43 Surgical Treatments for Movement Disorders
Surgical therapies for certain movement disorders are important treatment modalities, particularly in medically refractory cases where the patient has become significantly disabled. Early on, thousands of surgically induced brain lesions were performed between 1950 and 1970 after a serendipitous surgical “mistake” led to loss of a classic Parkinson disease (PD) tremor in one patient. Very rapidly an initial enthusiasm developed for this therapeutic modality. However, the introduction of levodopa in 1966 led to a significant cessation in the development of more sophisticated surgical treatment for PD. Subsequently our understanding of the physiology of movement disorders and our ability to better assess baseline and outcome data in these patients have markedly improved since the initial historical period. Concomitantly, it became clear that medical management would not provide long-term resolution of the classic PD in many patients. Today PD primarily includes mostly the idiopathic subset in contrast to the combined idiopathic as well as the postencephalitic variants present when surgical therapeutic methodologies were in their infancy. Currently there are several operations performed for the rather few neurologic disorders that are treated effectively by surgery (Table 43-1). These include very specific intentional destructive lesions targeting specific basal ganglia sites as well as deep brain stimulation (DBS) within the basal ganglia and thalamus.
Table 43-1 Summary of Current Best Procedures for Movement Disorders*
| Disorder | Procedures |
|---|---|
| Essential (familial) tremor | Vim Thalamic DBS or Thalamotomy (unilateral) |
| Parkinson disease | DBS either to the STN or Gpi or Pallidotomy (unilateral) |
| Dystonia | Gpi DBS |
* DBS, deep brain stimulation; Gpi, globus pallidus pars interna; STN, subthalamic nucleus; Vim, ventralis intermedius.
General Procedure
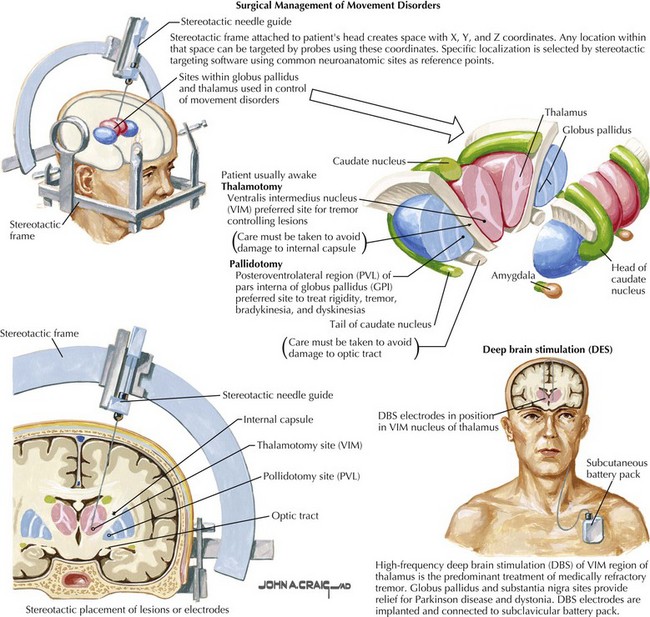
The operations are performed stereotactically, typically with the help of a frame affixed to the head of the patient (Fig. 43-1). This frame serves to create a space with x, y, and z coordinates that the head, and thus the brain, lies within. As such, any particular location within that space can be targeted using devices to place and hold a probe tip, recording electrode, or stimulating electrode at the desired coordinates. These systems are highly accurate, provided the surgeon is specifically trained and able to precisely focus the probe target within just 1 mm of an intended therapeutic site deep within the appropriate target brain structures including the basal ganglia, thalamus, or subthalamic brain structures. A system of stereotactic targeting software is used to define readily appreciated common brain structures as initial reference points. These systems typically use measurements based on the location of the anterior and posterior commissures.
During the intraoperative procedure, the patients are kept awake and monitored by a clinical neurophysiologist. As the probe is advanced, specific cell types are verified physiologically by having a clinical neurophysiologist make microelectrode recordings along the probe’s trajectory toward the intended subcortical target. Cells in certain specific areas have reproducible physiologic signatures vis-à-vis their firing rates and patterns. The utilization of these physiologic markers helps validate the exact location of the electrode (see Fig. 43-1).There are major albeit relatively uncommon risks associated with these procedures, the most significant being a debilitating or fatal intracerebral hemorrhage; currently, these complications still occur in approximately 0.5–3% of cases.
Operations Performed For Movement Disorders
Deep Brain Stimulation
In all instances when indicated, DBS four-contact electrodes may be placed bilaterally. This also includes settings where prior thalamotomies or pallidotomies have been unsuccessful. However, the stimulating electrodes need to be placed in a healthy area and not one previously lesioned. The electrodes are brought out through the brain and secured at the skull edge. A battery is placed in a small subcutaneous pocket just under the clavicle and tunneled to the electrode with a connecting wire (see Fig. 43-1).
Arle E, Shils JL, editors. Essential neuromodulation. Oxford: Elsevier, 2011.
Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. NEJM. 2003;349:1925-1934.
Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. NEJM. 2006;355:1978-1990.
Schuurman PR, Bosch DA, Bossuyt PMM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. NEJM. 2000;342:461-468.
2008 Yu H, Neimat JS; The treatment of movement disorders by deep brain stimulation. Neurotherapeutics. 2008;5(1):26-36.