CHAPTER 54 Surgical rehabilitation of Graves’ orbitopathy
Introduction
Graves’ orbitopathy (GO) is one of the phenotypic appearances of Graves’ disease, a multisystem disorder which usually leads to hyperthyroidism and goiter, less frequently to GO, and rarely to pretibial myxedema and acropachy. GO is the most frequent and important extrathyroidal expression of Graves’ disease. It may also be found, although less frequently, in patients with no present or past history of hyperthyroidism (so-called euthyroid or ophthalmic Graves’ disease), or in patients who are hypothyroid due to chronic autoimmune (Hashimoto’s) thyroiditis1,2. In most affected individuals GO is mild and self-limiting, and only in 3–5% of cases is it severe and potentially sight threatening1,3.
The exact pathogenesis of GO is unknown4–7. It is, however, worth highlighting the clear-cut link between the orbit and the thyroid, because this has important clinical and therapeutic implications8. In addition to endogenous (non-preventable) determinants, such as genetics, age- and gender-related factors9, GO occurrence and progression are influenced by environmental (preventable) factors, such as cigarette smoking, thyroid dysfunction, and different treatments for hyperthyroidism3,10. This implies that control or correction of these risk factors is an integral part of GO management.
The natural history of GO is of gradual increase in severity followed by a plateau phase then gradual improvement11. These are the active phases. The inactive phase follows with no change in severity. GO is thus self-limiting, although it often does not return to baseline. Treatment is aimed at accelerating recovery, preventing serious sequelae, and eventually functional and cosmetic rehabilitation.
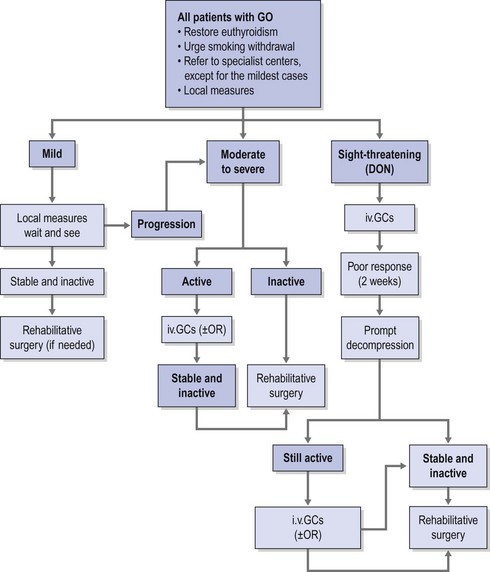
Therapeutic options consist of medical therapy, radiotherapy, surgery, or frequently a combination of these. Consensus as to indications and timing of these options has been reached by the EUGOGO consortium (Fig. 54.1)12,13.
Timing of surgery
The first rehabilitative step mainly consists of orbital bony decompression. It causes reduction in exophthalmos as well as reduction in upper and lower eyelid displacement14. It may positively influence extraocular muscle restrictions, but displacement and scarring of the soft orbital tissues caused by decompression surgery may also cause strabismus. Possible squint surgery should therefore follow orbital decompressions, but considering that vertical tropias may influence eyelid position, squint surgery should precede possible eyelid corrections. Finally, when necessary, the finishing touch can be given by eyebrow lift, forehead plasty, and blepharoplasty.
In short, surgical rehabilitation needs to respect the given order since the preceding step may influence the necessity and the extent of the step that follows. When all the steps are necessary, the entire rehabilitation may require between 1.5 and 2 years. In particular cases, exceptions are possible and the rehabilitation can be favorably speeded up by carrying out more than one procedure at the same time15. The traditional management algorithm has not been respected in only a few series16–20, and it has met with vigorous criticism21.
Orbital decompression
This positive feedback circle leads to an increase in the intraorbital pressure, which is first responsible for the progression of GO and later for its typical signs and symptoms5. Any surgical procedure aimed at decreasing the raised intraorbital pressure and its effects by means of enlargement of the bony orbit and/or removal of the orbital fat is defined as orbital decompression.
Orbital decompression is currently indicated for the treatment of optic neuropathy refractory to medical therapy, exposure keratopathy unresponsive to local measures and/or minor eyelid surgeries, disfiguring exophthalmos and symptoms. Eyeball subluxation (which may be a possible cause of acute optic neuropathy and exposure keratopathy) postural visual obscuration in patients with congestive inactive GO and recently onset choroidal folds due to eyeball indentation by enlarged extraocular muscles represent other functional indications for decompression surgery22.
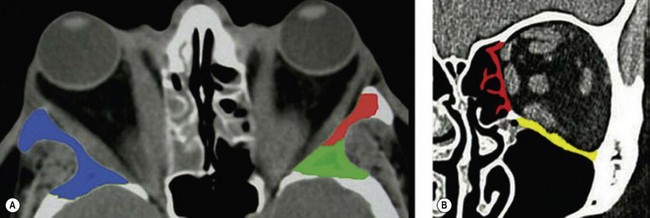
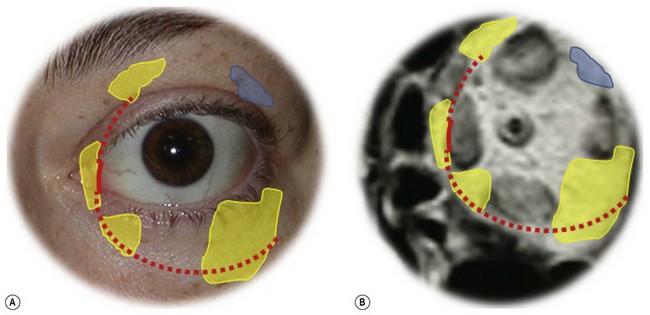
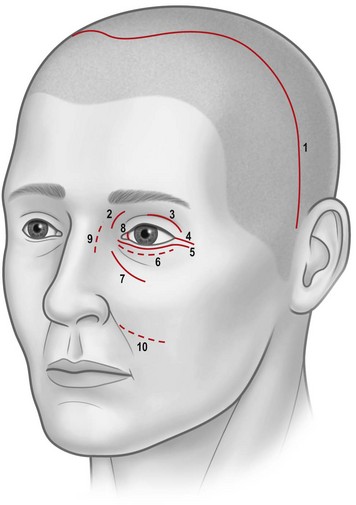
Osteotomies can involve the medial, and lateral orbital walls and the orbital floor (Fig. 54.2); lipectomies can be performed at the level of all the orbital quadrants (Fig. 54.3). Decompression surgery can be performed through several different surgical incisions (Fig. 54.4) preferably under general anesthesia.
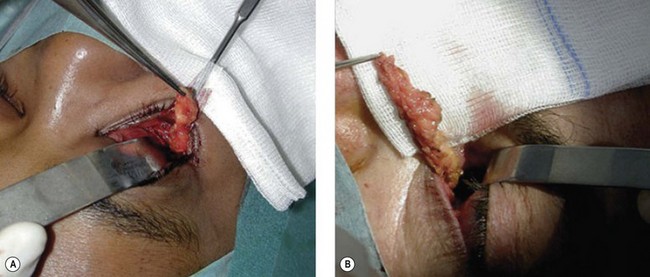
The inferior fornix incision is an extremely popular approach to orbital decompression. It can be extended medially into a transcaruncular incision and can be associated with an incision at the lateral canthus that permits the lower lid to swing outwards, thus easing the exposure of the lateral wall. This latter association, first described by McCord in 1981, is known as ‘swinging eyelid’23. This combination of periorbital incision permits an easy approach to the orbital floor, medial, and lateral walls and lipectomies from the inferior orbital quadrants.
The coronal approach is a more invasive procedure which, however, offers the widest access to the lateral orbital wall24, and it may have distinctive advantages in a number of situations including the presence of remarkable periorbital swelling or conjunctival chemosis, the necessity of minimizing the number of periorbital incisions, or the necessity of extensive manipulation of the lateral wall (including its rim)25,26. Through a coronal incision, brow lift, and correction of frontal/glabellar rhytids, which are often necessary in patients with GO, can be performed simultaneously with orbital decompression, thus favorably speeding up the timing of rehabilitative surgery25,27.
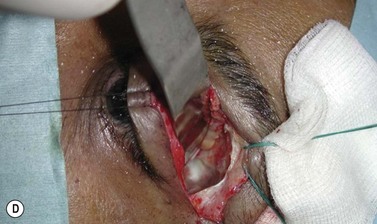
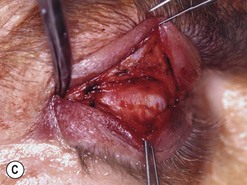
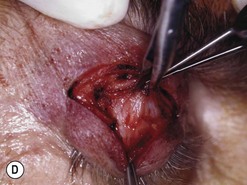
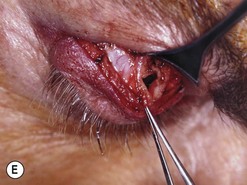
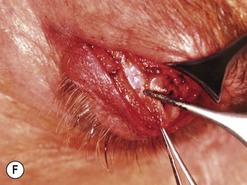
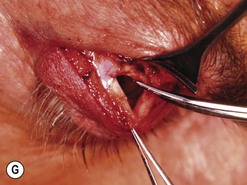
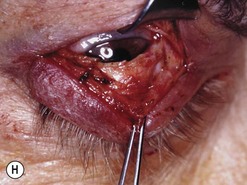
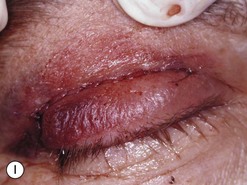
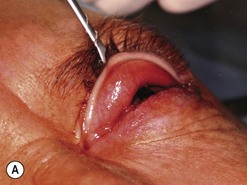
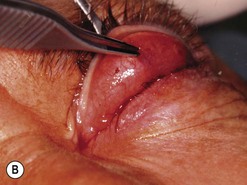
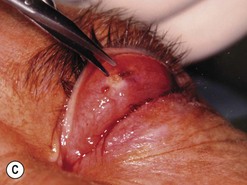
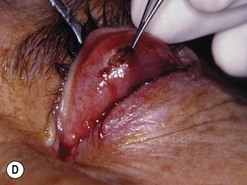
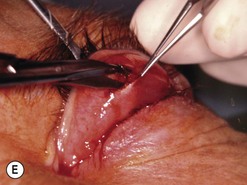
Orbital decompression by transinferior fornix/transcaruncular/swinging eyelid: surgical technique
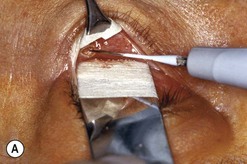
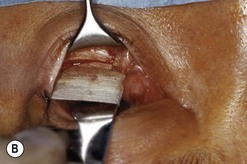
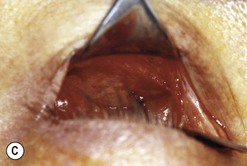
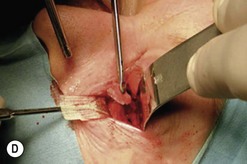
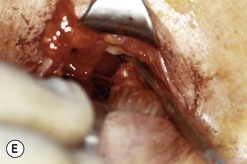
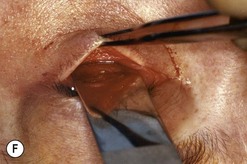
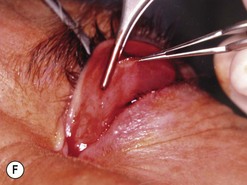
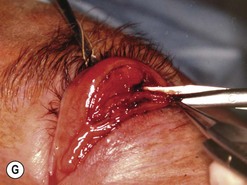
Fig. 54.5 Transinferior fornix decompression. Phases of surgery (A–F); for explanations see the main text.
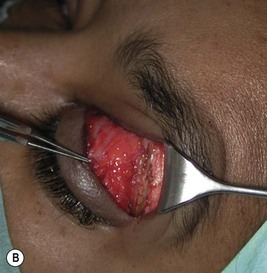
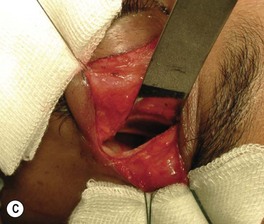
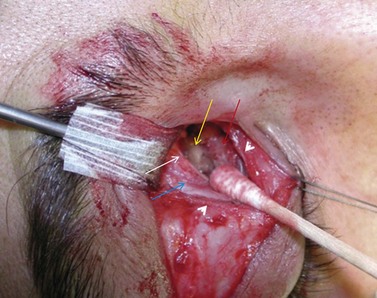
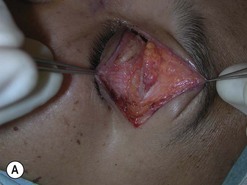
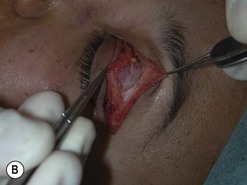
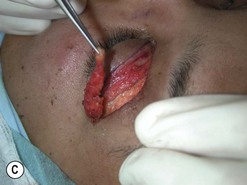
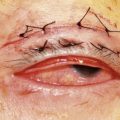
Orbital decompression by upper skin crease approach: surgical technique
 of the upper skin crease if the upper medial fat compartment is not to be addressed. On occasion it can be joined to the lateral canthotomy of a possible swinging eyelid approach to implement the exposure of the surgical site (Fig. 54.7A).
of the upper skin crease if the upper medial fat compartment is not to be addressed. On occasion it can be joined to the lateral canthotomy of a possible swinging eyelid approach to implement the exposure of the surgical site (Fig. 54.7A).Orbital decompression by coronal approach: surgical technique
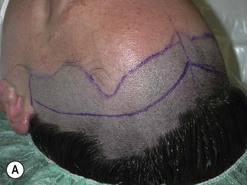
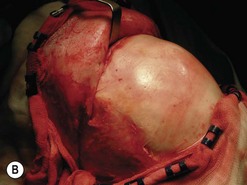
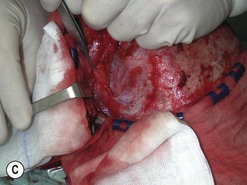
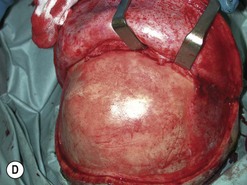
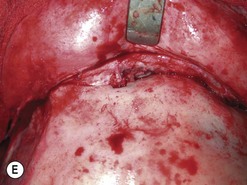
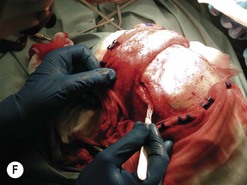
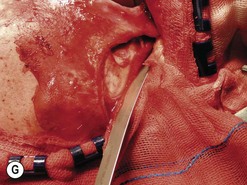
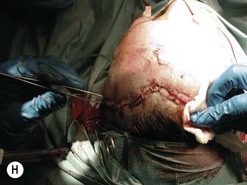
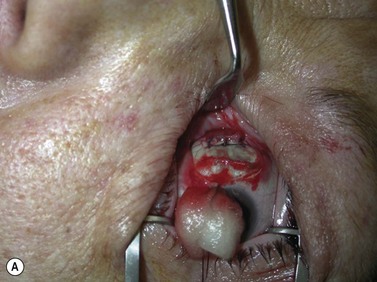
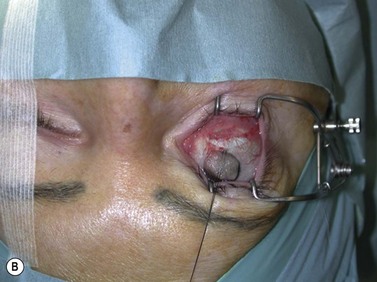
Fig. 54.9 Orbital decompression by coronal approach. Surgical phases (A–H); the explanation is in the text.
Possible complications of decompression surgery
Despite their invasiveness, bone and fat orbital decompressions are safe procedures. Common complications of this surgical approach are consecutive strabismus, infraorbital hypoesthesia and sinusitis, lower lid entropion, and eyeball dystopia. Leakage of cerebrospinal fluid, infections involving the central nervous system, damage to the eye and optic nerve or their vasculature, cerebral vasospasm, ischemia, and infarction are severe but rare events. Reactivation of GO after rehabilitative bony orbital decompression and infraorbital pain are other rare complications22.
In addition to complications common to orbital decompressions in general, different surgical approaches may carry the risk of specific complications. The coronal approach leaving the eyelid undisturbed is less likely than periorbital incisions to create complications, which may potentially be harmful to the eye. Periorbital scarring with iatrogenic lid retraction and cicatrical lagophthalmos, eyelid margin malpositions, and ptosis (although rare) are more likely to occur with periorbital incisions. On the other hand, temporal bossing, damage to the frontalis nerve, scarring and alopecia at the site of the scalp incision, or effects upon ischemic areas of the frontal flap after healing by secondary intention, may complicate the coronal approach25.
Most of the possible complications cannot be predicted and their prevention is based on recommendations which are not specific in nature, and which include careful manipulation of the orbital content, accurate dissection of the orbital fat, and avoidance of expandable hemostatic agents and/or extensive use of diathermy within the orbit22.
Complications with known pathogenesis such as sinusitis can be simply prevented by taking care to create adequate sinus aeration as a part of the surgical procedure at the time of bone decompression22.
The occurrence of other complications, namely sinuses atelectasis, infraorbital hypoesthesia or pain, eyeball dystopia leakage of cerebrospinal fluid, and possible consecutive infectious involvement of the central nervous system, can be reduced by means of accurate evaluation of preoperative imaging, adequate planning of surgical intervention, and the use of prophylactic antibiotics22.
Correction of lid retraction
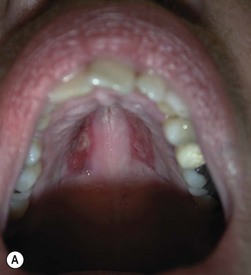
In GO, upper and lower lid retraction are due to a combination of inflammation, fibrosis, adrenergic stimulation, and restriction of the vertical rectus muscles. Exophthalmos also contributes in increasing the eyelid aperture by displacing either the upper or the lower lid14. Correction of upper or lower lid retraction implies recession of the lid retractors. Spacers are not essential for upper lid-lengthening procedures, but are necessary to provide height and the necessary stiffness to support the lower lid against gravity when it is severely retracted. A number of autologous, homologous, xenogenic, and synthetic materials have been used including ethanol-preserved donor sclera, upper lid tarsus, cartilage grafts, porous polyethylene, polytetrafluoroethylene or polyester meshes. Autogenous hard palate mucosal graft is relatively easy to obtain, is similar to lower lid tarsus in terms of thickness and stiffness, has a mucosal surface, has no risk of rejection, and undergoes minimal shrinkage following grafting. Ophthalmic complications of hard palate mucosal grafting are uncommon and morbidity at the donor site is rare29. Hard palate mucosal graft providing structural and epithelial elements represents an elective material for posterior lamella augmentation in lower lid lengthening. Homologous acellular dermal matrix with an appropriate consistency for posterior lamella augmentation is an optimal alternative to hard palate mucosa; unfortunately it is not readily available throughout Europe.
Lower eyelid lengthening
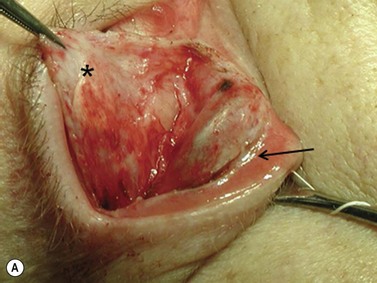
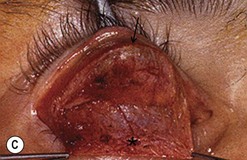
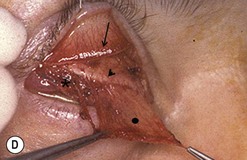
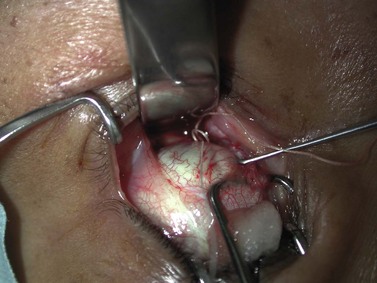
Hard palate mucosal graft harvesting: surgical technique
Local anesthesia is possible, but general anesthesia is preferred for the patient’s comfort.
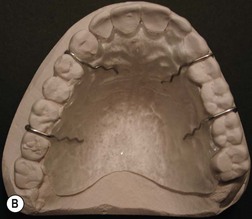
Postoperative care
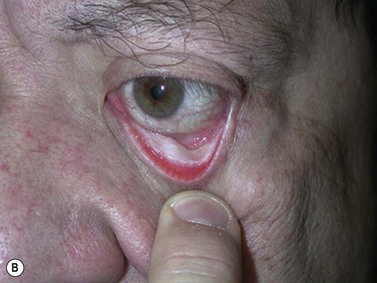
Broad-spectrum systemic antibiotics and antiseptic mouthwashes are recommended for 1 week after surgery. Soreness at the donor site for a few days after surgery is common especially during eating; custom-made acrylic palatal stents, which aid hemostasis and facilitate granulation, can increase the patient’s comfort (Fig. 54.10B).
Lower lid lengthening: surgical technique
Upper lid lengthening
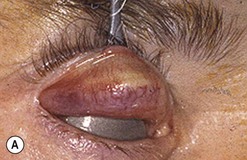
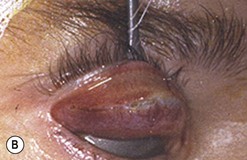
Sutureless transconjunctival müllerectomy: surgical technique
Free en bloc recession of conjunctiva–levator complex by anterior (blepharotomy) or sutureless posterior approach
Upper eyelid lengthening by means of blepharotomy was developed by Leo Koornneef, but because of his untimely death he was unable to publish his idea; a large series published by one of his fellows contributed to popularize the technique30.
Blepharotomy: surgical technique
Posterior approach: surgical technique
Squint surgery
Surgical techniques are similar to those described in Chapter 57; in the case of dysthyroid strabismus muscle recession is often generous. In such cases, in order to maintain the contact arch, extraocular muscle tendon elongation should be done. It can be attained by means of autologous or donor fascia grafts sutured between the recessed muscle tendon and the eye globe, or more simply by recessing the muscle by means of hang-back loops attained with sutures in 5.0 absorbable material (Fig. 54.15A).
Long-lasting dysthyroid restrictions are associated with conjunctival scarring; for this reason muscle recessions should preferably be done through a base fornix conjunctival incision, which permits conjunctival lengthening by means of its recession at the end of surgery (Fig. 54.15B).
Because of the enlarged GO muscles, retroequatorial miopexias can be difficult to perform, unless the ‘faden’ is passed through the sclera after having detached the muscle to be treated from its eye globe insertion. In many case this maneuver does not increase the surgical time as often in dysthyroid strabismus retroequatorial miopexias need to be associated with muscle recessions. A double passage of the ‘faden’ into the sclera is advisable in order to increase the strength of the suture the mechanical action of the oversized muscle (Fig. 54.16).
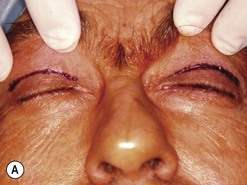
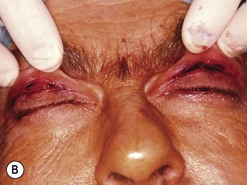
Cosmetic eyelid surgery
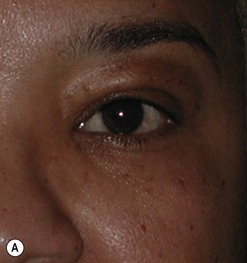
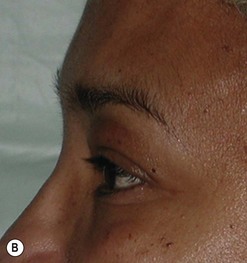
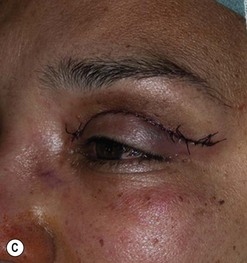
Cosmetic periorbital surgery in GO is substantially similar to that for the aging face (see Chapter 51). Skin removal should, however be more conservative in GO, as skin excess is often more apparent than real in the affected patients. Another difference concerns the treatment of the sub eye brow fat pad. Up until recently, in the aging patient, it was sculptured or resected with the unfortunate effect of taking away the natural fullness of the upper eyelid fold. Nowadays, sub-eyebrow fat repositioning is in general preferred in cosmetic surgery, while it continues to be sculptured or resected when, as in GO, it is pathologically enlarged (Fig. 54.17A–C). In light of the anti-vitamin-K action of commonly used thionamides such as methimazole in patients with GO, sculpturing or excision of the sub-brow fat pad can be assisted by the use of a CO2 laser (Fig. 54.18A–C).
1 Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21:168-199.
2 Perros P, Dickinson AJ. Ophthalmopathy. In: Braverman LE, Utiger RD, editors. Werner’s and Ingbar’s The Thyroid – A Fundamental and Clinical Text. 9th edn. Philadelphia: Lippincott Williams & Wilkins; 2005:474-487.
3 Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12:855-860.
4 Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24:802-835.
5 Bahn RS. Clinical review 157: Pathophysiology of Graves’ ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003;88:1939-1946.
6 Ludgate M, Baker G. Inducing Graves’ ophthalmopathy. J Endocrinol Invest. 2004;27:211-215.
7 Ajjan RA, Weetman AP. New understanding of the role of cytokines in the pathogenesis of Graves’ ophthalmopathy. J Endocrinol Invest. 2004;27:237-245.
8 Bartalena L, Wiersinga WM, Pinchera A. Graves’ ophthalmopathy: state of the art and perspectives. J Endocrinol Invest. 2004;27:295-301.
9 Perros P, Crombie AL, Matthews JN, et al. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf). 1993;38:367-372.
10 Bartalena L, Marcocci C, Pinchera A. Graves’ ophthalmopathy: a preventable disease? Eur J Endocrinol. 2002;146:457-461.
11 Rundle FF. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5:177-194.
12 Bartalena L, Baldeschi L, Dickinson JA, et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Eur J Endocrinol. 2008;158:273-285.
13 Bartalena L, Baldeschi L, Dickinson JA, et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid. 2008;18:333-346.
14 Baldeschi L, Wakelkamp IMMJ, Lindeboom R, et al. Early versus late orbital decompression in Graves’ orbitopathy: a retrospective study in 125 patients. Ophthalmology. 2006;113:874-878.
15 Baldeschi L. Rehabilitative surgery. In: Wiersinga WM, Kahaly GJ, editors. Graves’ Orbitopathy: A Multidisciplinary Approach – Questions and Answers. 2nd edn. Basel: Karger; 2010:167-170.
16 Michel O, Oberländer N, Neugebauer P, et al. Follow-up of transnasal orbital decompression in severe Graves’ ophthalmopathy. Ophthalmology. 2001;108:400-404.
17 Tremolada C, Tremolada MA. The ‘triple technique’ for treating stable Graves’ ophthalmopathy. Plast Reconstr Surg. 1997;100:40-48.
18 Ben Simon GJ, Mansury AM, Schwarcz RM, et al. Simultaneous orbital decompression and correction of upper eyelid retraction versus staged procedures in thyroid-related orbitopathy. Ophthalmology. 2005;112:923-932.
19 Richter DF, Stoff A, Olivari N. Transpalpebral decompression of endocrine ophthalmopathy by intraorbital fat removal (Olivari technique): experience and progression after more than 3000 operations over 20 years. Plast Reconstr Surg. 2007;120:109-123.
20 Olivari N. Endocrine ophthalmopathy: Surgical treatment. HNO. 2010;58:8-10. 12–4
21 Chang EL, Rubin PAD. Thyroid orbitopathy. Ophthalmology. 2006;113:718-719.
22 Baldeschi L. Orbital decompression. In: Wiersinga WM, Kahaly GJ, editors. Graves’ Orbitopathy: A Multidisciplinary Approach – Questions and Answers. 2nd edn. Basel: Karger; 2010:171-188.
23 McCord CD. Orbital decompression for Graves’ disease: exposure through lateral canthal and inferior fornix incision. Ophthalmology. 1981;88:533-541.
24 Goldberg RA, Weinberg DA, Schorr N, et al. Maximal 3-wall orbital decompression through a coronal approach. Ophthalmic Surg Lasers. 1997;28:832-843.
25 Baldeschi L. Small versus coronal incision orbital decompression in Graves’ orbitopathy. Orbit. 2010;29:177-182.
26 van der Wal KG, de Visscher JG, Boukes RJ, et al. Surgical treatment of Graves orbitopathy: a modified balanced technique. Int J Oral Maxillofac Surg. 2001;30:254-258.
27 Saks ND, Burnstine MA, Putterman AM. Glabellar rhytids in thyroid-associated orbitopathy. Ophthal Plast Reconstr Surg. 2001;17:91-95.
28 Shorr N, Baylis HI, Goldberg RA, et al. Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology. 2000;107:1459-1463.
29 Kim JW, Kikkawa DO, Lemke BN. Donor site complications of hard palate mucosal grafting. Ophthal Plast Reconstr Surg. 1997;13:36-39.
30 Hintschich C, Haritoglou C. Full thickness eyelid transsection (blepharotomy) for upper eyelid lengthening in lid retraction associated with Graves’ disease. Br J Ophthalmol. 2005;89:413-416.