Chapter 39 Surgical Treatment of Abdominal Aortic Aneurysms
Abdominal aortic aneurysms (AAAs) remain a leading cause of death in the elderly. In the United States, ruptured AAAs are the 15th leading cause of death overall and the 10th leading cause of death in men older than age 55.1 In addition, 30% to 40% of patients with ruptured AAAs die after reaching a hospital, but without operation.2 When combined with an operative mortality rate of 40% to 50%,3–7 this results in an overall mortality rate of 80% to 90% for AAA rupture.8–10 Unfortunately, this high mortality rate has not changed over the past 20 years despite improvements in operative technique and perioperative critical care management that have reduced the elective surgical mortality rate to less than 5% in most series.3 Ruptured aneurysms also impose a substantial financial burden on overall healthcare costs. One report estimated that as much as $50 million and 2000 lives could have been saved in 1 year if AAAs had been repaired prior to rupture.11 Another study showed that emergency operations for AAAs resulted in a mean financial loss to the hospital of $24,655 per patient.12 These data have significant implications in an era of healthcare cost containment. For all these reasons, AAAs remain a central focus for vascular surgeons and an important healthcare problem for all physicians.
Definition
Most aortic aneurysms are true aneurysms involving all layers of the aortic wall and are infrarenal in location. As shown by Pierce et al.,13 normal aortic diameter gradually decreases from the thorax (28 mm in men) to the infrarenal location (20 mm in men). At all anatomical levels, normal aortic diameter is approximately 2 mm larger in men than in women and increases with age and increased body surface area.13 Because the average infrarenal aortic diameter is 2 cm, using a 3-cm definition for an infrarenal AAA has been recommended, without the need to consider a more complicated definition based on factors such as gender or body surface area. Although such definitions are useful for large patient groups, in clinical practice with individual patients, defining an aneurysm based on a 50% or greater diameter enlargement compared with the adjacent nonaneurysmal aorta has been recommended.14 This is particularly true for patients with unusually small arteries, in whom even a 2.5-cm local dilation of the infrarenal aorta might be aneurysmal if the adjacent aorta were only 1.5 cm in diameter.
Decision Making for Elective Abdominal Aortic Aneurysm Repair
The choice between observation and elective surgical repair of an AAA for an individual patient at any given point should take into account the (1) rupture risk under observation, (2) operative risk of repair, (3) patient’s life expectancy, and (4) personal preferences of the patient.15,16 Two randomized trials have provided substantial information to assist with this decision-making process. The U.K. Small Aneurysm Trial was the first randomized trial to compare early surgery with surveillance of 4- to 5.5-cm diameter AAAs in 1090 patients aged 60 to 76.17 Those undergoing surveillance underwent repeat ultrasound every 6 months for AAAs 4 to 4.9 in diameter cm, and every 3 months for those 5 to 5.5 cm. If AAA diameter exceeded 5.5 cm, the expansion rate was more than 1 cm/yr, the AAA became tender, or repair of an iliac or thoracic aneurysm was necessary, elective surgical repair was recommended. At the initial report in 1998, after a mean 4.6 years’ follow-up, there was no difference in survival between the two groups. After 3 years, patients who had undergone early surgery had better late survival, but the difference was not significant. It was notable that more than 60% of patients randomized to surveillance eventually underwent surgery at a median time of 2.9 years. Rupture risk among those undergoing careful surveillance was 1% per year.
In 2002, the U.K. trial participants published results of long-term follow-up.18 At 8 years, there was a small survival advantage in the early surgery group (7.2% improved survival). However, the proportion of deaths due to rupture of an unrepaired AAA was low (6%). The early surgery group had a higher rate of smoking cessation, which may have contributed to a reduction in overall mortality. An additional 12% of surveillance patients underwent surgical repair during extended follow-up to bring the total to 74%. Fatal rupture occurred in only 5% of men but 14% of women in the surveillance group. Risk of rupture was more than four times higher for women than men. This prompted participants to recommend a lower-diameter threshold for elective AAA repair in women.
The Aneurysm Detection and Management (ADAM) study conducted at the U.S. Department of Veterans Affairs (VA) hospitals was published in 2002.19 In this trial, 1163 veterans (99% male) aged 50 to 79 with AAAs 4 to 5.4 cm in diameter were randomized to either surveillance or early surgery. Surveillance entailed ultrasound or computed tomography (CT) scan every 6 months, with elective surgery for expansion to 5.5 cm, expansion of greater than 0.7 cm in 6 months or greater than 1 cm in 1 year, or development of symptoms attributable to the AAA. Computed tomography was used for initial evaluation, with AAA diameter defined as the maximal cross-sectional measurement in any plane that was perpendicular to the aorta. Ultrasound was used for the majority of surveillance visits, but CT was used when the diameter reached 5.3 cm. Patients with severe heart or lung disease were excluded, as were those who were not likely to comply with surveillance. As in the U.K. trial, there was no survival difference between the two strategies after a mean follow-up of 4.9 years. Similarly, more than 60% of patients in the surveillance arm underwent repair. Initial AAA diameter predicted subsequent surgical repair in the surveillance group; 27% of those with AAAs initially 4 to 4.4 cm underwent repair during follow-up, compared with 53% of those with 4.5 to 4.9 cm, and 81% of those with 5- to 5.4-cm diameter AAAs. Operative mortality was 2.7% in the early surgery group and 2.1% in the surveillance group. Rupture risk in those undergoing surveillance was 0.6% per year. This trial confirmed the results of the U.K. trial, demonstrating lack of benefit of early surgery for AAAs 4 to 5.5 cm, even if operative mortality is low. Compliance with surveillance was high in both trials. More recently, Ouriel et al. reported results of 728 patients who were randomized to either ultrasound surveillance or early endovascular AAA repair (EVAR). Mean follow-up of 20 ± 12 months demonstrated no difference in AAA rupture, aneurysm-related death, or overall mortality between groups.20
Taken together, these two large randomized studies indicate that it is generally safe to wait for AAA diameter to reach 5.5 cm before performing surgery in selected men who are compliant with surveillance, even if their operative mortality is predicted to be low even in the endovascular era. However, compliance in these carefully monitored trials of selected patients was high. In another VA population, Valentine et al.21 reported that 32 of 101 patients undergoing AAA surveillance were noncompliant despite several appointment reminders, and 3 or 4 of these 32 patients experienced rupture. Additionally, the increased rupture risk for women seen in the U.K. trial highlights the need to individualize treatment on the basis of a careful assessment of individual patient characteristics (rupture risk, operative risk, life expectancy, and patient preferences).
Elective Operative Risk
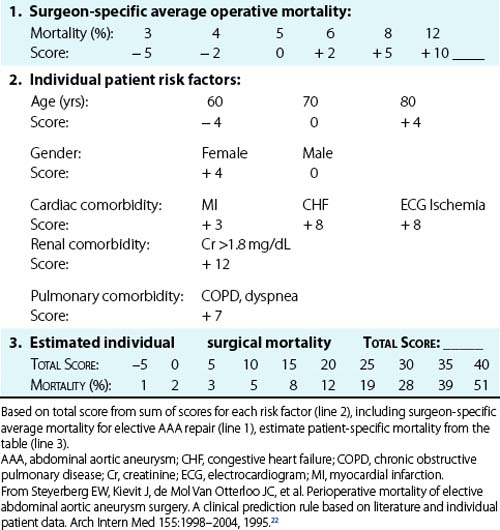
As expected, considerable variation in operative risk occurs among individual patients and depends on specific risk factors. A meta-analysis by Steyerberg et al.22 identified seven prognostic factors that were independently predictive of operative mortality with elective AAA repair and calculated the relative risk for these factors (Table 39-1). The most important risk factors for increased operative mortality were renal dysfunction (creatinine (Cr) > 1.8 mg/ dL), congestive heart failure (CHF) (cardiogenic pulmonary edema, jugular vein distension, or the presence of a gallop rhythm), and ischemic changes on resting electrocardiogram (ECG; ST depression >2 mm). Age had a limited effect on mortality when corrected for the highly associated comorbidities of cardiac, renal, and pulmonary dysfunction (mortality increased only 1.5-fold per decade). This explains the excellent results reported in multiple series in which selected octogenarians have undergone elective AAA repair, with mortality comparable to younger patients.23
Table 39-1 Independent Risk Factors for Operative Mortality After Elective Abdominal Aortic Aneurysm Repair
| Risk Factor | Odds Ratio* | 95% Confidence Interval |
|---|---|---|
| Cr > 1.8 mg/dL | 3.3 | 1.5-7.5 |
| CHF | 2.3 | 1.1-5.2 |
| ECG ischemia | 2.2 | 1-5.1 |
| Pulmonary dysfunction | 1.9 | 1-3.8 |
| Older age (per decade) | 1.5 | 1.2-1.8 |
| Female gender | 1.5 | 0.7-3 |
CHF, congestive heart failure; Cr, creatinine; ECG, electrocardiogram.
* Indicates relative risk compared with patients without that risk factor.
From Steyerberg EW, Kievit J, de Mol Van Otterloo JC, et al: Perioperative mortality of elective abdominal aortic aneurysm surgery. A clinical prediction rule based on literature and individual patient data. Arch Intern Med 155:1998–2004, 1995.22
On the basis of their analysis, Steyerberg et al.22 developed a clinical prediction rule to estimate the operative mortality for individual patients undergoing elective AAA repair (Table 39-2). This scoring system takes into account the seven independent risk factors plus the average overall elective mortality for a specific center. To demonstrate the impact of the risk factors on a hypothetical patient, it can be seen that the predicted operative mortality for a 70-year-old man in a center with an average operative mortality of 5% could range from 2% if no risk factors were present to more than 40% if cardiac, renal, and pulmonary comorbidities were all present. Obviously this would have a substantial impact on the decision to perform elective AAA repair. A similar Bayesian model for perioperative cardiac risk assessment in vascular patients has been reported by L’Italien et al.,24 which demonstrated the added predictive value of dipyridamole-thallium studies in patients with intermediate risk for cardiac death. This study also demonstrated the protective effect of coronary artery bypass surgery within the previous 5 years, which reduced the risk of myocardial infarction (MI) or death following AAA repair by 2.2-fold. Although this type of statistical modeling cannot substitute for experienced clinical judgment, it helps identify high-risk patients who might benefit from further evaluation, risk factor reduction, or medical management instead of surgery if AAA rupture risk is not high.
The review of Hallin et al.5 supports the findings of Steyerberg’s group that renal failure is the strongest predictor of mortality, with a four- to ninefold increased mortality risk. Cardiac disease (a history of either coronary artery disease [CAD], CHF, or prior MI) was associated with a 2.6- to 5.3-fold greater operative mortality risk. Older age and female gender appeared to be associated with increased risk, but the evidence was not as strong. Valuable data regarding predictors of operative risk have been generated by prospective trials. In the Canadian Aneurysm Study, overall operative mortality was 4.8%.25 Preoperative predictors of death were ECG evidence of ischemia, chronic pulmonary disease, and renal insufficiency. The randomized U.K. Small Aneurysm Trial found older age, lower forced expiratory volume in 1 second (FEV1), and higher Cr to be associated with mortality on univariate analysis.26 With multivariate analysis, the effect of age was diminished, whereas renal disease and pulmonary disease remained strong predictors of operative mortality. The predicted mortality ranged from 2.7% for younger patients with below average Cr and above average FEV1 to 7.8% in older patients with above average Cr and below average FEV1. The U.K. trialists noted that the Steyerberg prediction rule did not work well for the U.K. trial patients. However, they did not gather information on a history of CHF (one of the strongest predictors in Steyerberg’s analysis) in the randomized trial. Female gender has also been found to be associated with higher operative risk in several population-based studies using administrative data.3,22,27,28 However, these databases may suffer from inaccurate coding of comorbidities and thereby lack of ability to fully adjust for comorbid conditions.29 Gender has not been found to be associated with operative mortality in prospective trials.26,30
More recently, a study by Beck et al. from the Vascular Study Group of New England assessed risk factors associated with 1-year mortality following open AAA repair and EVAR. In this study, 1387 consecutive patients between 2003 and 2007 underwent elective AAA repair, including 748 who underwent open repair and 639 who underwent EVAR. Consistent with other studies, factors associated independently with 1-year mortality following open AAA repair included age (>70 years), chronic obstructive pulmonary disease (COPD), chronic renal insufficiency (Cr >1.8 mg/dL) and suprarenal aortic clamp site. Likewise, factors associated with 1-year mortality following EVAR included CHF and AAA diameter. One-year mortality correlated linearly with the number of risk factors present, and accordingly should be factored into decision making when considering elective AAA repair.31
Life Expectancy
Assessment of life expectancy is crucial to determine whether an individual patient will benefit from prophylactic repair of an AAA. Many patients with AAAs have been long-term smokers. Most AAA patients also have extensive comorbid disease, particularly CAD, COPD, hypertension, hyperlipidemia, cerebrovascular disease, and cancer.32–37 Many of these chronic conditions increase operative risk, as noted earlier. In addition, these factors impact life expectancy. Patients who survive elective AAA repair have a reduced life expectancy compared to age- and gender-matched populations.38–40 In 2001, Norman et al.41 reviewed 32 publications over 20 years that described long-term survival after AAA repair. They found that the mean 5-year survival after AAA repair was 70%, compared with 80% in the age- and gender-matched population without AAA. Predictors of late death after successful AAA repair include age, cardiac disease, chronic pulmonary disease, renal insufficiency, and continued smoking.38,42,43 The U.K. trialists found (after adjustment for age, gender, and AAA diameter but not cardiac disease) that both FEV1 and current smoking status (plasma cotinine) predicted late death.43
Surgical Decision Making
In patients with symptomatic AAAs, operative repair is nearly always appropriate because of the high mortality associated with rupture or thrombosis and the high likelihood of limb loss associated with peripheral embolism. Occasionally, high-risk patients or those with short life expectancies may choose to forego emergency repair of symptomatic AAAs, but in general, surgical decision making for symptomatic AAAs is straightforward. A contemporary analysis of outcomes of symptomatic AAAs by De Martino et al. from the Vascular Study Group of New England recently assessed 2386 AAA repairs in whom 1959 were elective, 156 were symptomatic, and 271 were ruptured. EVAR was successfully performed in 945 elective patients, 60 symptomatic patients, and 33 ruptured AAA patients, respectively. Hospital mortality was 1.7% for elective AAA, compared to 1.3% for the symptomatic cohort. One- and 4-year survival was determined to be 83% and 68%, respectively, among the symptomatic group, which compared favorably to the elective group with 89% and 73% 1- and 4-year survival.44
For those with asymptomatic AAAs, randomized trials have provided assurance that the typical male patient can generally be safely monitored with careful ultrasound surveillance until the AAA reaches 5.5 cm, at which time elective repair can be performed. However, decision analyses and cost-effectiveness modeling have previously demonstrated that individual patient rupture risk, operative risk, and life expectancy have to be considered to determine the optimal threshold for intervention.15,16,45,46 Both the U.K. and ADAM trials excluded patients who were considered “unfit” for repair, highlighting the fact that those with high operative risk and short life expectancy should have a threshold diameter greater than 5.5 cm. In the U.K. trial, the rupture risk for women was 4.5-fold higher than for men, prompting the authors to recommend a lower threshold for women than men, so it seems logical to consider other factors that may make rupture more likely during surveillance as well. In both randomized trials, 60% to 75% of patients undergoing surveillance eventually underwent AAA repair.19,47 In the U.K. trial, 81% of those with initial diameters 5 to 5.4 cm eventually underwent repair. Clearly, for many patients with this size AAA, the question is not whether to perform AAA repair but when. Therefore, in patients with AAA diameters approaching 5.5 cm whose life expectancy is expected to be more than 5 years and whose operative risk is estimated to be low, the patient should be informed that AAA repair would likely be required within the next few years. This subgroup of patients could be offered surgery at a time when it is convenient for them, with the understanding that waiting for expansion to 5.5 cm has little risk. In these cases, patient preference should weigh heavily in the decision-making process. For those with multiple risk factors for rupture, long life expectancy, and low operative risk, it would seem prudent to recommend AAA repair at less than 5.5 cm. Additionally, the ability of the patient to comply with careful surveillance should be considered. Although the recent randomized trials have provided a great deal of information to guide decision making, clinicians should not adopt a one-size-fits-all policy for treating patients with AAA. Moreover, with a progressively aging population in mind, quality-of-life assessments should likely be factored into decision-making analyses as well.
Preoperative Assessment
Patient Evaluation
A careful history, physical examination, and basic laboratory data are the most important factors for estimating perioperative risk and subsequent life expectancy. These factors may not only influence the decision to perform elective AAA repair, but they may focus preoperative management to reduce modifiable risk. Assessments of activity level, stamina, and stability of health are important and can be translated into metabolic equivalents to help assess both cardiac and pulmonary risks.48 Because COPD is an independent predictor of operative mortality,26,30 it should be assessed by pulmonary function studies as well as room air arterial blood gas measurement in patients who have apparent pulmonary disease. In some cases, preoperative treatment with bronchodilators and pulmonary toilet can reduce operative risk.49 In more extreme cases, pulmonary risk may substantially reduce life expectancy, and in these patients, formal pulmonary consultation may be helpful to estimate survival. Serum Cr is one of the most important predictors of operative mortality25 and must be assessed. The impact of other diseases such as malignancy on expected survival should also be carefully considered.
It is well established that patients with AAAs have a high prevalence of CAD. By performing routine preoperative coronary arteriography at the Cleveland Clinic in 1979, Hertzer et al.50 reported that only 6% of patients with AAAs had normal arteries; 29% had mild to moderate CAD, 29% had advanced compensated CAD, 31% had severe correctable CAD, and 5% had severe uncorrectable CAD. Furthermore, this study established that clinical prediction of the severity of CAD was imperfect because 18% of patients without clinically apparent CAD had severe correctable CAD on arteriography, compared with 44% of patients whose CAD was clinically apparent. This pivotal study has led to intense efforts to identify risk factors and algorithms that more accurately predict the presence of severe CAD that would justify its correction before AAA repair, or would lead to avoiding AAA repair. A number of clinical parameters such as angina, history of MI, Q-wave on ECG, ventricular arrhythmia, CHF, diabetes, and increasing age have been reported to increase the risk of postoperative cardiac events.51 Various combinations of these risk factors have been used to generate prediction algorithms for perioperative cardiac morbidity.48 In general, these algorithms identify low-risk, high-risk, or intermediate-risk patients. For high-risk patients, such as those with unstable angina, more sophisticated cardiac evaluation is required, whereas low-risk patients may undergo elective AAA repair without further testing. For intermediate-risk patients, who comprise the vast majority with AAAs, decision making is more difficult and may be assisted by additional cardiac testing.51
Aneurysm Evaluation
Most surgeons recommend a preoperative imaging study using CT scanning, magnetic resonance imaging or angiography (MRI/MRA), or arteriography. Contrast-enhanced CT appears to be the most useful study for preoperative AAA evaluation when considering information obtained, invasiveness, and cost (also see Chapter 14). This is particularly true for spiral CT scanning, with thin “slices” in the region of interest. This allows not only accurate size measurements but also accurate definition of the relationship of an AAA to visceral and renal arteries. Furthermore, CT scanning aids in identifying venous anatomical anomalies (e.g., retroaortic left renal vein, duplicated vena cava) or renal abnormalities (e.g., horseshoe or pelvic kidney) that would influence operative techniques and approach. Computed tomography is the technique of choice to identify suspected inflammatory aneurysms and may reveal unsuspected abdominal pathology such as associated malignancy or gallbladder disease. In centers with experience with these techniques, CT angiography has made percutaneous intraarterial angiography unnecessary in the vast majority of AAA patients. Moreover, in the EVAR era, CT is vital for case planning and accurate detailed anatomical assessment of aortic neck anatomy, iliac artery anatomy and tortuosity, and perirenal mural thrombus burden among other factors. In addition, three-dimensional (ED) modeling of contemporary CT scanning is useful prior to EVAR as well as open AAA repair and has largely supplanted the role of conventional angiography.
Magnetic resonance imaging is comparable with CT in terms of AAA measurement accuracy and other preoperative planning issues (also see Chapter 13). It avoids intravenous contrast, which may represent an advantage over CT for some patients. Because it is more expensive and time consuming, it also is not as widely used as CT. When MRA is included with this technique, however, it can significantly increase the value in patients where additional imaging would otherwise be required.
Surgical Treatment
For the past 40 years, AAAs have been repaired using the technique of endoaneurysmorrhaphy with intraluminal graft placement, as described by Creech.52 This procedure is described later in the section on transperitoneal approach. Development of this technique was based in part on the failure of previous “nonresective” operations now of only historical interest, including aneurysm ligation, wrapping, and attempts at inducing aneurysm thrombosis that yielded uniformly dismal results. Abdominal aortic aneurysm thrombosis by iliac ligation combined with axillobifemoral bypass enjoyed a brief resurgence in popularity for high-risk patients but demonstrated a high complication rate, including late aneurysm rupture, and an operative mortality rate comparable with conventional repair in similar patients.53–57 Thus this technique was similarly abandoned. As an alternative to standard open AAA repair, Shah and Leather et al.58 proposed exclusion of an AAA with bypass to reduce operative blood loss. However, this group has recently published long-term follow-up and no longer recommends this procedure owing to persistent flow in the excluded AAA sac and rupture in rare cases.59 In another attempt to reduce the invasiveness of open AAA repair, the use of laparoscopy as an adjunct has been suggested to assist AAA repair. This approach uses laparoscopic techniques to dissect the aneurysm neck and iliac arteries, followed by a standard endoaneurysmorrhaphy through a mini-laparotomy. Cohen et al.60 have reported their results in 20 patients to demonstrate the feasibility of this approach, but a clear benefit has not been shown; intraoperative, intensive care unit (ICU), and total hospital duration were comparable with conventional AAA repair. Further experience with this technique may identify a subgroup of patients for whom a laparoscopic-assisted AAA repair is advantageous.
EVAR (see Chapter 40) repair was introduced by Parodi in 1991 and has rapidly gained in popularity in the United States after reports of clinical trials and subsequent U.S. Food and Drug Administration (FDA) approval.61 Endovascular AAA repair has been shown to reduce operative morbidity, mortality, length of stay, and disability compared with open repair.62–65 Recovery time is shorter after endovascular repair than open repair,63,66 but endovascular repair may not be as durable.67–74 Frequent and lifelong surveillance is required after endovascular repair, along with reintervention or conversion to open repair in some. There appears to be a small ongoing risk of rupture after endografting as well. Decision analysis suggests that there is little difference in outcome between open and endovascular repair for most patients.72 However, endovascular AAA repair is usually recommended for those with good anatomy for EVAR or those with marginal anatomy but high operative risk for open surgery. Open surgery may be preferred for younger, healthier patients in whom there is little difference in operative risk between the two strategies, and for whom long-term durability is a concern, although contemporary stent grafts appear to have improved durability from their initial constructs and are now recommended for most patients with acceptable anatomy (see Chapter 40).
To date, there are several important randomized trials comparing open AAA repair with endovascular repair. Specifically, in the EVAR I and DREAM trials, patients were randomized to either open repair or EVAR. The EVAR I study demonstrated a 3% lower initial mortality associated with endovascular treatment, with a persistent associated reduction in AAA-related death at 4 years. However, there was no overall improvement in all-cause mortality between groups. Likewise, the DREAM trial demonstrated an operative mortality advantage associated with EVAR compared to open surgical repair, but 1-year survival was similar between groups. The EVAR II study randomized patients unfit for open AAA repair to either EVAR or no surgical therapy. This trial failed to demonstrate a survival advantage for the EVAR treatment group compared to the no treatment group. It should be noted, however, that most ruptures in the EVAR group occurred during a prolonged delay before surgery, making the results in this group appear worse. In addition, 27% of patients in EVAR II crossed over from the no treatment group to the EVAR group, potentially limiting the study’s findings.71,75–77 Likewise, the VA Open vs. Endovascular AAA repair (OVER) study randomized patients to either open AAA repair or EVAR. Results demonstrated diminished perioperative mortality in the EVAR group compared to the open repair group (0.5% vs. 3.0%). However, there was no observed difference in mortality at 2 years between groups. This study also demonstrated diminished median procedure times, blood loss, transfusion requirement, duration of mechanical ventilation, hospital length of stay, and ICU length of stay in the EVAR group.78
Perioperative Management
Preoperative intravenous antibiotics are administered to reduce the risk of prosthetic graft infection.79 Ample intravenous access, intraarterial pressure recording, and Foley catheter monitoring of urine output are routine. For patients with significant cardiac disease, pulmonary artery catheters are frequently used to guide volume replacement and vasodilator or inotropic drug therapy, both intraoperatively and in the early postoperative period. Mixed venous oxygen tension measurement, available with these catheters, can provide an additional estimate of global circulatory function. Transesophageal echocardiography (TEE) can be useful in certain patients to monitor ventricular volume and cardiac wall motion abnormalities and to guide fluid administration and use of vasoactive drugs. Despite the frequent use of pulmonary artery catheters, studies examining their use during AAA surgery have not demonstrated added value.80,81 However, these studies have usually excluded high-risk patients who are most likely to benefit from such monitoring. These techniques are not without risk, so selective use is probably more appropriate than routine application.
The volume of blood lost during AAA repair often requires blood replacement. Therefore, intraoperative autotransfusion as well as preoperative autologous blood donation has become popular, primarily to avoid the infection risk associated with allogeneic transfusion. Studies of the cost-effectiveness of such procedures, however, question their routine use.82–84 Autologous blood donation is less important for elderly patients in whom life expectancy is shorter than the usual time for development of transfusion-associated viral illness. Autologous blood donation does not appear to be cost-effective in elderly cardiovascular patients, because the allogenic blood pool has become safer and the transfusion requirement for elective AAA repairs lower.82 Intraoperative autotransfusion during AAA repair is widely used because of the documented safety of this technique.85 Because it is usually difficult to predict the volume of blood loss during AAA repair, most surgeons employ autotransfusion in case blood loss becomes extensive. Optimizing oxygen delivery to patients with reduced cardiac output by maintaining an adequate hematocrit appears beneficial in patients undergoing AAA repair. One study has shown that a postoperative hematocrit of less than 28% was associated with significant cardiac morbidity in vascular surgery patients.86
Maintenance of normal body temperature during aortic surgery is important to prevent coagulopathy, allow extubation, and maintain normal metabolic function. In a review of patients undergoing elective AAA repair, Bush et al.87 noted significantly more organ dysfunction (53% vs. 29%) and higher mortality (12% vs. 1.5%) in hypothermic patients (temperature <34.5 °C) compared with normothermic patients. The only predictor of intraoperative hypothermia was female gender, whereas prolonged hypothermia was related to initial hypothermia, indicating the difficulty in rewarming cold patients. A recent randomized trial found significantly reduced cardiac morbidity (1.4% vs. 6.3%) in patients who were normothermic (36.7 °C) rather than hypothermic (35.4 °C) intraoperatively.88 To prevent hypothermia, a recirculating warm forced-air blanket should be placed in contact with the patient, and intravenous fluids, including any blood returned from an autotransfusion device, should be warmed before administration.
The role of ischemic preconditioning in lowering the incidence of perioperative MI during open AAA repair remains undefined, although there are data to support its potential benefit. In the largest study to date, Ali et al. randomized 82 patients undergoing elective open AAA repair to receive remote ischemic preconditioning or not. The technique involves sequential clamping of each common iliac artery (CIA) for 10 minutes, followed by 10 minutes of respective reperfusion. The authors demonstrated that patients undergoing remote ischemic preconditioning had both diminished rates of postoperative MI and diminished critical care length of stay compared to the control groups.89
Anesthesia
Nearly all patients undergo general anesthesia for AAA repair. Supplemental use of continuous epidural anesthesia, begun immediately preoperatively and continued for postoperative pain control, is increasing in popularity.90 This technique allows a lighter level of general anesthesia to be maintained while controlling pain through the epidural blockade. Additional benefits may include a reduction in the sympathetic catecholamine stress response, which might decrease cardiac complications. One randomized trial comparing general anesthesia with combined general and epidural anesthesia demonstrated decreased deaths, cardiac events, infection, and overall complications.91 These benefits, however, were not observed in another randomized trial,92 suggesting that the details of perioperative management and patient selection may determine the impact of epidural anesthesia. Furthermore, it is possible that the major benefit of epidural anesthesia accrues in the postoperative period rather than intraoperatively.93
Perioperative β-adrenergic blockade remains somewhat more controversial, given recent findings of randomized controlled trials.94 Earlier studies by Pasternack et al.95 demonstrated that patients who underwent vascular surgery and received metoprolol immediately before operation had significantly lower heart rates and less intraoperative myocardial ischemia than untreated controls. Mangano et al.96 performed the first randomized placebo-controlled trial to assess the effect of atenolol (given intravenously immediately before and after surgery and orally during that hospitalization) in patients at risk for CAD who underwent noncardiac surgery. A significant reduction in mortality extending 2 years after discharge was observed in the atenolol-treated patients (3% vs. 14% 1-year mortality) because of reduction in death from cardiac causes. In a separate analysis, they noted that atenolol-treated patients had a 50% lower incidence of myocardial ischemia during the first 48 hours after surgery and a 40% lower incidence during postoperative days 0 to 7.97 Patients with perioperative myocardial ischemia were significantly more likely to die within 2 years after surgery. Poldermans et al.98 performed a randomized trial of perioperative β-blockade with bisoprolol in patients with abnormal dobutamine echocardiograms undergoing aortic or lower-extremity arterial reconstruction. They found that perioperative cardiac death was significantly reduced from 17% (placebo) to 3% (bisoprolol). Additionally, nonfatal MI occurred in 17% of those given placebo but in none of those given bisoprolol. A subsequent publication from the same authors demonstrated that during a mean follow-up of 22 months, cardiac events were significantly lower in those who had received perioperative β-blockade (12% vs. 32%).99
More recently, however, results from the POISE trial, a randomized controlled trial reflecting 190 hospitals, 23 countries, and an enrollment of 8351 patients, provided different results. This study compared the effects of perioperative extended release metoprolol succinate with a limited titration scheme to placebo among patients undergoing noncardiac surgery. Results demonstrated that there was a significant reduction in the composite endpoint of cardiovascular death, nonfatal MI, and nonfatal cardiac arrest among patients receiving perioperative β-blocker therapy. However, the study also revealed that there were more deaths and strokes among the treated group compared to placebo.94 Although these findings seemingly conflict, perioperative β-blocker use is valuable when titrated to heart rate, but not when applied at initial high dose or without respect to the patient’s hemodynamics.100
Given this knowledge, it has been suggested that β-blockers are underused, likely because of fears about use in patients with COPD or prior heart failure. However, chronic β-blocker usage is now known to improve outcomes in patients with heart failure.101,102 Additionally, Gottlieb et al.101 demonstrated that COPD should not be considered a contraindication for β-blockade. They found a 40% reduction in risk of death after MI in patients with COPD who were taking β-blockers compared with those who were not. In Mangano’s trial, the only exclusion criteria were preexisting ECG abnormalities that would preclude detection of new ischemic events. β-Blockers were withheld during the trial only for a heart rate of less than 55 beats/min, systolic blood pressure less than 100 mmHg, acute bronchospasm, current evidence of CHF, or third-degree heart block. The weight of evidence supports routine use of β-blockers for nearly all patients undergoing AAA repair.
Antiplatelet use remains common in this patient cohort, concordant with American College of Cardiology/American Heart Association (ACC/AHA) guidelines for noncardiac surgery. Associated bleeding risk with such agents, including aspirin and clopidogrel, remains controversial. In a recent study by the Vascular Study Group of New England, however, preoperative antiplatelet use (aspirin alone, clopidogrel alone, combined dual therapy) was not significantly associated with increased serious bleeding complications, measured as reoperation for bleeding across a spectrum of commonly performed vascular procedures including EVAR, open AAA repair, carotid endarterectomy, and lower-extremity bypass.103
Choice of Incision
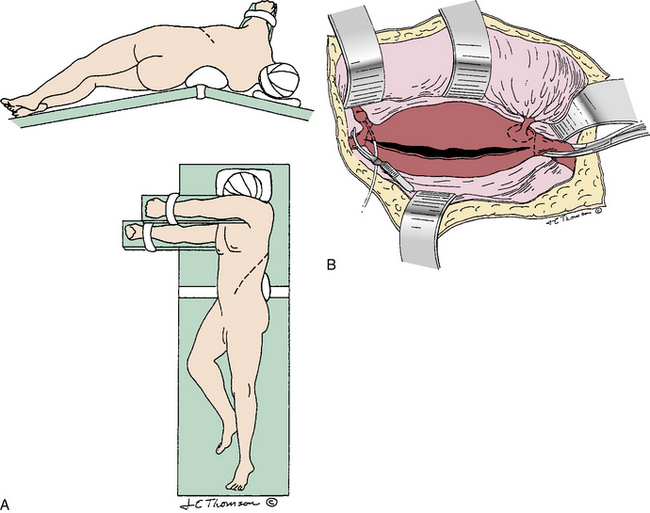
Abdominal aortic aneurysm repair can be accomplished through an anterior transperitoneal incision (midline or transverse; Fig. 39-1) or through a retroperitoneal approach (Fig. 39-2). Midline transperitoneal incisions can be performed rapidly and provide wide access to the abdomen, but they may be associated with more pulmonary complications due to postoperative splinting from upper abdominal pain. Transverse abdominal incisions just above or below the umbilicus require more time to open and close, but may be associated with fewer pulmonary complications and late incisional hernias, although this has not yet been proven. Retroperitoneal incisions, from the lateral rectus margin extending into the 10th or 11th intercostal space, afford good exposure of both the infrarenal and suprarenal aorta, but limit exposure of the contralateral renal and iliac arteries. In addition, this exposure does not allow access to intraabdominal organs unless the peritoneum is purposely opened. The left retroperitoneal approach is usually favored over the right for exposure of the upper abdominal aorta because the spleen is easier to mobilize and retract than the liver. The right retroperitoneal approach is used when specific abdominal problems, such as a stoma, preclude the left-sided approach.104
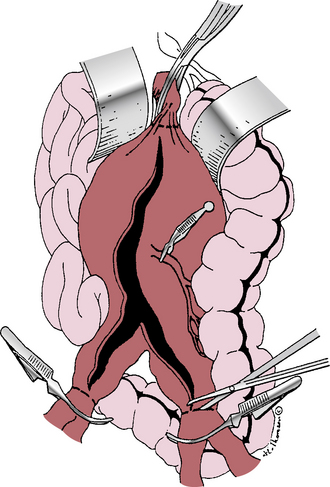
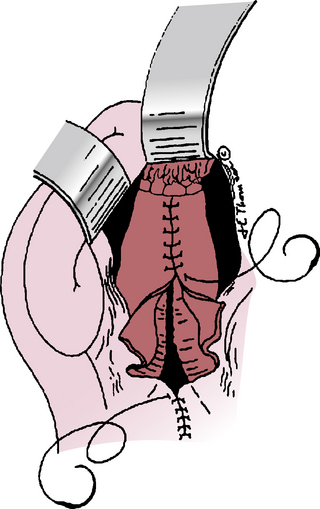
Figure 39-1 Transperitoneal abdominal aortic aneurysm (AAA) exposure, vascular clamps in place, incising the aneurysm.
In recent years, the left retroperitoneal approach has enjoyed a resurgence in popularity owing to suggestions that pulmonary morbidity, ileus, and intravenous fluid requirements are decreased postoperatively. Randomized trials have reached different conclusions about the potential advantages of retroperitoneal over transabdominal incisions, however. Sicard et al.105 reported more prolonged ileus, small-bowel obstruction, and overall complications after transabdominal compared with retroperitoneal aortic surgery, although pulmonary complications were similar. Cambria et al.106 found no differences in these incisions in terms of pulmonary complications, fluid or blood requirements, or other postoperative complications, except for slightly prolonged return to oral intake after the transperitoneal approach.
In the most recent randomized trial, Sieunarine et al.107 found no differences in operating time, cross-clamp time, blood loss, fluid requirement, analgesia requirement, gastrointestinal function, ICU stay, or hospital stay for transperitoneal versus retroperitoneal approaches for aortic surgery. In long-term follow-up, however, there were significantly more wound problems (hernias, bulging, and pain) in the retroperitoneal group. These results suggest that in most cases, the choice of incision for AAA repair is a matter of personal preference. However, both the transperitoneal and retroperitoneal approaches have advantages in certain patients. Relative indications for retroperitoneal exposure include a “hostile” abdomen due to multiple previous transperitoneal operations, an abdominal wall stoma, a horseshoe kidney, an inflammatory aneurysm, or anticipated need for suprarenal endarterectomy or anastomosis, mindful that the retroperitoneal approach provides facilitated access to the visceral aorta or even supraceliac aortic segments. Relative indications for a transperitoneal approach include a ruptured AAA, coexistent intraabdominal pathology, uncertain diagnosis, left-sided vena cava, large bilateral iliac artery aneurysms, or need for access to both renal arteries. Advantages of each approach make it advisable for surgeons to become proficient with both techniques.
Transperitoneal Approach
After entering the abdomen through a transperitoneal incision, the abdomen is thoroughly explored to exclude other pathology and assess extent of the aneurysm. The transverse colon is then retracted superiorly, and the ligament of Treitz is divided to allow retraction of the small bowel to the right. Exposure is greatly assisted using a fixed self-retaining retractor. A longitudinal incision is made in the peritoneum just to the left of the base of the small-bowel mesentery to expose the aneurysm. This incision extends from the inferior border of the pancreas proximally to the level of normal iliac arteries distally. Care must be taken to avoid the ureters, especially if exposure includes the iliac bifurcation where the ureters normally cross. Autonomic nerves to the pelvis course anterior to the proximal segment of the left CIA and should be retracted with associated retroperitoneal tissue rather than incised, to prevent sexual dysfunction in men. The left renal vein should be identified and retracted superiorly if necessary to fully expose the neck of the aneurysm. Care must be taken not to avulse renal vein tributaries, particularly a descending lumbar vein, frequently encountered to the left of the aorta, which must be divided before the left renal vein is mobile enough to allow upward retraction. Rarely, proximal exposure cannot be obtained without division of the left renal vein. In such cases, this should be done at its junction with the vena cava to maintain patency of collateral drainage via adrenal and gonadal branches. In a recent study by Sampson et al., 56 patients underwent left renal vein division and ligation during open aortic surgery; none developed directly related complications.108 If necessary, reanastomosis can be performed if renal vein engorgement suggests inadequate collateral drainage.
After obtaining adequate aortoiliac exposure, the normal aorta and iliac arteries are dissected sufficiently to place a vascular clamp proximal and distal to the aneurysm. Regardless of the proximal extent of an infrarenal AAA, it is desirable to construct the proximal aortic anastomosis near the renal arteries to avoid subsequent aneurysmal degeneration of residual infrarenal aorta. When an AAA approaches or involves the renal arteries, it can be safer to apply the cross-clamp proximal to the celiac artery, rather than between the renal arteries and the superior mesenteric artery (SMA). Green et al.109 demonstrated much higher operative mortality (32% vs. 3%) and renal failure requiring dialysis (23% vs. 3%) after infrarenal AAA repair when clamping was performed between the SMA and renal arteries rather than proximal to the celiac artery. They attributed this to the greater likelihood of dislodging atherosclerotic debris in the pararenal aorta as opposed to the supraceliac aorta, which is usually less diseased. Complications resulted from atheroembolization to the kidneys, legs, and intestine or injury to the aorta or renal arteries.
Others have also noted the relative safety of clamping the supraceliac aorta, which can easily be accessed by dividing the gastrohepatic ligament and the diaphragmatic crus.110 However, aortic clamping between the renal arteries and the SMA is also safe when performed in properly selected patients without extensive plaque in this region.111 Occasionally it is possible to obtain distal control of an AAA on the aorta, but usually aneurysmal changes or calcification in this location make iliac artery clamping preferred. A disease-free area of proximal aorta and iliac arteries should be identified for clamping to minimize the possibility of clamp injury or embolization of arterial debris. Some iliac arteries may be so diffusely calcified that clamping without injury is impossible. In such cases, internal occlusion with a balloon catheter or extension of the graft to the femoral arteries is required. In most cases, it is unnecessary to completely encircle the aorta and iliac arteries because vascular clamps can be placed in the anteroposterior direction, leaving the back wall undissected. This minimizes the likelihood of injury to both lumbar and iliac veins. Sometimes posterior arterial plaque necessitates placement of a vascular clamp transversely on either the aorta or iliac arteries, which then require careful posterior dissection precisely on the plane of the artery to avoid venous injury.
Abdominal aortic aneurysm repair can be accomplished with a straight (“tube”) graft in 40% to 50% of patients, without extension onto the iliac arteries.30,112 Although concern has been raised about the potential for future aneurysm development in the iliac arteries after tube graft repair of AAAs, late follow-up has shown that this is not clinically significant if the iliac arteries were not aneurysmal at the time of AAA repair.113 Extension to the iliac arteries with a bifurcated graft for AAA repair is necessary in the remaining 50% to 60% of patients because of aneurysmal involvement of the iliac arteries or severe calcification of the aortic bifurcation. Extension of the graft to the femoral artery level is indicated for severe concomitant iliac occlusive disease or rarely because of technical difficulties associated with a deep pelvic anastomosis. Iliac artery anastomoses are preferred, however, owing to decreased infection and pseudoaneurysm complications compared with femoral artery anastomoses.
Prosthetic grafts available for AAA repair include knitted Dacron, knitted Dacron impregnated with collagen or gelatin to decrease porosity, woven Dacron, and polytetrafluoroethylene (PTFE). There is no clear evidence that any of these graft types provides superior outcome. In a prospective randomized comparison of PTFE and Dacron, long-term patency was equivalent, but PTFE had a higher incidence of early graft failure and graft sepsis.114 In contrast, in a smaller trial with shorter follow-up, PTFE was found to be superior.115 Most surgeons prefer an impervious graft to avoid the need for preclotting and thus select impregnated knitted Dacron, PTFE, or woven Dacron.116 This not only saves time and more reliably prevents bleeding through the graft but also allows graft selection to be delayed until the aneurysm is opened so that a graft diameter corresponding to the inner diameter of the normal proximal aorta can be selected. It also allows delayed selection of a straight versus bifurcated graft that may not always be obvious before the aneurysm is open and the distal aorta can be carefully inspected.
Most surgeons use heparin anticoagulation during aortic cross-clamping to reduce lower-extremity thrombotic complications. Heparin dosage varies from 50 to 150 units/kg, based on personal preference. Activated clotting time (ACT) measurement is useful to determine the need for supplemental heparin in prolonged cases and the appropriate dose of protamine sulfate to reverse anticoagulation after declamping.117 The sequence for applying proximal and distal vascular clamps is selected to apply the initial clamp in the area of least atherosclerotic disease to reduce the risk of distal embolization. The aneurysm is opened longitudinally along its anterior surface, away from the inferior mesenteric artery (IMA) in case this requires later reimplantation. The proximal aorta is then incised horizontally at the level selected for proximal anastomosis (see Fig. 39-1). To avoid potential injury to posterior veins, this incision does not have to extend through the back wall of the aorta, although some surgeons prefer complete transection for better exposure. Intraluminal thrombotic material and atherosclerotic debris are extracted from the aneurysm sac, which usually discloses several backbleeding lumbar artery orifices that require suture ligation. If the IMA is patent, it should be controlled temporarily with a small vascular clamp (see Fig. 39-1) so its need for reimplantation can be assessed after the revascularization is completed. Inferior mesenteric artery revascularization may be advised if the hypogastric arteries are diseased or if one requires ligation for technical reasons.
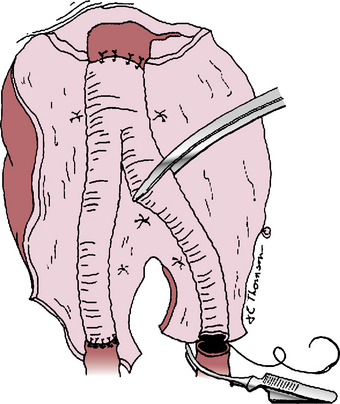
Once hemostasis within the opened aneurysm sac has been achieved, the proximal anastomosis is performed. There is often a distinct ring at the aneurysm neck that defines the appropriate level for this anastomosis. Usually polypropylene suture is used, taking large aortic “bites” and incorporating a double thickness of posterior aortic wall for added strength. If the aortic wall is friable, pledgets of Teflon or Dacron can be incorporated into the suture line. After completing the proximal anastomosis, the graft is clamped and the proximal aortic clamp released briefly to check for and correct any suture-line bleeding. If the distal anastomosis is to the aorta, a similar technique is used just above its bifurcation, suturing from within the lumen and encompassing both iliac artery orifices within the suture line. If iliac artery aneurysms exist, these are incised anteriorly so the limbs of a bifurcated graft can be sutured to the normal iliac artery beyond these aneurysms (Fig. 39-3). Often this requires graft extension to the CIA bifurcation, including the orifices of both the internal and external iliac arteries within the distal anastomosis. In rare instances, aneurysmal involvement of the distal CIA may preclude anastomosis to both the internal and external iliac artery orifices because these are widely separated. In such cases, an external iliac artery (EIA) anastomosis can be constructed, but care must be taken to preserve adequate pelvic blood flow, which may mean direct revascularization of at least one internal iliac artery (IIA). The need for internal iliac revascularization is usually assessed by the extent of back-bleeding, as discussed later in this chapter (isolated iliac aneurysms). For large aneurysms of the left iliac artery, medial reflection of the sigmoid mesocolon assists a retroperitoneal approach to the distal CIA and prevents unnecessary dissection of autonomic nerves crossing the proximal left common iliac artery. Before completing the distal anastomoses, arterial clamps are carefully removed, and vigorous irrigation is used to flush out any thrombus or debris.
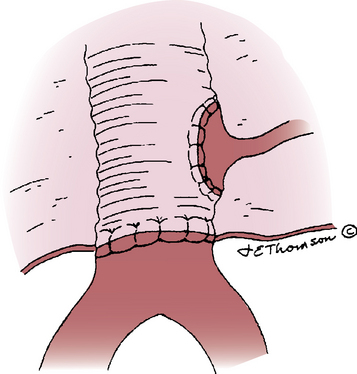
The IMA can be ligated with a transfixing suture applied to its internal orifice if it is small and not associated with known SMA occlusive disease, if it has good backflow on release of its vascular clamp, if the sigmoid colon and arterial pulsations are good, and if at least one IIA is patent. In questionable cases, Doppler signals from the sigmoid colon or an assessment of IMA stump pressure118 may be necessary to determine the need for IMA reimplantation. In the rare circumstances when sigmoid colon perfusion appears marginal, a circular cuff of the aortic wall around the IMA orifice is excised (Carrel patch) and anastomosed to the left side of the graft (Fig. 39-4). Next, the adequacy of lower-extremity blood flow is determined by visual inspection of the feet, palpation of distal pulses, or more sophisticated Doppler or pulse volume recording. If reduced blood flow is detected, intraoperative arteriography can differentiate thrombosis or embolism from peripheral vasoconstriction, which is relatively common if the procedure is prolonged and the patient is cold. Embolism or thrombosis requires prompt surgical correction, whereas vasoconstriction requires correction of any volume deficit and rewarming.
After assuring adequate intestinal and lower-extremity circulation, heparin is reversed with protamine sulfate if sufficient heparin has been given to justify reversal, and hemostasis is achieved. The aneurysm wall and retroperitoneum are then closed over the graft to provide a tissue barrier between the prosthesis and the adjacent intestine (Fig. 39-5). The aortic prosthesis and upper anastomosis must be isolated from the overlying duodenum during closure; if necessary, a pedicle of greater omentum can be interposed to achieve this purpose. The small bowel should be inspected carefully and replaced in its normal position before abdominal closure.
Retroperitoneal Approach
Proper patient positioning is essential to achieve optimal exposure using the retroperitoneal approach. For most infrarenal AAAs, a left retroperitoneal incision centered on the 11th or 12th rib is employed. The patient’s left shoulder is elevated at a 45- to 60-degree angle relative to the table, and the pelvis is positioned relatively flat. The table is flexed with the break positioned at a level midway between the iliac crest and the costal margin (see Fig. 39-2A). An air-evacuating “beanbag” is helpful to maintain proper positioning. Beginning at the lateral border of the left rectus muscle midway between the pubis and umbilicus, the skin incision is carried superiorly and then curved laterally up to the tip of the 11th or 12th rib. If extensive exposure of the right iliac artery is required, the incision can be extended inferolaterally into the right lower quadrant, or a separate right lower quadrant retroperitoneal incision can be used. The underlying lateral abdominal wall muscles are divided, exposing the underlying peritoneum and the anterior edge of the properitoneal fat layer at the lateral aspect of this exposure. Dissection in the retroperitoneal plane is then developed, either anterior or posterior to the left kidney, until the aorta is encountered.
For infrarenal aneurysm exposure, it is often sufficient to proceed anteriorly and leave the left kidney in its normal position. For juxtarenal or suprarenal aneurysms that require more cephalad exposure, the kidney is mobilized anteriorly to approach the aorta from behind the left renal artery (see Fig. 39-2B). If the need for higher exposure is anticipated, the incision should be directed more cephalad over the 9th or 10th rib and the shoulders positioned as perpendicularly as possible to the table. In this case, more table flexion is required to open the space between the pelvis and ribs, and the trunk is twisted so that the angle between the pelvis and the table is about 30 degrees. When approaching the aorta from behind the left renal artery, it is necessary to divide a large lumbar branch of the left renal vein to mobilize the kidney and renal vein anteriorly. The ureter must be identified and retracted medially with the kidney, taking care to separate it from the iliac bifurcation distally.
Medial mobilization of the peritoneal contents exposes the IMA, which usually is divided for more complete exposure of the aortic bifurcation and right renal artery, depending on the size of the AAA. Exposure is greatly assisted by using a fixed self-retaining retractor. If necessary, exposure of the right iliac artery and right renal artery is easier after opening and decompressing the AAA. Right iliac artery control is often best accomplished by using a balloon occlusion catheter after entering the aneurysm (see Fig. 39-2B). After achieving adequate exposure, repair of the AAAs is usually carried out as described earlier for the transperitoneal approach. The retroperitoneal technique does not normally afford an opportunity to inspect colonic and intestinal viability, but the peritoneum can be opened to accomplish this if any concern exists.
Associated Arterial Disease
Indications for concomitant mesenteric or renal artery revascularization during elective AAA repair are comparable with those used for isolated disease in these arteries. Occasionally, patients with asymptomatic high-grade stenoses of these arteries warrant “prophylactic” concomitant reconstruction if the patient is at low operative risk and the AAA repair proceeds uneventfully. Although the natural history of asymptomatic mesenteric artery stenosis is not well characterized, it appears that patients with critical disease of all three mesenteric arteries are at sufficiently high risk for future complications of mesenteric ischemia that concomitant revascularization is justified.119 Progression of renal artery stenosis has been better documented,120,121 but the ultimate clinical impact of such progression appears minimal in nonhypertensive patients with normal renal function.122 The adjacency of the renal arteries to the operative field for AAA repair has led some to recommend prophylactic repair of critical but asymptomatic renal artery stenoses.123 Although this may be appropriate in younger good-risk patients, it adds morbidity and mortality to the AAA repair, leading others to recommend the combined procedure only for standard indications of hypertension or ischemic nephropathy.124,125
Complications of Abdominal Aortic Aneurysm Repair
Despite improvements in the outcome of elective AAA repair, major complications occur and must be correctly managed or avoided to maintain the low mortality necessary to justify prophylactic AAA repair. Myocardial infarction is the leading single-organ cause of both early and late mortality in patients undergoing AAA repair25 and must be carefully assessed and managed to reduce mortality. In a recent review of patients undergoing elective AAA repair, however, Huber et al.84 found that multisystem organ failure (MSOF) caused more deaths (57%) than cardiac events (25%). Visceral organ dysfunction was the most common cause of MSOF, followed by postoperative pneumonia. However, most patients with MSOF had associated cardiac dysfunction that may have aggravated visceral ischemic injury. Several factors may be responsible for the emergence of MSOF as a more prominent cause of death following elective AAA repair. First, with modern techniques of intensive care, it is uncommon for patients to die with single-system failure (even cardiac) following AAA repair. Second, strict attention to cardiac risk in these patients may have reduced the relative impact of cardiac complications. Finally, older patients with more associated visceral and renal artery disease underwent AAA repair in this series and had the highest likelihood of MSOF postoperatively. The relative frequency of single-system complications following elective AAA repair is listed in Table 39-3.
Table 39-3 Early (30-Day) Complications After Elective Abdominal Aortic Aneurysm Repair*
| Complication | Frequency |
|---|---|
| Death | <5% |
| All cardiac | 15% |
| MI | 2%-8% |
| All pulmonary | 8%-12% |
| Pneumonia | 5% |
| Renal insufficiency | 5%-12% |
| Dialysis dependent | 1%-6% |
| DVT | 8% |
| Bleeding | 2%-5% |
| Ureteral injury | <1% |
| Stroke | 1% |
| Leg ischemia | 1%-4% |
| Colon ischemia | 1% |
| Spinal cord ischemia | <1% |
| Wound infection | <5% |
| Graft infection | <1% |
| Graft thrombosis | <1% |
* Estimated from the following surgical series: Johnston KW: Multicenter prospective study of nonruptured abdominal aortic aneurysm. Part II. Variables predicting morbidity and mortality. J Vasc Surg 9:437–447, 1989; Johnston KW, Scobie TK: Multicenter prospective study of nonruptured abdominal aortic aneurysms. I. Population and operative management. J Vasc Surg 7:69–81, 1988; Olsen PS, Schroeder T, Agerskov K, et al: Surgery for abdominal aortic aneurysms. A survey of 656 patients. J Cardiovasc Surg 32:636–642, 1991; AbuRahma AF, Robinson PA, Boland JP, et al: Elective resection of 332 abdominal aortic aneurysms in a southern West Virginia community during a recent five-year period. Surgery 109:244–251, 1991; Diehl JT, Cali RF, Hertzer NR, Beven EG: Complications of abdominal aortic reconstruction. An analysis of perioperative risk factors in 557 patients. Ann Surg 197: 49–56, 1983; and Richardson JD, Main KA: Repair of abdominal aortic aneurysms. A statewide experience. Arch Surg 126:614–616, 1991.DVT, deep vein thrombosis; MI, myocardial infarction. 25,30,112,152–154
Cardiac Complications
The majority of cardiac ischemic events occur within the first 7 days following surgery, during which time intensive care monitoring is appropriate for high-risk patients. Maximizing myocardial function with adequate preload, controlling oxygen consumption by the reduced heart rate and blood pressure product, ensuring adequate oxygenation, β-blockade, and establishing effective analgesia are important techniques for preventing myocardial ischemia postoperatively. Patients with cardiac dysfunction have a greater risk of MI when the postoperative hematocrit is below 28%, even though this is well tolerated by normal individuals.126 Postoperative epidural analgesia, in addition to providing excellent pain control, may reduce myocardial complications by decreasing the catecholamine stress response.91
Renal Failure
Although once common after infrarenal AAA repair, renal failure is now rare because of adequate volume replacement and maintenance of normal cardiac output and renal blood flow. Precautions are still required, however, to reduce the risk of this complication. Because of the renal toxicity of intravenous contrast, it is prudent to delay AAA repair following arteriography or contrast-enhanced CT to be certain that renal dysfunction has not been induced. A more likely cause of renal failure following infrarenal AAA repair is embolization of aortic atheromatous debris into the renal arteries during proximal aortic cross-clamping. Preoperative CT scanning may reveal pararenal atheromatous debris or thrombus, which should prompt temporary supraceliac cross-clamping until the infrarenal aorta is open. Preoperative renal insufficiency is the best predictor of postoperative renal failure,25,127 so special precautions are appropriate in such patients. Some evidence supports a beneficial effect of intravenous mannitol when given before aortic cross-clamping (≈︀ 25 g).127 Although some have advocated maintenance of higher urine volume using furosemide, the efficacy of this approach has not been proven and may hinder assessment of fluid balance by artificially increasing urine output.
Gastrointestinal Complications
Sigmoid colon ischemia following AAA repair is a rare but devastating complication that occurs after approximately 1% of elective AAA repairs.128,129 This may result from embolization into, or ligation of, the IMA or IIAs. Although the IMA is often chronically occluded, ligation too far from the aneurysm wall can obliterate important SMA collaterals. Fortunately, the abundance of collateral flow to the sigmoid colon usually prevents ischemia. Sigmoid ischemia is three to four times more likely following ruptured AAA repair, presumably due to the associated hypotension and shock added to the usual risk of this complication.128–130 Careful inspection of the sigmoid colon following graft placement is important and may be facilitated by Doppler insonation of the bowel wall and mesentery. Preoperatively, patent IMAs should be carefully inspected for back-bleeding following the aortic reconstruction, and ligated only when back-bleeding is pulsatile and colon viability is assured. In questionable circumstances, IMA reimplantation or direct internal iliac revascularization is indicated.131
Distal Embolization
Lower-extremity ischemia may occur after AAA repair, usually from embolization of aneurysmal debris that occurs during aneurysm mobilization or aortoiliac clamping. Usually such emboli are small (termed microemboli) and not amenable to surgical removal, and they result in transient patchy areas of dusky skin or “blue toes” (also see Chapter 47). This can result in persistent pain or skin loss, occasionally necessitating amputation. Management is largely expectant. Occasionally, larger emboli or distal intimal flaps, particularly in diseased iliac arteries, may require operative intervention. For this reason, the legs should be carefully inspected intraoperatively for ischemia after AAA repair, while the incision is still open and arterial access can be easily obtained if necessary.
Paraplegia
Paraplegia due to spinal cord ischemia is rare following infrarenal AAA repair. It can result when important spinal artery collateral flow via the IIAs or an abnormally low origin of the accessory spinal artery (arterial magna radicularis or artery of Adamkiewicz) is obliterated or embolized during AAA repair.132 Because the accessory spinal artery normally originates from the descending thoracic or upper abdominal aorta, this complication is much more common following thoracoabdominal aneurysm repair.
Impaired Sexual Function
Impotence or retrograde ejaculation due to injury of autonomic nerves during paraaortic dissection may result after AAA repair.133 The incidence of this complication is difficult to determine because of the multiple causes of impotence in this age group and frequent underreporting. In the recent ADAM trial in U.S. VA hospitals, 40% of men had impotence before AAA repair.134 Contrary to most other reports that asked patients retrospectively if they had impotence before AAA repair, in the ADAM trial, less than 10% developed new impotence in the first year after repair. However, the proportion reporting new impotence increased over time such that by 4 years after AAA repair, more than 60% reported having impotence, which underscores the multifactorial etiology of impotence in this age group. Careful preservation of nerves, particularly as they course along the left side of the infrarenal aorta, around the IMA, and cross the proximal left CIA has been shown to substantially reduce this complication, which has reportedly occurred in up to 25% of patients.135,136 Other possible causes of postoperative impotence include reduction in pelvic blood flow due to internal iliac occlusion or embolization. Sexual dysfunction is not confined to open AAA repair patients. A recent study by Pettersson et al. demonstrated that a group of patients, when asked about their respective sexual function following EVAR, reported increased postoperative impotence and ejaculatory function 1 year following AAA repair.137
Venous Thromboembolism
Pulmonary embolism (PE) and deep vein thrombosis (DVT) are less common after AAA repair than after other abdominal operations, perhaps because of intraoperative anticoagulation. Unrecognized DVT, however, can occur in up to 18% of untreated patients.138 Therefore, perioperative prophylaxis with intermittent pneumatic compression stockings or subcutaneous heparin or low-molecular-weight heparin (LMWH) is appropriate.
Functional Outcome
Williamson et al.139 recently reviewed their experience with open AAA repair with regard to functional outcome. They found that two thirds of patients experienced complete recovery at an average time of 4 months, whereas one third had not fully recovered at an average time of nearly 3 years. Additionally, 18% said they would not undergo AAA repair again after knowing the recovery process, despite appearing to understand the implications of AAA rupture. Eleven percent were initially discharged to a skilled nursing facility, with an average stay of 3.7 months. This is similar to a 9% rate of discharge to a facility other than home, as reported in a review of national administrative data by Huber et al.140 All patients in Williamson’s review were ambulatory preoperatively, but at a mean of 25 months’ follow-up, only 64% were fully ambulatory, whereas 22% required assistance, and 14% were nonambulatory. Although it is difficult to determine the extent of the disability that is due to the AAA repair, this report highlights the high rate of disability after open AAA repair. More research into long-term functional outcomes and quality-of-life assessment is clearly necessary.
Long-Term Survival
As noted previously, early (30-day) mortality after elective AAA repair in properly selected patients is 5% or less, whereas early mortality after ruptured AAA repair averages 54% (not including patients who died from rupture before repair).5,15 Five-year survival after successful AAA repair in modern series is approximately 70%, compared with approximately 80% in the age- and gender-matched general population.41,42,112,141–145 Ten-year survival after AAA repair is approximately 40%. Although survival is similar in men and women, women without AAA have longer survival than men. Therefore, survival relative to gender-specific norms is lower in women after AAA repair than in men.146 Survival after successful ruptured AAA repair versus successful elective repair was similar in one report147 but reduced in others.148,149 In a population-based analysis from Western Australia, survival after ruptured or elective AAA repair was similar for men but significantly reduced for women with ruptured AAA.146 Overall, survival after AAA repair is reduced compared with an age- and sex-matched population because of greater associated comorbidity in patients with aneurysms.33,142 Not surprisingly, systemic complications of atherosclerosis cause most late deaths after AAA repair in this predominately elderly male population. The cause of late deaths after AAA repair are cardiac disease (44%), cancer (15%), rupture of another aneurysm (11%), stroke (9%), and pulmonary disease (6%).141,142,150 Combining cardiac causes, aneurysmal disease, and stroke indicates that vascular complications account for two thirds of late deaths following AAA repair.
When outcome is stratified according to these risk factors, the 5-year survival rate improves to 84% in patients without heart disease, which is substantially better than the 54% survival rate observed in patients with known heart disease.141 Hypertension also reduces 5-year survival after AAA repair from 84% to 59%.141 In patients without hypertension or heart disease, late survival after AAA repair is identical to normal age-matched controls.142 Multivariate analysis indicates that uncorrected CAD is the most significant variable associated with late mortality after AAA repair, but that age, renal dysfunction, COPD, and peripheral occlusive disease also contribute.41,42,112,151 One analysis of coronary artery bypass grafting performed in preparation for AAA repair indicates that it may improve long-term survival in patients younger than age 70 but that older patients do not benefit from this aggressive approach.151 A recent prospective multicentered study identified not only age, cardiac, carotid, and renal disease as independent predictors of late mortality following elective AAA repair but also aneurysm extent, as judged by size, suprarenal extension, and external iliac involvement.143
1 Upchurch G.R.Jr, Schaub T.A. Abdominal aortic aneurysm. Am Fam Physician. 2006;73(7):1198–1204.
2 Bengtsson H., Nilsson P., Bergqvist D. Natural history of abdominal aortic aneurysm detected by screening. Br J Surg. 1993;80(6):718–720.
3 Heller J.A., Weinberg A., Arons R., et al. Two decades of abdominal aortic aneurysm repair: have we made any progress? J Vasc Surg. 2000;32(6):1091–1100.
4 Adam D.J., Mohan I.V., Stuart W.P., et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30(5):922–928.
5 Hallin A., Bergqvist D., Holmberg L. Literature review of surgical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2001;22(3):197–204.
6 Bown M.J., Sutton A.J., Bell P.R., et al. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg. 2002;89(6):714–730.
7 Ernst C.B. Abdominal aortic aneurysm. N Engl J Med. 1993;328(16):1167–1172.
8 Heikkinen M., Salenius J.P., Auvinen O. Ruptured abdominal aortic aneurysm in a well-defined geographic area. J Vasc Surg. 2002;36(2):291–296.
9 Kantonen I., Lepantalo M., Brommels M., et al. Mortality in ruptured abdominal aortic aneurysms. The Finnvasc Study Group. Eur J Vasc Endovasc Surg. 1999;17(3):208–212.
10 Bengtsson H., Bergqvist D. Ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 1993;18(1):74–80.
11 Pasch A.R., Ricotta J.J., May A.G., et al. Abdominal aortic aneurysm: the case for elective resection. Circulation. 1984;70(3 Pt 2):I1–I4.
12 Breckwoldt W.L., Mackey W.C., O’Donnell T.F.Jr. The economic implications of high-risk abdominal aortic aneurysms. J Vasc Surg. 1991;13(6):798–803. discussion 803–794
13 Pearce W.H., Slaughter M.S., LeMaire S., et al. Aortic diameter as a function of age, gender, and body surface area. Surgery. 1993;114(4):691–697.
14 Johnston K.W., Rutherford R.B., Tilson M.D., et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13(3):452–458.
15 Katz D.A., Littenberg B., Cronenwett J.L. Management of small abdominal aortic aneurysms. Early surgery vs. watchful waiting. JAMA. 1992;268(19):2678–2686.
16 Brewster D.C., Cronenwett J.L., Hallett J.W.Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37(5):1106–1117.
17 Brown L.C., Powell J.T. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg. 1999;230(3):289–296. discussion 296–287
18 Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352(9141):1649–1655.
19 Lederle F.A., Wilson S.E., Johnson G.R., et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437–1444.
20 Ouriel K., Clair D.G., Kent K.C., et al. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51(5):1081–1087.
21 Valentine R.J., Decaprio J.D., Castillo J.M., et al. Watchful waiting in cases of small abdominal aortic aneurysms–appropriate for all patients? J Vasc Surg. 2000;32(3):441–448. discussion 448–450
22 Steyerberg E.W., Kievit J., de Mol Van Otterloo J.C., et al. Perioperative mortality of elective abdominal aortic aneurysm surgery. A clinical prediction rule based on literature and individual patient data. Arch Intern Med. 1995;155(18):1998–2004.
23 Kazmers A., Perkins A.J., Jacobs L.A. Outcomes after abdominal aortic aneurysm repair in those > or = 80 years of age: recent Veterans Affairs experience. Ann Vasc Surg. 1998;12(2):106–112.
24 L’Italien G.J., Paul S.D., Hendel R.C., et al. Development and validation of a Bayesian model for perioperative cardiac risk assessment in a cohort of 1,081 vascular surgical candidates. J Am Coll Cardiol. 1996;27(4):779–786.
25 Johnston K.W. Multicenter prospective study of nonruptured abdominal aortic aneurysm. Part II. Variables predicting morbidity and mortality. J Vasc Surg. 1989;9(3):437–447.
26 Brady A.R., Fowkes F.G., Greenhalgh R.M., et al. Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: results from the UK Small Aneurysm Trial. On behalf of the UK Small Aneurysm Trial participants. Br J Surg. 2000;87(6):742–749.
27 Katz D.J., Stanley J.C., Zelenock G.B. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25(3):561–568.
28 Katz D.A., Cronenwett J.L. The cost-effectiveness of early surgery versus watchful waiting in the management of small abdominal aortic aneurysms. J Vasc Surg. 1994;19(6):980–990. discussion 990–981
29 Iezzoni L.I. Assessing quality using administrative data. Ann Intern Med. 1997;127(8 Pt 2):666–674.
30 Johnston K.W., Scobie T.K. Multicenter prospective study of nonruptured abdominal aortic aneurysms. I. Population and operative management. J Vasc Surg. 1988;7(1):69–81.
31 Beck A.W., Goodney P.P., Nolan B.W., et al. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009;49(4):838–843. discussion 843–834
32 Lederle F.A., Johnson G.R., Wilson S.E., et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160(10):1425–1430.
33 Newman A.B., Arnold A.M., Burke G.L., et al. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134(3):182–190.
34 Rodin M.B., Daviglus M.L., Wong G.C., et al. Middle age cardiovascular risk factors and abdominal aortic aneurysm in older age. Hypertension. 2003;42(1):61–68.
35 Singh K., Bonaa K.H., Jacobsen B.K., et al. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromso Study. Am J Epidemiol. 2001;154(3):236–244.
36 Tornwall M.E., Virtamo J., Haukka J.K., et al. Life-style factors and risk for abdominal aortic aneurysm in a cohort of Finnish male smokers. Epidemiology. 2001;12(1):94–100.
37 Wilmink A.B., Quick C.R. Epidemiology and potential for prevention of abdominal aortic aneurysm. Br J Surg. 1998;85(2):155–162.
38 Johnston K.W. Nonruptured abdominal aortic aneurysm: six-year follow-up results from the multicenter prospective Canadian aneurysm study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994;20(2):163–170.
39 Batt M., Staccini P., Pittaluga P., et al. Late survival after abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 1999;17(4):338–342.
40 Aune S., Amundsen S.R., Evjensvold J., et al. Operative mortality and long-term relative survival of patients operated on for asymptomatic abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1995;9(3):293–298.
41 Norman P.E., Semmens J.B., Lawrence-Brown M.M. Long-term relative survival following surgery for abdominal aortic aneurysm: a review. Cardiovasc Surg. 2001;9(3):219–224.
42 Hertzer N.R., Mascha E.J., Karafa M.T., et al. Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg. 2002;35(6):1145–1154.
43 Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19(6):636–642.
44 De Martino R.R., Nolan B.W., Goodney P.P., et al. Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg. 2010;52(1):5–12 e11.
45 Michaels J.A. The management of small abdominal aortic aneurysms: a computer simulation using Monte Carlo methods. Eur J Vasc Surg. 1992;6(5):551–557.
46 Schermerhorn M.L., Birkmeyer J.D., Gould D.A., et al. Cost-effectiveness of surgery for small abdominal aortic aneurysms on the basis of data from the United Kingdom small aneurysm trial. J Vasc Surg. 2000;31(2):217–226.
47 Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445–1452.
48 Eagle K.A., Berger P.B., Calkins H., et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery–executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg. 2002;94(5):1052–1064.
49 Fagevik Olsen M., Hahn I., Nordgren S., et al. Randomized controlled trial of prophylactic chest physiotherapy in major abdominal surgery. Br J Surg. 1997;84(11):1535–1538.
50 Hertzer N.R., Young J.R., Kramer J.R., et al. Routine coronary angiography prior to elective aortic reconstruction: results of selective myocardial revascularization in patients with peripheral vascular disease. Arch Surg. 1979;114(11):1336–1344.
51 Eagle K.A., Coley C.M., Newell J.B., et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110(11):859–866.
52 Creech O.Jr. Endo-aneurysmorrhaphy and treatment of aortic aneurysm. Ann Surg. 1966;164(6):935–946.
53 Hollier L.H., Reigel M.M., Kazmier F.J., et al. Conventional repair of abdominal aortic aneurysm in the high-risk patient: a plea for abandonment of nonresective treatment. J Vasc Surg. 1986;3(5):712–717.
54 Inahara T., Geary G.L., Mukherjee D., et al. The contrary position to the nonresective treatment for abdominal aortic aneurysm. J Vasc Surg. 1985;2(1):42–48.
55 Karmody A.M., Leather R.P., Goldman M., et al. The current position of nonresective treatment for abdominal aortic aneurysm. Surgery. 1983;94(4):591–597.
56 Lynch K., Kohler T., Johansen K. Nonresective therapy for aortic aneurysm: results of a survey. J Vasc Surg. 1986;4(5):469–472.
57 Schwartz R.A., Nichols W.K., Silver D. Is thrombosis of the infrarenal abdominal aortic aneurysm an acceptable alternative? J Vasc Surg. 1986;3(3):448–455.
58 Shah D.M., Chang B.B., Paty P.S., et al. Treatment of abdominal aortic aneurysm by exclusion and bypass: an analysis of outcome. J Vasc Surg. 1991;13(1):15–20. discussion 20–12
59 Darling R.C.3rd, Ozsvath K., Chang B.B., et al. The incidence, natural history, and outcome of secondary intervention for persistent collateral flow in the excluded abdominal aortic aneurysm. J Vasc Surg. 1999;30(6):968–976.
60 Kline R.G., D’Angelo A.J., Chen M.H., et al. Laparoscopically assisted abdominal aortic aneurysm repair: first 20 cases. J Vasc Surg. 1998;27(1):81–87. discussion 88
61 Parodi J.C., Palmaz J.C., Barone H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(6):491–499.
62 Moore W.S., Brewster D.C., Bernhard V.M. Aorto-uni-iliac endograft for complex aortoiliac aneurysms compared with tube/bifurcation endografts: results of the EVT/Guidant trials. J Vasc Surg. 2001;33(Suppl 2):S11–S20.
63 Matsumura J.S., Brewster D.C., Makaroun M.S., et al. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2003;37(2):262–271.
64 Zarins C.K., White R.A., Schwarten D., et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg. 1999;29(2):292–305. discussion 306–298
65 Lee W.A., Carter J.W., Upchurch G., et al. Perioperative outcomes after open and endovascular repair of intact abdominal aortic aneurysms in the United States during 2001. J Vasc Surg. 2004;39(3):491–496.
66 Aquino R.V., Jones M.A., Zullo T.G., et al. Quality of life assessment in patients undergoing endovascular or conventional AAA repair. J Endovasc Ther. 2001;8(5):521–528.
67 Bernhard V.M., Mitchell R.S., Matsumura J.S., et al. Ruptured abdominal aortic aneurysm after endovascular repair. J Vasc Surg. 2002;35(6):1155–1162.
68 Harris P.L., Vallabhaneni S.R., Desgranges P., et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32(4):739–749.
69 Holzenbein T.J., Kretschmer G., Thurnher S., et al. Midterm durability of abdominal aortic aneurysm endograft repair: a word of caution. J Vasc Surg. 2001;33(2 Suppl):S46–S54.
70 Schermerhorn M.L., Finlayson S.R., Fillinger M.F., et al. Life expectancy after endovascular versus open abdominal aortic aneurysm repair: results of a decision analysis model on the basis of data from EUROSTAR. J Vasc Surg. 2002;36(6):1112–1120.
71 Prinssen M., Verhoeven E.L., Buth J., et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607–1618.
72 Blankensteijn J.D., de Jong S.E., Prinssen M., et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352(23):2398–2405.
73 Zarins C.K., White R.A., Fogarty T.J. Aneurysm rupture after endovascular repair using the AneuRx stent graft. J Vasc Surg. 2000;31(5):960–970.
74 Ohki T., Veith F.J., Shaw P., et al. Increasing incidence of midterm and long-term complications after endovascular graft repair of abdominal aortic aneurysms: a note of caution based on a 9-year experience. Ann Surg. 2001;234(3):323–334. discussion 334–325
75 Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365(9478):2179–2186.
76 Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365(9478):2187–2192.
77 Rutherford R.B. Randomized EVAR trials and advent of level I evidence: a paradigm shift in management of large abdominal aortic aneurysms? Semin Vasc Surg. 2006;19(2):69–74.
78 Lederle F.A., Freischlag J.A., Kyriakides T.C., et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302(14):1535–1542.
79 Kaiser A.B., Clayson K.R., Mulherin J.L.Jr, et al. Antibiotic prophylaxis in vascular surgery. Ann Surg. 1978;188(3):283–289.
80 Bender J.S., Smith-Meek M.A., Jones C.E. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg. 1997;226(3):229–236. discussion 236–227
81 Ziegler D.W., Wright J.G., Choban P.S., et al. A prospective randomized trial of preoperative “optimization” of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery. 1997;122(3):584–592.
82 Birkmeyer J.D., AuBuchon J.P., Littenberg B., et al. Cost-effectiveness of preoperative autologous donation in coronary artery bypass grafting. Ann Thorac Surg. 1994;57(1):161–168. discussion 168–169
83 Goodnough L.T., Monk T.G., Sicard G., et al. Intraoperative salvage in patients undergoing elective abdominal aortic aneurysm repair: an analysis of cost and benefit. J Vasc Surg. 1996;24(2):213–218.
84 Huber T.S., Carlton L.C., Irwin P.B., et al. Intraoperative autologous transfusion during elective infrarenal aortic reconstruction. J Surg Res. 1997;67(1):14–20.
85 Ouriel K., Shortell C.K., Green R.M., et al. Intraoperative autotransfusion in aortic surgery. J Vasc Surg. 1993;18(1):16–22.
86 Nelson A.H., Fleisher L.A., Rosenbaum S.H. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med. 1993;21(6):860–866.
87 Bush H.L.Jr, Hydo L.J., Fischer E., et al. Hypothermia during elective abdominal aortic aneurysm repair: the high price of avoidable morbidity. J Vasc Surg. 1995;21(3):392–400. discussion 400–392
88 Frank S.M., Fleisher L.A., Breslow M.J., et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127–1134.
89 Ali Z.A., Callaghan C.J., Lim E., et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116(11 Suppl):I98–I105.
90 Mason R.A., Newton G.B., Cassel W., et al. Combined epidural and general anesthesia in aortic surgery. J Cardiovasc Surg (Torino). 1990;31(4):442–447.
91 Yeager M.P., Glass D.D., Neff R.K., et al. Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology. 1987;66(6):729–736.
92 Baron J.F., Bertrand M., Barre E., et al. Combined epidural and general anesthesia versus general anesthesia for abdominal aortic surgery. Anesthesiology. 1991;75(4):611–618.
93 Raggi R., Dardik H., Mauro A.L. Continuous epidural anesthesia and postoperative epidural narcotics in vascular surgery. Am J Surg. 1987;154(2):192–197.
94 Devereaux P.J., Yang H., Yusuf S., et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
95 Pasternack P.F., Grossi E.A., Baumann F.G., et al. Beta blockade to decrease silent myocardial ischemia during peripheral vascular surgery. Am J Surg. 1989;158(2):113–116.
96 Mangano D.T., Layug E.L., Wallace A., et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335(23):1713–1720.
97 Wallace A., Layug B., Tateo I., et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology. 1998;88(1):7–17.
98 Poldermans D., Boersma E., Bax J.J., et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
99 Poldermans D., Boersma E., Bax J.J., et al. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J. 2001;22(15):1353–1358.
100 Fleisher L.A., Beckman J.A., Brown K.A., et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120(21):e169–e276.
101 Gottlieb S.S., McCarter R.J., Vogel R.A. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998;339(8):489–497.
102 Cleland J.G., McGowan J., Clark A., et al. The evidence for beta blockers in heart failure. BMJ. 1999;318(7187):824–825.
103 Stone D.H., Goodney P.P., Schanzer A., et al. Clopidogrel is not associated with major bleeding complications during peripheral arterial surgery. J Vasc Surg. 2011;54(3):779–784.
104 Chang B.B., Paty P.S., Shah D.M., et al. The right retroperitoneal approach for abdominal aortic surgery. Am J Surg. 1989;158(2):156–158.
105 Sicard G.A., Reilly J.M., Rubin B.G., et al. Transabdominal versus retroperitoneal incision for abdominal aortic surgery: report of a prospective randomized trial. J Vasc Surg. 1995;21(2):174–181. discussion 181–173
106 Cambria R.P., Brewster D.C., Abbott W.M., et al. Transperitoneal versus retroperitoneal approach for aortic reconstruction: a randomized prospective study. J Vasc Surg. 1990;11(2):314–324. discussion 324–315
107 Sieunarine K., Lawrence-Brown M.M., Goodman M.A. Comparison of transperitoneal and retroperitoneal approaches for infrarenal aortic surgery: early and late results. Cardiovasc Surg. 1997;5(1):71–76.
108 Samson R.H., Lepore M.R.Jr, Showalter D.P., et al. Long-term safety of left renal vein division and ligation to expedite complex abdominal aortic surgery. J Vasc Surg. 2009;50(3):500–504. discussion 504
109 Green R.M., Ricotta J.J., Ouriel K., et al. Results of supraceliac aortic clamping in the difficult elective resection of infrarenal abdominal aortic aneurysm. J Vasc Surg. 1989;9(1):124–134.
110 Breckwoldt W.L., Mackey W.C., Belkin M., et al. The effect of suprarenal cross-clamping on abdominal aortic aneurysm repair. Arch Surg. 1992;127(5):520–524.
111 Nypaver T.J., Shepard A.D., Reddy D.J., et al. Repair of pararenal abdominal aortic aneurysms. An analysis of operative management. Arch Surg. 1993;128(7):803–811. discussion 811–803
112 Olsen P.S., Schroeder T., Agerskov K., et al. Surgery for abdominal aortic aneurysms. A survey of 656 patients. J Cardiovasc Surg (Torino). 1991;32(5):636–642.
113 Provan J.L., Fialkov J., Ameli F.M., et al. Is tube repair of aortic aneurysm followed by aneurysmal change in the common iliac arteries? Can J Surg. 1990;33(5):394–397.
114 Polterauer P., Prager M., Holzenbein T., et al. Dacron versus polytetrafluoroethylene for Y-aortic bifurcation grafts: a six-year prospective, randomized trial. Surgery. 1992;111(6):626–633.
115 Lord R.S., Nash P.A., Raj B.T., et al. Prospective randomized trial of polytetrafluoroethylene and Dacron aortic prosthesis. I. Perioperative results. Ann Vasc Surg. 1988;2(3):248–254.
116 Piotrowski J.J., McCroskey B.L., Rutherford R.B. Selection of grafts currently available for repair of abdominal aortic aneurysms. Surg Clin North Am. 1989;69(4):827–836.
117 Mabry C.D., Thompson B.W., Read R.C. Activated clotting time (ACT) monitoring of intraoperative heparinization in peripheral vascular surgery. Am J Surg. 1979;138(6):894–900.
118 Ernst C.B., Hagihara P.F., Daugherty M.E., et al. Inferior mesenteric artery stump pressure: a reliable index for safe IMA ligation during abdominal aortic aneurysmectomy. Ann Surg. 1978;187(6):641–646.
119 Thomas J.H., Blake K., Pierce G.E., et al. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg. 1998;27(5):840–844.
120 Tollefson D.F., Ernst C.B. Natural history of atherosclerotic renal artery stenosis associated with aortic disease. J Vasc Surg. 1991;14(3):327–331.
121 Zierler R.E., Bergelin R.O., Davidson R.C., et al. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens. 1996;9(11):1055–1061.
122 Dean R.H., Benjamin M.E., Hansen K.J. Surgical management of renovascular hypertension. Curr Probl Surg. 1997;34(3):209–308.
123 Cambria R.P., Brewster D.C., L’Italien G., et al. Simultaneous aortic and renal artery reconstruction: evolution of an eighteen-year experience. J Vasc Surg. 1995;21(6):916–924. discussion 925
124 Benjamin M.E., Hansen K.J., Craven T.E., et al. Combined aortic and renal artery surgery. A contemporary experience. Ann Surg. 1996;223(5):555–565. discussion 565–557
125 Williamson W.K., Abou-Zamzam A.M.Jr, Moneta G.L., et al. Prophylactic repair of renal artery stenosis is not justified in patients who require infrarenal aortic reconstruction. J Vasc Surg. 1998;28(1):14–20. discussion 20–12
126 Nennhaus H.P., Javid H. The distinct syndrome of spontaneous abdominal aortocaval fistula. Am J Med. 1968;44(3):464–473.
127 Miller D.C., Myers B.D. Pathophysiology and prevention of acute renal failure associated with thoracoabdominal or abdominal aortic surgery. J Vasc Surg. 1987;5(3):518–523.
128 Jarvinen O., Laurikka J., Salenius J.P., et al. Mesenteric infarction after aortoiliac surgery on the basis of 1752 operations from the National Vascular Registry. World J Surg. 1999;23(3):243–247.
129 Bast T.J., van der Biezen J.J., Scherpenisse J., et al. Ischaemic disease of the colon and rectum after surgery for abdominal aortic aneurysm: a prospective study of the incidence and risk factors. Eur J Vasc Surg. 1990;4(3):253–257.
130 Welling R.E., Roedersheimer L.R., Arbaugh J.J., et al. Ischemic colitis following repair of ruptured abdominal aortic aneurysm. Arch Surg. 1985;120(12):1368–1370.
131 Ernst C.B. Prevention of intestinal ischemia following abdominal aortic reconstruction. Surgery. 1983;93(1 Pt 1):102–106.
132 Szilagyi D.E., Hageman J.H., Smith R.F., et al. Spinal cord damage in surgery of the abdominal aorta. Surgery. 1978;83(1):38–56.
133 DePalma R.G., Levine S.B., Feldman S. Preservation of erectile function after aortoiliac reconstruction. Arch Surg. 1978;113(8):958–962.
134 Lederle F.A., Johnson G.R., Wilson S.E., et al. Quality of life, impotence, and activity level in a randomized trial of immediate repair versus surveillance of small abdominal aortic aneurysm. J Vasc Surg. 2003;38(4):745–752.
135 Flanigan D.P., Schuler J.J., Keifer T., et al. Elimination of iatrogenic impotence and improvement of sexual function after aortoiliac revascularization. Arch Surg. 1982;117(5):544–550.
136 Weinstein M.H., Machleder H.I. Sexual function after aorto-iliac surgery. Ann Surg. 1975;181(6):787–790.
137 Pettersson M., Mattsson E., Bergbom I. Prospective follow-up of sexual function after elective repair of abdominal aortic aneurysms using open and endovascular techniques. J Vasc Surg. 2009;50(3):492–499.
138 Olin J.W., Graor R.A., O’Hara P., et al. The incidence of deep venous thrombosis in patients undergoing abdominal aortic aneurysm resection. J Vasc Surg. 1993;18(6):1037–1041.
139 Williamson W.K., Nicoloff A.D., Taylor L.M.Jr, et al. Functional outcome after open repair of abdominal aortic aneurysm. J Vasc Surg. 2001;33(5):913–920.
140 Huber T.S., Wang J.G., Derrow A.E., et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(2):304–310. discussion 310–301
141 Crawford E.S., Saleh S.A., Babb J.W.3rd, et al. Infrarenal abdominal aortic aneurysm: factors influencing survival after operation performed over a 25-year period. Ann Surg. 1981;193(6):699–709.
142 Hollier L.H., Plate G., O’Brien P.C., et al. Late survival after abdominal aortic aneurysm repair: influence of coronary artery disease. J Vasc Surg. 1984;1(2):290–299.
143 Koskas F., Kieffer E. Long-term survival after elective repair of infrarenal abdominal aortic aneurysm: results of a prospective multicentric study. Association for Academic Research in Vascular Surgery (AURC). Ann Vasc Surg. 1997;11(5):473–481.
144 Soreide O., Lillestol J., Christensen O., et al. Abdominal aortic aneurysms: survival analysis of four hundred thirty-four patients. Surgery. 1982;91(2):188–193.
145 Vohra R., Reid D., Groome J., et al. Long-term survival in patients undergoing resection of abdominal aortic aneurysm. Ann Vasc Surg. 1990;4(5):460–465.
146 Norman P.E., Semmens J.B., Lawrence-Brown M.M., et al. Long-term relative survival after surgery for abdominal aortic aneurysm in western Australia: population based study. BMJ. 1998;317(7162):852–856.
147 Stonebridge P.A., Callam M.J., Bradbury A.W., et al. Comparison of long-term survival after successful repair of ruptured and non-ruptured abdominal aortic aneurysm. Br J Surg. 1993;80(5):585–586.
148 Kazmers A., Perkins A.J., Jacobs L.A. Aneurysm rupture is independently associated with increased late mortality in those surviving abdominal aortic aneurysm repair. J Surg Res. 2001;95(1):50–53.
149 Cho J.S., Gloviczki P., Martelli E., et al. Long-term survival and late complications after repair of ruptured abdominal aortic aneurysms. J Vasc Surg. 1998;27(5):813–819. discussion 819–820
150 Hertzer N.R. Fatal myocardial infarction following abdominal aortic aneurysm resection. Three hundred forty-three patients followed 6–11 years postoperatively. Ann Surg. 1980;192(5):667–673.
151 Reigel M.M., Hollier L.H., Kazmier F.J., et al. Late survival in abdominal aortic aneurysm patients: The role of selective myocardial revascularization on the basis of clinical symptoms. J Vasc Surg. 1987;5(2):222–227.
152 AbuRahma A.F., Robinson P.A., Boland J.P., et al. Elective resection of 332 abdominal aortic aneurysms in a southern West Virginia community during a recent five-year period. Surgery. 1991;109(3 Pt 1):244–251.
153 Diehl J.T., Cali R.F., Hertzer N.R., et al. Complications of abdominal aortic reconstruction. An analysis of perioperative risk factors in 557 patients. Ann Surg. 1983;197(1):49–56.
154 Richardson J.D., Main K.A. Repair of abdominal aortic aneurysms. A statewide experience. Arch Surg. 1991;126(5):614–616.