CHAPTER 60 SURGICAL TECHNIQUES FOR THORACIC, ABDOMINAL, PELVIC, AND EXTREMITY DAMAGE CONTROL
Injury severity and spectrums of injury have continually evolved, resulting in greater and different challenges for the modern trauma surgeon. High energy blunt trauma, with resultant multisystem organ injury, as well as increasingly sophisticated firearms with greater wounding capacity, has resulted in greater severity of injury. Despite the fact that these injury patterns are more likely to result in the death of a patient, improvements in prehospital transport and trauma resuscitation have allowed more moribund patients to reach the hospital alive but in extremis. Damage control surgery, addressing the life-threatening injuries immediately but delaying definitive repair until the metabolic and physiologic perturbations have been corrected, has evolved to address this population of patients.
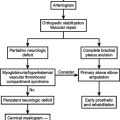
Indications for damage control strategy are multiple. The goal of the damage control procedure is to preserve life in the face of devastating injuries with profound hemorrhagic shock. As described by Moore et al., they include an inability to achieve hemostasis resulting from ongoing coagulopathy, a technically difficult or inaccessible major venous injury, a time-consuming procedure in the face of under-resuscitated shock, and a need to address other life-threatening injuries. These indications have been expanded to include hemodynamically unstable patients with high-energy blunt torso trauma or multiple penetrating injuries, or any trauma patient presenting in shock with hypothermia and coagulopathy (Table 1). Most commonly applied to the abdomen, damage control approaches have now been applied successfully to both devastating thoracic and orthopedic injuries.
Table 1 Indications for Damage Control Procedures
Adapted with permission from Rotondo MF, Zonies DH: The damage control sequence and underlying logic. Surg Clin North Am 77:761–777, 1997.
INITIAL RESUSCITATION CONCERNS
A systematic approach to the initial management of the injured patient has been promulgated by the Advanced Trauma Life Support course of the American College of Surgeons Committee on Trauma. The primary and secondary surveys as described in that course allow the rapid identification of life-threatening injuries and allow the surgeon to prioritize subsequent operative management of the unstable trauma patient. Patients with exsanguinating hemorrhage should be expeditiously transported to the operating room, where a decision regarding the initiation of damage control should be made early in the operative course, based on the patient’s physiologic status, body temperature, and intravascular volume status (see Table 1). In patients with obvious ongoing resuscitation requirements, a central venous catheter should be placed for aggressive volume resuscitation. Given the multiple factors that predispose these patients to coagulopathy, early consideration should be given to the administration of coagulation factors (fresh frozen plasma and cryoprecipitate) and platelets, in addition to the standard crystalloid and packed red blood cell resuscitation.
PHASE I: DAMAGE CONTROL OPERATION
Damage Control Laparotomy
There are several options available for the management of major vascular injuries. Some venous injuries will respond to packing. Ongoing bleeding, however, requires direct surgical intervention. Many abdominal vascular injuries can be managed with simple ligation of the bleeding vessel (Table 2). Ligation, however, is not tolerated in aortic or proximal superior mesenteric artery injuries, and is not technically feasible in retrohepatic caval injuries. These injuries are typically initially approached by an attempt at repair or the placement of a temporary intraluminal shunt, with planned repair at a second operation (phase 3). Commonly, Argyle carotid shunts and Javid shunts have been used for this purpose. Chest tubes may be used when larger conduits are necessary. The shunts should be secured using umbilical tapes, vessel loops, or suture, and do not require anticoagulation to maintain patency. Another technique for the management of exsanguinating vascular injury is the use of endoluminal balloon catheters to obtain proximal and distal control of hemorrhage. The catheters are inserted into the vessel at the site of injury, and the balloon inflated. This technique allows repair of the injured vessel in a relative dry operative field.
Table 2 Abdominal Vessel Ligation and Expected Complications
| Vessel | Complication | Recommendations |
|---|---|---|
| Celiac axis | None | |
| Splenic artery | None if the short gastric vessels are intact | |
| Common hepatic artery | None if the portal vein is intact, possible gallbladder ischemia | Cholecystectomy (may be done at second look) |
| Superior mesenteric artery | Bowel ischemia | Second-look procedure |
| Superior mesenteric vein | Bowel ischemia | Second-look procedure |
| Portal vein | Bowel ischemia | Second-look procedure |
| Suprarenal inferior vena cava | Possible renal failure | Wrap and elevate legs, assess for compartment syndrome |
| Infrarenal inferior vena cava | Lower extremity edema | Wrap and elevate legs, assess for compartment syndrome |
| Left renal vein (proximal) | None | |
| Right renal vein | Renal ischemia | Nephrectomy |
| Common and external iliac artery | Lower extremity ischemia | Ipsilateral calf and sometimes thigh fasciotomies or extra-anatomic bypass |
| Common and external iliac vein | Lower extremity edema | Wrap and elevate legs |
| Internal iliac artery | None | |
| Internal iliac vein | None |
Adapted with permission from Shapiro MB, Jenkins DH, Schwab CW, Rotondo MF: Damage control: collective review. J Trauma 49:969–978, 2000.
Candidates for damage control surgery often have associated hollow viscus injury. The goal in management of these injuries is the control of contamination. Intestinal lacerations may be controlled by linear stapling or by stapled resection. After enterectomy, the gastrointestinal tract is left in discontinuity, and the decision to perform an anastomosis or stoma is postponed until the patient is stabilized and able to return to the operating room for definitive management (phase 3). Associated biliary or pancreatic injuries can often be managed with judicious placement of closed suction drains, with plans to address the injury at the second procedure (phase 3).
Damage Control Orthopedics
Pelvic ring disruptions continue to be a significant source of both morbidity and mortality after trauma. Pelvic fractures account for 3%–8% of all skeletal fractures and most commonly result from high-energy trauma, with motor vehicle crashes being the predominant mechanism of injury. During the acute phase, the goal of treatment should be the control of hemorrhage. The retroperitoneum if intact can contain up to 4 liters of blood, and bleeding from the associated vascular injuries will continue until physiologic tamponade is obtained. However, if the retroperitoneal space is disrupted, pelvic fractures can lead to uncontrolled hemorrhage and increase the risk of exsanguination.
COMPLICATIONS FOLLOWING DAMAGE CONTROL SURGERY
Immediate
Unplanned reoperation may be necessary in the patient with ongoing postoperative hemorrhage despite aggressive resuscitation and correction of the lethal triad. Indications for return to the operating room within the first 24 hours after a damage control procedure are listed in Table 3.
Asensio JA, Demetriades D, Berne JB, et al. Stapled pulmonary tractotomy: a rapid way to control hemorrhage in penetrating pulmonary injuries. J Am Coll Surg. 1997;185(5):486-487.
Asensio JA, McDuffie L, Petrone P, et al. Reliable variables in the exsanguinated patient which indicate damage control and predict outcome. Am J Surg. 2001;182:743-751.
Asensio JA, Petrone P, Roldan G. Has evolution in awareness of guidelines for institution of damage control improved outcome in the management of the post-traumatic open abdomen? Arch Surg. 2004;139:209-214.
Abikhaled JA, Granchi RS, Wall MJ, et al. Prolonged abdominal packing is associated with increased morbidity and mortality. Am Surg. 1997;63:1109-1113.
Barker DE, Kaufman HJ, Smith LA, et al. Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma. 2000;48:201-206.
Garner GB, Ware DN, Cocanour CS, et al. Vacuum-assisted wound closure provides early fascial reapproximation in trauma patients with open abdomens. Am J Surg. 2001;182:630-638.
Gentilello LM, Cobean RA, Offner PJ, et al. Continuous arteriovenous re-warming: rapid reversal of hypothermia in critically ill patients. J Trauma. 1992;32:316-327.
Gentilello LM, Jurkovich GJ, Stark MS, Hassantash SA, O’Keefe GE. Is hypothermia in the victim of major trauma protective or harmful? A randomized, prospective study. Ann Surg. 1997;226:439-449.
Giannoudis PV, Pape H-C. Damage control orthopaedics in unstable pelvic ring injuries. Injury. 2004;35:671-677.
Granchi T, Schmittling Z, Vasquez J, et al. Prolonged use of intraluminal arterial shunts without systemic anticoagulation. Am J Surg. 2000;180:493-497.
Gregory JS, Flancbaum L, Townsend MC, et al. Incidence and timing of hypothermia in trauma patients undergoing operations. J Trauma. 1991;31:795-800.
Jurkovich GJ, Greiser WB, Luterman A, et al. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27:1019-1024.
Karmy-Jones R, Jurkovich GJ, Shatz DV, et al. Management of traumatic lung injury: a Western Trauma Association multi-center review. J Trauma. 2001;51:1049-1053.
Martin RR, Byrne M. Postoperative care and complications of damage control surgery. Surg Clin North Am. 1997;77:929-942.
Moore EE, Burch JM, Franciose RJ, et al. Staged physiologic restoration and damage control surgery. World J Surg. 1998;22:1184-1191.
Offner PJ, de Souza AL, Moore EE, et al. Avoidance of abdominal compartment syndrome in damage-control laparotomy after trauma. Arch Surg. 2001;136:676-681.
Pape H-C, Stalp M, Griensven M, Weiberg A, Dahlweit M, Tscherne H. Optimal timing for secondary surgery in polytrauma patients: an evaluation of 4314 serious-injury cases. Chirurg. 1999;70:1287-1293.
Raeburn CD, Moore EE, Biffl WL, et al. The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am J Surg. 2001;182:542-546.
Reed RLII, Bracey AW, Hudson JD, et al. Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141-152.
Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin North Am. 1997;77:761-777.
Scalea TM, Boswell SA, Scott JD, Mitchell KA, Kramer ME, Pollak AN. External fixation as a bridge to intramedullary nailing for patients with multiple injuries and with femur fractures: damage control orthopedics. J Trauma. 2000;48:613-623.
Schreiber MA. Damage control surgery. Crit Care Clin. 2004;20:101-118.
Shapiro MB, Jenkins DH, Schwab CW, Rotondo MF. Damage control: collective review. J Trauma. 2000;49:969-978.
Wittmann DH, Aprahamian C, Gergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies using zippers, slide fastener and velcro analogue for temporary abdominal closure. World J Surg. 1990;14:218-226.