Chapter 71 Surgical Treatment of Moyamoya Disease in Adults
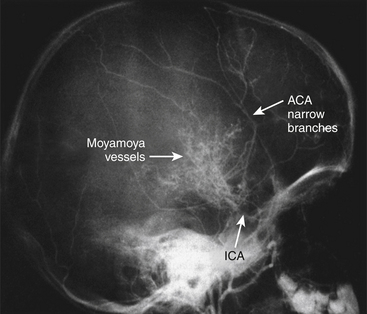
Moyamoya disease is a chronic, cerebrovascular occlusive disease, in which the terminal portions of the intracranial internal carotid arteries and the initial segments of the middle and anterior cerebral arteries progressively become narrowed or occluded. Due to this phenomenon, reduced blood flow to the brain is produced, and tiny collateral vessels at the base of the brain enlarge to become collateral pathways. These vessels are called “moyamoya vessels” because the angiographic appearance of these vessels resemble the “cloud” or “puff” of cigarette smoke, which is described as “moya-moya” in the Japanese language; also, “moya-moya” is the Japanese word to describe a hazy appearance or an unclear idea about something.1
In the 1950s, leading Japanese neurosurgeons began to notice a new clinical entity that came to be called moyamoya disease. Since its etiology was unknown, it was named in various ways. Takeuchi and Shimizu described it as a hypoplasia of bilateral internal carotid arteries.2 Later, Suzuki and Takaku described in detail the angiographic appearance and development of this disease,1 and gave it the name moyamoya disease. Kudo named it officially as the spontaneous occlusion of the circle of Willis3 (Fig. 71-1).
Clinical Findings and Preoperative Assessment
Symptoms and signs of moyamoya disease include brain ischemia and hemorrhage. Initial symptoms in moyamoya disease, both juvenile (under age 15 years) and adult cases considered together, are most frequently motor disturbances. In the experience of Suzuki.4 these were found in 36% of patients, followed by intracranial hemorrhage in 25%, headache in 20%, and convulsions in 6%. This is similar to the experience reported by Yamaguchi et al.,5 who reported motor disturbances in 62.7% of males and 53.8% of females, disturbances of consciousness in 28.1% of males and 34.6% of females, signs of meningeal irritation in 10.3% of males and 20.5% of females, and speech disturbances 16.7% of males and 14% of females.
Among the juvenile cases, motor disturbances, including monoparesis, paraparesis, and hemiparesis are found in 60%, and in these juvenile cases, some 20% show motor disturbances indicative of transient brain ischemia.4
If we also included other symptoms thought to be due to brain ischemia, such as sensory disturbances and mental and psychic disorders, then 85% of these juvenile cases show symptoms of brain ischemia. Intracranial hemorrhage was seen in only 4% of juvenile cases.4
In other Japanese reported experiences,4,6,7 the onset of adult cases was accompanied by intracranial hemorrhage in 43% of patients, and symptoms due to brain ischemia,8,9 including motor, mental, and psychic disturbances, were seen in 20% of these adult cases.
Moyamoya disease is basically diagnosed both by clinical symptoms and angiographic findings.
Neuroimaging
The widespread availability of magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) as useful and safe imaging methods has led to the increasing use of these methods for primary imaging in patients with symptoms suggestive of moyamoya.10–12 An acute infarct is more likely to be detected with the use of diffusion-weighted imaging, whereas a chronic infarct is more likely to be seen with T1- and T2-weighted imaging. Diminished cortical blood flow due to moyamoya can be inferred from fluid-attenuated inversion recovery (FLAIR) sequences showing linear high signals that follow a sulcal pattern, which is called the “ivy sign.”13
The finding most suggestive of moyamoya on MRI is reduced flow voids in the internal, middle, and anterior cerebral arteries coupled with prominent flow voids through the basal ganglia and thalamus from moyamoya-associated collateral vessels. These findings are virtually diagnostic of moyamoya,14 and also called “the sign of termite nest.”15
When we search for the classical findings of moyamoya disease, both studies, catheter angiography and MRA, show the terminal portions of the intracranial internal carotid arteries and the initial segments of the middle and anterior cerebral arteries progressively become narrowed or occluded. According to the report by Suzuki et al.1 that named this disease, tiny collateral vessels at the base of the brain enlarge to become collateral pathways. These vessels are called “moyamoya vessels” because the angiographic appearance of these vessels resemble the “cloud” or “puff” of cigarette smoke, which is described as “moya-moya” in the Japanese language.
Suzuki and Takaku1 classified the development of moyamoya disease into six stages. According to this classification, many patients fall into stage 3. Fukuyama and Umezu16 then further divided stage 3 into three.
Stage 1: Narrowing of carotid fork.
Stage 2: Initiation of the “moyamoya vessels”; dilatation of the intracerebral main arteries.
Stage 3: Intensification of the “moyamoya vessels”; nonfilling of the anterior and middle cerebral arteries.
Stage 4: Minimization of the “moyamoya vessels”; disappearance of the posterior cerebral artery.
Stage 5: Reduction of the “moyamoya vessels”; the main arteries arising from the internal carotid artery disappear.
Stage 6: Disappearance of the “moyamoya vessels”; the original moyamoya vessels at the base of the brain are completely missing and only the collateral circulation from the external carotid artery is seen (Fig. 71-2).
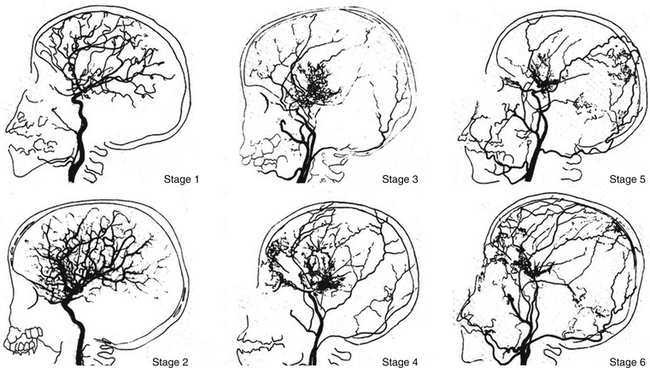
FIGURE 71-2 Angiographic staging of moyamoya disease.
(From Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288-299.)
Inconveniences of this classification are as follows: Many cases belong to stages 3 to 5, especially to stage 3. There are few cases in stages 1 and 6. Stages of moyamoya disease are not strongly related to clinical symptoms. In stages 1 and 6, there are no moyamoya vessels on cerebral angiography, which is not moyamoya disease by definition. There is some doubt that vascular dilatation in stage 2 really exists.
CBF, positron-emission CT or single-photon CT, or xenon inhalation CT are commonly used to obtain greater detail. Recently, perfusion x-ray CT and MRI with contrast materials have been used for this purpose.17,18
Emergency Treatment
In the acute stage, the treatment is the same as for brain infarction or spontaneous intracerebral hemorrhage due to other etiologies.19 In the event of ventricular hemorrhage, an external ventricular drainage operation is performed if the patient presents in acute evolution with signs of intracranial hypertension.20,21
In the case of intracerebral hemorrhage (ICH), initial medical treatment is indicated if the hemorrhage totals less than 25 cc in volume. If the hemorrhagic volume totals more than 25 cc, is associated with a lobar topography, and demonstrates mass effect over the midline structures, then surgical evacuation is indicated.20 In patients with ICH, infusion of osmotic agents is frequently used to control the intracerebral pressure and edema, as well as administration of anticonvulsants to control seizures, is also required.21
Bypass surgery in the acute stage of the disease is not indicated.19
Treatment in the Chronic Stage
Patients with Cerebral Ischemia
There is no consensus on medical treatment with aspirin, other antiplatelet agents, anticoagulants, vasodilators, or corticosteroids to prevent future ischemic attacks in patients with chronic disease.22
Surgical Anastomosis
In order to eliminate ischemic symptoms or to prevent recurrent ischemic stroke, bypass surgery is accepted as the treatment of choice. Site of the bypass is also determined occasionally by the results of the examination of cerebral blood flow (Xe133-CT scan, SPECT, PET scan).22 The rationale to initiate some form of revascularization follows:
Clinical picture: As already described, there are mainly different forms of ischemic stroke in children (juvenile cases), and hemorrhagic and ischemic stroke in young adults.
Laboratory studies: The following studies may be indicated in patients with moyamoya disease: In a patient with stroke of unclear etiology, a hypercoagulability profile may be helpful. Significant abnormality in any of the following is a risk factor for ischemic stroke: protein C, protein S, antithrombin III, homocysteine, and factor V Leiden. Erythrocyte sedimentation rate (ESR) can be obtained as part of the initial workup of a possible vasculitis. However, a normal ESR does not rule out vasculitis.
Imaging studies: Cerebral angiography is the criterion standard for diagnosis. The following findings support the diagnosis:
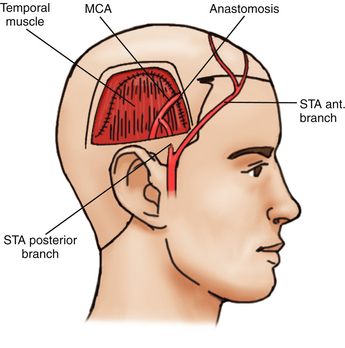
Vascular anastomoses are classified as direct or indirect. In direct anastomosis, the superficial temporal artery (STA) in the scalp is dissected and anastomosed with the middle cerebral artery (MCA) on the brain surface under microsurgery. This surgical technique gives the patient a high cerebral blood flow (CBF) immediately after the surgery.23–26 However, in this disease the diameters of the cortical arteries are very small, and the anastomotic technique requires for its proper implementation cortical arteries of at least 1 mm in diameter.
In the indirect anastomosis, the periosteum, dura mater, or a slice of the temporal muscle is placed over the brain surface, in anticipation of the development of new spontaneous anastomoses between extra and intracranial circulation. Some time is required to establish such anastomoses that also function with utility. Thus, the brain parenchyma is provided with collateral circulation through these structures, the STA, deep temporal artery, middle meningeal artery, and anterior meningeal artery. During the surgical technique (synangiosis), these arteries must be preserved. In some cases, and to ensure close contact between the STA and galea surrounding the cerebral cortex, the extirpation of the pia mater is done in zones or “windows,” suturing the edges of the galea to the piamater.27 While using these indirect techniques, a high increase of cerebral blood flow does not develop immediately; early revascularization is frequently observed between 3 to 6 months following the intervention, especially in cases that course with cerebral ischemia.
It is common to add an indirect bypass more or less when a direct bypass is scheduled.23 In other situations, multiple burr-hole surgery is performed, in which multiple small holes are made on the skull bone, in anticipation of the development of spontaneous anastomoses.28,29 Other less frequent surgeries are omental transplantation30 and omental transposition.31
In moyamoya disease, usually both cerebral hemispheres are ischemic; thus bypass surgery is required bilaterally. First, one-sided operation for the hemisphere that is more ischemic is performed, and then bypass surgery for the opposite side is scheduled 2 or 3 months later.
The indirect revascularization techniques most widely used are the encephalo-duro-arterio-synangiosis (EDAS) and the encephalo-myo-synangiosis (EMS).32–34 There are many modes of indirect anastomoses. Such techniques reviewed by Matsushima et al.35 are summarized in Table 71-1.
Table 71-1 Procedures Using Different Tissues for Indirect Anastomoses
Patients with Intracerebral Hemorrhage
Donor vessel (the STA) should be selected with an external diameter not less than 1 mm because vessels of smaller diameter have a high percentage of occlusion, deliver a low blood flow, are not useful, and are more difficult to anastomose.23–26 To prevent mechanical vasospasm, it is useful to apply topical diluted papaverine or nimodipine.
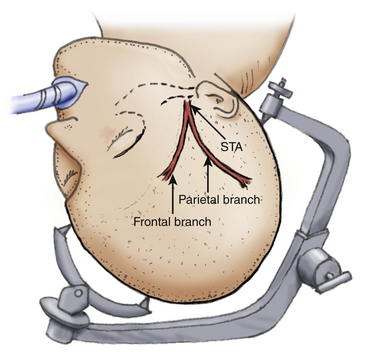
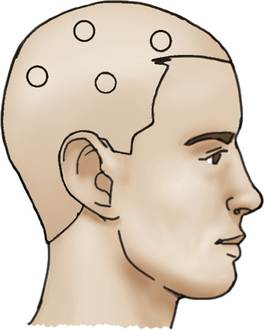
The patient is placed on the operating table with the head rotated toward the contralateral side and the temporal bone is parallel to the floor, kept in position by the head holder with three points. After the scalp is shaved, the standard Doppler ultrasound is used over the donor artery and correlated with the preoperative angiography to locate the most suitable branch of the STA. The skin is painted with its branches using a marker pen. Usually there are two branches of the STA, the frontal and parietal. Both must be marked during the proceedings (Fig. 71-3).
Direct Bypass Surgery
Superficial Temporal Artery–Middle Cerebral Artery
The first bypass STA-MCA as treatment for moyamoya disease and other ischemic cerebrovascular pathologies, was performed in 1972 by Yasargil,24 and since then, several clinical series have been reported showing good results in the immediate postoperative period with an isolated direct surgical technique25,26 or combined with other indirect revascularization techniques.23,35
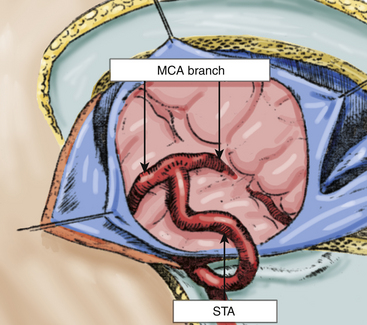
The dura mater is opened widely relative to the craniotomy, and the microscope is installed to select the recipient artery, which should ideally have an outer diameter of 1 mm or more. We must also take into account the orientation of the recipient artery and the location of the branch of the MCA in relation to the sylvian fissure. As closer branches allow better blood flow back to the internal carotid artery bifurcation, it is best to choose an M3 branch of the MCA emerging from the sylvian fissure. The exposure of the recipient vessel is performed with a meticulous dissection of the surrounding arachnoid membrane, over a segment from 6 to 10 mm in length, around the direction of the artery. Small collaterals that emerge from the recipient artery are coagulated and cut. It is preferable to place a small piece of plastic underneath the elected branch of the MCA, which increases visibility in the operating field.
Subsequently, under a microscope, the anastomosis is performed using 10-0 monofilament suture. Sutures are placed first at the corners of the diamond-shaped incision and then five interrupted sutures are placed over the distal wall edges. The procedure is repeated with five interrupted sutures over the nearest wall. The intima are always included in the suture to avoid any increased tension at the sutured site (Fig. 71-4).
We recommended using interrupted sutures.23–25,36,37 but some authors prefer a continuous suture.38 Proceed to remove the first distal clip and then the proximal clip of the recipient artery. Finally, the temporary clip on the STA is removed. Classically, it is recommended that the time of temporary clipping of the recipient artery should be no more than 30 minutes. After removing the clips, Cottonoids are applied with gentle pressure on the site of the anastomosis. If a higher rate of bleeding occurs at any time during the anastomosis with a higher rate of bleeding, then an additional suture must be placed. Minor oozing usually ceases with Surgicel (vegetal oxidized cellulose mesh, Ethicon®) and pressure on the anastomosed site. Once the anastomosis is complete, Doppler can be used to examine anastomotic patency.
Indirect Bypass Surgery
Encephalo-Duro-Arterio-Synangiosis
The encephalo-duro-arterio-synangiosis (EDAS), described by Matsushima et al.,32,33 is an alternative to the STA-MCA bypass. EDAS is an indirect way to increase collateral blood flow to the ischemic brain. This indirect technique does not increase cerebral blood flow immediately. Rather, indirect techniques are most often used in cases of cerebral ischemia and are associated with revascularization between 6 to 12 months following intervention, specially in cases that occur with cerebral ischemia.15,23,32,33,35
In this surgical technique, the STA is dissected, as in the technique described above, but the vessel is left in continuity, not sectioned. Then, an incision is made over the temporalis muscle. A diamond-shaped or biconvex craniotomy is performed with two holes using an automatic drill and with the directionality of the craniotomy site mirroring the direction of the underlying STA. Taking care to avoid damage to vessels of the extraintracranial collateral circulation, some authors advocate creating little windows15,33 in the dura mater and pia-arachnoid to allow for greater, closer contact between the area of the STA and the cerebral cortex.
Next, the cuff of connective tissue surrounding the STA is sutured to the edges of the dura mater with monofilament 5-0 interrupted sutures. It is necessary to be careful that the path of the artery, sutured to the dura mater, is not curved on the edges of the craniotomy, which must be lowered to prevent this complication. Topical papaverine may be used to prevent mechanical vasospasm, and Doppler imaging can be used once the craniotomy has been repositioned to verify that there is adequate distal flow through the artery. After fixation of the craniotomy, the temporalis muscle, galea, and skin are sutured in a conventional manner (Figs. 71-5 and 71-6).
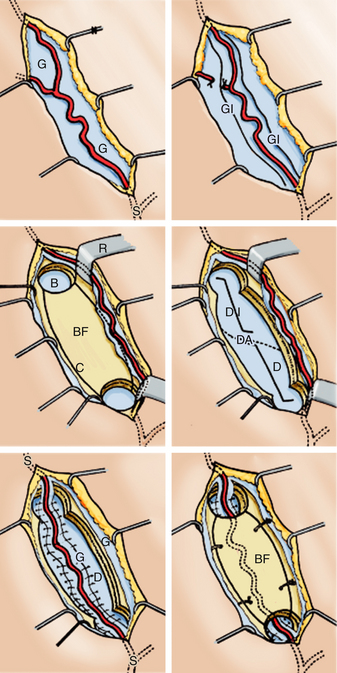
FIGURE 71-6 Shows the stages of encephalo-duro-arterio-synangiosis (EDAS), described by Matsushima et al.
(From Matsushima Y, Fukai M, Tanaka K, et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol. 1981;15:313-320.)
The authors advocate that in the EDAS, temporary clipping over the branches of the MCA is not required. The extraintracranial spontaneous anastomoses that develop through the dura mater generally have good patency, and this procedure is technically much easier to perform than the STA-MCA procedure.15,32,33 Furthermore, EDAS can be performed in cases where a donor or recipient artery of appropriate size is not available, which may occur as a function of the underlying disease.
Matsushima et al. reported their results treating moyamoya disease in 38 pediatric cases (70 hemispheres). In these cases, 100% of revascularization was obtained, with most patients showing improvement in symptoms due to cerebral ischemia.32,33
Encephalo-Myo-Synangiosis
In the encephalo-myo-synangiosis (EMS), a flap of the temporalis muscle is sutured to the edges of the dural surgical opening so that the muscle is positioned closer to the brain surface. As in the EDAS, a frontotemporal craniotomy is performed and the arachnoid over the brain surface in question is opened as widely as possible. Next, the edges of the dura mater are sutured to the edges of the muscle flap.
Neovascularization occurs from muscle to the brain parenchyma, providing greater collateral blood flow to the brain. As in the EDAS, the EMS is technically simpler to perform than the direct STA-MCA bypass and does not require identification of a recipient artery. Additionally, the EMS can be combined with a direct STA-MCA bypass in some patients23,34 (Fig. 71-7).
However, this procedure has been associated with an increased risk in some patients to developing an epileptogenic focus.23,34,35 Yet, several series have shown that EMS improves the clinical condition of patients and promotes revascularization in the region of the MCA in 70% to 80% of all patients.26,34
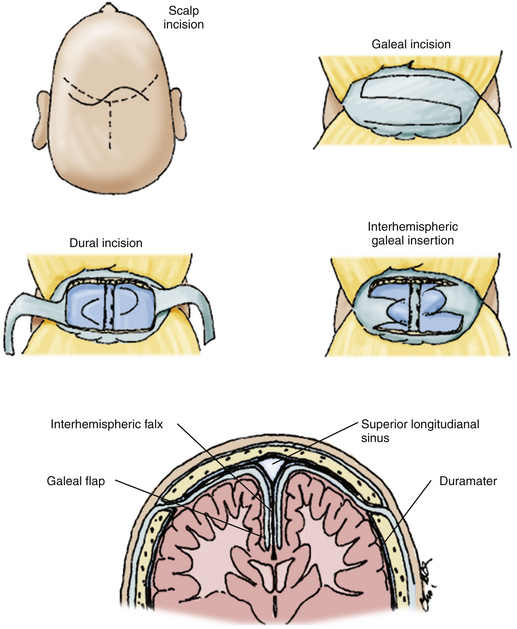
EDAS plus Encephalo-Galeo-Synangiosis
The EDAS is performed according to the technique described by Matsushima and Inaba.33 This surgery is performed in two stages, initially on the more hemodynamically affected cerebral hemisphere, with an average time between the first and second procedure being 6 to 8 months. To further increase collateral circulation in the territory of the anterior cerebral arteries, EDAS is performed with encephalo-galeo-synangiosis (EGS) in the bifrontal region as detailed below.39
Finally, the craniotomy is fixed, and the skin and galea are closed via a conventional technique39 (Fig. 71-8).
Omental Transplantation
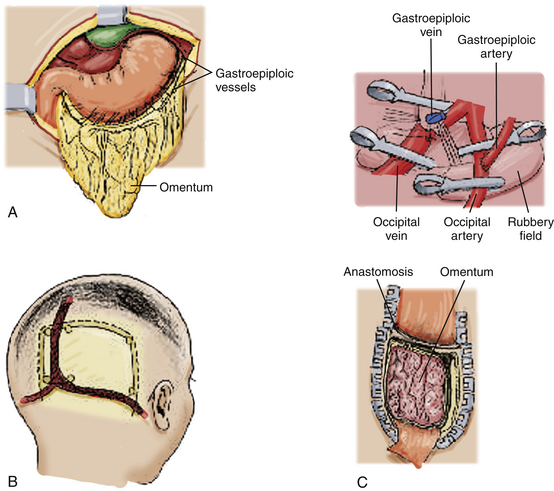
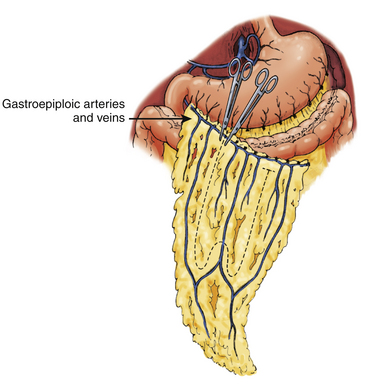
Using microsurgical techniques, end-to-end anastomoses or end-to-side anastomoses between the STA (or occipital artery) and the gastroepiploic artery, as well as between the STV (or occipital vein) and the gastroepiploic vein are established, using 10-0 monofilament suture. First, arterial anastomosis is performed. Next, a temporary clip is placed on the donor artery during venous anastomosis. After this set of anastomoses has been secured, the omentum is spread over the cerebral surface and under the edges of the exposed dura mater. The graft is sutured to the edges of the dura mater, and the craniotomy plate is fixed, taking care not to compress the vascular pedicle30 (Fig. 71-9).
Omental Transposition
This surgical technique involves extending the omentum in order to reach the skull, while it remains attached to its natural vascular pedicle. Advantages to such an approach include preserving lymphatic drainage and avoiding an additional vascular anastomosis. The omentum’s gastroepiploic pedicle is left to its normal anatomy and the omentum is able to reach the skull by dividing the elongated omentum with incisions in the form of an “L” (Fig. 71-10).
The omentum is then tunneled subcutaneously, passing through the subcutaneous chest, neck, and scalp, including behind the ear. The omentum, with its pedicle, must not be under tension after the tunneling, nor should it be angled in any segment of the subcutaneous tunnel. Thus, several incisions are made in the skin along the tunnel to facilitate graft passage. Once inside the skull, the omentum is placed on the brain surface under the edges of the exposed dura mater. The graft is sutured to the edges of the dura mater, and the craniotomy plate is fixed, taking care not to compress the vascular pedicle31 (Fig. 71-11).
Multiple Burr-Holes Operation
To avoid injury to the STA over the burr holes, the trajectory of the STA is located by Doppler. After creating two to four burr holes over each cerebral hemisphere, the dura mater and arachnoid are widely opened, preserving the meningeal arteries with the use of a microscope. Then, the skin is sutured tightly closed with 2-0 monofilament28,29 (Fig. 71-12).
Surgical Complications
With the reviewed surgical techniques, the incidence of surgical complications is very low; however, the incidence of complications is not zero. Postoperative complications include bleeding from the surgical site (subcutaneous, epidural, subdural, and intracerebral), anemia, cerebral infarction (due to perioperative hypotension, hypocapnia due to hyperventilation, and prolonged, temporary clipping of arteries), transient ischemic attacks, scalp necrosis, surgical wound infection, and convulsive seizures.19,23,33,35
The benefits of sugery usually do not appear immediately. However, the frequency of ischemic events tends to gradually decline.23,27,29,33–35 The postoperative course of patients is variable and depends on increase in cerebral blood flow and surgical method, among other factors.15,19,22,24,27,35
General anesthesia for moyamoya disease has been associated with a relatively low risk of a new stroke, both during bypass surgery as well as during cerebral angiography, in adults.26,27,33–35
General Results of Surgical Bypass
The purpose of using surgical anastomoses, either directly or indirectly, is to prevent recurrent cerebrovascular disease, not to reverse the effects of already completed strokes. In cases of ischemic stroke, most neurosurgeons with experience in the field believe that bypass presents an important modifier of the natural history of moyamoya disease. Indeed, many moyamoya patients with a history of ischemic events experience a reduction or disappearance of symptoms following surgery, correlated in the majority of series with good to excellent revascularization on imaging.15,19,23,24,33–35
Endo M., Kawano N., Misayaka Y., Yada K. Cranial burr hole for revascularization in moyamoya disease. J Neurosurg. 1989;71:180-185.
Fujiwara H., Momoshima S., Kuribayashi S. Leptomeningeal high signal intensity (ivy sign) on fluid-attenuated inversion-recovery (FLAIR) MR images in moyamoya disease. Eur J Radiol. 2005;55:224-230.
Goldsmith H.S. Brain and spinal cord revascularization by omental transposition. Neurolog Res. 1994;16:159-162.
Hirano M., Sakurai S. Psychiatric symptoms and signs associated with spontaneous occlusion of the circle of Willis. A case report and studies of 379 cases in Japan. Psychiatr Med (Tokyo). 1972;14:329-338.
Houkin K., Ishikawa T., Yoshimoto T., Abe H. Direct and indirect revascularization for moyamoya disease—surgical techniques and peri-operative complications. Clin Neurol Neurosurg. 1997;99(Suppl 2):142-145.
Ikesayki K., Matsushima T., Kubawara Y., et al. Cerebral circulation and oxigen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J Neurosurg. 1994;81:843-850.
Karasawa J., Kikuchi H., Furuse S., et al. A surgical treatment of “moyamoya” disease—encephalo-myo-synangiosis. Neurol Med Chir. 1977;17:29-37.
Karasawa J., Touho H., Ohnishi H. Cerebral revascularization using omental transplantation for childhood moyamoya disease. J Neurosurg. 1993;79(2):192-196.
Kim S.K., Wang K.C., Kim I.O., et al. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo (periostial) synangiosis in pediatric moyamoya disease. Neurosurgery. 2002;50:88-96.
Kudo T. Spontaneous occlusion of the circle of Willis: a disease apparently confined to Japanese. Neurology. 1968;18:485-496.
Little J.R., Salerno T.A. Continuous suturing for microvascular anastomosis—technical note. J Neurosurg. 1978;48:1042-1045.
Matsushima T., Inoue T., Katsuta T., et al. An indirect revascularization method in the surgical treatment of moyamoya disease—various kinds of indirect procedures and a multiple combined indirect procedure. Neurol Med Chir (Tokyo). 1998;38(Suppl):297-302.
Matsushima Y., Inaba Y. Moyamoya disease in children and its surgical treatment. Introduction of a new surgical procedure and its follow-up angiograms. Childs Brain. 1984;11(3):155-170.
McDonald C., Carter B.S., et al. Medical management of increased intracranial pressure after spontaneous intracerebral hemorrhage. Neurosurg Clin North Am.. 2002;13(3):335-338.
Quintana L. Experiencia de 20 años en el manejo de la enfermedad moyamoya. Rev Chil Neurocirug. 2004;23:30-36.
Scott R.M., Smith E.R. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;19;360(12):1226-1237.
Suzuki J. Epidemiology and symptomatology. In: Suzuku J., editor. Moyamoya Disease. Berlin and Heidelberg: Springer-Verlag; 1986:7-16.
Suzuki J., Takaku A. Cerebrovascular “moyamoya” disease showing abnormal net-like vessels in base of brain. Arch Neurol (Chicago). 1969;20:288-299.
Takeuchi K., Shimizu K. Hypogenesis of bilateral internal carotid arteries. No To Shinkei. 1957;9:37-43.
Takeuchi S., Tanaka R., Ishii R., et al. Cerebral hemodynamics in patients with moyamoya disease. A study of regional cerebral blood flow by the Xe-133 inhalation method. Surg Neurol. 1985;23:468-474.
Yamada I., Matsushima Y., Suzuki S. Moyamoya disease: diagnosis with three-dimensional time-of-flight MR angiography. Radiology. 1992;184:773-778.
Yamada I., Suzuki S., Matsushima Y. Moyamoya disease: comparison of assessment with MR angiography and MR imaging versus conventional angiography. Radiology. 1995;196:211-218.
Yasargil M.G., Yonekawa Y. Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery. 1977;1:22.
Yonekawa Y., Yasargil M.G. Extra-intracranial arterial anastomosis: clinical and technical aspects. Results. In: Krayenbühl H., editor. Advances and Technical Standards in Neurosurgery. Zürich, Wien, and New York: Springer-Verlag; 1977:47-78.
1. Suzuki J., Takaku A. Cerebrovascular “moyamoya” disease showing abnormal net-like vessels in base of brain. Arch Neurol (Chicago). 1969;20:288-299.
2. Takeuchi K., Shimizu K. Hypogenesis of bilateral internal carotid arteries. No To Shinkei. 1957;9:37-43.
3. Kudo T. Spontaneous occlusion of the circle of Willis: a disease apparently confined to Japanese. Neurology. 1968;18:485-496.
4. Suzuki J. Epidemiology and symptomatology. In: Suzuki J., editor. Moyamoya Disease. Berlin, Heidelberg: Springer-Verlag; 1986:7-16.
5. Yamaguchi T., Tashiro T., Minematsu K., Kitamura K. Summary of Japanese survey of occlusion of the circle of Willis. In: Reports by the Research Committee on Spontaneous Occlusion of the Circle of Willis. Japanese Ministry of Health and Welfare, Tokyo, 1980.
6. Higashi K., Hatano M., Maza T. Disease with abnormal intracranial vascular network complicated with intracerebral haematoma. J Neurol Neurosurg Psychiatry. 1974;37:365-369.
7. Hori T., Fukushima T., Tsuchida T., et al. A case of cerebral juxta-basal telangiectasia with intracerebral hematoma. Brain Nerve (Tokyo). 1971;23:1403-1408.
8. Hayasaki K. A case of schizophrenic state combined with intracranial rete mirabile vascularity. Brain Nerve (Tokyo). 1970;22:1319-1323.
9. Hirano M., Sakurai S. Psychiatric symptoms and signs associated with spontaneous occlusion of the circle of Willis. A case report and studies of 379 cases in Japan. Psychiatr Med (Tokyo). 1972;14:329-338.
10. Yamada I., Suzuki S., Matsushima Y. Moyamoya disease: comparison of assessment with MR angiography and MR imaging versus conventional angiography. Radiology. 1995;196:211-218.
11. Katz D.A., Marks M.P., Napel S.A., et al. Circle of Willis: evaluation with spiral CT angiography, MR angiography, and conventional angiography. Radiology. 1995;195:445-449.
12. Takanashi J.I., Sugita K., Niimi H. Evaluation of magnetic resonance angiography with selective maximum intensity projection in patients with childhood moyamoya disease. Eur J Paediatr Neurol. 1998;2:83-89.
13. Fujiwara H., Momoshima S., Kuribayashi S. Leptomeningeal high signal intensity (ivy sign) on fluid-attenuated inversion-recovery (FLAIR) MR images in moyamoya disease. Eur J Radiol. 2005;55:224-230.
14. Yamada I., Matsushima Y., Suzuki S. Moyamoya disease: diagnosis with three-dimensional time-of-flight MR angiography. Radiology. 1992;184:773-778.
15. Quintana L. Experiencia de 20 años en el manejo de la enfermedad moyamoya. Rev Chil Neurocirug. 2004;23:30-36.
16. Fukuyama Y., Umezu R. Clinical and cerebral angiographic evolution of idiopathic progressive occlusive disease of the circle of Willis (“moyamoya” disease) in children. Brain Dev. 1985;7:21-37.
17. Ikesaki K., Matsushima T., Kubawara Y., et al. Cerebral circulation and oxigen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J Neurosurg. 1994;81:843-850.
18. Takeuchi S., Tanaka R., Ishii R., et al. Cerebral hemodynamics in patients with moyamoya disease. A study of regional cerebral blood flow by the Xe-133 inhalation method. Surg Neurol. 1985;23:468-474.
19. Wanifuchi H., Takeshita M., Izawa M., et al. Management of adult moyamoya disease. Neurol Med Chir (Tokyo). 1993;33:300-305.
20. Abdulrauf S.I., Furlan A.J., Awad I. Primary intracerebral hemorrhage and subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 1999;8(3):146-150.
21. McDonald C., Carter B.S. Medical management of increased intracranial pressure after spontaneous intracerebral hemorrhage. Neurosurg Clin North Am. 2002;13(3):335-338.
22. Scott R.M., Smith E.R. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360(12):1226-1237.
23. Houkin K., Ishikawa T., Yoshimoto T., Abe H. Direct and indirect revascularization for moyamoya disease—surgical techniques and peri-operative complications. Clin Neurol Neurosurg. 1997;99(Suppl 2):142-145.
24. Yasargil M.G., Yonekawa Y. Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery. 1977;1:22.
25. Karasawa J., Kikuchi H., Furuse S., et al. Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg. 1978;49:679-688.
26. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (“moyamoya” disease). Clin Neurol Neurosurg. 1997;99:S238-S240.
27. Adelson P.D., Scott R.M. Pial synangiosis for moyamoya syndrome in children. Pediatr Neurosurg. 1995;23(1):26-33.
28. Endo M., Kawano N., Misayaka Y., Yada K. Cranial burr hole for revascularization in moyamoya disease. J Neurosurg. 1989;71:180-185.
29. Kawaguchi T., Fujita S., Hosoda K., et al. Multiple burr hole operation for adult moyamoya disease. J Neurosurg. 1996;84:468-476.
30. Karasawa J., Touho H., Ohnishi H. Cerebral revascularization using omental transplantation for childhood moyamoya disease. J Neurosurg. 1993;79(2):192-196.
31. Goldsmith H.S. Brain and spinal cord revascularization by omental transposition. Neurolog Res. 1994;16:159-162.
32. Matsushima Y., Fukai M., Tanaka K., et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol. 1981;15:313-320.
33. Matsushima Y., Inaba Y. Moyamoya disease in children and its surgical treatment. Introduction of a new surgical procedure and its follow-up angiograms. Childs Brain. 1984;11(3):155-170.
34. Karasawa J., Kikuchi H., Furuse S., et al. A surgical treatment of “moyamoya” disease—encephalo-myo-synangiosis. Neurol Med Chir. 1977;17:2937.
35. Matsushima T., Inoue T., Katsuta T., et al. An indirect revascularization method in the surgical treatment of moyamoya disease—various kinds of indirect procedures and a multiple combined indirect procedure. Neurol Med Chir (Tokyo). 1998;38(suppl):297-302.
36. Crowell R.M. Direct brain revascularization. In: Schmiedek H.H., Sweet W.H. Current Techniques in Operative Neurosurgery. New York: Grune & Stratton; 1977:1-18.
37. Yonekawa Y., Yasargil M.G. Extra-Intracranial arterial anastomosis: clinical and technical aspects.Results. In: Krayenbühl H., editor. Advances and Technical Standards in Neurosurgery. New York, Zurich, Wien: Springer-Verlag; 1977:47-78.
38. Little J.R., Salerno T.A. Continuous suturing for microvascular anastomosis—technical note. J Neurosurg. 1978;48:1042-1045.
39. Kim S.K., Wang K.C., Kim I.O., et al. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo (periostial) synangiosis in pediatric moyamoya disease. Neurosurgery. 2002;50:88-96.