Chapter 49 Surgical Therapy for Pain
• Chronic pain syndromes are of two types: cancer-related pain and chronic benign pain.
• In general, the various surgical procedures that are available for the management of the chronic pain syndromes can be categorized into lesional surgery, stimulation procedures, and corrective surgery (microvascular decompression of the trigeminal nerve).
• The efficacy of a number of pain control procedures is difficult to assess from the literature in the absence of class 1 or class 2 evidence. Individual experience and the conjoint services of a pain control program are necessary to evaluate and treat patients with chronic pain.
• Lesional procedures for the control of pain syndromes in order of ascending pain pathway are as follows: peripheral neurectomy, dorsal rhizotomy and dorsol root ganglionectomy, sympathectomy, dorsal root entry zone leions, anterolateral cordotomy, mesencephalotomy, and cingulotomy.
• Trigeminal neuralgia can be treated by a variety of surgical techniques: peripheral neurectomy, radiofrequency rhizotomy, glycerol injection, balloon compression, microvascular decompression, trigeminal nerve root resection, and radiosurgery. These measures are used when medical treatment has failed.
• Stimulation techniques (neuromodulation procedures) for chronic pain control include deep brain stimulation, motor cortex stimulation, spinal cord stimulation, and peripheral nerve stimulation. The indications for and results of these techniques are different.
• Intrathecal and intraventricular administration of opioid drugs may also be used for pain control in some patients.
Pain is one of the most common symptoms experienced by cancer patients and probably the one that maximally influences the quality of life. More than 75% of patients with advanced disease complain of severe pain.1 The majority of these patients can benefit from treatment with oral opioids,1 probably because of the nociceptive nature of pain in such cases. Other options are rehabilitation techniques, transcutaneous electrical nerve stimulation (TENS), acupuncture, and physical therapies.2–5 For medically intractable pain from malignant disease, intrathecal administration of drugs can be taken into account, but considering the possible development of addiction and tolerance, which can turn into serious impairment of the overall quality of life,6 these patients may be considered for neuroablative procedures.7,8
Chronic benign pain, on the other hand, can result from a multitude of causes and, despite the individual predominance of nociceptive or neuropathic features, in the majority of cases it is a combination of both, with the significant addition of psychopathological features. For these reasons, chronic pain from nonmalignant disease remains the major challenge to pain doctors and still today there is great uncertainty as to the proper surgical and nonsurgical treatment.9,10
For many years, surgical therapy for pain has relied on ablative or “lesional” procedures, consisting of disruption or interruption of anatomical structures and pathways involved in the transmission of painful sensations. However, the rates of complications, pain recurrence, and the development of deafferentation pain due to such procedures were noted to be rather high; over time, these techniques benefited by several improvements, thanks to the introduction of microsurgery and to a more extensive knowledge of the anatomical functional correlations between pain modulative systems.
Although the patients’ selection criteria for ablative procedures have become increasingly selective (also with the aid of thermocoagulation and of CO2 laser), the development of neuromodulation procedures (by means of electrical stimulation or of intrathecal administration of drugs) has led to a decrease in the indications for such surgery.6
Lesional Surgery
Lesional procedures for the treatment of refractory pain have their own rationale in the causative role of a given nuclear or axonal structure in the generation of altered nociceptive information. After the identification of the putative structure responsible for the transmission of established painful symptoms, this structure is ablated or “lesioned” by means of different techniques. It is important to consider neuromodulative procedures before proceeding to ablative techniques, and of course the indication for such surgery strictly depends on the prognosis quoad vitam of the individual patient, the previous pharmacological or surgical attempts already administered, the overall medical condition, and the patient’s choice. Before considering a patient for an ablative procedure, conservative nonsurgical therapy (or neuromodulation procedures) must have been attempted, maximized, and failed.11
Peripheral Neurectomy
Known from the sixteenth century, thanks to Ambroise Paré, neurectomy consists of the resection of a portion or the whole of one or more peripheral nerves. It is an easy and simple procedure, performed under local anesthesia, but its long-term failure rate makes its indications in chronic pain management very limited today.12 In fact, adjacent intact sensory nerves can reinnervate the anesthetic region, thus causing pain recurrence; a new pain sensation due to the denervation hypersensitivity and painful postoperative neuromas may develop, too.12,13
Neurectomy is indicated in those cases in which pain is limited to the nervous distribution of a specific nerve; such cases are rare and it is thus preferable (given the innervation’s juxtaposition of different dermatomes) to perform peripheral neurotomies in steps. Painful neuromas following peripheral nerve injuries may benefit from neuroma excision and neurectomy, if conservative nonsurgical treatment has not succeeded.13,14 Proximal neurectomy in amputee patients with painful stump neuromas can be of benefit, but nonspecific stump pains or phantom limb pains do not respond to nerve resection.12,15 Chest wall cancer pain and postherpetic neuralgia also do not benefit from neurectomy.
In some rare cases it is possible to perform a trial infiltration of long-acting anesthetic, eventually followed by neurolysis with percutaneous thermocoagulation or with injection of neurodestructive agents, such as phenol or alcohol;16 potential action sites are the posterior ramus of the second cervical nerve in the case of occipital neuralgia and the posterior rami of the spinal intercostal nerves in the case of drug-refractory intercostal neuralgia.
Dorsal Rhizotomy and Dorsal Root Ganglionectomy
Dorsal rhizotomy implies the sectioning of the dorsal nerve roots. Extradural, percutaneous, and radiofrequency methods have been described as well as partial rhizotomy.17–20 The procedure was indicated especially in the thoracic and sacral regions for malignant pain,21 but because of the high rate of pain recurrence22,23 and development of dysesthesic or deafferentation pain24 the procedure has been all but abandoned these days.
Performed first in 1966 by Scoville,25 based on the discovery that a certain number of axons in the ventral root were unmyelinated afferent fibers from the dorsal root ganglion,26–28 dorsal root ganglionectomy showed some long-term pain relief in the treatment of chest wall pain, known as the postthoracotomy syndrome.11 Some authors suggest it for sciatica, too, but there is some controversy about this indication.
Dorsal Root Entry Zone Lesions
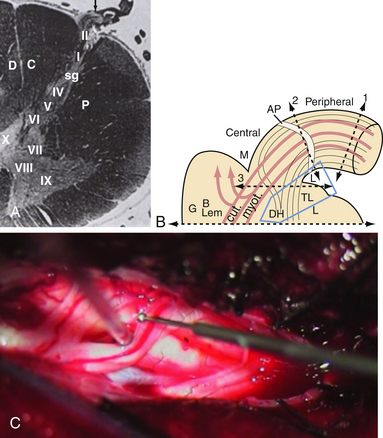
The dorsal root entry zone (DREZ) is an anatomical entity that includes the central portion of dorsal root, the tract of Lissauer (TL), and layers I to V of the dorsal horn, where the afferent fibers synapse with the origins of the sensory pathways.29 The TL plays an important role in the intersegmental modulation of the nociceptive afferents. It is located dorsolaterally to the dorsal horn and it is divided in two parts: (1) the medial part receives nociceptive afferents directed to the dorsal horn and transmits the excitatory effects of each dorsal root to the adjacent segment;30,31 (2) the lateral part receives longitudinal endogenous propriospinal fibers interconnecting different levels of substantia gelatinosa (SG) and thus conveying the inhibitory influence of the SG into the neighboring metameres.31 The SG, through synaptic connections with the dendrites of some neurons of the spinoreticulothalamic (STR) tract, exerts a strong modulating effect on the nociceptive input (Fig. 49.1A). When the large lemniscal afferents in peripheral nerves or dorsal roots are altered, there is reduction of the inhibitory control of the dorsal horn, hence resulting in excessive firing of the dorsal horn neurons and causing deafferentation pain.32–35
The first DREZ open surgery procedure was performed by Sindou in 1972 on a patient with chronic pain due to Pancoast syndrome.36 Soon after him, in 1974, Nashold started using radiofrequency thermocoagulation.37,38 Later, DREZ procedures were performed with laser39,40 and with the use of ultrasound probes.41,42
The procedure known as microsurgical DREZotomy (MDT) was again accomplished by Sindou29 and involves several segments of the lateral portion of the dorsal horn (where C fibers predominate), meaning the destruction of the LT and laminae I to V of Rexed (Fig. 49.1B and C). By sparing the medial portion of the dorsal horn where large A fibers enter, Sindou tried to avoid complete abolition of tactile and proprioceptive sensation, preserving antinociception and preventing deafferentation.43 Intraoperative sensory evoked potentials (SEPs) during MDT are recommended for identification of the spinal cord segments and can also be helpful in monitoring the surgical procedure itself.44–46 Sindou developed special microelectrodes for intraoperative microelectrophysiology studies and performed microdialysis studies in the dorsal horn as well.47,48
MDT has several indications. Some cancer pain patients may benefit from MDT. Good candidates are patients with long life expectancy, sufficient general conditions to undergo surgery and general anesthesia, and topographically delimited pain due to well-localized tumors. MDT from C7 to T2 can be performed for Pancoast syndrome.49 Malignancies with circumscribed invasion of the thorax, of the abdomen wall, of the perineal floor, or lumbosacral roots or plexus are other indications for MDT. For more extensive cancer, intrathecal opioids or high cervical anterolateral cordotomy is preferable.29
Patients with chronic deafferentation pain from plexus avulsion are tremendously difficult to manage for pain doctors. Even if in some cases initial conservative therapy may be of some help, standard analgesics and narcotics are usually ineffective and medical management is limited to antidepressant, sedative, and anticonvulsant medications combined with psychological support.50 DREZ lesion surgery that is not limited to the avulsed segments but is extended to the adjacent roots is instead recommended for plexus avulsion pain. The results are extremely good, with more than 85% of patients reporting good to excellent pain control after brachial avulsion,6,36,51–54 although pain relief is fairly lower for conus medullaris root avulsion.55 Mortality rate is low,6,51 and morbidity consists of transient sensory deficit and mild weakness.6 DREZ lesion surgery for lumbosacral avulsion carries less than 10% risks of bladder and sexual dysfunction.55
Pain following spinal cord injuries (SCIs) can have a multitude of causes, and hence rigorous patient assessment is mandatory. MDT is indicated for burning pain with radicular distribution at the level of the lesion or for patients complaining mainly of allodynia and electric shock–like border-zone pain. Conversely, pain in the totally or almost totally anesthetic area below the lesion, especially in the perineosacral region, is not favorably influenced by MDT.29 These patients should be referred to rehabilitation services.
Neurostimulation techniques are the first treatment option for relief of pain due to peripheral nerve injuries and to limb amputation; if these methods fail, DREZ lesion surgery can be considered for both. In peripheral nerve injuries, DREZ surgery may be particularly useful when the predominant component of pain is paroxysmal (electrical shooting pain), associated with allodynia, hyperalgesia, or both.29 After limb amputation two types of pain may develop and may coexist: pain in the phantom limb and pain in the stump. The former may be reduced by DREZotomy, while the latter is inconstantly influenced.29
Finally, MDT can be performed also for severe occipital neuralgia, unbearable laterocervical pain, and postherpetic pain, but the procedure has been performed only in small subgroups of patients and has had a high recurrence rate.56,57 Based on the fact that muscle tone was decreased in the operated areas after MDT,36,58 Sindou applied it also for treatment of harmful spasticity with reasonably good results.59,60–62
In 1978 Nashold described nucleus caudalis tractotomy/nucleotomy, also known as caudalis DREZotomy, in which DREZ lesion surgery is extended to the trigeminothalamic system for treatment of facial deafferentation pain such as trigeminal postherpetic neuralgia and facial anesthesia dolorosa.10,63 The results are not as good as those for the other major indications of DREZotomy, but almost 50% of patients may obtain pain relief, and it should be considered that these patients are typically not responding to standard narcotic therapies. For patients without prominent allodynia or hyperpathia, on the other hand, neurostimulation procedures are preferred.6
Provided that rigorous selection criteria are applied, DREZ surgery can achieve very good pain control in some intractable painful syndromes. Sindou proposed a schematic decision-making process for neuropathic pain.56 Accurate DREZ surgery requires good knowledge of the radicular innervation and of the surgical anatomy of the spinal cord and roots.56
Sympathectomy
Sympathectomy means the interruption of the paravertebral sympathetic ganglion chain at a chosen level. Used since the end of the nineteenth century for several diseases,64 it is still indicated for the treatment of a wide group of conditions sympathetically maintained and in some cases of vascular diseases.65–67
The term causalgia (“burning pain”) was first introduced by Mitchell in 1864 to describe a syndrome characterized by burning pain following a partial peripheral nerve injury caused by a high-velocity missile. Its classic triad includes burning pain (associated with allodynia and hyperpathia), autonomic dysfunction, and trophic changes.68 The severe form is known as major causalgia and implies always a high-velocity missile injury, and minor causalgia is reserved for less severe forms including also some described after nonpenetrating trauma.69 The median and sciatic nerves and the brachial plexus are most commonly affected.
Reflex sympathetic dystrophy (RDS)70 consists of many pain syndromes that result from a variety of causes that may or may not include direct nerve trauma. The autonomic nervous system was thought to be implicated, although the “reflexive nature” is still to be demonstrated and the distrophic features are not always present. Sudek’s atrophy and Raynaud’s syndrome have been attributed to sympathetically mediated disorders, too.
In order to reduce confusion the term complex regional pain syndrome (CPRS) was coined to describe a symptom complex and not a particular syndrome or a medical entity.71 The expression sympathetic maintained or mediated pain embraces a spectrum of conditions in which the main symptom, pain, may be associated with vasospasticity and dystrophic features and can be relieved by interrupting the sympathetic outflow to a body region.66,72–74
Upper extremity and upper thoracic sympathectomy implies dividing the chain below the first thoracic ganglion and resecting the T2 ganglion either with open surgery or endoscopically75,76 or percutaneously by chemical, electrical, or radiofrequency techniques.77,78
Open surgical sympathectomy carries high success rates, up to 100%, and low complication rates, between 2.5% and 5%.6,51,68,79 Pain recurrence is possible, but its rates vary considerably, ranging between 0% and 33% in many series reported.64,67,68,80 Conversely, it is well recognized that best results are obtained early in the course of the disease before the development of trophic changes,64,66,73,74 and therefore, rigorous early assessment by a pain specialist is needed.10
Cordotomy Procedures
Anterolateral Cordotomy
The basis for AC resides in the neuroanatomy of the ascending pathways transmitting nociceptive information. A detailed description of this anatomy is not the aim of this chapter, and the reader can find more extensive information in other publications.81,82 Both clinical and experimental observation in the last century provided evidence that the anterolateral quadrant of the spinal cord contained mainly contralateral ascending nociceptive pathways.83–89 This fnding suggested the use of AC for the control of pain. The pathways reaching suprasegmental levels throughout the anterolateral quadrant of the spinal cord are the spinothalamic tract (STT), the spinoreticular tract (SRT), the ventral spinocerebellar pathway, the spino-olivar pathway, and the propriospinal pathways.90,91 The STT and SRT are the tracts mainly involved in pain transmission. The SRT, also termed paleospinothalamic tract, provides indirect connection between the spinal cord and the thalamus,92 and the STT, also termed the neospinothalamic tract, provides a more direct monosynaptic connection mainly to the lateral thalamus.83 The primary terminations of the SRT are the intralaminar nuclei of the thalamus,93 thus being primarily related to the aversive and alerting aspect of pain. The STT participates in both the sensory discrimination aspects of pain projecting to the lateral thalamus and in the aversive aspects of pain projecting to the intralaminar thalamus, thus representing the main target of AC. Unfortunately, the STT is not a discrete, separate bundle of fibers, but is diffusely intermixed with other ascending and descending systems (this being responsible for many of the unwanted side effects and complications that can accompany AC). The general organization of the anterolateral quadrant (AQ) ascending sensory system is that lower spinal dermatomes are represented more dorsally and laterally, whereas the higher dermatomes are represented more anteriorly and medially.90,94,95 The descending spinoreticular pathways are also located in the AQ of the spinal cord, and they mediate myriad automatic functions.
The ideal candidates for AC are cancer patients with unilateral, localized pain. Even unilateral upper body pain, such as that experienced by patients with lung carcinoma, mesothelioma, or Pancoast tumor, can be treated by a cervical percutaneous approach.96,97 Bilateral cordotomy can be proposed only for cases with bilateral pain in abdominal, pelvic, or lower extremity regions, and pain in the upper trunk or extremity is considered a contraindication because of the high risk of respiratory complications.96,97 Nonmalignant pain can be treated even if it is probably preferable to use a morphine pump.
Open Cordotomy
The procedure is performed with the patient in the prone position. Neurophysiological monitoring can be done with electromyography (EMG) activity recorded in the lower extremities during spinal cord stimulation prior to the section.98 Removal of the spinous processes and the lamina bilaterally at T2-T3 more widely to the side of the AC is performed. The dura is opened under microscopic view. The arachnoid over the lateral spinal cord and associated nerves are dissected carefully in order to identify the dentate ligament, which is divided. The cord is then rotated gently in order to visualize the ventral roots. Using microtechnique, the pia over the AQ is opened at an avascular area from the level of the dentate ligament posteriorly to the level of the ventral roots anteriorly. At this point a cordotomy electrode can be inserted into the white matter of the AQ. Neurophysiological monitoring can be performed to identify the corticospinal tract. Higher frequency of stimulation can be used to activate ascending fibers if the wakeup test is performed, as advocated by some authors.98 The transverse diameter of the spinal cord is measured, and a right angle probe with blunt extremity that will reach the midline is used to make the incision. The probe is swept anteriorly and a large anterolateral quadrant lesion is completed. The dura is therefore closed in a watertight fashion and the remainder of the wound is closed in a standard fashion.
Percutaneous Cordotomy
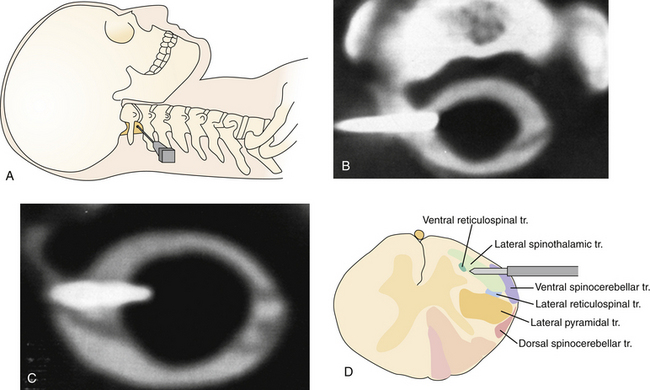
Percutaneous AC is superior to conventional open methods. It is performed with the patient under local anesthesia, requires no incision, and allows excellent functional monitoring prior to completion. In addition, with the patient awake in response to neuromodulation, the functional characteristics of the region of the spinal cord to be sectioned can be studied in more detail. It is also more likely to provide pain relief for symptoms above T5 than the open cordotomy. This technique has been pioneered by Mullan and associates,99 and later modified by the use of radiofrequency, image guidance, and functional mapping.100–102
Contrast material should be administered in the subarachnoid space. This could be done preoperatively (20-30 minutes before) by a lumbar puncture (in this case the patient should be maintained in the Trendelenburg position before the procedure) or at the beginning of the procedure by needle insertion at the C1-C2 level. The patient is placed in a supine position in the computed tomography (CT) unit with the head slightly flexed. Under local anesthesia and intravenous sedation if needed, the needle is advanced from the lateral neck into the C1-C2 intradural space. Lateral fluoroscopy allows identification of the appropriate site to start the needle trajectory toward the spinal canal and the final position of the needle (Fig. 49.2A). Once the dura is penetrated, cerebrospinal fluid (CSF) flow will be evident after stylette removal. At this point, an axial scanogram by CT allows the measurement of the spinal cord and is used to better understand intradural needle positioning. The tip diameter of the needle should be guided anterior to the dentate ligament and posterior to the anterior cord (1 mm anterior for lumbar fibers and 2-3 mm anterior for thoracic and cervical fibers). The needle in its ideal position is nearly perpendicular to the spinal cord (Fig. 49.2B). Once the position of the needle is satisfactory, the electrode is passed through the spinal needle into the spinal canal and the spinal cord. Penetration of the pia results in a brief increase in local pain. Correct positioning of the electrode can be performed by CT scan (Fig. 49.2C and D). Now electrical stimulation is performed to adjust the electrode position. Stimulation at low frequency (2-5 Hz) is used to evoke motor responses. Sensory stimulation is done with higher frequency (100 Hz). Placement of the electrode in the STT and SRT area is confirmed by the occurrence of contralateral sensory phenomena, usually a feeling of warmth or cold; with higher stimulus strength, a painful sensation can be evoked.103 When adequate electrode positioning has been performed, the initial lesion is made by using an electrode with a 2-mm exposed tip applying 30 to 40 mA for 30 to 60 seconds.101 After this lesion, the patient is examined to evaluate the area of analgesia obtained. Further lesions can be performed in cases of incomplete results in the target area by increasing the time of passing current or advancing the electrode tip in small increments of 0.5, 1 mm. If the procedure is performed satisfactorily, an ipsilateral Horner’s syndrome usually occurs.
The more recent literature is almost exclusively related to percutaneous procedures. The results are better for pain related to malignancies. In experienced hands, the initial pain control is achieved in 90% of patients. However, the level of analgesia falls with time, reaching 40% of patients at 2 years.104
The complications resulting from AC are related to the damage of the STT and SRT and of all the other adjacent tracts (Fig. 49.2D). Damage to STT and SRT can lead to painful dysesthesias. Damage to nearby structures can result in (a) respiratory failure and death, (b) bowel, bladder, and sexual disfunction, (c) hypotension, (d) ipsilateral weakness, (e) mirror pain, or (f) ataxia. In cervical cordotomies, respiratory dysfunction can be the principal complication. This is particularly true for patients with preexistent respiratory disorders and in cases of bilateral cordotomies. In this last situation, respiratory functions should therefore be carefully observed in the postoperative period. In the past, with the use of open cordotomy, higher rates of complications were observed. Two reasons can be recognized as the cause of complications: (1) needle mislocalization, which is more difficult with the use of CT-guided AC; and (2) involuntary enlargement of the sectioned area, which is less frequent with the use of recently developed electrodes and small thermic lesions. Therefore, percutaneous AC offers a reduction in the percentage of complications.
Cingulotomy
Cingulotomy has been successfully used to treat intractable cancer pain.105–108 The cingulate cortex is a key component of the limbic system, and its anterior cingulate gyrus plays an important role in the integration of nociceptive, motor, affective, and memory functions.109 Experimental studies on animals and also functional studies in human volunteers demonstrated a role of cingulate gyrus in control of contralateral nociceptive information.110–115
Results of bilateral cingulotomy are variable. It can assure an improvement of pain control in selected cases.116 However, even after MRI-guided localization of the cingulate cortex, complications related to behavioral changes or cognitive deficit can occur.116
Mesencephalotomy
After the first attempts by Walker117 and Drake and McKenzie,118 the procedure was modified by the application of stereotactic technique119 and avoidance of the medial lemniscus. With these changes, it was possible to reduce the incidence of severe dysesthesia and to reduce the mortality rate.
The first attempts in open surgery were to perform a section of the lateral spinothalamic pathways, but these resulted in unacceptable risks. Stereotactic procedures were therefore applied to interrupt nociceptive pathways with more precision. Looking for an ideal target for pain control, Shieff and Nashold120,121 elucidated that the procedures worked better and with fewer side effects when the lesion involved the multisynaptic reticulospinal pathways, sparing the spinothalamic tract, which was the original target.
Stereotactic procedure is performed to complete the sectioning, following target selection.
Gybels and Sweet122 reported pain relief in 86% of patients, usually lasting for the remaining part of the patient’s life. Mortality rates ranged from 1.8% to 8%, although it can be difficult to attribute deaths to the procedure itself and not to the disease. Oculomotor dysfunction is a relatively frequent complication. Dysesthesia can occur in 15% to 21% of cases.
Mesencephalotomy can be a valuable option for the management of cancer pain in selected cases.
Percutaneous Techniques for Trigeminal (and Glossopharyngeal) Neuralgia
The first clear description of trigeminal neuralgia (TN) was given in 1671.123 John Fothergill,124 in a paper published in 1773, described the typical features of TN, including its paroxysmal nature and association with triggering factors such as eating, speaking, or touching the face. In 1756, the French surgeon Nicholas André coined the term tic douloureux to describe at least three patients with TN treated by neurectomy.125–128
The clinical features of the so-called classic idiopathic TN have been well documented and are now universally recognized. One of the pillars in our understanding of this syndrome is the classification that Eller and colleagues proposed in 2005.129 These authors classified TN on the basis of two broad categories: the patient’s history and seven specific diagnostic criteria. Following Eller’s classification, TN type 1 identifies the classic idiopathic form, also known as the typical form of TN, and from now on we will refer to TN as this entity. TN is a chronic pain syndrome whose patients suffer from idiopathic episodes of spontaneous facial pain. This pain, which is experienced in one or more divisions of the trigeminal nerve, expresses itself as paroxysms of brief and excruciatingly intense bouts of stabbing, electrical shocks. These paroxysms of TN can arise spontaneously or in response to gentle tactile stimulation of a trigger point on the face or in the oral cavity. They may also be triggered by such natural activities as chewing, speaking, swallowing, touching the face, or brushing the teeth. TN pain is almost always experienced unilaterally, although there have been some relatively rare reports describing bilateral signs and symptoms.129 TN type 1 pain typically occurs after conspicuously obvious pain-free intervals that can last for weeks, months, and years. The neurological examination is almost always normal, although cases have been reported in which a slight degree of sensory loss has been described.130 The diagnosis of TN is always based on the patient’s clinical history. In terms of its pathophysiology, the features of classic TN are currently thought to be related to a compression of the trigeminal nerve root, usually by a blood vessel, at or near the root entry zone of the trigeminal nerve.131–134
The incidence of TN is about 2.3 to 4.5 per 100,000 new cases per year.135 Age at onset is variable, but the incidence increases in the fifth and sixth decades of life.
From the time of its first recognition, TN has been treated in a variety of ways.136,137 Today several medical and surgical treatments are available.
Glossopharyngeal neuralgia (GN) is a spasmodic, lancinating, and paroxysmal pain that starts in the posterior throat or base of the tongue and often radiates down the throat and side of the neck. Often pain is triggered by swallowing or yawning. Typically, cocainization of the area reduces pain and has been proposed to confirm diagnosis.138 Seldom, hypotension, syncope, and cardiac irregularities may accompany GN.139,140 Isolated GN occurs at a rate of 1 case in every 70 to 100 cases of TN;141 concurrence of TN and GN is also possible.142
Percutaneous Injection of Neurodestructive Substances
In 1912 Hartel proposed the procaine injection, and later in 1914 the alcohol injection, of the gasserian ganglion for TN.143,144 In 1940 Harris reported a series of 2500 cases treated through this method.145 However, the high rate of postinjection paresthesia and anesthesia dolorosa and some reported cases of major complications due to the diffusion of alcohol in the posterior fossa, along with a high pain recurrence rate, caused the technique to be abandoned.
Conversely, glycerol injection of the ganglion, first introduced by Hakanson in the 1970s,146 occasionally is still performed today. The procedure is relatively safe, can be repeated if necessary, and is well tolerated, but it may cause transient sensory loss, corneal anesthesia, and dysesthesia.147,148 The main drawback is the rate of pain recurrence, which is reported to be up to 10% in the early postoperative period, up to 50% at 3 years, and 90% at 6 years.147,149,150
Percutaneous Trigeminal Radiofrequency Thermocoagulation
Even though it had been tried since the beginning of the twentieth century,151,152 it was not until the mid-1960s, with the modifications of the technique by Sweet, that this procedure became popular. Sweet’s modifications consisted of electrophysiological stimulation for precise localization, reliable radiofrequency current for nerve destruction, intermittent patient sedation with short-acting intravenous drugs allowing intraoperative patient assessment, and temperature monitoring to control precisely the configuration of the site.94,153,154 With these modifications the use of radiofrequency rhizotomy spread widely and it is still commonly used.
The rationale is based on the fact that pain fibers are carried by unmyelinated C-fibers or thinly myelinated Aδ-fibers that are blocked at a lower temperature than those of larger Aα- and β-fibers carrying deep and tactile sensations.155–157 Thus, radiofrequency-induced heat will destroy the former fibers, sparing the latter ones.
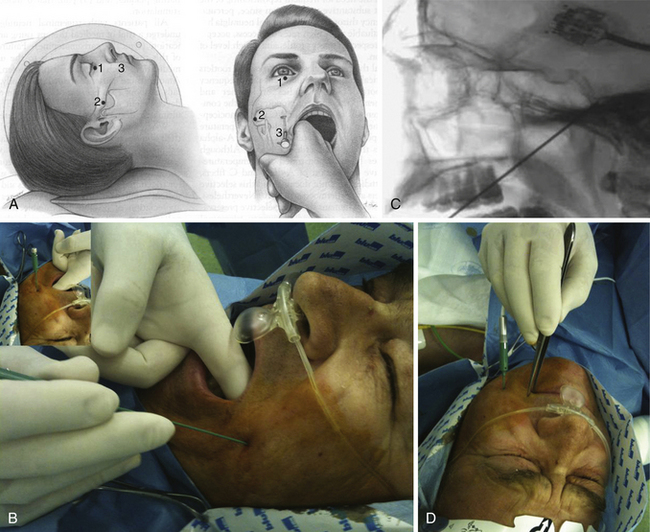
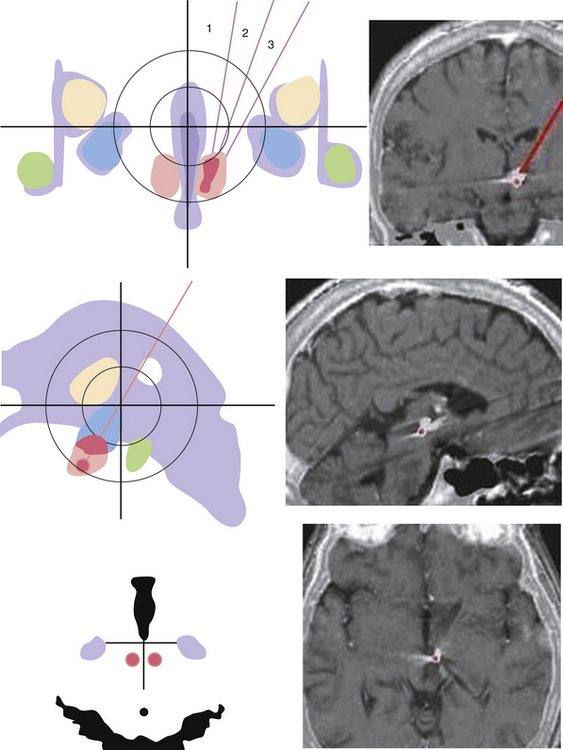
The technique consists of the introduction of a needle under fluoroscopy at the level of the gasserian ganglion through the foramen ovale, entering 2.5 cm lateral to the oral commissure and aiming toward the medial aspect of the pupil (Fig. 49.3A and B). Several methods have been described to place the needle into the foramen ovale;158–160 careful checking under fluoroscopy (Fig. 49.3C) and considerable training and experience are mandatory to avoid internal carotid artery injuries or penetrating the foramina adjacent to the foramen ovale. Once at the target, confirmed by outflow of CSF, the electrode can be inserted.
With the patient under mild sedation, an average of three lesions at 60 to 70 degrees for 90 seconds are usually done. The goal is analgesia in the primarily affected trigeminal division, hypalgesia in correspondence of trigger points, and mild hypalgesia in the secondarily affected trigeminal divisions. After each lesion, clinical assessment of facial sensitivity, corneal reflex, and strength of the muscles of mastication is mandatory (Fig. 49.3D).
The procedure does not require general anesthesia, can be performed for patients not suitable for open surgery (the elderly, those with comorbid conditions, etc.), and can be repeated if necessary. Immediate pain relief can be obtained in up to 98% of patients,147,161 but the long-term recurrence rate may be particularly variable: 25% to 37% at 5 years, 25% to 80% after 10 years,162,163 or 27% overall (early and late recurrence) in a study with an average follow-up period of 9 years.164 Almost all patients develop numbness in the face after the procedure, but in most cases this is tolerable. The mortality rate is extremely low;147,165 loss of corneal reflex occurs in 3.5% to 7%; and cheratitis is seen in less than 3%.147,148 Dysesthesia develops in 6% to 24% of patients, but anesthesia dolorosa occurs in only 0.2% to 4%, with higher risk in more complete or extensive procedures.147,150,161,164 Trigeminal motor function may be impaired, but most of the time this effect is transient.164 When patients are asked to rate the procedure and the outcome, the majority of them are usually satisfied.162,164
By the same technique, the glossopharyngeal nerve can be reached percutaneously at the foramen lacerum, allowing a postganglionar sectioning of the fibers.166
Percutaneous Trigeminal Balloon Compression
The idea of compressing with open surgery the root of the trigeminal nerve to treat TN was first proposed around 1950, but the results were not encouraging.167,168 Later, Mullan proposed the gasserian ganglion compression through a percutaneus approach, reporting good results.169–171
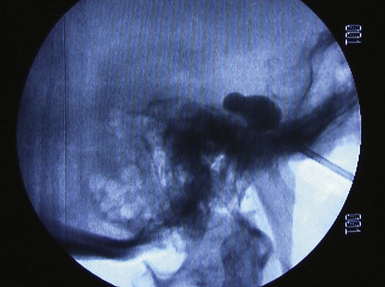
The procedure is performed in the same way as for radiofrequency thermocoagulation until the ganglion is reached. Then a balloon catheter is inserted. A characteristic pear figure appears under fluoroscopy when the balloon is properly inflated with contrast agent in Meckel’s cave (Fig. 49.4). The goal is to compress the nerve at 1100 mm Hg or 1.3 to 1.5 atm for 1 minute.
Balloon compression is particularly indicated for first trigeminal division pain, because it carries a lower risk of corneal reflex loss and cheratitis compared to radiofrequency thermocoagulation. Nevertheless, during the procedure the patient may develop bradycardia and hypotension172 and a pacemaker may be required; the compression is mild and this leads to a higher rate of pain recurrence. The initial rate of pain relief is 92% and the overall recurrence rate is 26%; postoperative numbness and mild to moderate hypesthesia occur in about 50% to 60% of patients; and anesthesia dolorosa and corneal anesthesia are extremely rare, less than 0.1% of cases. On the other hand, masseter weakness can occur in about 10% of patients.147
Microvascular Decompression for Trigeminal (and Glossopharyngeal) Neuralgia
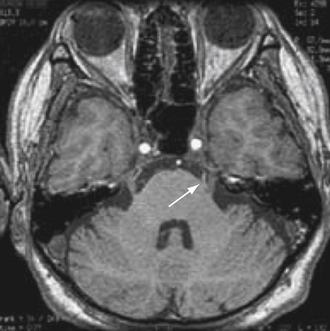
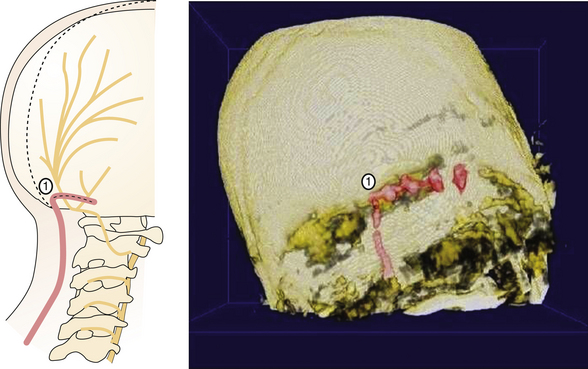
Dandy173 was the fist to propose that there might be a causal relationship between pain paroxysms and the compression of the trigeminal root. This compression is typically caused by adjacent arterial loops, although on occasion, tumors, arteriovenous malformations, and aneurysms located in this region have been known to cause this kind of compression. Though Dandy was the first to describe the role played by compression in 1929, it was not until the 1950s that any kind of therapeutic relief became obtainable by decompressing the trigeminal nerve in TN patients.174,175 The notion that a microvascular compression might be causing TN has gained much support from the work of Jannetta,134,176–178 who was able not only to find a compressing vascular contact in a high percentage of TN patients but also to demonstrate that prolonged pain relief could be obtained by decompressive surgery, without causing any sensory loss. Patients suffering from typical idiopathic TN usually are found to have a blood vessel that is in close contact with the trigeminal root entry zone (TREZ). This finding is typically made during surgery, during the exploration of the cerebellopontine angle (CPA), or radiologically by MRI176,178–180 (Fig. 49.5).
Microvascular decompression (MVD), also known as Jannetta’s procedure, is a nondestructive treatment designed to resolve the compressive conflict between the trigeminal nerve and a blood vessel. Today, it is widely recognized as the primary therapeutic option in the treatment of TN.178,181–184
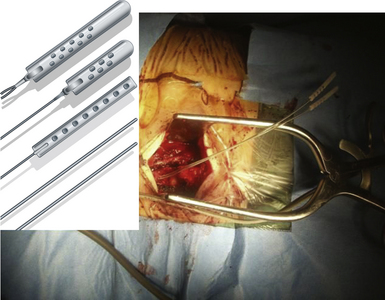
The procedure is performed in the operating room under general anesthesia. With the patient in the sitting or lateral position, a small retrosigmoid craniectomy is done, allowing for exposure of the transverse sinus, the sigmoid sinus, and the junction between the two. Intradurally, by means of opening the CPA cistern, the CSF drainage facilitates a natural cerebellar relaxation, creating a perfect surgical corridor to the fifth cranial nerve and to the seventh to eighth cranial nerve complex. The TREZ is the most common site of vascular compression. It is the locus of transition from central to peripheral myelin and the most vulnerable part of the nerve. After the neurovascular conflict is identified (Fig. 49.6A), a sharp dissection of the arachnoidal bands that fix the artery into its position allows for moving the nerve away from the artery. Many methods have been advocated for keeping the vessel separated from the nerve, including using pieces of muscle, fascia, periosteum, or subcutaneous fat. Our experience is that these strategies usually have little effect. This kind of tissue is usually reabsorbed and favors adhesions, fibrosis, and arachnoiditis, as has been observed during reoperations in patients in whom neuralgia has recurred.185–189 Most authors dealing with MVD now use synthetic implants, particularly Ivalon sponge,187,190–192 Dacron knitted material,189,193 or Teflon felt.178,193–196 In most of these reports, a small piece of synthetic material has been interposed between the nerve and the vessel. Though they are considered biocompatible, Ivalon, Dacron, and Teflon may, in fact, generate granulomas and carry the consequent risk of distorting or irritating the trigeminal root and, in so doing, producing a recurrence of pain.197–199 The so-called sling retraction technique183,193,200,201 has been proposed as an alternative by introducing the concept of a noncompressive technique for MVD (Fig. 49.6B), with good results.201
The results with this technique are excellent, with up to 98% of patients reporting immediate pain relief and more than 85% and 80% of patients pain-free without medication at the 5 and 9 years’ follow-up, respectively.150,202 The mortality rate is less than 1%.150,192,203 The facial numbness rate is only 2%; the rate of dysesthesia, anesthesia dolorosa, corneal anesthesia, and trigeminal motor disfunction approximates 0%. The overall cranial nerve deficit is about 3%;150,204 fourth and seventh cranial nerve deficit with diplopia and facial weakness may occur, although such effects are usually transient. Deafness has been reported as well. CSF leak, wound infection, and meningitis can occur with an overall rate of 10%,150 but only a few cases require second surgery or prolonged hospitalization.
Similar results in terms of pain relief and complication rate can be obtained with MVD for GN.205,206
Last, when negative or equivocal exploration is encountered during MVD, partial trigeminal rhizotomy can be performed. The technique was first proposed by Dandy.207 It consists of a partial section of the nerve in the portion adjacent to the brainstem, starting at its posterior inferior margin. This allows the surgeon to spare most of the facial sensibility, corneal reflex, and motor function. Results are rather good, with more than 80% experiencing pain relief, albeit sensory deficit and dysesthesia are obviously higher than with MVD.208 This procedure can be effective also in patients with demyelinating or neurodegenerative diseases and some consider it the last line of defense for patients who have proved to have a recalcitrant form of TN and have failed all previous treatments.
Neuromodulation Procedures
Deep Brain Stimulation
The first report of deep brain stimulation (DBS) procedures affecting perception of pain dates back to the 1950s, when Pool observed an analgesic effect by stimulating the septum pellucidum and the columns of the fornices during a psychosurgery intervention;209 subsequently, the same author and Heath reported an antinociceptive effect after the stimulation of the paraseptal regions.210,211 The first report of thalamic DBS for control of pain was by Mazars in 1960.212 In the 1970s, Hosobuchi and Richardson were the first to report a case of thalamic and periaqueductal gray (PAG) and periventricular gray (PVG) stimulation.213–216 According to the gate theory by Melzack and Wall217 thalamic stimulation induced paresthesias in the pain-affected areas.
Another cerebral region that led to pain alleviation and also paresthesias when stimulated was the posterior limb of the internal capsule.218 The first report of stimulation of the centromedian nucleus of the thalamus is due to Andy.219 Although the stimulation of PAG and PVG does not preclude paresthesias, it can lead to “pleasant” sensations in the patient. Another potential target is the centromedianus parafascicular complex of the thalamus, which has been used by Andy for the treatment of dyskinesias.
Many clinical observations would tend toward a greater efficacy of the stimulation of PAG/PVG regions in the treatment of nociceptive pain, and of VPL/VPM complexes in the treatment of neuropathic pain; however, as stated earlier, the number of patients treated is still too low, and it is not possible to draw any definitive conclusion without controlled, prospective, and randomized studies. It is also necessary to consider the clinical situations of “mixed” pain (with both nociceptive and neuropathic components) and this once again emphasizes the importance of an accurate patient selection (some authors consider the future use of simultaneous implantation of both thalamic and periventricular electrodes220).
Painful syndromes that can be treated with DBS include anesthesia dolorosa, postischemic pain, thalamic pain, brachial plexus avulsion, postherpetic neuralgia, postcordotomy dysesthesia, medullary lesions, failed back surgery syndrome, cancer-related pain, and peripheral neuropathic pain.221 Other important indications for DBS (the target being in this case the posterior hypothalamic region, pHyp) are refractory trigeminal autonomic cephalalgias (TACs), including chronic cluster headache (CCH). DBS of the pHyp was the first application in which the choice of target was motivated by neuroimaging functional data. Activation of the pHyp during cluster headache pain attacks was observed during positron emission tomography (PET) studies;222 this original observation led to the placement of deep brain electrodes within the pHyp to inhibit the pathologically activated neuronal pool in patients with CCH (Fig. 49.7).
Series reported in the literature include 50 patients affected by CCH.223–229 Five patients affected with TN due to demyelinating disease,230 two patients affected by short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT),231,232 one patient affected by chronic paroxysmal hemicrania,233 and four patients affected by neuropathic pain of the face230 have been reported in the literature.
With regard to CCH, in the Belgian group,228 whose mean follow-up period was 14.5 months, two out of four patients were pain-free, one patient had a dramatic reduction of pain attacks to less than three per month, and one patient had only transient clinical benefit. In the open-phase period of the study reported by the French multicenter study,226 the mean weekly attack frequency decreased by 48.4%, and 6 out of 11 patients were considered as “responders” (decrease of at least 50% in the frequency of weekly attacks). In the German group,223 three out of these six patients were reported to be almost completely attack-free (one mean pain attack per month) after a follow-up period ranging from 9 to 17 months. Of the two patients reported by the British group233 one patient only reported infrequent pain attacks (seven injections of sumatriptan) at 11 months’ follow-up, and the other patient reported a decrease in attack frequency from daily to weekly with “massive reduced severity.” In the U.S. group,229 three out of five patients could be considered responders because of “50% reduction in headache frequency intensity, or both”; the follow-up period ranged from 6 to 12 months in their study. As far as our group is concerned,227 the mean follow-up time was 4 years; a state of persistent freedom from painful attacks was still present in 10 of 18 patients (62%). Four patients (25%) still required prophylactic drugs to prevent the attacks. In the last 2 years of follow-up, three patients no longer benefited from stimulation despite several changes in parameters. In these three patients, the disease changed from the chronic to the episodic form. Two patients affected by SUNCT who underwent posterior hypothalamic DBS have been reported in the literature to date. The first patient was reported by our group in 2005.231 The preoperative frequency of pain bouts ranged from 70 to 300 per day; after surgery, a complete and definitive remission of symptoms has been confirmed at the last clinical examination, performed at 5-year follow-up. The second patient with drug-refractory SUNCT treated with hypothalamic DBS was reported by Lyons and co-workers in 2009;232 a 63% reduction in the mean number of daily attacks (133 attacks per day preoperatively vs. 45 attacks per day) was observed in the first postoperative month; at 12-month follow-up a further improvement was observed, with 80% in reduction in frequency of attacks (25 attacks per day).
Only one patient affected by chronic paroxysmal hemicrania (CPH) submitted to posterior hypothalamic DBS has been reported to date.233 One of the main criteria for diagnosis of CPH is an absolute response to indomethacin administration; in this patient, however, this drug was discontinued because of the onset of iatrogenic gastritis superimposed on a preexisting Barrett’s esophagus; given the refractoriness of symptoms, the patient underwent pHyp DBS. After a 27-month follow-up period she was reported to be symptom-free. At our institute, we implanted with pHyp DBS three patients affected by secondary neuropathic trigeminal pain (the conditions leading to the painful condition were a posterior mandibular carcinoma with subsequent radical transmandibular tumor resection in one patient, a minor dental procedure in the second, and a nasopharyngeal carcinoma in the third patient).230 None of the three patients had reduction in painful symptoms at the last clinical examination despite several attempts in modification of stimulation parameters. At our institution five multiple sclerosis (MS) patients affected by refractory TN were submitted to pHyp DBS;39 three patients had beneficial effects within 24 hours from the procedure; all patients reported a reduction of paroxysmal pain attacks within the ophthalmic branch after surgery. Three patients complained of recurrent pain in the second and in the third branch (but, importantly, not in the first) and underwent further radiofrequency thermorhizotomies.
The other two patients reported relief of pain in all three trigeminal branches by combining stimulation with analgesics and did not necessitate further surgical procedures.
The overall percentage of patients with CCH treated with pHyp considered to be responders is 63%.234
Motor Cortex Stimulation
In 1991 Tsubokawa introduced the epidural motor cortex stimulation for the treatment of central deafferentation pain, and his group concluded that sensitive cortex stimulation was not effective for this purpose. He revealed that such technique was able to inhibit burst-like thalamic discharges that had been previously demonstrated to be altered in animal models of deafferentation pain. He also demonstrated the safety of this procedure with particular regard to eventual postoperative development of seizures.235
The mechanism of action of MCS is still poorly understood; the comprehension of its physiological effects strictly depends on that of the anatomical connections between the motor cortex and several cortical, subcortical, brainstem, and spinal cord structures. These connections can now be revealed through animal studies and different neuroradiological studies, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Some SPECT studies demonstrated a normalization of the local cortical somatosensory-motor (SI-MI) circuit236 after MCS; this observation could be in line with Tsubokawa’s original view, according to which MCS activates non-nociceptive neurons of primary sensory cortex through both orthodromic and antidromic axonal pathways interconnecting MI and SI, thus leading to activation of surrounding nociceptive inhibition in SI.
Other studies demonstrated that cortical stimulation is able to increase the blood flow to the ipsilateral thalamus, thus modulating the long reverberating thalamocortical loop, and overriding the pathological phenomena of disrupted oscillation and temporal synchronization.237
Several painful conditions have been treated with MCS up to now, such as thalamic pain (postictal or posthemorrhagic), postischemic bulbar pain which accompanies Wallenberg’s syndrome, neuropathic facial (trigeminal) pain, phantom limb pain, deafferentation pain, postherpetic neuralgia, spinal cord injuries, and pain due to avulsion of the brachial plexus. One of the first reports of the efficacy of MCS for treatment of central pain is by Tsubokawa (“good” control of symptoms with MCS in 65% of patients with thalamic and putaminal postischemic and posthemorrhagic pain) and by Katayama who performed MCS in three patients who had been submitted to DBS of the VPL nucleus of the thalamus for pain related to Wallenberg’s syndrome; after DBS, these patients reported a worsening of symptoms, but they presented a 60% improvement after MCS. Treatment of facial neuropathic pain has been indicated as the major indication for MCS, in part because of the relatively large cortical representation of the face; and in fact, different authors report a 60% pain improvement after a mean follow-up period of 1 year.238–242
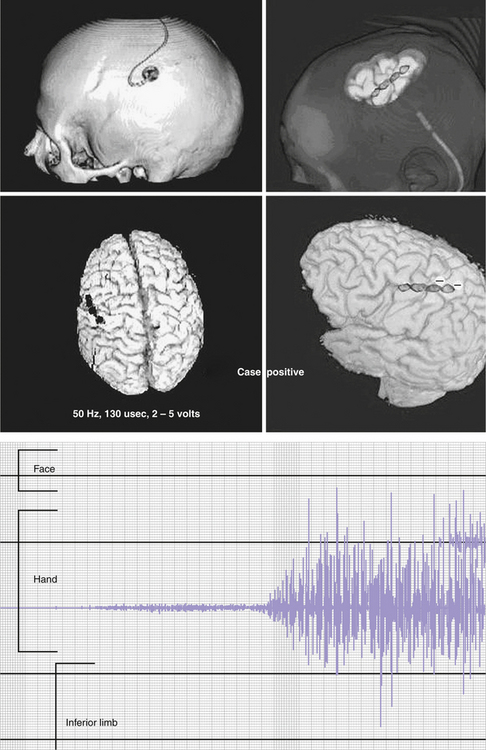
Some authors perform MCS by sliding the plate electrode through a simple bur hole centered on the posterior portion of the previously localized precentral gyrus, whereas others perform a craniotomy. Once positioned, the abovementioned integrative methodologies can be used, thus permitting a refinement of the positioning according to the case. In general, it is preferable to position the active contacts just above the area of the motor strip representing the affected part of the body (Fig. 49.8) given that the lower limb representation lies in the medial surface of the hemisphere (anterior portion of the paracentral lobule). MCS is usually performed for facial or upper limb pain; nonetheless, reports exist of placement of the electrode subdurally over the paracentral lobule, or epidurally, very close to the sagittal sinus; in these cases, 6 out of 12 patients with lower limb pain improved by 40% to 50%.243
After the procedure, the implanted pulse generators connected to the intracranial electrodes are activated at various settings, which vary according to the institution, the physician’s preferences, and the results obtained. They range from 1 to 5 V, 15 to 130 Hz, 60 to 450 microseconds. The configuration more often used is bipolar rather than monopolar; when in bipolar mode and in those cases of electrodes placed perpendicular to the central sulcus, the most anterior contacts are set as cathodes.244
As reported by Fontaine and associates243 only three studies, comprising 10 patients, reported postoperative CT or MRI for determining the exact location of the electrode;239,244,245 in seven of these patients, postoperative imaging was obtained to rule out electrode misplacement and in the other three the electrodes were thought to be located in the preoperatively determined target with neuronavigation systems and then assessed with intraoperative stimulation.
Spinal Cord Stimulation
Spinal cord stimulation (SCS) was first introduced (with the name stimulation of the dorsal column of the spine) by Shealy in 1967;246 initially, though, satisfactory and definitive results were not always achieved, partly because of continuous and indiscriminate use of the method and also because of poor choice of indications.
Better results were obtained beginning in the 1970s, when more accurate patient selection and advancement in technological equipment allowed this technique to gain a wider acceptance.247
The procedure consists of the positioning of an electrode (with various shapes, dimensions, and number of contacts) in the epidural spinal space; the site of the implant depends on the site of pain. It is generally placed at the cervical or middle-lower thoracic level (Fig. 49.9). The internal pulse generator (IPG) is positioned in the subcutaneous tissue (generally at the abdominal level) and connected to the electrode through tunneled extension leads.
The mechanism of action of SCS is still debated; numerous theories have been proposed, the first of which has obviously been the “gate” theory of Melzack and Wall. New physiological mechanisms have been hypothesized, such as the “orthodromic theory” (the electrical impulses conveyed by the fasciculum gracilis and cuneatus activate suprasegmental centers such as PAG and nucleus of raphe magnum, which exert inhibitory control on pain perception via descending pathways), the “collision current theory” (the current traveling along the dorsal roots enters in collision with orthodromic stimuli traveling through Aδ- and C-fibers, blocking them), and the “inhibitory neurotransmitter” theory, according to which the analgesic effect could be due to the stimulation-induced release of endogenous opioids at the level of the dorsal horns, or to the fact that stimulation leads to an increase of the concentrations of neuroactive substances in CSF such as norepinephrine, GABA, substance P, adenosine, and glycine. In particular, the release of inhibitory amino acids such as GABA and glycine could limit the release of excitatory amino acids such as aspartate and glutamate and could act on their own receptors (especially GABAB receptors) at various spinal levels, restoring normal GABAergic activity within the spinal cord.
Probably different mechanisms play a role in the analgesic effect of SCS, and as stated by Linderoth248 the final common effect could be a suppressive effect of current on wide-dynamic range and hyperexcitable neurons located on the dorsal horn of the spinal cord.
Most of the studies report a clinical efficacy of SCS in 50% to 70% of the patients treated (responders: reduction of at least 50% in intensity of pain). SCS has been reported to be superior to surgical reexploration in failed back surgery syndrome in a study by North and co-workers.249
Peripheral Nerve Stimulation
The first report of PNS for pain dates back to 1967, when Wall and Sweet inserted an electrode into their own infraorbital foramina and observed a decrease in painful sensitivity during the period of stimulation.250 In the same period Shelden implanted electrodes around the mandibular nerve in three patients and delivered electric current through an implanted receiver at a frequency of 14,000 Hz; again, temporary pain decrease was obtained.251 Weiner and Reed in 1999 described the first percutaneous insertion of an electrode in proximity to the occipital nerves.252 This report paved the way for the clinical application of ONS, which is now employed for several refractory painful conditions. Slavin and Burchiel subsequently performed the PNS approach for refractory pain in the trigeminal and occipital territories.253 Subsequently, other reports were published reporting the efficacy and different surgical techniques of this procedure in different syndromes.254–256
The basic principle of PNS is the vicinity of the placed electrodes to the area of referred pain. The characteristics of pain should dictate the appropriate placement of the electrode(s); as stated by Barolat,257 in case of mild allodynia, the implanted electrode or electrodes should be still better placed in the painful area; in cases of severe allodynia, the electrodes should be placed on each side of the allodynic region, so as to “circumvent” it. Barolat states that with this technique allodynic symptoms are likely to be significantly reduced.
The most frequent indications for PNS are axial lumbar pain (which is typically not well controlled by SCS), thoracic pain, shoulder pain, atypical facial pain, inguinal pain, and more extensively, several headache conditions. According to Barolat257 the preferred location of the implanted electrodes should be the superficial layer of the subcutaneous tissue, because a more superficial location could lead to lack of perceived paresthesias (considered to be essential, at least as predictive factors, for effective pain relief, also taking into account the “gate control” theory) and a deeper location could lead to undesired muscle contractions (in cases of proximity of the lead to the muscular fascia). The usual preoperative and noninvasive techniques employed before the operation are TENS and anesthetic peripheral nerve block; nonetheless, there is no consensus about the predictive values of such modalities with regard to subsequent PNS.
PNS is a relatively simple technique performed by tunneling a cylindrical electrode through the skin to the affected area of the body and connecting it to an internal pulse generator. The difficulty of the procedure depends on the amount of adipose tissue of the subject and on the extension of the painful area; furthermore, the complexity and the different dysesthetic features of given regions in a given patient can require careful preoperative planning, taking into account bony prominences and possible sites of skin erosion or lead decubitus; different trajectories can in fact be required to address these important operative issues.
Occipital Nerve Stimulation, Peripheral Trigeminal Stimulation, and Vagal Nerve Stimulation
ONS consists of stimulating the great occipital nerve (GON) by means of implanted paddle-type or wire-type electrodes that are positioned in the suboccipital region under the subcutaneous tissue and above the splenium fascial plane. One or two electrodes can be positioned according to the symptoms and to the patient’s and clinician’s preference. The configuration of such electrodes varies according to the different numbers of contacts and different distance between them. Bilateral implanting procedure implies the use of two symmetrical electrodes positioned about 1 cm below the inion and extending from 1 to 5 cm laterally to the midline, to ensure proper coverage of GONs, which emerge from the splenium muscle’s fascia at this level (Fig. 49.10). As for other PNS procedures, the implantation is completed after positioning of the IPGs in the subcutaneous tissue, more often in the infraclavicular or paraumbilical regions. In several centers a trial-stimulation period is performed before definitive implant of the system; during this period, the electrodes, which are sutured to the skin in a sterile fashion, are connected to external pulse generators. Patients reporting more than 50% decrease in pain intensity or painful attack intensity are considered “responders” and recommended for implantation of IPGs.
The main indications for performing ONS are migraine,258,259 transformed migraine,260 cluster headache,261,262 hemicrania continua,263 cervicogenic headaches,255 and occipital neuralgia,264 the latter being the most common so far. Slavin and colleagues264 reported on 14 consecutive patients with intractable occipital neuralgia treated with ONS. Ten patients were considered responders after the trial stimulation period and proceeded with system internalization. At the last clinical follow-up examinations, seven patients (70%) continued to benefit from the procedure and were able to reduce the daily intake of prophylactic drugs, whereas three patients had their hardware removed because of loss of beneficial effect or occurrence of infection.
Matharu258 reported results in eight patients submitted to ONS for chronic migraine, who all described the clinical outcome at least as “good,” with pain reduction ranging from 75% to 90%.
Popeney and Alo260 reported on 25 patients with transformed migraine implanted with C1 through C3 peripheral nerve stimulation. The evaluation was carried out using the migraine disability assessment (MIDAS); symptoms had been refractory to conventional treatments for at least 6 months in this series. Prior to stimulation, all patients experienced severe disability (scored as grade IV on the MIDAS). After stimulation, the average improvement in the MIDAS score was 88.7%, and all of the patients reported that their headaches were well controlled after the implantation procedure.
Rodrigo-Royo and associates255 reported on the results concerning four patients with drug-refractory, long-persistent, and severe pain in the occipital region, who were treated with electrical stimulation of the C1, C2, and C3 peripheral nerves. Results were considered good in all patients; the authors observed the disappearance of continuous pain in all patients and the improvement in intensity and frequency of pain bouts, with subsequent reduction or discontinuation of pharmacological therapy. Burns and colleagues263 reported on six patients suffering from hemicrania continua submitted for ONS. At long-term follow-up, four of the patients reported 80% to 95% improvement, one patient reported 30% improvement, and one patient reported worsening of symptoms.
Bartsch and Goadsby265 in 2003 reported that stimulation of nociceptive afferent C-fibers of the dura mater of the rat can lead to a sensitization of second-order cervical spinal neurons. The authors proposed that such a mechanism could be involved in the referral of pain from trigeminal to cervical structures in patients affected by CCH and chronic migraine. Piovesan in 2003266 highlighted the concept of “trigeminocervical complex,” thus hypothesizing the convergence of nociceptive information in trigeminal and cervical territories at the level of the neurons in the trigeminal nucleus caudalis, extending to the C2 spinal segment. Based on this study, Magis and co-workers261 reported on the results of eight patients with refractory CCH submitted to ONS. Two patients were pain-free after a follow-up period of 16 and 22 months; three patients reported 90% reduction in attack frequency, and two patients reported improvement of about 40%. Switching off the IPGs led to recurrence and increase of attack frequency in all the patients. The mean time elapse from implantation to the onset of beneficial effects was reported to be 2 months.
Burns263 reported 14 patients with drug-refractory CCH submitted to bilateral implantation of ONS electrodes; the median follow-up period was 17.5 months, and 10 out of 14 patients benefited from the procedure. Three patients reported improvement of 90% or better, three reported a moderate improvement (40% or better), and four reported 20% to 30% improvement. Most of the patients reported relapse of pain attacks when the device was switched off; interestingly in one patient the ONS system helped in aborting acute attacks.
Slavin267 reported on a case series of 30 patients submitted to a trial period of peripheral nerve stimulation for refractory craniofacial pain, of whom only 22 experienced more than 50% improvement in pain intensity and were subsequently implanted with the complete system. Three patients received infraorbital subcutaneous electrodes, four received supraorbital subcutaneous electrodes, 13 received occipital electrodes, one patient had a combination of infraorbital and occipital stimulation system, and one patient had a combination of supraorbital and occipital stimulation. Follow-up evaluations were carried on for a mean time period of 35 months. At the last clinical examination, the systems had been removed in five patients because of improvement in pain intensity, persistence of symptoms, and infection; 17 patients continued to use the PNS device; three of them had an improvement in pain of less than 50%, whereas 14 reported an improvement of 50% and were considered responders. Reed268 reported seven patients affected by refractory chronic migraine headaches who received a combined occipital nerve–supraorbital nerve neurostimulation system. In this study, the relative responses to the ONS system alone and to both ONS and supraorbital systems were evaluated. The follow-up period ranged from 1 to 35 months; all patients reported full therapeutic response only to combined supraorbital and occipital neurostimulation, whereas only a partial response was provided by the ONS system alone. The authors conclude that in patients affected by chronic migraine the clinical benefit may be better in those implanted with both systems.
Migraine is often comorbid with a large spectrum of diseases, including epilepsy, stroke, and above all, psychiatric disorders,269 especially major depression; depressive episodes can influence pain’s processing and perception and are associated with a poorer clinical outcome in cephalalgic patients. Given that vagal nerve stimulation (VNS), approved by the U.S. Food and Drug Administration as an adjunctive treatment for drug-refractory depressive disorder,270 has been shown to be effective in controlling episodic migraine,271,272 our group273 published preliminary data about the employment of VNS for refractory chronic daily headache (CDH) with comorbid depression.
In our case four patients were selected for implantation of this device; all of them had a clinical history of CDH lasting at least 2 years, were refractory to conservative treatments for both headache and major depression, and suffered from severe disability on activities of daily living. None of the patients had psychotic features, heart disease, or lung diseases that could contraindicate chronic stimulation of the left vagus nerve. The intensity of stimulation used ranged from 1 to 2.25 mA. Two of the patients showed a significant improvement for both headache and depression after 1 to 3 months from the start of stimulation. The remaining two patients showed limited or no clinical benefit after VNS. Another study by Mauskop274 reported improvement after VNS in two patients with CCH and in two patients with chronic migraine.
Systems of Spinal Intrathecal Release of Opioids
The discovery of opioid receptors at the spinal cord level paved the way for this procedure; in 1976 Yaksh and Rudy obtained a reduction of the hind-limb withdrawal reflex in rats exposed to algic stimuli and concomitantly submitted to subarachnoid administration of opioids.275 The analgesic effect of opioids at the spinal level is thought to be mediated by inhibitory interneurons that act to reduce the nociceptive transmission at higher levels.276 Intrathecal opioid (morphine chlorhydrate or hydromorphone) therapy is usually performed for pain of nociceptive type, whereas neuropathic pain is sometimes considered a secondary indication, both because of the efficacy of SCS and for the higher opioid concentrations required in this case. Nonetheless, there are reports about the possible efficacy of IT opioids in neuropathic pain.277 Other types of agents are available for this indication (even if still less studied and tested than morphine).
Classical indications for opioid IT therapy are failed back surgery syndrome, cancer pain, axial spinal pain, complex regional pain syndrome, arachnoiditis, poststroke pain, and spinal cord injury. Other prerequisites are the compliance of the patient and the possibility of performing regular clinical follow-up examinations, which are mandatory for the variation of the daily dosage.
Nonopioid drugs, as mentioned earlier, have been proposed, both alone or in combination with morphine, for IT therapy. They include clonidine, tizanidine, ziconotide, glutamate antagonists, benzodiazepines, tricyclic antidepressants, butamin, and nitric oxide synthetase inhibitors.221 Most of these drugs have been tested only on experimental models. Hydromorphone can be used in cases of tolerance to morphine, and IT clonidine can be added to IT opioids in cases of tolerance development or if unsatisfactory results are obtained with one drug alone; in particular, two early studies report the safety and efficacy of this combination in noncancer and in cancer pain.278,279 Baclofen and bupivacaine can be used as a third-line approach, too, alone or in combination to first-line agents.280
For morphine chlorhydrate, initial daily dosage is usually 2 to 3 mg, but it can be varied according to the patient’s needs and to the eventual onset of side effects (dosage spectrum is quite wide, ranging from 1 to about 60 mg/day). For clonidine, the recommended dosage range is 10 to 10,000 µg per day.281 In the current literature, the percentage of pain relief is reported to be 60% to 80% for malignant pain and 60% to 65% for nonmalignant pain.281
Franzini A., Messina G., Cordella R., et al. Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29(2):E13.
Jannetta P.J., McLaughlin M.R., Casey K.F. Technique of microvascular decompression. Technical note. Neurosurg Focus. 2005;18(5):E5.
Linderoth B.G., Meyerson B.A. Spinal cord stimulation: mechanisms of action. In: Burchiel K.J., editor. Surgical Management of Pain. New York: Thieme; 2002:505-526.
Sindou M. Microsurgical DREZotomy. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques. 3rd ed. Philadelphia: WB Saunders; 1995:1613-1622.
Tasker R.R. Percutaneous cordotomy. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques: Indications, Methods and Results. Orlando: Grune & Stratton; 1995:1595-1611.
Please go to expertconsult.com to view the complete list of references.
1. Fields H.L., editor. Cancer Pain. Core Curriculum for Professional Education in Pain. Seattle: IASP Press, 1995.
2. Arbit E. Management of Cancer Related Pain. Mt. Kisko, NY: Futura; 1993.
3. Patt R.B. Cancer Pain. Philadelphia: JB Lippincott; 1993.
4. Doyle D., Hanks G.W.C., MacDonald M., editors. Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press, 1995.
5. Basmajian J.V. Manipulation, Traction and Massage, 3rd ed, Baltimore: Williams & Wilkins, 1985.
6. Coffey R.J. Neurosurgical management of intractable pain. In Youmans J.R., editor: Neurological Surgery, 4th ed, Philadelphia: WB Saunders, 1996.
7. Loeser J.D. Introduction: ablative neurosurgical operations. In: Bonica J.J., editor. The Management of Pain. Philadelphia: Lea & Febiger; 1990:2040-2043.
8. Poletti C.E. Open cordotomy and medullary tractotomy. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques. 3rd ed. Philadelphia: WB Saunders; 1995:1557-1571.
9. Max M.B., Portenoy R.K., Laska E.M., editors. The Design of Analgesic Trials. New York: Raven Press, 1991.
10. Consensus Conference on the Neurosurgical Management of Pain. Meeting report. Neurosurgery. 1994;34:756-761.
11. Steel T.R., Burchiel K.J. Ablative neurosurgical techniques in the treatment of chronic pain: overview. In: Burchiel K.J., editor. Surgical Management of Pain. New York: Thieme; 2002:633-646.
12. Loeser J.D., Sweet W.H., Tew J.M., van Loveren H. Neurosurgical operation involving peripheral nerve. In: Bonica J.J., editor. The Management of Pain. Philadelphia: Lea & Febiger; 1990:2044-2066.
13. Pagni C.A. Central pain and painful anesthesia. In: Krayenbhul H., Maspes P.E., Sweet W.H., editors. Pain: Its Neurosurgical Management. Part II: Central Procedures. Basel: Karger; 1977:132-257.
14. Burchiel J.K., Johans T.J., Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78:714-719.
15. Sunderland S. Nerves and Nerve Injuries. Edinburgh: Churchill Livingstone; 1972. 486-503
16. Marshall K.A. Managing cancer pain: basic principles and invasive treatment. Mayo Clin Proc. 1996;71:472-477.
17. Doubloon D. Root surgery. In: Wall P.D., Melzack R., editors. Textbook of Pain. 3rd ed. Edinburgh: Churchill Livingstone; 1994:1055-1065.
18. Uematsu S., Udvarhelyi G.B., Benson D.W., Siebens A.A. Percutaneous radiofrequency rhizotomy. Surg Neurol. 1974;2:319-325.
19. Sindou M., Fisher G., Goutelle A., Allegre G.E. Microsurgical selective posterior rhizotomy. Pain. 1981;1(Suppl):354.
20. Stolker R.J., Vervest A.C.M., Groen J.T. The treatment of chronic thoracic segmental pain by radiofrequency percutaneous partial rhizotomy. J Neurosurg. 1994;80:986-992.
21. Crue B.L., Todd E.M. A simplified technique of sacral rhizotomy for pelvic pain. J Neurosurg. 1964;21:835-837.
22. Loeser J.D. Dorsal rhizotomy for relief of chronic pain. J Neurosurg. 1972;36:745-750.
23. Onofrio B.M., Campa H.K. Evaluation of rhizotomy: review of 12 years experience. J Neurosurg. 1972;36:751-755.
24. Pagni C.A., Lanotte M., Canavero S. How frequent is anesthesia dolorosa following spinal posterior rhizotomy? A retrospective analysis of fifteen patients. Pain. 1993;54:323-327.
25. Scoville W.B. Extradural spinal sensory rhizotomy. J Neurosurg. 1966;25:94-95.
26. Coggleshell R.E., Applebaum M.L., Fazen M., et al. Un-myelinated axons in human ventral roots, a possible explanation for the failure of dorsal rhizotomy to relieve pain. Brain. 1975;98:157-166.
27. Coggleshell R.E. Afferent fibers in the ventral root. Neurosurgery. 1979;4:443-448.
28. Hosobuchi Y. The majority of un-myelinated afferent axons in human ventral roots probably conduct pain. Pain. 1980;8:167-180.
29. Sindou M. Microsurgical DREZotomy. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques. 3rd ed. Philadelphia: WB Saunders; 1995:1613-1622.
30. Rand R. Further observations on Lissauer’s tractolysis. Neurochirurgica. 1960;3:151-168.
31. Denny-Brown D., Kirk E.J., Yanagisawa N. The tract of Lissauer in relation to sensory transmission in the dorsal horn of spinal cord in the macaque monkey. J Comp Neurol. 1973;151:175-200.
32. Loeser J.D., Ward A.A.Jr., White L.E.Jr. Chronic deafferentation of human spinal cord neurons. J Neurosurg. 1968;29:48-50.
33. Jeanmonod D., Sindou M., Magnin M., Baudet M. Intraoperative unit recordings in the human dorsal horn with a simplified floating microelectrode. Electroencephalogr Clin Neurophysiol. 1989;72:450-454.
34. Loeser J.D., Ward A.A.Jr., White L.E.Jr. Some effects of deafferentation of neurons. J Neurosurg. 1967;17:629-636.
35. Albe-Fessard D., Lombard M.C. Use of an animal model to evaluate the origin of a protection against deafferentation pain. Bonica J.J., Lindblom U., Iggo A., et al, editors. Pain Research and Therapy. Vol. 5. New York: Raven Press; 1983:691-700.
36. Sindou M. Etude de la Jonction Radiculo-medullairePosterieure: La Radicellotomie Posterieure Selective dans la Chirurgie de la Douleur (thesis). Lyons, France: University Claude-Bernard; 1972.
37. Nashold B.S., Urban B., Zorub D.S. Phantom pain relief by focal destruction of substantia gelatinosa of Rolando. Bonica J.J., Albe-Fessard D., editors. Advances in Pain Research and Therapy. Vol. 1. New York: Raven Press; 1976:959-963.
38. Nashold B.S., Ostdahl P.H. Dorsal root entry zone lesions for pain relief. J Neurosurg. 1979;51:59-69.
39. Levy W.J., Nutkiewicz A., Ditmore M., Watts C. Laser induced dorsal root entry zone lesions for pain control: report of three cases. J Neurosurg. 1983;59:884-886.
40. Powers S.K., Adams J.E., Edwards S.B., et al. Pain relief from dorsal root entry zone lesions made with argon and carbon dioxide microsurgical lasers. J Neurosurg. 1984;61:841-847.
41. Kandel E.I., Ogleznev K.J.A., Dreval O.N. Destruction of posterior root entry zone as a method for treating chronic pain in traumatic injury to the brachial plexus. Vopr Neurochir. 1987;6:20-27.
42. Dreval O.N. Ultrasonic DREZ-operations for treatment of pain due to brachial plexus avulsion. Acta Neurochir (Wien). 1993;122:76-81.
43. Jeanmonod D., Sindou M. Somatosensory function following dorsal root entry zone lesions in patients with neurogenic pain or spasticity. J Neurosurg. 1991;74:916-932.
44. Jeanmonod D., Sindou M., Mauguiere F. The human cervical and lumbo-sacral evoked electrospinogram: data from intra-operative spinal cord surface recordings. Electroencephalogr Clin Neurophysiol. 1991;80:477-479.
45. Turano G., Sindou M., Mauguiere F. SCEP monitoring during spinal surgery for pain and spasticity. In: Dimitrijevic M.R., Halter J.A., editors. Atlas of Human Spinal Cord Evoked Potentials. Boston: Butterworth Heinemann; 1995:1-180.
46. Sindou M., Turano G., Pantieri R., et al. Intraoperative monitoring of spinal cord SEPs, during microsurgical DREZotomy (MDT) for pain, spasticity and hyperactive bladder. Stereotact Func Neurosurg. 1994;62:164-170.
47. Guenot M., Hupe J.M., Mertens P., et al. Microelectrode recordings during microsurgical DREZotomy. Stereotact Func Neurosurg. 1996-1997;67:1-2. 56 (abstract 210)
48. Mertens P., Ghaemmaghami C., Perret-Liaudet A., et al. in vivo amino-acids concentrations in human dorsal horn studied by microdialysis during DREZotomy: methodology and preliminary results. Stereotact Func Neurosurg. 1996-1997;67:1-2. 58 (abstract 213)
49. Sindou M., Lapras C. Neurosurgical treatment of pain in the Pancoast-Tobias syndrome: selective posterior rhizotomy and open antero-lateral C2-cordotomy. Bonnica J.J., Ventafrida V., Pagni C.A., editors. Advances in Pain Research and Therapy. Vol. 4. New York: Raven Press; 1982:199-209.
50. Pagni C.A. Central pain and painful anesthesia: pathophysiology and treatment of sensory deprivation syndromes due to central and peripheral nervous system lesions. Prog Neurol Surg. 1976;8:132-257.
51. Nashold J.R.B., Nashold B.S. Microsurgical DREZotomy in treatment of deafferentation pain. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques. 3rd ed. Philadelphia: WB Saunders; 1995:1623-1636.
52. Gorecki J.P., Burt T., Wee A. Relief from chronic pelvic pain through surgical lesion of the conus medullaris dorsal root entry zone. Stereotact Func Neurosurg. 1992;59:69-75.
53. Campbell J.N., Solomon C.T., James C.S. The Hopkins experience with lesions of the dorsal horn (Nashold’s operation) for pain for avulsion of the brachial plexus. Appl Neurophysiol. 1988;51:198-205.
54. Freidman A.H., Bullit E. Dorsal root entry zone lesions in the treatment of pain following brachial plexus avulsion, spinal cord injury and herpes zoster. Appl Neurophysiol. 1988;51:170-174.
55. Sampson J.H., Cashman R.E., Nashold B.S., Friedman A.H. Dorsal root entry zone lesions for intractable pain after trauma to the conus medullaris and cauda equina. J Neurosurg. 1995;82:28-34.
56. Sindou M. Dorsal root entry zone lesions. In: Burchiel K.J., editor. Surgical Management of Pain. New York: Thieme; 2002:701-713.
57. Friedman A.H., Nashold B.S. Dorsal root entry zone lesions for the treatment of postherpetic neuralgia. Neurosurgery. 1984;15:969-970.
58. Sindou M., Fisher G., Goutelle A., Mansuy L. La radicellotimie posterieure selective:premiers resultats dans la chirurgie de la douleur. Neurochirurgie. 1974;20:391-408.
59. Sindou M., Fisher G., Goutelle A., et al. La radicellotimie posterieure selective dans la traitment des spasticites. Rev Neurol. 1974;130:201-215.
60. Sindou M., Millet M.F., Mortamais J., Eyssette M. Results of selective posterior rhizotomy in the treatment of painful and spastic paraplegia secondary to multiple slerosis. Appl Neurophysiol. 1982;45:335-340.
61. Sindou M., Mifsud J.J., Boisson D., Goutelle A. Selective posterior rhizotomy in the dorsal root entry zone for treatment of hyperspasticity and pain in the hemiplegic upper limb. Neurosurgery. 1986;18:587-595.
62. Sindou M., Jeanmonod D., Mertens P. Microsurgical DREZotomy for the treatment of spasticity and pain in the lower limbs. Neurosurgery. 1989;24:655-670.
63. Bernard E.J., Nashold B.S., Caputi F., et al. Nucleus caudalis DREZ lesions for facial pain. Br J Neurosurg. 1987;1:81.
64. Wilkinson H.A. Sympathectomy for pain. In: Youmans, editor. Neurological Surgery. 4th ed. Philadelphia: WB Saunders; 1996:3489-3499.
65. Dohn D.F., Sava G.M. Sympathectomy for vascular syndromes and hyperhidrosis of upper extremities. Clin Neurosurg. 1978;25:637-650.
66. Gybels J.M., Sweet W.H. Neurosurgical Treatment of Persistent Pain. New York: Karger; 1984. 1045-1061
67. Hardy R.W. Surgery of the sympathetic nervous system. Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques: Indications, Methods and Results. Vol. 2. New York: Grune & Stratton; 1982:1045-1061.
68. Bonica J.J. Causalgia and other reflex sympathetic dystrophies. In: Bonica J.J., editor. The Management of Pain. Philadelphia: Lea & Febiger, 1990.
69. Sternschein M.J., Mayers S.J., Frewin D.B., et al. Causalgia. Arch Phys Med Rehabil. 1975;56:58-63.
70. Schott G.D. An unsympathetic view of pain. Lancet. 1995;345:634-636.
71. Ochoa J.L. Reflex? Sympathetic? Dystrophy triple questioned again. Mayo Clin Proc. 1995;70:1124-1125.
72. Dawson D.M., Katz M. Reflex sympathetic dystrophy. Neurol Chron. 1993;8:1-6.
73. Schwartzman R.J., McLellan T.L. Reflex sympathetic dystrophy: a review. Arch Neurol. 1987;44:555-561.
74. White J.C., Sweet W.H. Pain: Its Mechanism and Neurosurgical Control. Springfield, IL: Charles C Thomas; 1955.
75. Kao M.C. Video endoscopic sympathectomy using a fiberoptic CO2 laser to treat palmar hyperhidrosis. Neurosurgery. 1992;30:131-135.
76. Lee K.H., Hwang P.Y.K. Video endoscopic sympathectomy for palmar hyperhidrosis. J Neurosurg. 1996;84:484-486.
77. Wilkinson H.A. Percutaneous radiofrequency upper thoracic sympathectomy: a new technique. Neurosurgery. 1984;15:811-814.
78. Wilkinson H.A. Percutaneous radiofrequency upper thoracic sympathectomy. J Neurosurg. 1996;38:715-725.
79. Robertson D.P., Simpson R.K., Rose J.E., Garza J.S. Video-assisted endoscopic thoracic ganglionectomy. J Neurosurg. 1993;79:238-240.
80. White J.C., Silverstone B. Pain and related phenomena including causalgia. In: Woodhall B., Beebe G.W., editors. Peripheral Nerve Regeneration. Medical Monograph. Washington, DC: U.S. Government Printing Office; 1956:311-347.
81. Hodge C.J.Jr., Apkarian A.V. The spinothalamic tract. Crit Rev Neurobiol. 1990;5:363-397.
82. Willis W.D.Jr. The Pain System. Basel: Karger; 1985.
83. Brown-Sequard C.E. De la transmission des impressions sensitives dans la moelle epiniere. C R Hebd Seanc Acad Sci Paris. 1850;1:700.
84. Brown-Sequard C.E. Recherches cliniques et experimentales sur les entrecroisements des conducteurs servant aux mouvements voluntaires. Arch Physiol Norm Pathol. 1860;21:219.
85. Brown-Sequard C.E. Physiology and pathology of the nervous system; and on the treatment of organic nervous affections. Lancet. 1868;2:659-662.
86. Brown-Sequard C.E. Physiology and pathology of the nervous system; and on the treatment of organic nervous affections. Lecture 1. On spinal hemiplegia. Lancet. 1868;2:593-596.
87. Gowers W.R. A case of unilateral gunshot injury to the spinal cord. Trans Clin Soc Lond. 1878;11:24-32.
88. Spiller W.G. The location within the spinal cord of the fibers for temperature and pain sensations. J Nerv Ment Dis. 1905;32:318-320.
89. Spiller W.G., Martin E. The treatment of persistent pain of organic origin in the lower part of the body by division of the anterolateral column of the spinal cord. JAMA. 1912;58:1489-1490.
90. Brodal A. Neurological Anatomy. New York: Oxford University Press; 1969. 1
91. Kerr F.W. The ventral spinothalamic tract and other ascending systems of the ventral funiculus of the spinal cord. J Comp Neurol. 1975;159:335-356.
92. Kevetter G.A., Willis W.D. Collateralization in the spinothalamic tract: new methodology to support or deny phylogenetic theories. Brain Res Rev. 1984;7:1-4.
93. Willis W.D. The origin and destination of pathways involved in pain transmission. In: Wall P.D., Melzack R., editors. Textbook of Pain. New York: Churchill Livingstone; 1984:88-99.
94. White J.C., Sweet W.H. Pain and the Neurosurgeon: A Forty-Year Experience. Springfield, IL: Charles C Thomas; 1969. 1
95. Iseki H., Amano K., Kawamura H., et al. Somatotopic arrangement of lateral spinothalamic tract in percutaneous cervical cordotomy. Appl Neurophysiol. 1982;45:484-491.
96. Kanpolat Y., Caglar S., Akyar S., Temiz C. CT-guided pain procedures for intractable pain in malignancy. Acta Neurochir. 1998;64:88-91.
97. Kanpolat Y., Savas A., Caglar S., et al. Computerized tomography—guided percutaneous bilateral selective cordotomy. Neurosurg Focus. 1997;2:1-5.
98. Poletti C.E. Open cordotomy and medullary tractotomy. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques: Indications, Methods and Results. Orlando, FL: Grune & Stratton; 1988:1557-1571.
99. Mullan S., Harper P.V., Hekmatpanah J., et al. Percutaneous interruption of spinal-pain tracts by means of strontium-90 needle. J Neurosurg. 1963;20:931-939.
100. Tasker R.R. Percutaneous cordotomy: the lateral high cervical technique. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques: Indications, Methods and Results. Orlando, FL: Grune & Stratton; 1988:1191-1205.
101. Tasker R.R. Percutaneous cordotomy. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques: Indications, Methods and Results. Orlando, FL: Grune & Stratton; 1995:1595-1611.
102. Kanpolat Y., Deda H., Akyar S., Bilgic S. CT-guided percutaneous cordotomy. Acta Neurochiur Suppl (Wien). 1989;46:67-68.
103. Mayer D.J., Price D.D., Becker D.P., Young H.F. Threshold for pain from anterolateral quadrant stimulation as a predictor of success of percutaneous cordotomy for relief of pain. J Neurosurg. 1975;43:445-447.
104. Rosomoff Hl, Carroll F., Brown J., Sheptak P. Percutaneous radiofrequency cervical cordotomy: technique. J Neurosurg. 1965;23:639-644.
105. Sprangler W.J., Cosgrove G.R., Ballantine H.T., et al. Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease. Neurosurgery. 1996;38:1071-1076.
106. Hassenbusch S.J., Pillay P.K., Barnett G.H. Radiofrequency cingulotomy for intractable cancer pain using stereotaxis guided by magnetic resonance imaging. Neurosurgery. 1990;27:220-223.
107. Davis K.D., Hutchison W.D., Lozano A.M., Dostrovsky J.O. Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain. 1994;59:189-199.
108. Pillay P.K., Hassenbusch S.J. Bilateral MRI-guided stereotactic cingulotomy for intractable cancer pain. Stereotact Funct Neurosurg. 1992;59:33-38.
109. Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279-306.
110. Horsley V.A., Schafer E.A. A record of experiments upon the functions of the cerebral cortex. Philos Trans R Soc Lond. 1988;179B:1-45.
111. Vaccarino A.L., Melzack R. Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain. 1989;39:213-219.
112. Sikes R.W., Vogt B.A. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992;68:1720-1732.
113. Casey K.L., Minoshima S., Berger K.L., et al. Positron emission tomographic analysis of cerebral structures activated by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802-807.
114. Jones A.K.P., Brown W.D., Friston K.J., et al. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc R Soc Lond [Biol]. 1991;244:39-44.
115. Talbot J.D., Marrett S., Evans A.C., et al. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355-1358.
116. Wong E.T., Gunes S., Gaughan E., et al. Palliation of intractable cancer pain by MRI-guided cingulotomy. Clin J Pain. 1997;13:260-263.
117. Walker A.E. Relief of pain by mesencephalic tractotomy. Arch Neurol Psychiatry. 1942;48:865-883.
118. Drake C.G., McKenzie K.G. Mesencephalic tractotomy for pain. Experience with six cases. J Neurosurg. 1953;10:457-462.
119. Spiegel E.A., Wycis H.T. Mesencephalotomy in treatment of intractable facial pain. AMA Arch Neurol Psychiatry. 1953;69:1-13.
120. Shieff C., Nashold B.S.J. Stereotactic mesencephalotomy. Neurosurg Clin North Am. 1990;1:825-839.
121. Shieff C., Nashold B.S.J. Stereotactic mesencephalic tractotomy for the relief of thalamic pain. Br J Neurosurg. 1987;1:305-310.
122. Gybels J.M., Sweet W.H. Neurosurgical Treatment of Persistent Pain. Pain and Headache. Vol. 11. Basel: Karger; 1989.
123. Lewy F.H. The first authentic case of major trigeminal neuralgia and some comments on the history of this disease. Ann Med Hist. 1938;10:247-250.
124. Fothergill J. Of a painful affection of the face. Medical observations and inquires by a society of physicians. Med Observ Inquir. 1773;5:129-142.
125. André N. Observations Pratiques sur les Maladies de l’Urethre, et sur Plusieurs Faits Convulsifs, & la Guérison de Plusieurs Maladies Chirurgicales Avec la Décomposition d’un Remède Propre à Réprimer la Dissolution Gangréneuse & Cancéreuse, & à la Réparer; Avec des Principes qui Pourront Servir à Employer les Différens Caustiques. Paris: Delaguette; 1756.
126. Brown J.A., Coursaget C., Preul M.C., et al. Mercury water and cauterizing stones: Nicolas André and tic douloureux. J Neurosurg. 1999;90:977-981.
127. Stookey B., Ransohoff J. Trigeminal Neuralgia: Its History and Treatment. Springfield, IL: Charles C Thomas; 1959.
128. Fields W.S., Lemak N.A. Trigeminal neuralgia: historical background, etiology, and treatment. Barrow Neurol Inst Q. 1987;3(2):47-56.
129. Eller J.L., Raslan A.M., Burchiel K.T. Trigeminal neuralgia: definition and classification. Neurosurg Focus. 2005;18(5):E3.
130. Elias W.J., Burchiel K.J. Trigeminal neuralgia and other craniofacial pain syndromes: an overview. Semin Neurosurg. 2004:59-69.
131. Fromm G.H., Sessle B.J. Trigeminal Neuralgia: Current Concepts Regarding Pathogenesis and Treatment. Boston: Butterworth- Heinemann; 1991.
132. Haines S.J., Jannetta P.J., Zorub D.S. Microvascular relations of the trigeminal nerve. An anatomical study with clinical correlation. J Neurosurg. 1980;52:381-386.
133. Hamlyn P.J., King T.T. Neurovascular compression in trigeminal neuralgia: a clinical and anatomical study. J Neurosurg. 1992;76:948-954.
134. Jannetta P.J. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(Suppl):159-162.
135. Zakrzewska J.M. Trigeminal Neuralgia. London: WB Saunders; 1995. 19
136. Wilkins R.H. Historical perspectives. In: Rovit R.L., Murali R., Jannetta P.J., editors. Trigeminal Neuralgia. Baltimore: Williams & Wilkins; 1990:1-25.
137. Rushton J.G. Trigeminal neuralgia: one hundred fifty years of nonsurgical treatment. Minn Med. 1957;40:702-706.
138. Adson A.W. The surgical treatment of glossopharyngeal neuralgia. Arch Neurol. 1924;12:487-506.
139. Weinstein R.E., Herec D., Freidman J.H. Hypotension due to glossopharyngeal neuralgia. Arch Neurol. 1986;43:90-92.
140. Ferrante L., Artico M., Nardacci B., et al. Glossopharyngeal neuralgia with cardiac syncope. Neurosurgery. 1995;36:58-63.
141. Youmans J.R., editor. Neurological Surgery, 2nd ed, Philadelphia: WB Saunders, 1982.
142. Bruyn G.W. Glossopharyngeal neuralgia. Handbook Clin Neurol. 1986;4:459-473.
143. Hartel F. Die Leitungsanasthesie und Injektionsbehandlung des ganglion Gasseri und der trigeminusstamme. Arch Klin Chir. 1912;100:193-292.
144. Hartel F. Ueber die intracranielle Injektionsbehandlung der Trigeminusneuralgie. Med Klin. 1914;10:582-584.
145. Harris W. An analysis of 1433 cases of paroxysmal trigeminal neuralgia (trigeminal-tic) and the end-results of gasserian alcohol injection. Brain. 1940;63:209-224.
146. Hakanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981;9:638-646.
147. Brown J.A., Chittum C.J., Sabol D., Gouda J.J. Percutaneous balloon compression of the trigeminal nerve for treatment of trigeminal neuralgia. Neurosurg Focus. 1996;1(2):1-8. (Article 4)
148. Lunsford L.D. Trigeminal neuralgia: treatment by glycerol rhizotomy. In: Wilkins R.H., Rengachary S.S., editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996:3953-3959.
149. Sweet R.H. Trigeminal neuralgia: problems as to cause and consequent conclusions regarding treatment. In: Wilkins R.H., Rengachary S.S., editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996:3931-3943.
150. Taha J.M., Tew J.M. Comparison of surgical treatments for trigeminal neuralgia: reevaluation of radiofrequency rhizotomy. Neurosurgery. 38, 1996. 865–671
151. Rethi A. Die elektrolytische Behandlung der Trigeminusneuralgien. Munch Med Wochensch. 1913;60:295-296.
152. Kirschner M. Die Punktionstechnik und die Elektrokoagulation des Ganglion Gasseri: uber “gezielte” Operationen. Arch Klin Chir. 1933;176:581-620.
153. Sweet W.H., Wespic J.C. Controlled thermocoagulation of trigeminal ganglion and rootlets for differential destruction of pain fibers. Part 1: trigeminal neuralgia. J Neurosurg. 1974;40:143-156.
154. Gybels J.M., Sweet W.H. Neurosurgical Treatment of Persistent Pain: Physiological and Pathological Mechanisms of Human Pain. Basel: Karger; 1989.
155. Letcher F.S., Goldring S. The effect of radiofrequency current and heat on peripheral nerve action potential in the cat. J Neurosurg. 1968;29:42-47.
156. Brodkey J.S., Miyazaki Y., Ervin F.R., Mark V.H. Reversible heat lesions with radiofrequency current. J Neurosurg. 1964;21:49-53.
157. Frigyesi T., Siegfried J., Broggi G. The selective vulnerability of evoked potentials in the trigeminal sensory root to graded thermocoagulation. Exp Neurol. 1975;49:11-21.
158. Tew J.M.Jr., Taha J.M. Percutaneous rhizotomy in the treatment of intractable facial pain (trigeminal, glossopharyngeal, and vagus nerves). In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques. Philadelphia: WB Saunders; 1996:3386-3403.
159. Nugent R. Radiofrequency treatment of trigeminal neuralgia using a cordotomy-type electrode: a method. Neurosurg Clin North Am. 1997;8:41-52.
160. Brown J., Gouda J. Percutaneous balloon compression of the trigeminal nerve. Neurosurg Clin North Am. 1997;8:53-62.
161. Broggi G., Franzini A., Lasio G., et al. Long term results of percutaneous retrogasserian thermorhizotomy for “essential” trigeminal neuralgia: considerations in 1000 consecutive patients. Neurosurgery. 1990;27(5):783-787.
162. Taha J.M., Tew J.M.Jr., Bruncher C.R. A prospective 15-year follow-up of 154 consecutive patients with trigeminal neuralgia treated by percutaneous stereotactic radiofrequency thermal rhizotomy. J Neurosurg. 1995;83:989-993.
163. Menzel J., Piotrowski W., Penzholz H. Long-term results of gasserian ganglion electrocoagulation. J Neurosurg. 1975;42:140-143.
164. Nugent G.R. Trigeminal neuralgia: treatment by percutaneous electrocoagulation. In: Wilkins R.H., Rengachary S.S., editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996:3945-3951.
165. Sweet W.H. The treatment of trigeminal neuralgia (tic douloureux). N Engl J Med. 1986;315:174-177.
166. Broggi G., Siegfried J. Percutaneous differential radiofrequency rhizotomy of glossopharyngeal nerve in facial pain due to cancer. Bonica J.J., editor. Advances in Pain Research and Therapy. Vol. 2. New York: Raven Press; 1979:469-473.
167. Shelden C.H., Pudenz R.H., Freshwater D.B., Crue B.L. Compression rather than decompression for trigeminal neuralgia. J Neurosurg. 1955;12:123-126.
168. Shelden CH. Compression procedure for trigeminal neuralgia: review and clinical appraisal of ten years’ experience. Presented at 26th Annual Meeting of the American Academy of Neurological Surgery. Key Biscayne, FL: 1964.
169. Mullan S., Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59:1007-1012.
170. Lichtor T., Mullan J.F. A 10-years follow-up review of percutaneous microcompression of the trigeminal ganglion. J Neurosurg. 1990;72:49-54.
171. Brown J.A., Preul M.C. Percutaneous trigeminal ganglion compression for trigeminal neuralgia: experience in 22 cases: patients and review of the literature. J Neurosurg. 1989;70:900-904.
172. Dominguez J., Lobato R.D., Rivas J.J., et al. Changes in systolic blood pressure and cardiac rhythm induced by therapeutic compression of the trigeminal ganglion. Neurosurgery. 1994;34:422-427.
173. Dandy W.E. Concerning the cause of trigeminal neuralgia. Am J Surg. 1934;24:447-455.
174. Gardner W.J., Miklos M.V. Response of trigeminal neuralgia to decompression of sensory root; discussion of cause of trigeminal neuralgia. JAMA. 1959;170(15):1773-1776.
175. Gardner W.J. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg. 1963;19:947-958.
176. Jannetta P.J. Trigeminal neuralgia and hemifacial spasm—etiology and definitive treatment. Trans Am Neurol Assoc. 1975;100:89-91.
177. Jannetta P.J. Microsurgical approach to the trigeminal nerve for tic douloureux. Prog Neurol Surg. 1976;7:180-200.
178. Jannetta P.J., McLaughlin M.R., Casey K.F. Technique of microvascular decompression. Technical note. Neurosurg Focus. 2005;18(5):E5.
179. Chun-Cheng Q., Qing-Shi Z., Ji-Qing Z., Zhi-Gang W. A single-blinded pilot study assessing neurovascular contact by using high-resolution MR imaging in patients with trigeminal neuralgia. Eur J Radiol. 2009;69(3):459-463.
180. Jannetta P.J. Trigeminal and glossopharyngeal neuralgia. Curr Diagn. 1971;3:849-850.
181. Allende R., Teja S., Alleyne C.H.Jr. Microvascular decompression for trigeminal neuralgia. Neurology. 2001;57:1093.
182. Barker F.G., Jannetta P.J., Bissonnette D.J., et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077-1084.
183. Hitotsumatsu T., Matsushima T., Inoue T. Microvascular decompression for treatment of trigeminal neuralgia, hemifacial spasm, and glossophyaryngeal neuralgia: three surgical approach variations: technical note. Neurosurgery. 2003;53:1436-1443.
184. Zhang K.W., Zhao Y.H., Shun Z.T., et al. Microvascular decompression by retrosigmoid approach for trigeminal neuralgia: experience in 200 patients. Ann Otol Rhinol Laryngol. 1990;99:129-130.
185. Jannetta P.J., Bissonnette D.J. Management of the failed patient with trigeminal neuralgia. Clin Neurosurg. 1985;32:334-347.
186. Klun B. Microvascular decompression and partial sensory rhizotomy in the treatment of trigeminal neuralgia: personal experience with 220 patients. Neurosurgery. 1992;30:49-52.
187. Rath S.A., Klein H.J., Richter H.P. Findings and long-term results of subsequent operations after failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1996;39:933-940.
188. Szapiro J.Jr., Sindou M., Szapiro J. Prognostic factors in microsurgical decompression for trigeminal neuralgia. Neurosurgery. 1985;17:920-929.
189. Yamaki T., Hashi K., Niwa J., et al. Results of reoperation for failed microvascular decompression. Acta Neurochir (Wien). 1992;115:1-7.
190. Apfelbaum R.I. Surgery for tic douloureux. Clin Neurosurg. 1983;31:351-368.
191. Liao J., Cheng W., Chang C., et al. Reoperation for recurrent trigeminal neuralgia after microvascular decompression. Surg Neurol. 1997;47:562-570.
192. Jannetta P.J. Microsurgical management of trigeminal neuralgia. Arch Neurol. 1985;42:800.
193. Sindou M., Amrani F., Mertens P. Microsurgical vascular decompression in trigeminal neuralgia. Comparison of two technical modalities and physiopathologic deductions. A study of 120 cases [in French]. Neurochirurgie. 1990;36:16-26.
194. Ammar A., Lagenaur C., Jannetta P.J. Neural tissue compatibility of Teflon as an implant material for microvascular decompression. Neurosurg Rev. 1990;13:299-303.
195. McLaughlin M.R., Jannetta P.J., Clyde B.L., et al. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90:1-8.
196. Ma Z., Li M., Cao Y., Chen X. Keyhole microsurgery for trigeminal neuralgia, hemifacial spasm and glossopharyngeal neuralgia. Eur Arch Otorhinolaryngol. 2010;267:449-454.
197. Cho D.Y., Chang C.G.S., Wang Y.C., et al. Repeat operations in failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1994;35:665-670.
198. Megerian C.A., Busaba N.Y., McKenna M.J., Ojemann R.G. Teflon granuloma presenting as an enlarging, gadolinium enhancing, posterior fossa mass with progressive hearing loss following microvascular decompression. Am J Otol. 1995;16:783-786.
199. Vitali A.M., Sayer F.T., Honey C.R. Recurrent trigeminal neuralgia secondary to Teflon felt. Acta Neurochir (Wien). 2007;149:719-722.
200. Sindou M., Mertens P., Amrani F. Does microsurgical vascular decompression for trigeminal neuralgia work through a neo-compressive mechanism? Anatomical-surgical evidence for a decompressive effect. Acta Neurochir Suppl (Wien). 1991;52:127-129.
201. Sindou M., Leston J.M., Decullier E., Chapuis F. Microvascular decompression for trigeminal neuralgia: the importance of a noncompressive technique—Kaplan-Meier analysis in a consecutive series of 330 patients. Neurosurgery. 2008;63(4 Suppl 2):341-351.
202. Keller J.T., van Loveren H. Pathophysiology of the pain of trigeminal neuralgia and atypical facial pain: a neuroanatomical perspective. Clin Neurosurg. 1985;32:275-293.
203. Burchiel K.J., Clarke H., Haglund M., et al. Long-term efficacy of microvascular decompression in trigeminal neuralgia. J Neurosurg. 1988;69:35-38.
204. Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques, 2nd ed, Philadelphia: WB Saunders, 1988.
205. Ferroli P., Fioravanti A., Schiariti M., et al. Microvascular decompression for glossopharyngeal neuralgia: a long-term retrospectic review of the Milan-Bologna experience in 31 consecutive cases. Acta Neurochir. 2009;151:1245-1250.
206. Sampson J.H., Grossi P.M., Asaoka K., et al. Microvascular decompression for glossopharyngeal neuralgia: long-term effectiveness and complication avoidance. Neurosurgery. 2004;54:884-890.
207. Dandy W.E. The treatment of trigeminal neuralgia by the cerebellar route. Ann Surg. 1932;96:787-795.
208. Bederson J.B., Wilson C.B. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71:359-367.
209. Pool J.L. Psychosurgery in older people. J Am Geriatr Soc. 1954;2:456-466.
210. Pool J., Clark W., Hudson P., Lombardo M. Hypothalamic-Hypophyseal Interrelationships. Springfield, IL: Charles C Thomas; 1956.
211. Heath R., Mickle W. Evaluation of 7 years’ experience with depth electrode studies in human patients. In: Ramey E., O’Doherty D., editors. Electrical Studies in Unanesthetized Brain. New York: Harper & Brothers; 1960:214-217.
212. Mazars G., Roger R., Mazars Y. Results of the stimulation of the spinothalamic fascicles and their bearing on the physiopathology of pain. Rev Prat. 1960;103:136-138.
213. Hosobuchi Y., Adams J.E., Linchitz R. Pain relief by electrical stimulation of the central grey matter in humans and its reversal by naloxone. Science. 1977;197:183-186.
214. Richardson D.E., Akil H. Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47:178-183.
215. Richardson D.E., Akil H. Pain reduction by electrical brain stimulation in man. Part 2: chronic self-administration in the periventricular grey matter. J Neurosurg. 1977;47:184-194.
216. Mazars C. Intermittent stimulation of nucleus ventralis posteromedialis for intractable pain. Surg Neurol. 1975;4:93-95.
217. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971-978.
218. Fields H.L., Adams J.E. Pain after cortical injury relieved by electrical stimulation of the internal capsule. Brain. 1974;97:169-178.
219. Andy O.J. Parafascicular-center median nuclei stimulation for intractable pain and dyskinesia (painful-dyskinesia). Appl Neurophysiol. 1980;43:133-134.
220. Nandi D., Aziz T.Z. Deep brain stimulation in the management of neuropathic pain and multiple sclerosis tremor. J Clin Neurophysiol. 2004;21:31-39.
221. Raslan A.M., McCartney S., Burchiel K.J. Management of chronic severe pain: cerebral neuromodulatory and neuroablative approaches. Acta Neurochir Suppl. 2007;97(Pt 2):17-26.
222. May A., Bahra A., Büchel C., et al. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275-278.
223. Bartsch T., Pinsker M.O., Rasche D., et al. Hypothalamic deep brain stimulation for cluster headache: experience from a new multicase series. Cephalalgia. 2008;28(3):285-295.
224. Brittain J.S., Green A.L., Jenkinson N., et al. Local field potentials reveal a distinctive neural signature of cluster headache in the hypothalamus. Cephalalgia. 2009;29(11):1165-1173.
225. D’Andrea G., Nordera G.P., Piacentino M. Effectiveness of hypothalamic stimulation in two patients affected by intractable chronic cluster headache. Neurology. 2006;66(Suppl 2):A140.
226. Fontaine D., Lazorthes Y., Mertens P., et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11(1):23-31.
227. Leone M., Franzini A., Broggi G., et al. Lessons from 8 years’ experience of hypothalamic stimulation in cluster headache. Cephalalgia. 2008;28(7):787-797.
228. Schoenen J., Di Clemente L., Vandenheede M., et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128(Pt 4):940-947.
229. Sillay K.A., Sani S., Starr P.A. Deep brain stimulation for medically intractable cluster headache. Neurobiol Dis. 2010;38(3):361-368.
230. Franzini A., Leone M., Messina G., et al. Neuromodulation in treatment of refractory headaches. Neurol Sci. 2008;29(Suppl 1):S65-S68.
231. Leone M., Franzini A., D’Andrea G., et al. Deep brain stimulation to relieve drug-resistant SUNCT. Ann Neurol. 2005;57(6):924-927.
232. Lyons M.K., Dodick D.W., Evidente V.G. Responsiveness of short-lasting unilateral neuralgiform headache with conjunctival injection and tearing to hypothalamic deep brain stimulation. J Neurosurg. 2009;110(2):279-281.
233. Walcott B.P., Bamber N.I., Anderson D.E. Successful treatment of chronic paroxysmal hemicrania with posterior hypothalamic stimulation: technical case report. Neurosurgery. 2009;65(5):E997.
234. Franzini A., Messina G., Cordella R., et al. Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29(2):E13.
235. Tsubokawa T., Katayama Y., Yamamoto T., et al. Treatment of thalamic pain by chronic motor cortex stimulation. PACE. 1991;14:131-134.
236. Canavero S., Bonicalzi V. Cortical stimulation for central pain. J Neurosurg. 1995;83:1117.
237. Canavero S. Dynamic reverberation. A unified mechanism for central and phantom pain. Med Hypotheses. 1994;42:203-207.
238. Ebel H., Rust D., Tronnier V., et al. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien). 1996;138:1300-1306.
239. Herregodts P., Stadnik T., De Ridder F., D’Haens J. Cortical stimulation for central neuropathic pain: 3-D surface MRI for easy determination of the motor cortex. Acta Neurochir Suppl. 1995;64:132-135.
240. Meyerson B.A., Lindblom U., Linderoth B., et al. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl. 1993;58:150-153.
241. Nguyen J.P., Keravel Y., Feve A., et al. Treatment of deafferentation pain by chronic stimulation of the motor cortex: report of a series of 20 cases. Acta Neurochir Suppl. 1997;68:53-60.
242. Rainov N.G., Fels C., Heidecke V., Burket W. Epidural electrical stimulation of the motor cortex in patients with facial neuralgia. Clin Neurol Neurosurg. 1997;99:205-209.
243. Fontaine D., Hamani C., Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110(2):251-256.
244. Nguyen J.P., Lefaucheur J.P., Decq P., et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82(3):245-251.
245. Sol J.C., Casaux J., Roux F.E., et al. Chronic motor cortex stimulation for phantom limb pain: correlations between pain relief and functional imaging studies. Stereotact Funct Neurosurg. 2001;77(1-4):172-176.
246. Shealy C.N., Mortimer J.T., Reswick J.B. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489-491.
247. Kumar K., Wilson J.T. Factors affecting spinal cord stimulation outcome in chronic benign pain with suggestions to improve success rate. Acta Neurochir Suppl. 2007;97(1):91-99.
248. Linderoth B.G., Meyerson B.A. Spinal cord stimulation: mechanisms of action. In: Burchiel K.J., editor. Surgical Management of Pain. New York: Thieme; 2002:505-526.
249. North R.B., Kidd D.H., Farrokhi F., Piantadosi S.A. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-106.
250. Wall P.D., Sweet W.H. Temporary abolition of pain in man. Science. 1967;155:108-109.
251. Shelden C.H. Depolarization in the treatment of trigeminal neuralgia. Evaluation of compression and electrical methods; clinical concept of neurophysiological mechanism. In: Knighton R.S., Dumke P.R., editors. Pain. Boston: Little, Brown; 1966:373-386.
252. Weiner R.L., Reed K.L. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217-221.
253. Slavin K.V., Burchiel K.J. Use of long-term nerve stimulation with implanted electrodes in the treatment of intractable craniofacial pain. J Neurosurg. 2000;92:576.
254. Dunteman E. Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation. 2002;5:32-37.
255. Rodrigo-Royo M.D., Azcona J.M., Quero J., et al. Peripheral neurostimulation in the management of cervicogenic headaches: four case reports. Neuromodulation. 2005;8:241-248.
256. Slavin K.V., Wess C. Trigeminal branch stimulation for intractable neuropathic pain: technical note. Neuromodulation. 2005;8:7-13.
257. Barolat G. Peripheral subcutaneous stimulation for intractable pain. In: Lavano A., Landi A., La Notte M., editors. Handbook of Stereotactic and Functional Neurosurgery. Turin, Italy: Minerva Medica; 2010:144-149.
258. Matharu M.S., Bartsch T., Ward N., et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220-230.
259. Rogers L.L., Swidan S. Stimulation of the occipital nerve for the treatment of migraine: current state and future prospects. Acta Neurochir Suppl. 2007;97(Pt 1):121-128.
260. Popeney C.A., Alo K.M. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43:369-375.
261. Magis D., Allena M., Bolla M., et al. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6(4):314-321.
262. Leone M., Franzini A., Cecchini A.P., et al. Stimulation of occipital nerve for drug-resistant chronic cluster headache. Lancet Neurol. 2007;6(4):289-291.
263. Burns B., Watkins L., Goadsby P.J. Treatment of hemicrania continua by occipital nerve stimulation with a bion device: longterm follow-up of a crossover study. Lancet Neurol. 2008;7(11):1001-1012.
264. Slavin K.V., Nersesyan H., Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:112-119.
265. Bartsch T., Goadsby P.J. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126:1801-1813.
266. Piovesan E.J., Kowacs P.A., Oshinsky M.L. Convergence of cervical and trigeminal sensory afferents. Curr Pain Headache Rep. 2003;5:377-383.
267. Slavin K.V., Colpan M.E., Munawar N., et al. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurg Focus. 2006;21(6):E5.
268. Reed K.L., Black S.B., Banta C.J.2nd, Will K.R. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia. 2010;30(3):260-271.
269. Jette N., Patten S., Williams J., et al. Comorbidity of migraine and psychiatric disorders—a national population-based study. Headache. 2008;48(4):501-516.
270. Nahas Z., Marangell L.B., Husain M.M., et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66(9):1097-1104.
271. Hord E.D., Evans M.S., Mueed S., et al. The effect of vagus nerve stimulation on migraines. J Pain. 2003;4(9):530-534.
272. Multon S., Schoenen J. Pain control by vagus nerve stimulation: from animal to man and back. Acta Neurol Belg. 2005;105(2):62-67.
273. Cecchini A.P., Mea E., Tullo V., et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci. 2009;30(Suppl 1):S101-S104.
274. Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82-96.
275. Yaksh T.L., Rudy T.A. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357-1358.
276. Yaksh T.L., Al-Rhodan N.R.F., Jensen T.S. Sites of action of opiates in production of analgesia. Prog Brain Res. 1988;77:371-394.
277. Leiphart J.W., Dills C.V., Zikel O.M., et al. A comparison of intrathecally administered narcotic and nonnarcotic analgesics for experimental chronic neuropathic pain. J Neurosurg. 1995;82:595-599.
278. Tamsen A., Gordth T. Epidural clonidine produces analgesia. Lancet. 1984;2:231-232.
279. Coombs D.W., Saunders R.L., Fratkin J.D., et al. Continuous intrathecal hydromorphone and clonidine for intractable cancer pain. J Neurosurg. 1986;64:890-894.
280. Hassenbusch S.J., Portenoy R.K., Cousins M., et al. Polyanalgesic Consensus Conference 2003: an update on the management of pain by intraspinal drug delivery—report of an expert panel. J Pain Symp Manage. 2004;27(6):540-563.
281. Rainov N.G., Heidecke V. Management of chronic back and leg pain by intrathecal drug delivery. Acta Neurochir Suppl. 2007;97(Pt 1):49-56.