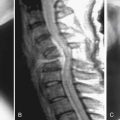
27 Surgical Treatment Modalities for Cervical Stenosis: Central Cord Syndrome and Other Spinal Cord Injuries in the Elderly
KEY POINTS
Introduction
Patients presenting following cervical spinal cord injuries with disproportionate weakness of the hands and arms and relative preservation of lower extremity strength are often categorized as having either a cruciate paralysis or, more commonly, acute central cervical spinal cord injury (Box 27-1).
Acute central cord syndrome was first described in 1954 by Schneider, in a case series of 8 patients with neurological deficits following hyperextension injury to the cervical spine and a review of another 6 cases reported in the literature with a similar injury mechanism and neurological presentation. 1
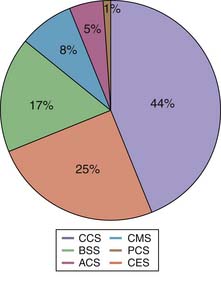
It is estimated that the annual incidence of SCI varies between 11.5 to 53.4 per million population.2 In the United States, the annual incidence is around 40 per million. Central cord syndrome is the most common spinal cord injury pattern.3 The relative distribution of other types of SCI is shown in Figure 27-1.
Mechanism
Based on radiographic, operative, and postmortem findings, Schneider postulated that during forceful hyperextension the anterior osteophytes and the bulging of the ligamentum flavum caused significant anteroposterior narrowing of the spinal canal and contusion of the spinal cord (Figure 27-2). He noted in the postmortem findings that the maximum injury was in the central part of the spinal cord. The mechanism of central cord syndrome is a hyperextension injury, often on a background of long-standing cervical spondylosis, with no bony or ligamentous injury. Hyperextension of the cervical spine causes overlapping of the laminae and buckling of the ligamentum flavum, reducing the canal diameter by a further 2 to 3 mm. This compromise may cause significant acute cord compression in an aging spondylotic cervical spine with preexisting discoligamentous and osseous compression. It is estimated that this mechanism of hyperextension accounts for around 50% of cases of acute traumatic central cord syndrome (ATCCS). The other mechanisms are fractures and/or subluxations and herniated nucleus pulposus.4 The most common mechanism of injury in the elderly population is a low-energy ground-level fall with impact on the head or chin causing forceful hyperextension of the neck. Typically this low-energy impact does not cause any bony injury.
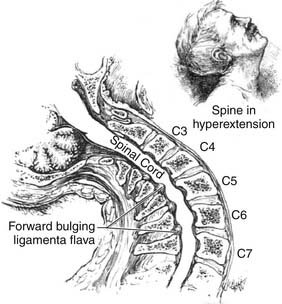
Figure 27-2 Original drawing of the hyperextension mechanism from Schneider’s paper in 1954.
(From Schneider RC, Cherry G, Pantek H: The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine [part 1], J Neurosurg 11:546-577, 1954.)
Definition of Central Cord Syndrome
Central cord syndrome was described by Schneider in 1954: ‘‘It is characterized by disproportionately more motor impairment of the upper than the lower extremities, bladder dysfunction, usually urinary retention, and varying degrees of sensory loss below the level of the lesion.’’ (Box 27-2).1
Incidence and Age
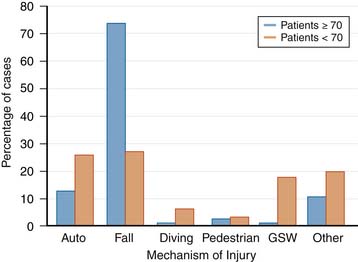
The average age of spinal cord injury (SCI) has increased from 28.7 to 38 years, and the percentage of spinal cord injury in people over 60 years of age has increased from 4.7% to 11.5%. After the age of 45 years, falls are the most common cause of cervical spinal cord injury, increasing in incidence with advancing age. In one series, 74% of injuries in patients 70 years of age or older were caused by falls (Figure 27-3).5 SCI in the elderly typically results from a low-energy injury sustained from a fall at ground level. There is a bimodal pattern of the age distribution in ATCCS patients. The increase in age has had an impact on the pathogenesis, functional deficit, recovery, and rehabilitation of patients with spinal cord injuries.6 In general, older patients demonstrate less recovery from spinal cord injury compared to younger patients (Box 27-3).7 Experimental animal studies have shown that increased age increases the area of pathology and amount of demyelination, while demonstrating a significantly lower amount of endogenous remyelination following induced SCI.6 These studies have demonstrated that abnormalities in myelination and functional deficits secondary to SCI are age-related.
Advances in medicine seen over the past five decades have resulted in a dramatic increase in life expectancy following spinal cord injury. At present, the available data would suggest the mortality among spinal cord injury patients is around 3.8% in the first year post-injury, 1.6% in the second year post-injury, and approximately 1.2% a year over the next 10 years.8 The chronological age, degree of injury severity, injury completeness, and neurological level are the most important predictors of mortality. The annual mortality rate varies between 0.4% and 0.5%.
Basic Science
Pathophysiology of Acute Traumatic Central Cord Syndrome (ATCCS)
The peripheral compressive forces exerted on the spinal cord cause concussion, and contusion of the spinal cord results in a primary and secondary neuronal injury following the acute trauma. MRI and histopathological studies have shown that ATCCS is predominantly a white matter injury. Intramedullary hemorrhage, as was previously thought in the original descriptions, is not a necessary feature of the syndrome. However, rarely and in more severe trauma, bleeding into the central part of the cord may cause ATCCS, portending a less favorable prognosis. These histological changes reflect stasis of axoplasmic flow and both intracellular and extracellular edematous injury. The secondary injury is facilitated by a cascade of events mediated by systemic and local vascular insults, electrolyte shifts, edema, and excitotoxicity. The pathophysiological changes can progress in the first few days after the injury, both proximally and distally.9
Theory of Somatotopic Organization of Corticospinal Tracts (Neuroanatomical Theory)
Although the theory had been that central cord injury results in deficits that reflect the somatotopic organization of the corticospinal tracts (CST), recent axonal tracing data from primates and imaging-pathological data from humans have essentially discredited this theory.10 This theory was based on early work done by Foerster (1937) and Schneider (1954), where it was postulated that the somatotopic organization of the human corticospinal tract (CST) resulted in the central injury of the spinal cord affecting the more medially organized CST fibers of the hand within the lateral columns in preference to the more laterally placed lower limb fibers. Foerster presented no evidence for his theory of somatotopic organization of the CST tracts. There has been no neuroanatomic evidence presented even subsequently to support these assertions.
Theory of Increased Upper Limb and Hand Functional Representation of CST (Functional Theory)
The more recent theory postulated is that the CST in the human is more important for hand and arm function than it is for the lower extremity. It is believed that the CST has assumed a relatively greater importance for movement, particularly hand function, as humans ascend the phylogenetic scale, with more CST fibers synapsing with anterior horn cells innervating upper limb and hand function. Therefore any injury of the cervical cord involving the CST will preferentially affect more upper limb and hand function, as this pathway may be predominantly devoted to the motor function of the arms and hands in humans. The afferent fibers carried in the lateral and ventral descending pathways can mediate lower limb voluntary motor activity, and both must be substantially damaged to cause lower limb paralysis. More recent studies looking at the MRI scan findings in ATCCS have also concluded that the injury to the corticospinal tracts is global in nature and not confined to its medial part as has been described in the past. 10,11 All these more recent studies have suggested that the functional differentiation of the descending pathways of the upper and lower is the reason for the disproportionate involvement of upper limbs and hands in central cord syndrome.
Neurological and Functional Recovery of Central Cord Syndrome in the Elderly
The influence of age alone on the neurological recovery is difficult to ascertain from the literature due to the heterogeneity of the outcome measures and the use of historical controls. In a more recent study, age alone has not been shown to be an independent predictor of poor neurological recovery following ATCCS. However, increased age has been shown to have an adverse effect as measured by the Functional Independence Measure (FIM).12 Improved FIM has also been shown to improve with formal education, absence of spasticity, and with surgical treatment.
Imaging in Acute Traumatic Spinal Cord Injury13
Imaging Modalities Used to Assess Cervical Spine Injury (Box 27-4)
MRI Findings in Traumatic SCI
Cord Deformation and Signal Change within the Cord
Edema of the cord is seen as areas of high signal intensity on proton-density and T2-weighted images. The area of increased signal is directly proportional to the severity of the injury. In the first week following injury, intramedullary hemorrhage is seen as low signal intensity on T2-weighted images and is centered in the region of cord edema. After the first week, the area of intramedullary hemorrhage consists mainly of methemoglobin, which is seen as an area of high signal intensity on T1-weighted images.14 The presence of intramedullary hemorrhage not only reflects on the severity of the injury but also on the poor prognosis for neurological recovery.15
Treatment
Spontaneous, though usually incomplete, recovery of neurological function in the setting of central cord syndrome is well recognized. There is no convincing evidence that acute decompressive surgery improves the neurological outcome of this group at this stage, although this remains an area of controversy. The University of Maryland, led by Aarabi, is currently undertaking a clinical trial to examine this question. However, in the absence of more definitive data, delayed surgical treatment of the stenosis once the neurological improvement has plateaued, or earlier if no significant neurological improvement occurs, is a reasonable algorithm for treating this subgroup. In patients with normal cervical sagittal balance, decompression through laminectomy, with or without posterior instrumented fusion, is an option. In cervical spines without instability, an open-door expansile cervical laminoplasty is another surgical intervention that can be safely applied with promising results. In kyphotic cervical spines, it is recommended that the decompression be performed from an anterior approach, with multilevel discectomies or corpectomies with internal fixation.
There is Class II and class III evidence to support early surgical decompression and stabilization in ATCCS caused by ligamentodiscal injuries and unstable skeletal injuries, the objective being relief of acute compression, stabilization, and correction of spinal alignment, thus preventing further secondary injury to the spinal cord. Early decompressive surgery in this group is more effective and has been shown to reduce length of ICU stay and improve overall motor recovery. The exact timing of surgery in this setting is, however, debatable. Currently there is no standard regarding the timing of decompressive surgery after SCI. The proposed guidelines, based on the recent literature, state that decompressive surgery can be safely performed in the first 24 hours of injury in a hemodynamically stable patient (Box 27-5). There is preliminary evidence that early decompression within 24 hours of injury may improve the neurological recovery.16–18
1. Schneider R.C., Cherry G., Pantek H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine (part1). J. Neurosurg.. 1954;11:546-577.
2. Sekhon L.H., Fehlings M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2-12.
3. McKinley W., Santos K., Meade M., Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J. Spinal Cord. Med.. 2007;30:215-224.
4. Aarabi B., Koltz M., Ibrahimi D. Hyperextension cervical spine injuries and traumatic central cord syndrome. Neurosurg. Focus. 2008;25:E9.
5. Fassett D., Harrop J., Maltenfort M., Jeyamohan S., Ratliff J., Anderson D., Hilibrand A., Albert T., Vaccaro A., Sharan A. Mortality rates in geriatric patients with spinal cord injuries. J. Neurosurg. Spine. 2007;7:277-281.
6. Siegenthaler M.M., Ammon D.L., Keirstead H.S. Myelin pathogenesis and functional deficits following SCI are age-associated. Exp. Neurol.. 2008;213:363-371.
7. Furlan J., Bracken M., Fehlings M. Is age a key determinant of mortality and neurological outcome after acute traumatic spinal cord injury? Neurobiol. Aging. 2008.
8. Krause J.S., Carter R.E., Pickelsimer E.E., Wilson D. A prospective study of health and risk of mortality after spinal cord injury. Arch. Phys. Med. Rehab.. 2008;89:1482-1491.
9. Tator C.H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995.
10. Pappas C.T., Gibson A.R., Sonntag V.K. Decussation of hind-limb and fore-limb fibers in the monkey corticospinal tract: relevance to cruciate paralysis. J. Neurosurg.. 1991;75:935-940.
11. Collignon F., Martin D., Lénelle J., Stevenaert A. Acute traumatic central cord syndrome: magnetic resonance imaging and clinical observations. J. Neurosurg. Spine. 2002;supplement:29-33.
12. Dvorak M.F., Fisher C.G., Hoekema J., Boyd M.C., Noonan V. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: a long-term follow-up. Spine. 2005;31(11):2011-2303.
13. Lammertse D., Dungan D., Dreisbach J., Falci S., Flanders A., Marino R., Schwartz E. Rehabilitation NIoDa: Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J. Spinal Cord Med.. 2007;30(20):205-214.
14. Flanders A.E., Schaefer D.M., Doan H.T., Mishkin M.M. Acute cervical spine trauma: correlation of MR imaging findings with degree of neurologic deficit. Radiology. 1990;177(1):25-33.
15. Flanders A.E., Spettell C.M., Tartaglino L.M., Friedman D.P., Herbison G.J. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649-655.
16. Fehlings M.G., Sekhon L.H., Tator C.H. The role and timing of decompression in acute spinal cord injury: what do we know? What should we do? Spine. 2001;26:S101-10.
17. Fehlings M.G. Perrin: The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence,. Spine. 2006;31(11 supplement):28-35.
18. Harrop J.S., Sharan A.D., Ratliff J. Central cord injury: pathophysiology, management, and outcomes. Spine J. 2006;6:S198-S206.