long term psychosocial functioning of childhood acute lymphoblastic leukemia survivors treated by intrathecal methotrexate with or without cranial radiation. Cancer. 1998;82:208-218.
19 Moore IM, Kramer JH, Wara W, et al. Cognitive function in children with leukemia. Effect of radiation dose and time since irradiation. Cancer. 1991;68:1913-1917.
20 Smibert E, Anderson V, Godber T, et al. Risk factors for intellectual and educational sequelae of cranial irradiation in childhood acute lymphoblastic leukaemia. Br J Cancer. 1996;73:825-830.
21 Waber DP, Turek J, Catania L, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia. Findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol. 2007;25:4914-4921.
22 Mulhern RK, Fairclough D, Ochs J. A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy, or no cranial irradiation. J Clin Oncol. 1991;9:1348-1356.
23 Mulhern RK, Kepner JL, Thomas PR, et al. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation. A Pediatric Oncology Group study. J Clin Oncol. 1998;16:1723-1728.
24 Waber DP, Shapiro BL, Carpentieri SC, et al. Excellent therapeutic efficacy and minimal late neurotoxicity in children treated with 18 grays of cranial radiation therapy for high-risk acute lymphoblastic leukemia. A 7-year follow-up study of the Dana-Farber Cancer Institute Consortium Protocol 87-01. Cancer. 2001;92:15-22.
25 Lee AW, Foo W, Chappell R, et al. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;40:35-42.
26 Lee AW, Kwong DL, Leung SF, et al. Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys. 2002;53:75-85.
27 Teo PM, Leung SF, Chan AT, et al. Final report of a randomized trial on altered-fractionated radiotherapy in nasopharyngeal carcinoma prematurely terminated by significant increase in neurologic complications. Int J Radiat Oncol Biol Phys. 2000;48:1311-1322.
28 Jen YM, Hsu WL, Chen CY, et al. Different risks of symptomatic brain necrosis in NPC patients treated with different altered fractionated radiotherapy techniques. Int J Radiat Oncol Biol Phys. 2001;51:344-348.
29 Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma. Initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267-2276.
30 Corn BW, Yousem DM, Scott CB, et al. White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83-02). Cancer. 1994;74:2828-2835.
31 Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis. Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499-508.
32 Sause WT, Scott C, Krisch R, et al. Phase I/II trial of accelerated fractionation in brain metastases RTOG 85-28. Int J Radiat Oncol Biol Phys. 1993;26:653-657.
33 Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases. A report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39:571-574.
34 Lawrence RY, Li AX, Naqa IE, et al. Radiation dose volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20-S27.
35 Jian JJ, Cheng SH, Tsai SY, et al. Improvement of local control of T3 and T4 nasopharyngeal carcinoma by hyperfractionated radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys. 2002;53:344-352.
36 Schoenfeld GO, Amdur RJ, Morris CG, et al. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;71:377-385.
37 Daly ME, Chen AM, Bucci MK, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2007;67:151-157.
38 Hoppe BS, Wolden SL, Zelefsky MJ, et al. Postoperative intensity-modulated radiation therapy for cancers of the paranasal sinuses, nasal cavity, and lacrimal glands. Technique, early outcomes, and toxicity. Head Neck. 2008;30:925-932.
39 Nishimura H, Ogino T, Kawashima M, et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys. 2007;68:758-762.
40 Noel G, Habrand JL, Mammar H, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base. The Centre de Protontherapie D’Orsay experience. Int J Radiat Oncol Biol Phys. 2001;51:392-398.
41 Weber DC, Rutz HP, Pedroni ES, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base. The Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys. 2005;63:401-409.
42 Wenkel E, Thornton AF, Finkelstein D, et al. Benign meningioma. Partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363-1370.
43 Debus J, Hug EB, Liebsch NJ, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39:967-975.
44 Debus J, Hug EB, Liebsch NJ, et al. Dose-volume tolerance of the brainstem after high-dose radiotherapy. Front Radiat Ther Oncol. 1999;33:305-314.
45 Merchant TE, Chitti RM, Li C, et al. Factors associated with neurological recovery of brainstem function following postoperative conformal radiation therapy for infratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2010;76:496-503.
46 Uy NW, Woo SY, Teh BS, et al. Intensity-modulated radiation therapy (IMRT) for meningioma. Int J Radiat Oncol Biol Phys. 2002;53:1265-1270.
47 Mayo C, Yorke ED, Merchant TE. Radiation-associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76:S36-S41.
48 Schultheiss TE. The radiation dose-response of the human spinal cord. Int J Radiat Oncol Biol Phys. 2008;71:1455-1459.
49 Marucci L, Niemierko A, Liebsch NJ, et al. Spinal cord tolerance to high-dose fractionated 3D conformal proton-photon irradiation as evaluated by equivalent uniform dose and dose volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;59:551-555.
50 Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42-S49.
51 van den Bergh AC, Schoorl MA, Dullaart RP, et al. Lack of radiation optic neuropathy in 72 patients treated for pituitary adenoma. J Neuroophthalmol. 2004;24:200-205.
52 Jiang GL, Tucker SL, Guttenberger R, et al. Radiation-induced injury to the visual pathway. Radiother Oncol. 1994;30:17-25.
53 Aristizabal S, Caldwell WL, Avila J. The relationship of time-dose fractionation factors to complications in the treatment of pituitary tumors by irradiation. Int J Radiat Oncol Biol Phys. 1977;2:667-673.
54 Parsons JT, Bova FJ, Fitzgerald CR, et al. Radiation optic neuropathy after megavoltage external-beam irradiation. Analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:755-763.
55 Kline LB, Kim JY, Ceballos R. Radiation optic neuropathy. Ophthalmology. 1985;92:1118-1126.
56 Bhandare N, Monroe AT, Morris CG, et al. Does altered fractionation influence the risk of radiation-induced optic neuropathy? Int J Radiat Oncol Biol Phys. 2005;62:1070-1077.
57 Martel MK, Sandler HM, Cornblath WT, et al. Dose-volume complication analysis for visual pathway structures of patients with advanced paranasal sinus tumors. Int J Radiat Oncol Biol Phys. 1997;38:273-284.
58 Mackley HB, Reddy CA, Lee SY, et al. Intensity-modulated radiotherapy for pituitary adenomas. The preliminary report of the Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;67:232-239.
59 Mayo CS, Martel MK, Marks LB, et al. Tolerance of the optic nerves and chiasm to radiation. Int J Radiat Oncol Biol Phys. 2010;76:S28-S35.
60 Bhandare N, Antonelli PJ, Morris CG, et al. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67:469-479.
61 Oh YT, Kim CH, Choi JH, et al. Sensory neural hearing loss after concurrent cisplatin and radiation therapy for nasopharyngeal carcinoma. Radiother Oncol. 2004;72:79-82.
62 Pan CC, Eisbruch A, Lee JS, et al. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2005;61:1393-1402.
63 Honore HB, Bentzen SM, Moller K, et al. Sensori-neural hearing loss after radiotherapy for nasopharyngeal carcinoma. Individualized risk estimation. Radiother Oncol. 2002;65:9-16.
64 Chen WC, Jackson A, Budnick AS, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106:820-829.
65 van der Putten L, de Bree R, Plukker JT, et al. Permanent unilateral hearing loss after radiotherapy for parotid gland tumors. Head Neck. 2006;28:902-908.
66 Merchant TE, Gould CJ, Xiong X, et al. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int J Radiat Oncol Biol Phys. 2004;58:1194-1207.
67 Bhandare N, Jackson A, Eisbruch A, et al. Radiotherapy; sensorineural hearing loss; ototoxicity. Int J Radiat Oncol Biol Phys. 2010;76:S50-S57.
68 Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577-587.
69 Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695-704.
70 Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg. 2003;27:832-837.
71 Chao KS. Protection of salivary function by intensity-modulated radiation therapy in patients with head and neck cancer. Semin Radiat Oncol. 2002;12:20-25.
72 Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy. Initial results. Int J Radiat Oncol Biol Phys. 2001;49:907-916.
73 Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055-1069.
74 Amosson CM, Teh BS, Van TJ, et al. Dosimetric predictors of xerostomia for head-and-neck cancer patients treated with the smart (simultaneous modulated accelerated radiation therapy) boost technique. Int J Radiat Oncol Biol Phys. 2003;56:136-144.
75 Maes A, Weltens C, Flamen P, et al. Preservation of parotid function with uncomplicated conformal radiotherapy. Radiother Oncol. 2002;63:203-211.
76 Munter MW, Karger CP, Hoffner SG, et al. Evaluation of salivary gland function after treatment of head-and-neck tumors with intensity-modulated radiotherapy by quantitative pertechnetate scintigraphy. Int J Radiat Oncol Biol Phys. 2004;58:175-184.
77 Pacholke HD, Amdur RJ, Morris CG, et al. Late xerostomia after intensity-modulated radiation therapy versus conventional radiotherapy. Am J Clin Oncol. 2005;28:351-358.
78 Roesink JM, Schipper M, Busschers W, et al. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer. Implications for future trials. Int J Radiat Oncol Biol Phys. 2005;63:1006-1009.
79 Portaluri M, Fucilli FI, Castagna R, et al. Three-dimensional conformal radiotherapy for locally advanced (Stage II and worse) head-and-neck cancer. Dosimetric and clinical evaluation. Int J Radiat Oncol Biol Phys. 2006;66:1036-1043.
80 Bussels B, Maes A, Flamen P, et al. Dose-response relationships within the parotid gland after radiotherapy for head and neck cancer. Radiother Oncol. 2004;73:297-306.
81 Braam PM, Roesink JM, Moerland MA, et al. Long-term parotid gland function after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:659-664.
82 Roesink JM, Moerland MA, Battermann JJ, et al. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2001;51:938-946.
83 Li Y, Taylor JM, Ten Haken RK, et al. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:660-669.
84 Saarilahti K, Kouri M, Collan J, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol. 2006;78:270-275.
85 Jellema AP, Doornaert P, Slotman BJ, et al. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol. 2005;77:164-171.
86 Deasy JO, Moiseenko W, Marks LB, et al. Radiation therapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58-S63.
87 Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer. Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425-1439.
88 Sanguineti G, Adapala P, Endres EJ, et al. Dosimetric predictors of laryngeal edema. Int J Radiat Oncol Biol Phys. 2007;68:741-749.
89 Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007;68:750-757.
90 Rancati T, Schwarz M, Allen AM, et al. Radiation dose volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys. 2010;76:S64-S69.
91 Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289-1298.
92 Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiother Oncol. 2007;85:74-82.
93 Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle. A dose-effect relationship. Radiother Oncol. 2007;85:64-73.
94 Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110-1118.
95 Kocak Z, Evans ES, Zhou SM, et al. Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:635-638.
96 Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non–small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323-329.
97 Ten Haken RK, Martel MK, Kessler ML, et al. Use of Veff and iso-NTCP in the implementation of dose escalation protocols. Int J Radiat Oncol Biol Phys. 1993;27:689-695.
98 Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity. A dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650-659.
99 Yorke ED, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non–small cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54:329-339.
100 Willner J, Jost A, Baier K, et al. A little to a lot or a lot to a little? An analysis of pneumonitis risk from dose-volume histogram parameters of the lung in patients with lung cancer treated with 3-D conformal radiotherapy. Strahlenther Onkol. 2003;179:548-556.
101 Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys. 2003;55:724-735.
102 Kim TH, Cho KH, Pyo HR, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology. 2005;235:208-215.
103 Fay M, Tan A, Fisher R, et al. Dose-volume histogram analysis as predictor of radiation pneumonitis in primary lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1355-1363.
104 Yorke ED, Jackson A, Rosenzweig KE, et al. Correlation of dosimetric factors and radiation pneumonitis for non–small cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672-682.
105 Piotrowski T, Matecka-Nowak M, Milecki P. Prediction of radiation pneumonitis: dose-volume histogram analysis in 62 patients with non–small cell lung cancer after three-dimensional conformal radiotherapy. Neoplasma. 2005;52:56-62.
106 Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non–small cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2006;66:1399-1407.
107 Hope AJ, Lindsay PE, El Naqa I, et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys. 2006;65:112-124.
108 Tsujino K, Hirota S, Kotani Y, et al. Radiation pneumonitis following concurrent accelerated hyperfractionated radiotherapy and chemotherapy for limited-stage small cell lung cancer: Dose-volume histogram analysis and comparison with conventional chemoradiation. Int J Radiat Oncol Biol Phys. 2006;64:1100-1105.
109 Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non–small cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94-102.
110 Bradley JD, Hope A, El Naqa I, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985-992.
111 Schallenkamp JM, Miller RC, Brinkmann DH, et al. Incidence of radiation pneumonitis after thoracic irradiation. Dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67:410-416.
112 Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose. An analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1-9.
113 Armstrong J, Raben A, Zelefsky M, et al. Promising survival with three-dimensional conformal radiation therapy for non–small cell lung cancer. Radiother Oncol. 1997;44:17-22.
114 Tsujino K, Hirota S, Endo M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110-115.
115 Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non–small cell lung cancer (NSCLC). Predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075-1086.
116 Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311. A phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non–small cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318-328.
117 Hayman JA, Martel MK, Ten Haken RK, et al. Dose escalation in non–small cell lung cancer using three-dimensional conformal radiation therapy. Update of a phase I trial. J Clin Oncol. 2001;19:127-136.
118 Narayan S, Henning GT, Ten Haken RK, et al. Results following treatment to doses of 92.4 or 102.9 Gy on a phase I dose escalation study for non–small cell lung cancer. Lung Cancer. 2004;44:79-88.
119 Rosenzweig KE, Mychalczak B, Fuks Z, et al. Final report of the 70.2-Gy and 75.6-Gy dose levels of a phase I dose escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable non–small cell lung cancer. Cancer J. 2000;6:82-87.
120 Seppenwoolde Y, De Jaeger K, Boersma LJ, et al. Regional differences in lung radiosensitivity after radiotherapy for non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:748-758.
121 Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258-1267.
122 Liao ZX, Komaki RR, Thames HDJr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non–small cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(3):775-781.
123 Sura S, Gupta V, Yorke E, et al. Intensity-modulated radiation therapy (IMRT) for inoperable non–small cell lung cancer. The Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol. 2008;87:17-23.
124 Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640-645.
125 Miles EF, Larrier NA, Kelsey CR, et al. Intensity-modulated radiotherapy for resected mesothelioma, The Duke experience. Int J Radiat Oncol Biol Phys. 2008;71:1143-1150.
126 Rice DC, Smythe WR, Liao Z, et al. Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2007;69:350-357.
127 Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70-S76.
128 De Jaeger K, Seppenwoolde Y, Boersma LJ, et al. Pulmonary function following high-dose radiotherapy of non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:1331-1340.
129 Choi NC, Kanarek DJ. Toxicity of thoracic radiotherapy on pulmonary function in lung cancer. Lung Cancer. 1994;10(Suppl 1):S219-S230.
130 Miller KL, Zhou SM, Barrier RCJr, et al. Long-term changes in pulmonary function tests after definitive radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:611-615.
131 Abratt RP, Willcox PA. The effect of irradiation on lung function and perfusion in patients with lung cancer. Int J Radiat Oncol Biol Phys. 1995;31:915-919.
132 Marks LB, Fan M, Clough R, et al. Radiation-induced pulmonary injury. Symptomatic versus subclinical endpoints. Int J Radiat Biol. 2000;76:469-475.
133 Marks LB, Munley MT, Bentel GC, et al. Physical and biological predictors of changes in whole-lung function following thoracic irradiation. Int J Radiat Oncol Biol Phys. 1997;39:563-570.
134 Allen AM, Henning GT, Ten Haken RK, et al. Do dose-volume metrics predict pulmonary function changes in lung irradiation? Int J Radiat Oncol Biol Phys. 2003;55:921-929.
135 Fan M, Marks LB, Hollis D, et al. Can we predict radiation-induced changes in pulmonary function based on the sum of predicted regional dysfunction? J Clin Oncol. 2001;19:543-550.
136 Fan M, Marks LB, Lind P, et al. Relating radiation-induced regional lung injury to changes in pulmonary function tests. Int J Radiat Oncol Biol Phys. 2001;51:311-317.
137 Mah K, Van Dyk J. Quantitative measurement of changes in human lung density following irradiation. Radiother Oncol. 1988;11:169-179.
138 Marks LB, Munley MT, Spencer DP, et al. Quantification of radiation-induced regional lung injury with perfusion imaging. Int J Radiat Oncol Biol Phys. 1997;38:399-409.
139 Ghobadi G, Hogeweg LE, Faber H, et al. Quantifying local radiation-induced lung damage from computed tomography. Int J Radiat Oncol Biol Phys. 2010;76(2):548-556.
140 Borst GR, De Jaeger K, Belderbos JS, et al. Pulmonary function changes after radiotherapy in non–small cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys. 2005;62:639-644.
141 Gagliardi G, Lax I, Rutqvist LE. Partial irradiation of the heart. Semin Radiat Oncol. 2001;11:224-233.
142 Adams MJ, Hardenbergh PH, Constine LS, et al. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55-75.
143 Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949-1955.
144 Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214-223.
145 Evans ES, Prosnitz RG, Yu X, et al. Impact of patient-specific factors, irradiated left ventricular volume, and treatment set-up errors on the development of myocardial perfusion defects after radiation therapy for left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2006;66:1125-1134.
146 Das SK, Baydush AH, Zhou S, et al. Predicting radiotherapy-induced cardiac perfusion defects. Med Phys. 2005;32:19-27.
147 Carr ZA, Land CE, Kleinerman RA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842-850.
148 Gagliardi G, Lax I, Ottolenghi A, et al. Long-term cardiac mortality after radiotherapy of breast cancer—application of the relative seriality model. Br J Radiol. 1996;69:839-846.
149 Eriksson F, Gagliardi G, Liedberg A, et al. Long-term cardiac mortality following radiation therapy for Hodgkin’s disease. Analysis with the relative seriality model. Radiother Oncol. 2000;55:153-162.
150 Tripp P, Malhotra HK, Javle M, et al. Cardiac function after chemoradiation for esophageal cancer. Comparison of heart dose-volume histogram parameters to multiple gated acquisition scan changes. Dis Esophagus. 2005;18:400-405.
151 Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707-714.
152 Martel MK, Sahijdak WM, Ten Haken RK, et al. Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys. 1998;40:155-161.
153 Heidenreich PA, Hancock SL, Lee BK, et al. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743-749.
154 Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831-2837.
155 Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878-1886.
156 King V, Constine LS, Clark D, et al. Symptomatic coronary artery disease after mantle irradiation for Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1996;36:881-889.
157 Gagliardi G, Constine LS, Moiseenko W, et al. Radiation-associated heart injury. Int J Radiat Oncol Biol Phys. 2010;76:S77-S85.
158 Kahn D, Zhou S, Ahn SJ, et al. “Anatomically-correct” dosimetric parameters may be better predictors for esophageal toxicity than are traditional CT-based metrics. Int J Radiat Oncol Biol Phys. 2005;62:645-651.
159 Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with non–small cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:337-341.
160 Qiao WB, Zhao YH, Zhao YB, et al. Clinical and dosimetric factors of radiation-induced esophageal injury. Radiation-induced esophageal toxicity. World J Gastroenterol. 2005;11:2626-2629.
161 Maguire PD, Sibley GS, Zhou SM, et al. Clinical and dosimetric predictors of radiation-induced esophageal toxicity. Int J Radiat Oncol Biol Phys. 1999;45:97-103.
162 Ahn SJ, Kahn D, Zhou S, et al. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335-347.
163 Werner-Wasik M, Yorke ED, Deasy JO, et al. Radiation-associated esophageal toxicity. Int J Radiat Oncol Biol Phys. 2010;73:S86-S93.
164 Eifel PJ, Levenback C, Wharton JT, et al. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1995;32:1289-1300.
165 Cosset JM, Henry-Amar M, Burgers JM, et al. Late radiation injuries of the gastrointestinal tract in the H2 and H5 EORTC Hodgkin’s disease trials. Emphasis on the role of exploratory laparotomy and fractionation. Radiother Oncol. 1988;13:61-68.
166 Mak AC, Rich TA, Schultheiss TE, et al. Late complications of postoperative radiation therapy for cancer of the rectum and rectosigmoid. Int J Radiat Oncol Biol Phys. 1994;28:597-603.
167 Pan CC, Dawson LA, McGinn CJ, et al. Analysis of radiation-induced gastric and duodenal bleeds using the Lyman-Kutcher-Burman model. Int J Radiat Oncol Biol Phys. 2003;57:S217.
168 Kavanagh B, Pan CC, Dawson LA, et al. Radiation dose volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101-S107.
169 Roeske JC, Bonta D, Mell LK, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69:201-207.
170 Baglan KL, Frazier RC, Yan D, et al. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176-183.
171 Schultheiss TE, Lee WR, Hunt MA, et al. Late GI and GU complications in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:3-11.
172 Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer. Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005;61:1019-1034.
173 Peeters ST, Hoogeman MS, Heemsbergen WD, et al. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys. 2006;66:11-19.
174 Jackson A, Skwarchuk MW, Zelefsky MJ, et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys. 2001;49:685-698.
175 Skwarchuk MW, Jackson A, Zelefsky MJ, et al. Late rectal toxicity after conformal radiotherapy of prostate cancer (I): multivariate analysis and dose-response. Int J Radiat Oncol Biol Phys. 2000;47:103-113.
176 Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124-1129.
177 Wachter S, Gerstner N, Goldner G, et al. Rectal sequelae after conformal radiotherapy of prostate cancer. Dose-volume histograms as predictive factors. Radiother Oncol. 2001;59:65-70.
178 Fiorino C, Sanguineti G, Cozzarini C, et al. Rectal dose-volume constraints in high-dose radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57:953-962.
179 Fiorino C, Cozzarini C, Vavassori V, et al. Relationships between DVHs and late rectal bleeding after radiotherapy for prostate cancer: analysis of a large group of patients pooled from three institutions. Radiother Oncol. 2002;64:1-12.
180 Cozzarini C, Fiorino C, Ceresoli GL, et al. Significant correlation between rectal DVH and late bleeding in patients treated after radical prostatectomy with conformal or conventional radiotherapy (66.6-70.2 Gy). Int J Radiat Oncol Biol Phys. 2003;55:688-694.
181 Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097-1105.
182 Huang EH, Pollack A, Levy L, et al. Late rectal toxicity. Dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:1314-1321.
183 Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151-1161.
184 Kupelian PA, Reddy CA, Carlson TP, et al. Dose/volume relationship of late rectal bleeding after external beam radiotherapy for localized prostate cancer: absolute or relative rectal volume? Cancer J. 2002;8:62-66.
185 Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1297-1308.
186 Tucker SL, Dong L, Cheung R, et al. Comparison of rectal dose-wall histogram versus dose-volume histogram for modeling the incidence of late rectal bleeding after radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:1589-1601.
187 Boersma LJ, van den Brink M, Bruce AM, et al. Estimation of the incidence of late bladder and rectum complications after high-dose (70-78 GY) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys. 1998;41:83-92.
188 Michalski JM, Gay H, Jackson A, et al. Radiation induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123-S129.
189 Russell AH, Clyde C, Wasserman TH, et al. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases. Results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27:117-123.
190 Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781-788.
191 Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810-821.
192 Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma. Dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156-162.
193 Cheng JC, Wu JK, Lee PC, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60:1502-1509.
194 Kim TH, Kim DY, Park JW, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225-231.
195 Pan CC, Kavanagh B, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94-S100.
196 Bianchetti MG, Kanaka C, Ridolfi-Luthy A, et al. Persisting renotubular sequelae after cisplatin in children and adolescents. Am J Nephrol. 1991;11:127-130.
197 McCune JS, Friedman DL, Schuetze S, et al. Influence of age upon Ifosfamide-induced nephrotoxicity. Pediatr Blood Cancer. 2004;42:427-432.
198 Dewit L, Verheij M, Valdes Olmos RA, et al. Compensatory renal response after unilateral partial and whole volume high-dose irradiation of the human kidney. Eur J Cancer. 1993;29A:2239-2243.
199 Kim TH, Somerville PJ, Freeman CR. Unilateral radiation nephropathy—the long-term significance. Int J Radiat Oncol Biol Phys. 1984;10:2053-2059.
200 Willett CG, Tepper JE, Orlow EL, et al. Renal complications secondary to radiation treatment of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 1986;12:1601-1604.
201 Kost S, Dorr W, Keinert K, et al. Effect of dose and dose-distribution in damage to the kidney following abdominal radiotherapy. Int J Radiat Biol. 2002;78:695-702.
202 Welz S, Hehr T, Kollmannsberger C, et al. Renal toxicity of adjuvant chemoradiotherapy with cisplatin in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;69:1429-1435.
203 Varlotto JM, Gerszten K, Heron DE, et al. The potential nephrotoxic effects of intensity modulated radiotherapy delivered to the para-aortic area of women with gynecologic malignancies. Preliminary results. Am J Clin Oncol. 2006;29:281-289.
204 Flentje M, Hensley F, Gademann G, et al. Renal tolerance to nonhomogenous irradiation: comparison of observed effects to predictions of normal tissue complication probability from different biophysical models. Int J Radiat Oncol Biol Phys. 1993;27:25-30.
205 Jansen EP, Saunders MP, Boot H, et al. Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:781-785.
206 Dawson LA, Kavanagh BD, Paulino AC, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys. 2010;76:S108-S115.
207 Viswanathan AN, Yorke ED, Marks LB, et al. Radiation-associated bladder injury. Int J Radiat Oncol Biol Phys. 2010;76:S116-S122.
208 Wallner KE, Merrick GS, Benson ML, et al. Penile bulb imaging. Int J Radiat Oncol Biol Phys. 2002;53:928-933.
209 Kao J, Turian J, Meyers A, et al. Sparing of the penile bulb and proximal penile structures with intensity-modulated radiation therapy for prostate cancer. Br J Radiol. 2004;77:129-136.
210 Brown MW, Brooks JP, Albert PS, et al. An analysis of erectile function after intensity modulated radiation therapy for localized prostate carcinoma. Prostate Cancer Prostatic Dis. 2007;10:189-193.
211 van der Wielen GJ, Hoogeman MS, Dohle GR, et al. Dose-volume parameters of the corpora cavernosa do not correlate with erectile dysfunction after external beam radiotherapy for prostate cancer. Results from a dose-escalation trial. Int J Radiat Oncol Biol Phys. 2008;71:795-800.
212 Selek U, Cheung R, Lii M, et al. Erectile dysfunction and radiation dose to penile base structures: a lack of correlation. Int J Radiat Oncol Biol Phys. 2004;59:1039-1046.
213 Fisch BM, Pickett B, Weinberg V, et al. Dose of radiation received by the bulb of the penis correlates with risk of impotence after three-dimensional conformal radiotherapy for prostate cancer. Urology. 2001;57:955-959.
214 Wernicke AG, Valicenti R, Dieva K, et al. Radiation dose delivered to the proximal penis as a predictor of the risk of erectile dysfunction after three-dimensional conformal radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60:1357-1363.
215 Mangar SA, Sydes MR, Tucker HL, et al. Evaluating the relationship between erectile dysfunction and dose received by the penile bulb: using data from a randomised controlled trial of conformal radiotherapy in prostate cancer (MRC RT01, ISRCTN47772397). Radiother Oncol. 2006;80:355-362.
216 Roach M, Winter K, Michalski JM, et al. Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406. Findings from a prospective, multi-institutional, phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys. 2004;60:1351-1356.
217 Roach M, Nam J, Gagliardi G, et al. Radiation dose volume effects and the penile bulb. Int J Radiat Oncol Biol Phys. 2010;76:S130-S134.