39 Surgical site infection and antimicrobial prophylaxis
Epidemiology
In England, there are over nine million operations and interventions undertaken each year. Over 50% of these are performed as day cases and many patients are admitted on the day of surgery (Health and Social Care Information Centre, 2009). Healthcare-associated infections (HCAIs), including surgical site infections, complicate around 7% of all hospital admissions (HIS/ICNA, 2007). Surgical site infections are of major clinical importance because they account for 14–16% of all healthcare-associated infections (HPS, 2009; Public Accounts Committee, 2009) and are associated with considerable morbidity and mortality. One-third of peri-operative deaths are related to surgical site infections (Astagneau et al., 2001). It has been estimated that surgical site infections double the length of hospital stay (Coello et al., 2005). While surgical site infections can be common in some procedures, the incidence can be minimised by the care provided before and after the operation, together with the skill of the surgeon (HPS, 2009).
Surveillance
Monitoring the incidence of surgical site infections is hampered by the lack of agreed measuring systems. In particular, to monitor the rates of surgical site infection within an organisation, or to benchmark between organisations, there needs to be a standard approach to diagnosis. Criteria for such a definition have been developed by the Centres for Disease Control and Prevention (CDC) (Mangram et al., 1999) and these are presented in Table 39.1. More detailed surgical site infection scoring systems have been developed but these are time consuming to use.
Table 39.1 Criteria for defining surgical site infection (Mangram et al., 1999)
| Type | Level | Signs and symptoms |
|---|---|---|
| Superficial incisional | Skin and subcutaneous tissue | Localised (Celsian) signs such as redness, pain, heat or swelling at the site of the incision or by the presence of pus within 30 days |
| Deep incisional | Fascial and muscle layers | Presence of pus or an abscess, fever with tenderness of the wound, or a separation of the edges of the incision exposing the deeper tissues within 30 days (or 1 year if an implant used) |
| Organ or space infection | Any part of the anatomy other than the incision that is opened or manipulated during the surgical procedure, for example, joint or peritoneum | Loss of function of a joint, abscess in an organ, localised peritonitis or collection. Ultrasound or CT scans confirm infection. Within 30 days (or 1 year if implant is used) |
Mandatory surveillance for surgical site infections in orthopaedic surgery in the UK was introduced in 2003. In addition, Scotland monitors most other common procedures (http://www.hps.scot.nhs.uk) while in Wales caesarian section is also monitored (http://www.wales.nhs.uk). England has a voluntary reporting system for a broader range of operations (http://www.hpa.org.uk). All report their findings annually. Many surgical site infections, for example, those involving prosthetic joints, often develop late (>28 days post-operation), so post-discharge surveillance schemes are essential. Patients need to be aware how a surgical site infection may present after discharge from hospital. Surveillance of surgical site infections and feedback to the surgical team has been shown to reduce rates of infection (Gastmeier et al., 2005).
Risk factors
Surgical site infections can be categorised into three groups: superficial incisional, deep incisional and organ or space (Fig. 39.1) Whether a wound infection occurs after surgery depends on a complex interaction between the following:
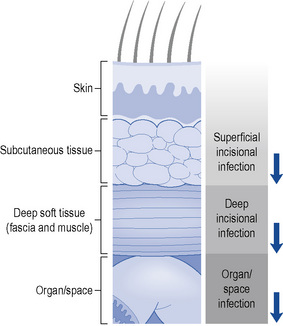
Fig. 39.1 Schematic representation of the anatomical classification of surgical site infection
(Horan et al., 1992). Reproduced with permission from the University of Chicago Press.
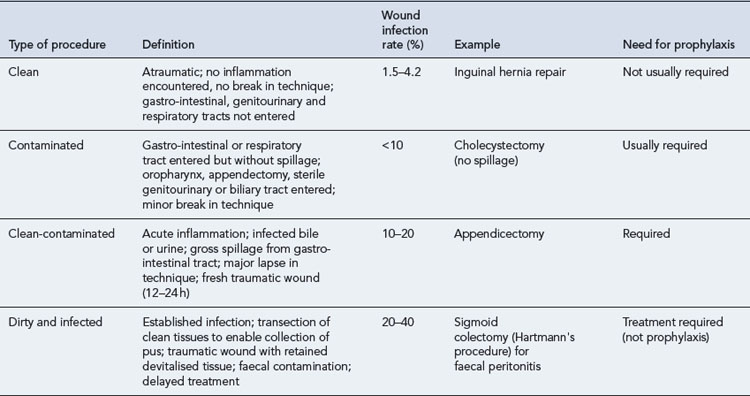
A system to stratify operative wounds by the expected level of bacterial contamination (Table 39.2) was developed to help predict likely infection rates (Mangram et al., 1999). A number of other factors have also been found to affect the incidence of surgical site infection and are discussed below.
Prosthetic implants
Medical implants have a detrimental effect on host defences such that a lower bacterial count is needed to initiate infection. Hence, there is a greater risk of infection during implant surgery. Bacteria growing on an abiotic surface, such as a prosthetic hip implant or heart valve, together with a protective layer of microbial polymers are known as a biofilm (Donlan and Costerton, 2002). Antimicrobials are frequently ineffective against micro-organisms growing in biofilms, making treatment of implant infections problematic and their prevention even more important.
Duration of surgery
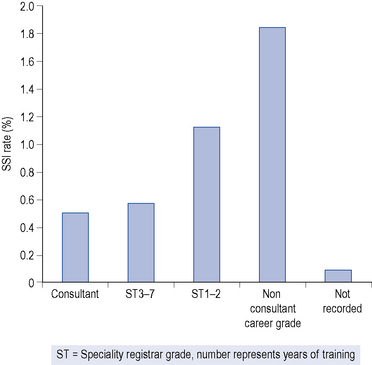
The longer the operation, the greater is the risk of wound infection. This, in turn, may be influenced by the experience (Fig. 39.2) speed and skill of the surgeon and is additional to the classification of the operation by risk of infection, for example, clean, contaminated, dirty or infected.
Patient related factors
Table 39.3 American Society of Anesthesiology (ASA) classification of physical status (Mangram et al., 1999)
| ASA score | Physical status |
|---|---|
| 1 | A normal healthy patient |
| 2 | A patient with mild systemic disease |
| 3 | A patient with a severe systemic disease that limits activity but is not incapacitating |
| 4 | A patient with an incapacitating systemic disease that is a constant threat to life |
| 5 | A moribund patient that is not expected to survive 24 h with or without operation |
For each surgical procedure, a score of 0–3 is allocated to represent the number of risk factors present. Patients with a score of 0 are at the lowest risk of developing a surgical site infection, while those with a score of 3 have the greatest risk (Table 39.4). Use of this risk index allows comparison of similar patient groups in terms of surgical site infection risk over time. The risk index is a significantly better predictor of surgical-wound risk than the traditional wound classification system and performs well across a broad range of operative procedures.
Table 39.4 Risk index based on presence of co-morbidity and duration of operation (Culver et al., 1991)
| Risk index | Infection rate (%) |
|---|---|
| 0 | 1.5 |
| 1 | 2.9 |
| 2 | 6.8 |
| 3 | 13.0 |
Other factors
There are a number of other risk factors that may increase the risk of a surgical site infection (Table 39.5) for an individual patient but the impact has not been quantified to the extent of those risk factors discussed above.
Table 39.5 Patient and operative risk factors for surgical site infection
| Patient risk factors | Operative risk factors |
|---|---|
| Advanced age | Tissue ischaemia |
| Malnutrition | Lack of haemostasis |
| Obesity | Tissue damage, for example, crushing by surgical instruments |
| Concurrent infection | Presence of necrotic tissue |
| Diabetes mellitus | Presence of foreign bodies including surgical materials |
| Liver impairment | |
| Renal impairment | |
| Immune deficiency states | |
| Prolonged preoperative stay | |
| Blood transfusion | |
| Smoking |
Smoking
Smoking increases the risk of developing a wound infection (Myles et al., 2002). The mechanism is not known but tobacco use may delay wound healing via the vasoconstricting effects of nicotine and thus increase the risk of infection (Myles et al., 2002).
Diabetes mellitus
Long-term diabetes does not appear to have any impact on the risk of developing a surgical site infection. However, peri-operative fluctuations in blood glucose for up to 48 h have been shown to double the infection risk in cardiac patients (Latham et al., 2001).
Age
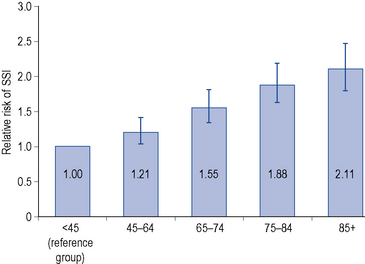
Increasing age is associated with an increased risk of surgical site infection. However, there is debate whether age serves simply as a marker for underlying disease or whether the decline in immune function with age is the significant factor. A study of 72,000 patients in the USA, which adjusted for hospital type, procedure, duration, wound class and physical status of the patient, showed a 1.1% increase in surgical site infection per year of age from the age of 18 to 65 years, but a 1.2% decrease in individuals over 65 years (Kaye et al., 2005). In contrast, the findings of the English surgical site infection surveillance scheme (Fig. 39.3) indicated that the chance of getting a surgical site infection were 37% higher for a 65-year-old person compared to a 45-year-old person (HPA, 2008).
Pathogenesis
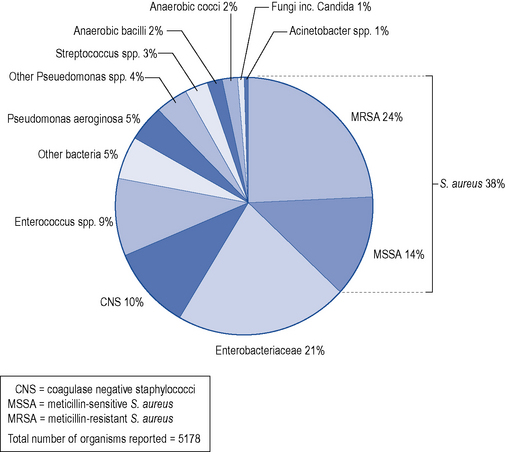
Development of a surgical site infection depends on survival of the contaminating micro-organism in a wound site at the end of a surgical procedure; the pathogenicity and number of these micro-organisms; and the host’s immune response. Most micro-organisms are from the host (endogenous), but are occasionally introduced via surgical instruments, the environment or contaminated implants (exogenous). The likely invading micro-organism varies according to the type of surgery (Table 39.6). Data for England from 2003 to 2007 has shown that the predominant organism was Staphylococcus aureus, which accounted for 38% of all surgical site infections (Fig. 39.4); 64% of these were caused by a meticillin-resistant strain (MRSA). The proportion of surgical site infections caused by S. aureus was highest in hip hemiarthroplasty (57%), followed by limb amputation (54%) and open reduction of long bone fracture (52%). Enterobacteriaceae (coliforms) caused the second largest group of infections, accounting for 21% of all surgical site infections. These were the prominent causes of surgical site infections in three categories: large bowel surgery (33%), coronary artery bypass graft (32%) and small bowel surgery (30%).
Table 39.6 Likely pathogens in post-operative wound infections
| Category of surgery | Most likely pathogen(s) |
|---|---|
| Clean | |
| Cardiac/vascular/orthopaedic Breast | Coagulase-negative staphylococci, S. aureus, Gram-negative bacilliS. aureus |
| Clean-contaminated | |
| Burns | S. aureus, Pseudomonas aeruginosa |
| Head and neck Gastro-intestinal tract | S. aureus, Streptococcus spp., anaerobes (from oral cavity)Coliforms, anaerobes (Bacteroides fragilis) |
| Urogenital tract | Coliforms, Enterococcus spp. |
| Dirty | |
| Ruptured viscera | Coliforms, anaerobes (B. fragilis) |
| Traumatic wound | S. aureusStreptococcus pyogenes, Clostridium spp. |
Prevention of surgical site infection
The evidence that supports interventions to minimise surgical site infection has been highlighted in national guidelines (NICE, 2008a) and categorised into four areas: information to patients, preoperative phase, peri-operative phase and post-operative phase (Table 39.7). When selecting antimicrobial prophylaxis regimens or evaluating potential prophylaxis failures, it is important to ensure that all four aspects of prevention have been addressed.
Table 39.7 Recommendations for the prevention and treatment of surgical site infections (NICE, 2008a)
| Category | Recommendation |
|---|---|
| Information for patients and carers | How to recognise a surgical site infection and what to do |
| Preoperative phase | Patient preparation: pre-op washing, hair removal, nasal MRSA decontamination and bowel preparation |
| Antimicrobial prophylaxis guidance | |
| Staff preparation and theatre movement | |
| Intra-operative phase | Operating team preparation |
| Patient skin preparation | |
| Maintaining patient homeostasis | |
| Wound dressings | |
| Post-operative phase | Dressing and cleansing the wound |
| Antimicrobial treatment for surgical site infection | |
| Debridement of surgical site infections |
Antimicrobial prophylaxis
In the early 1960s, it was demonstrated, using a guinea-pig model, that surgical-wound infection could be reduced by administration of an antimicrobial just before an incision was made, but the beneficial effect disappeared if antimicrobial administration was delayed by 3–4 h after the incision (Burke, 1961). Since then, many clinical trials have indicated the benefit of maintaining adequate antimicrobial tissue levels from the time of initial surgical incision until closure.
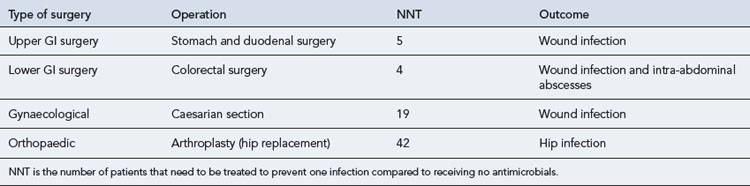
The number of patients that need to be treated with antimicrobial agents to prevent one infection in the different types of surgery are presented in Table 39.8.
Choice of antimicrobial
The majority of clinical trials that have demonstrated the benefit of antimicrobial prophylaxis are outdated, and probably do not reflect current surgical practice. First and second generation cephalosporins (cefazolin and cefuroxime) have been the mainstay of agents studied (Bratzler and Houck, 2004). There are advantages and disadvantages with using cephalosporins. The advantages include a low anaphylaxis risk, but they have the disadvantage of excessive or inadequate spectrum of cover depending on the operation (Morgan, 2006) and a strong association with Clostridium difficile infection. Many antimicrobials used in prophylaxis have not been extensively studied in clinical trials, but are selected on a theoretical basis of their antimicrobial spectrum (see Tables 39.9–39.11).
| Surgical site infection for a skin wound at any site | |
| S. aureus | Highly variable (30–60% susceptible) to flucloxacillin therefore MRSA screening essential |
| Beta haemolytic Streptococci (BHS) | 90% susceptible to penicillins, macrolides or clindamycin |
| Additional pathogens by site of infection | |
| Head and neck surgery | |
| Oral anaerobes | 95% susceptible to metronidazole or co-amoxiclav |
| Operations below the diaphragm | |
| Anaerobes | 95% susceptible to metronidazole or co-amoxiclav |
| E. coli and other Enterobacteriaceae | 80–90% of E. coli sensitive to cefuroxime, co-amoxiclav (or other β-lactam with inhibitor combination) or gentamicin |
| Insertion of a prosthesis, graft or shunt | |
| Coagulase-negative Staph (CNS) | Two-thirds of CNS are methicillin-resistant, but β-lactams may still be used but preferably with a second agent with staphylococcal cover, for example, gentamicin, or a glycopeptide used instead. See above for S. aureus. |
| S. aureus, diphtheroids | |
(adapted from SIGN, 2008)
Table 39.10 Micro-organisms commonly isolated from surgical site infections and prophylactic antimicrobials used in common surgical procedures (Prtak and Ridgway, 2009)
| Surgical procedure | Most common micro-organisms | Examples of prophylactic IV antimicrobials |
|---|---|---|
| Gastro-intestinal | Bowel flora | |
If patient has previously had MRSA or is at high risk (e.g. nursing home resident), use teicoplanin or other gylcopeptide.
For β-lactam allergy, replace co-amoxiclav or cefuroxime with teicoplanin +/− ciprofloxacin.
Table 39.11 Suggested cephalosporin-free antimicrobial prophylaxis for surgical site infection
| Type of surgery | Suggested antimicrobials | Alternatives for penicillin allergy |
|---|---|---|
| Cardiothoracic | Flucloxacillin +/– gentamicin | Teicoplanin or Co-trimoxazole |
| ENT, maxillofacial and oral | Amoxicillin + metronidazole or Co-amoxiclav | Clarithromycin +/– metronidazole or Clindamycin |
| Gynaecology | Gentamicin + metronidazole | |
| Lower GI | Gentamicin + metronidazole | |
| Obstetrics | Co-amoxiclav | Clarithromycin +/– metronidazole or Clindamycin |
| Orthopaedic | Flucloxacillin +/– gentamicin | Teicoplanin or Co-trimoxazole |
| Thoracic | Flucloxacillin or Co-amoxiclav | |
| Upper GI | Gentamicin | |
| Urology | Gentamicin | |
| Vascular | Flucloxacillin +/– gentamicin (+metronidazole for amputations) | Co-trimoxazole or Teicoplanin |
GI = gastro-intestial
(adapted from SIGN, 2008)
Timing and duration
The optimum time to administer prophylactic antimicrobials before incision is probably 30 min, but national recommendations vary from less than 30 min (SIGN, 2008) to 60 min (NICE, 2008a) prior to incision. Figure 39.5 represents the two major studies undertaken to identify the optimum time for administration of prophylaxis. Both studies determined that post-incision administration of antimicrobials significantly increased the risk of surgical site infection.
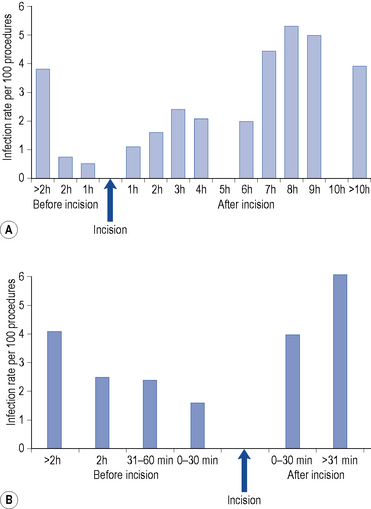
(Steinberg et al., 2009). Taken from Mandell et al. (2010). Reproduced with kind permission from Elsevier.
Historically, the only occasion where antimicrobial administration has been delayed to after the incision is Caesarian section, where antimicrobials are given after cross clamping the umbilical cord to prevent drug delivery to the neonate. However, it is recognised that this does not provide the mother with adequate tissue levels at the time of incision and two studies have shown that antimicrobials can be given safely before incision without adversely affecting the neonate (Sullivan et al., 2007; Thigpen et al., 2005).
When a tourniquet is used during orthopaedic procedures to minimise bleeding, the antimicrobial should be infused before inflating the tourniquet. This ensures adequate tissue levels are achieved at the site of surgery (Bratzler and Houck, 2004).
Repeat doses
Although single dose prophylaxis regimens are widely advocated (DH/HPA, 2008; NICE, 2008a; SIGN, 2008), many surgeons continue to use prolonged courses of ‘prophylaxis’ often for several days, without a clear evidence base. For some procedures, the optimum duration of prophylaxis is not known and 24–48 h prophylaxis is considered acceptable, for example, for open heart surgery (SIGN, 2008).
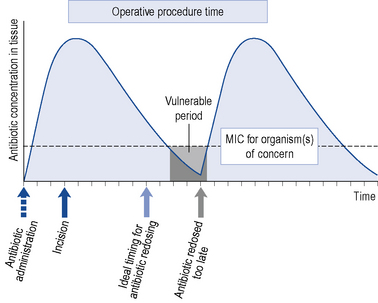
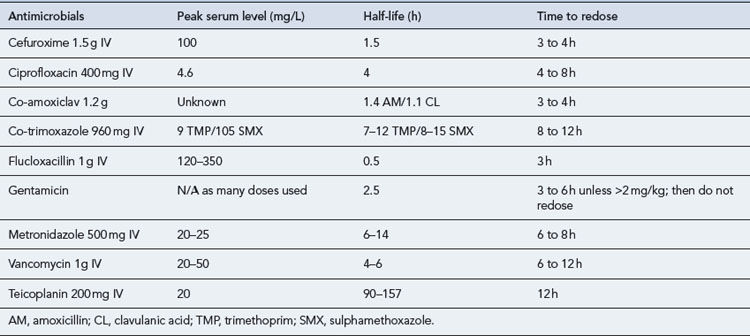
A schematic model for the tissue concentration time profile of an antimicrobial agent used to prevent surgical site infection is presented in Fig. 39.6. After an initial dose of the antimicrobial agent, tissue concentrations reach their peak rapidly, with a subsequent decline over time. The goal of prophylaxis is for the antimicrobial tissue concentration to remain above the minimum inhibitory concentration (MIC) for the specific pathogens at the time of incision and throughout the procedure. The antimicrobial should be readministered during prolonged procedures to prevent a period where tissue concentrations are below the MIC (grey area). Failure to readminister antimicrobials appropriately may result in a period during which the wound is vulnerable. Recommendations for peri-operative re-dosing schedules are presented in Table 39.12. General guidance is to repeat doses of antimicrobials at intervals of 1–2 half-lives.
β-Lactam allergy
Penicillin and cephalosporin antibiotics have been the cornerstone of antimicrobial prophylaxis to prevent surgical site infections. Patients reported to be allergic to β-lactam antibiotics or other antimicrobials need to be carefully assessed, as alternatives may not be optimal. Alternatives are often glycopeptides, for example, teicoplanin or vancomycin, which are more expensive, often need to be given by infusion (vancomycin) and can increase selection for resistant bacteria. The prevalence of penicillin allergy in the general population is unknown. The incidence of self reported penicillin allergy ranges from 1% to 10%, with the frequency of life-threatening anaphylaxis estimated at 0.01–0.05% (or 1–5 in 10,000). More than 80% of patients with a self reported allergy to penicillin have no evidence of IgE antibodies on skin testing. Important details of an allergic reaction include signs, symptoms, severity, history of prior reaction, time course of allergic event, temporal proximity to administered drug, route of administration, other medication being taken and adverse events to other medication (Park and Li, 2005). Reactions to penicillins and other β-lactams occur because of allergy to the parent compound or the metabolites. The cross sensitivity between penicillins and cephalosporins is unknown, but has been variably reported to be up to 10%. Early cephalosporin preparations were contaminated with penicillins probably leading to an over estimate of cross sensitivity (Saxon et al., 1987). As the generation of the cephalosporin increases, the likelihood of cross sensitivity decreases (Pichichero and Casey, 2007). Those with a penicillin allergy showed an increased risk of allergic reaction to a first generation cephalosporin. First generation cephalosporins share a similar side chain to penicillin and amoxicillin. However, cross sensitivity to second and third generation cephalosporins was lower. The different side chains appear to play a more dominant role than the β-lactam ring in allergy.
Recent prospective studies have shown that the cross-reactivity to carbapenems and monobactams is very small. It is around 1% for imipenem and meropenem, and no cross-reaction has been reported for aztreonam (Frumin and Gallagher, 2009).
The increased use of penicillins rather than first or second generation cephalosporins for surgical site infection prophylaxis is increasing the potential for adverse reactions. In addition, the current nomenclature for penicillin combinations, for example, co-amoxiclav, can often make it more difficult for staff to recognise penicillin containing antimicrobials. Current guidance on the use of β-lactams in patients with penicillin allergy is detailed in Table 39.13.
Table 39.13 Guidance on the use of β-lactam antibiotics in patients with penicillin allergy recommended by BNF (Joint Formulary Committee, 2010)
| Allergic reaction | Action |
|---|---|
| Immediate hypersensitivity reaction to a penicillin | Avoid penicillins and cephalosporins |
| Minor rash – localised or widespread but delayed (>72 h) | Avoid penicillins, but cephalosporins are safe to use |
Topical or local antimicrobial prophylaxis
In vascular surgery, synthetic grafts bonded with or soaked in rifampicin are frequently used, despite evidence showing that there was no decrease in infection rates at 1 month and 2 years (Stewart et al., 2006). There is some evidence to support the local delivery of gentamicin into wounds via collagen fleece impregnated with gentamicin and further research into this was recommended (NICE, 2008a); however, two recent randomised controlled trials have shown it not to be efficacious (Bennett-Guerrero et al., 2010a,b) The use of topical cefotaxime in contaminated surgery has been shown not to decrease peritonitis, and should not be used.
Answer
NICE guidelines no longer recommend prophylaxis for patients at risk of endocarditis undergoing investigations involving the oral, gastro-intestinal or urogenital tract, in the absence of local infection (NICE, 2008b). The usual prophylaxis for this regimen should be recommended. If the patient had a urinary tract infection, this should ideally be treated before surgery.
Astagneau P., Rioux C., Golliot F., et al. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J. Hosp. Infect.. 2001;48:267-274.
Bennett-Guerrero E., Pappas T.N., Koltun W.A., et al. Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N. Engl. J. Med.. 2010;363:1038-1049.
Bennett-Guerrero E., Ferguson T.B., Lin M., et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. J. Am. Med. Assoc.. 2010;304:755-762.
Bratzler D.W., Houck P.M. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin. Infect. Dis.. 2004;38:1706-1715.
Burke J. The effective period of preventative antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168.
Classen D., Evans R.S., Pestotnik S.L., et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N. Engl. J. Med. 326. 1992;5:281-286.
Coello R.C.A., Wilson J., Ward V., et al. Adverse impact of surgical site infections in English hospitals. J. Hosp. Infect.. 2005;60:93-103.
Culver D.H., Horan T.C., Gaynes R.P., et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. Am. J. Med.. 1991;91:S152-S157.
Department of Health and Health Protection Agency. Clostridium difficile infection: how to deal with the problem. DH/HPA, London. Available at http://www.hpa.nhs.uk/web/HPAwebFile/HPAweb_C/1232006607827, 2008.
Donlan R.M., Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev.. 2002;15:167-193.
Frumin J., Gallagher J.C. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann. Pharmacother.. 2009;43:304-315.
Gastmeier P.S.D., Brandt C., et al. Reduction of orthopaedic wound infections in 21 hospitals. Arch. Orthop. Trauma Surg.. 2005;125:526-530.
Gilbert D.N., Moellering R.C., Eliopoulos G.M., et al. Sanford Guide to Antimicrobial Therapy, 39th ed. Virginia: Sperryville; 2009.
Health and Social Care Information Centre. Hospital episodes statistics (England): main procedures and interventions: Summary, 2008–09. London: Department of Health; 2009. Available at http://www.hesonline.nhs.uk
Health Protection Scotland. Surveillance of surgical site infection. 2009 Annual report. Glasgow: Health Protection Scotland; 2009. Available at http://www.documents.hps.scot.nhs.uk/hai/sshaip/publications/ssi/ssi-2008.pdf
HIS/ICNA. The third prevalence survey of healthcare associated infections in acute hospitals 2006. Hospital Infection Society and Infection Control Nurses Association. Available at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_078389.pdf, 2007.
Health Protection Agency. Surveillance of healthcare associated infections report 2008. HPA, London. Available at http://www.hpa.nhs.uk/web/HPAwebFile/HPAweb_C/1216193833496, 2008.
Horan T.C., Culver D.H., Gaynes R.P. Surgical Site Infection (ssi) risk stratum rates: What they are and how to compare them using aggregated data from the National Nosocomial Infections Surveillance (NNIS). System. Am. J. Infect. Control 21. 1993;2:99.
Joint Formulary Committee. British National Formulary, 60th ed. London: British Medical Association and Royal Pharmaceutical Society; 2010.
Kaye K.S., Schmit K., Pieper C., et al. The effect of increasing age on the risk of surgical site infection. J. Infect. Dis.. 2005;191:1056-1062.
Latham R., Lancaster A.D., Covington J.F., et al. The association of diabetes and glucose control with surgical site infections among cardiothoracic surgery patients. Infect. Control Hosp. Epidemiol.. 2001;22:607-612.
Mandell G.L., Bennett J.E., Dolin R. Principles and Practice of Infectious Diseases, seventh ed. Philadelphia: Elsevier; 2010.
Mangram A.J.H.T., Pearson M.L., et al. Guideline for prevention of surgical site prevention, 1999. Hospital Infection Control Practices Advisory Committee. Infect. Control Hosp. Epidemiol.. 1999;20:250-255.
Morgan M. Surgery and cephalosporins: a marriage made in heaven or time for divorce? Intern. J. Surg.. 2006:8. Available at http://www.ispub.com/journal/the_internet_journal_of_surgery/volume_8_number_1/article/surgery_and_cephalosporins_a_marriage_made_in_heaven_or_time_for_divorce.html
Myles P.S., Iacono G.A., Hunt J.O., et al. Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: smokers versus nonsmokers. Anesthesiology. 2002;97:842-847.
National Institute for Health and Clinical Excellence. Surgical site infection: prevention and treatment of surgical site infection. Clinical Guideline 74. London: NICE; 2008. Available at http://www.nice.org.uk/nicemedia/pdf/CG74NICEGuideline.pdf
National Institute for Health and Clinical Excellence. Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. London: NICE; 2008. Available at http://www.nice.org.uk/nicemedia/pdf/CG64NICEguidance.pdf
Park M.A., Li J.T. Diagnosis and management of penicillin allergy. Mayo Clin. Proc.. 2005;80:405-410.
Pichichero M.E., Casey J.R. Safe use of selected cephalosporins in penicillin-allergic patients: a meta-analysis. Otolaryngol. Head Neck Surg.. 2007;136:340-347.
Prtak L.E., Ridgway E.J. Prophylactic antibiotics in surgery. Surgery (Oxford). 2009;27:431-434.
Publics Account Committee. Reducing healthcare associated infections in hospitals in England. Commons House. London: Hansard. 2009.
Saxon A., Beall G.N., Rohr A.S., et al. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann. Intern. Med.. 1987;107:204-215.
Scottish Intercollegiate Guideline Network. Antibiotic prophylaxis in surgery. Edinburgh: SIGN; 2008. Available at http://www.sign.ac.uk/pdf/sign104.pdf
Steinberg J.P., Braun B.I., Hellinger W.W., et al. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the trial to reduce antimicrobial prophylaxis errors. Annals of Surgery. 2009;250(1):10-16.
Stewart A., Eyers P.S., Earnshaw J.J. Prevention of infection in arterial reconstruction. Cochrane Database of Systematic Reviews, Issue 3, Art No. CD003073. DOI: 10.1002/14651858.CD003073.pub2. Available at http://onlinelibrary.wiley.com/o/cochrane/clsysrev/articles/CD003073/frame.html, 2006.
Sullivan S.A., Smith T., Chang E., et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. Am. J. Obstet. Gynecol.. 2007;196:455e1-455e5.
Thigpen B.D., Hood W.A., Chauhan S., et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am. J. Obstet. Gynecol.. 2005;192:1864-1868. Discussion pp. 1868–1871
Anderson D.J., Kaye K.S., Classen D., et al. Strategies to prevent surgical site infections in acute care hospitals. Infect. Control Hosp. Epidemiol.. 2008;29(s1):S51-S56.
Nelson R.L., Glenny A.M., Song F. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database of Systematic Reviews, Issue 1, Art No. CD001181. DOI: 10.1002/14651858.CD001181.pub3. Available at http://onlinelibrary.wiley.com/o/cochrane/clsysrev/articles/CD001181/frame.html, 2009.