CHAPTER 335 Surgical Management of Traumatic Brain Injury
Approximately 1.5 million head injuries occur every year in the United States, with 250,000 patients requiring hospitalization and 52,000 dying of the injury.1 At least 5.3 million Americans, 2% of the U.S. population, are currently living with disabilities resulting from traumatic brain injury (TBI).2,3 Furthermore, TBI is the leading cause of death and disability in children and adults 1 to 44 years of age.1 When compared with most severe medical conditions, TBI has a predilection for the young. This magnifies the economic impact of TBI as one considers the cost of long-term care coupled with the loss of productivity from an active sector of the workforce. Even with this growing appreciation of the consequences of TBI, the number of injuries continues to climb. Hospitalization rates for TBI have increased from 79 per 100,000 in 2002 to 87.9 per 100,000 in 2003.1 In “newly mechanized” countries such as Vietnam, Columbia, China, and Brazil, the numbers are much higher, and greater effort toward prevention of TBI is needed.
Management of TBI presents extensive challenges for neurosurgeons worldwide. The complexity of the injury, which is usually associated with trauma to other organ systems, makes decisions about medical or surgical management critical to patient outcome. Gennarelli and coworkers showed that the overall mortality is 3 times higher in trauma patients with head injury than in those without intracranial trauma.4 Their study also showed that among head-injured patients, the cause of death was attributed to brain injury in 67.8%, to extracranial injuries in 6.6%, and to both cranial and extracranial trauma in 25.6%. The severity of the head injury still remains the strongest predictor of overall outcome in multiply traumatized individuals.4–6 In the past, many neurosurgeons regarded surgery for posttraumatic intracranial hematoma (ICH) to be unrewarding. This pessimism was based on the belief that outcome is determined principally by the magnitude of the initial injury and that it frequently remains poor despite optimal surgery. In fact, in the Traumatic Coma Data Bank series, only 37% of comatose patients underwent surgery for the removal of ICH.7 Although the mortality rate after severe head injury remains as high as 30% to 35% in some series, it has fallen to as low 20% in centers that treat brain injury frequently and aggressively.
The most dangerous consequence of severe TBI is the development of ICH, also known as “mass lesion,” which accounts for approximately 70% of the causes of clinical deterioration. One publication reported the incidence of ICH to be 25% to 45% in patients with severe TBI, 3% to 12% in those with moderate TBI, and 1 in 500 in those with mild TBI.8 Consequently, prompt and aggressive identification of ICH is of paramount importance during the evaluation of a patient with severe TBI. At least 25% of patients with mass lesions will clinically deteriorate in the initial 2 to 3 days after injury.9 This is especially true for contusions, notoriously known to blossom, as opposed to epidural hematoma (EDH) and subdural hematoma (SDH). The most commonly encountered ICHs are SDH, EDH, intraparenchymal hematoma and contusions, and posterior fossa hematoma. Surgery plays a vital role in the management of patients with severe TBI and concomitant ICH. Additionally, other complications of TBI that may warrant surgical intervention include depressed skull fractures, sinus injuries, and intractable intracranial hypertension requiring decompressive craniectomy. In general, the decision to operate is based on (1) findings on computed tomography (CT), (2) clinical neurological status, (3) clinical deterioration, and (4) extent of extracranial injury. High-volume lesions (>50 cc) customarily undergo surgery, whereas small lesions (<25 cc) are usually managed with conservative therapy. Once the indications for surgery have been met, early and urgent surgical intervention is suggested to prevent further neurological decline, minimize perilesional edema, improve the local metabolic environment, and attenuate evolving ischemic changes. Interestingly, blood constituents were shown to worsen focal ischemia in a study comparing equal volumes of hematoma with an inert fluid used to generate experimental intracerebral mass lesions.10 However, the ischemia was not improved after removal of the hematoma. Mass lesions can also alter cerebral metabolism, and their removal has been shown to improve jugular venous saturation indices.11
Conservative Management
Only a third of patients who sustain severe head injuries are candidates for craniotomy, and hence a majority of patients are managed by nonsurgical means. The strategy for nonsurgical management of TBI is focused on prevention of secondary injury after TBI. Medical interventions are targeted at controlling intracranial pressure (ICP), ensuring adequate blood flow and oxygen delivery, correcting and then maintaining a healthy metabolic environment, and minimizing edema. Improvements in early resuscitation, diagnosis, neurophysiologic monitoring, and emergency surgical treatment of head injury victims may be reaching a plateau in terms of further reducing mortality and morbidity. Refinements in neurocritical care combined with technologic advancements in diagnostic and monitoring devices continue to offer new avenues for enhancing outcome. This subject is covered fully in Chapter 334.
About two thirds of patients with severe head injury have no significant mass lesion on the initial CT scan. At least 25% of patients with mass lesions will clinically deteriorate in the initial 2 to 3 days after injury.9 This is especially true for contusions, notoriously known to “blossom,” especially when occult or overt coagulopathy is present. At least 80% of patients with severe TBI have high ICP, and in the majority who die, high ICP is the cause of death.12–15 For this reason, ICP monitoring and ICP-directed intensive care therapy are advocated for all patients older than 40 years whose Glasgow Coma Scale (GCS) score remains 8 or lower at 6 or more hours after injury. In patients younger than 40 years, ICP monitoring can be based on the status of the basal cisterns as demonstrated on good-quality CT. Effaced cisterns are associated with high ICP in more than 70% of patients and mandate ICP monitoring. In patients younger than 40 with patent cisterns, normal findings on CT, and the absence of hypoxic or ischemic episodes, ICP monitoring can be deferred and repeat CT performed in 6 to 12 hours.14 Among the most difficult neurotrauma patients to manage are those whose GCS scores are between 8 and 14 and who have mass lesions of intermediate size. Frequently, they are restless, combative, uncooperative, or intoxicated, thus making GCS assessment very difficult. In such circumstances, it is usually best to intubate, sedate, and ventilate the patient in an intensive care setting and carry out ICP monitoring. Appropriate management of these moderate TBIs and medium-sized hematomas is the biggest difference that clinicians can make. If ICP is persistently greater than 20 to 25 mm Hg, surgical evacuation is indicated even for small mass lesions because associated brain swelling is extremely common.
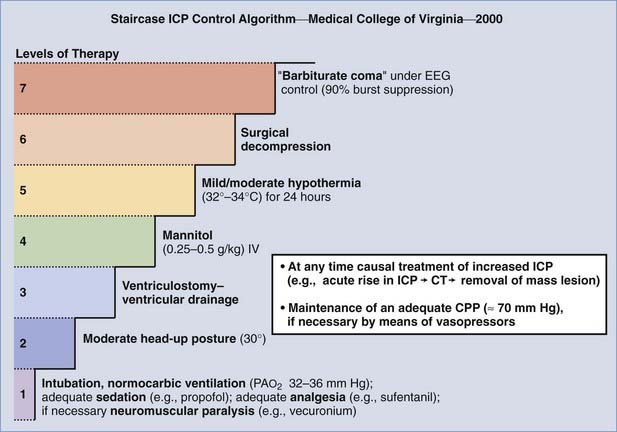
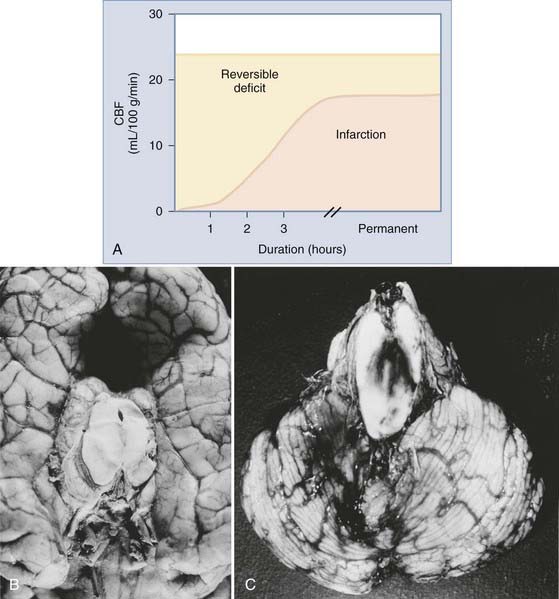
The Medical College of Virginia first proposed a “staircase” of therapeutic steps to optimally treat elevated ICP (Fig. 335-1). The early, aggressive management of ICP by surgical or medical means is directed primarily at optimizing cerebral blood flow (CBF) and preventing cerebral ischemia and the consequent irreversible neurological deficits and mortality (Fig. 335-2A). Nonsurgical management is detailed in the third edition of the “Guidelines for the Management of Severe Traumatic Brain Injury.”16 Uncal herniation with midbrain compression and Duret’s hemorrhages of the brainstem are the usual postmortem findings in patients who succumb to untreatable intracranial hypertension (Fig. 335-2B and C). Management decisions in individual patients must take into account a number of factors, such as extracranial injuries, the age of the patient, preexisting conditions, and the presence of associated intracerebral contusions or hemisphere swelling. In patients with intraparenchymal lesions, such as contusions and intracerebral hematomas, management decisions are more complex and difficult given the risk for coagulopathy and bleeding. Some recent studies have provided guidelines for the management of SDH and extradural hematoma in the relatively uncommon situation of a fully conscious patient. Nonoperative management should be considered only if the patient is fully conscious, the extra-axial mass lesion is the single dominant lesion (i.e., there are no multiple contusions or potentially significant contralateral mass lesions that may be preventing midline shift), and there are no features of a mass effect, such as a midline shift greater than 3 mm, or basal cistern effacement.17 If these criteria are met in a conscious patient with an acute subdural hematoma (aSDH) less than 10 mm at its thickest point, conservative treatment has been shown to be successful in most cases.18 Similarly, deep-seated tentorial or interhemispheric SDHs (Fig. 335-3) and small extradural hematomas in a stable, conscious patient frequently do not require surgical evacuation. However, when doubt exists and consciousness is depressed, the neurosurgeon should always monitor ICP or remove mass lesions in such patients.
Indications for the Evacuation of Intracranial Hematomas
Until recently, the role of surgery in the management of TBI was often based on subjective criteria or previous experience of the surgeon. Most would agree that surgical evacuation is always indicated for a mass lesion when there is a decline in the patient’s level of consciousness, the development of new focal signs, and severe and worsening headache, nausea, or vomiting. In unconscious, noncommunicating, or sedated and ventilated patients, surgical evacuation is always indicated when there is a decline in neurological status (this may be revealed only by the development of new “brainstem” signs) and a sustained increase in ICP (e.g., >25 mm Hg). CT should be done urgently to evaluate progression of the ICH and brain swelling, and blood gas analysis should be performed to exclude the onset of hypoxemia, if appropriate. An increase in the size of an ICH on CT is also an indication for surgical removal.19
In 2006, a joint venture of the Congress of Neurological Surgeons and the Brain Trauma Foundation published the “Guidelines for the Surgical Management of Traumatic Brain Injury.”20 The publication is the product of an extensive review of the literature dating from 1975 to 2001. The authors applied the principles of “evidence-based medicine” throughout the evaluation process, but the paucity of well-designed, randomized controlled trials for surgical lesions prohibited classification of the literature with the “level of evidence” categories now customary in constructing guidelines. Instead, the results are presented as “literature-based recommendations.” Five primary complications of TBI warranting surgical consideration were identified: acute epidural hematomas (aEDHs), aSDHs, traumatic parenchymal lesions, posterior fossa mass lesions, and depressed cranial fractures. Worthy of special consideration are temporal lobe hematomas (Fig. 335-4), which are especially treacherous and dangerous. They may cause brainstem compression at low ICP with little midline shift, and the threshold to operate should thus be much lower. Finally, in patients who require operative intervention, urgent and rapid evacuation of the mass lesion ensures the best outcome because ischemic brain damage is dependent on the duration of ischemia. The specific recommendations in the aforementioned document are presented in the following sections.
Acute Epidural Hematoma
Timing
Important caveat: EDH in the middle fossa/inferotemporal lobe should have a lower threshold for surgery. Hematomas in this location can cause brainstem compression at low ICP with little midline shift.17,21 Additionally, one report found anisocoria, suggestive of impending herniation, to occur in patients with ICP as low as 18 mm Hg.22
Acute Subdural Hematoma
Note: SDH is considered acute if it is diagnosed within 14 days of TBI.
Indications for Surgery
Intraparenchymal Hemorrhage and Contusions
Indications for Surgery
Timing and Methods
Posterior Fossa Mass Lesions/Hemorrhages
Indications for Surgery
Evaluation of Relevant Findings on Computed Tomography
Posttraumatic Mass Volume Measurement in Patients with Traumatic Brain Injury
The preceding guidelines recommend using a modified ellipsoid method, better remembered as the “ABC/2” method, to estimate the volume of an ICH.23 This method has the following steps:
Radiographic Correlates of Outcome
Standard CT protocols acquire 5-mm slices from the foramen magnum to the sella and then 10-mm slices above the sella, parallel to the orbitomeatal line.20 Chesnut and colleagues identified three early CT parameters that correlate with outcome24:
Preoperative Preparation
Frequently, surgery in the setting of TBI is done under emergency conditions, but nevertheless, every effort should be made to continue resuscitative efforts and optimize a patient’s neurophysiologic status while preparing for surgery—all of which may need to be accomplished in less than 10 minutes. Certain goals have been found to correlate with improved outcome in the setting of severe TBI and are outlined in the third edition of the “Guidelines for the Management of Severe Traumatic Brain Injury.”16 These goals include maintaining mean arterial pressure greater than 70 mm Hg (>90 mm Hg until cerebral perfusion pressure [CPP] can be measured), CPP higher than 60 mm Hg, euthermia, eucapnia, oxygen saturation greater than 93%, PaO2 of 95 to 100 mm Hg, ICP higher than 20 mm Hg, and a serum sodium concentration of 135 to 145 mEq. All patients with severe head injury should be intubated to protect the airway, ensure adequate oxygenation, and control PaCO2 at the appropriate level. Prophylactic hyperventilation or the use of mannitol is no longer recommended unless the patient exhibits focal neurological signs (contralateral weakness, ipsilateral anisocoria or “blown pupils,” decerebrate or decorticate posturing). If this is the case, the patient is best hyperventilated to a PaCO2 of 30 to 32 mm Hg and given 1 g/kg of mannitol immediately while being taken to the operating room.
Finally, preoperative assessment must always rule out coagulopathic states. This is especially important in view of the increase in TBI among the growing elderly population. The frequent use of anticoagulant and antiplatelet drugs, often for marginal indications, can generate dangerous operative conditions. Qualitative platelet disorders have been described in those with chronic alcoholism and chronic aspirin ingestion. The recent development plus use of recombinant factor VIIa (rFVIIa) has provided a new option in the management strategy for these patients. rFVIIa is thought to induce hemostasis within 10 minutes of administration and has been used successfully to decrease hematoma expansion in patients with hypertensive hemorrhage and trauma.25,26 Recently, rFVIIa was shown to decrease the time needed to normalize coagulopathic trauma patients and allow earlier surgical intervention.27 By decreasing the time to invention it is hoped that outcomes can be improved as well. However, rFVIIa is very expensive, has been linked to increased thromboembolic complications, and has a relatively short half-life of 2 to 3 hours. Research is still ongoing to determine the optimal dose of rFVIIa in patients with TBI. However, in coagulopathic patients requiring urgent normalization for emergency surgery, such as those with herniation, it is suggested that 20 to 40 mg of rFVIIa, 5 to 10 mg of vitamin K intravenously, platelets, and fresh frozen plasma (FFP) be administered as determined by preoperative laboratory work. Because of its short half-life, rFVIIa may need to be redosed if further normalization is necessary based on the partial thromboplastin time (PTT) and international normalization ratio (INR). Finally, in addition to its hemostatic actions, rFVIIa was unexpectedly shown to be neuroprotective by reducing hippocampal neuronal degeneration, as well as the extent of traumatic axonal injury, in an animal model of TBI.28
Before or during preparation for craniotomy, the following checklist should be completed:
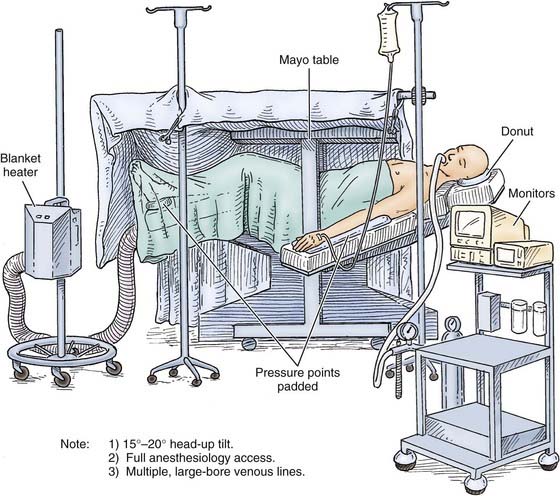
Figure 335-5 shows how a patient is positioned for a craniotomy. In general, the head is placed on a horseshoe or “doughnut” headrest, turned to the opposite side while avoiding any constriction of the neck veins, and elevated above the level of the heart. A sandbag placed beneath the ipsilateral shoulder makes turning the head easier and also relaxes the tissues in the neck. The pressure points should be padded carefully. Unless deterioration is rapid, the scalp should be shaved and prepared as for any other neurosurgical procedure.
Strict attention to anesthetic techniques is vital to avoid hypercapnia and further elevation of ICP. All inhaled anesthetics have a potential cerebral vasodilator effect and may therefore increase ICP. They also lower cerebral metabolism, with the exception of nitrous oxide (N2O). Thus, a beneficial dissociation of CBF and the cerebral metabolic rate of oxygen (CMRO2) may occur with their use.29–31 N2O was used widely in the past to maintain anesthesia, and its rapid action allows easy control of the depth of anesthesia. However, because there is evidence that N2O may increase ICP as a result of vasodilation and because the interaction of N2O with volatile and intravenous anesthetics is complex, N2O should be avoided in surgery after acute central nervous system trauma. Similarly, the use of volatile agents (halothane, enflurane, sevoflurane, desflurane, and isoflurane) has had its drawbacks. Use of halothane for surgery on intracranial lesions has decreased in recent years, although it may be safe in low concentrations (≈0.5%), because it can also increase ICP and uncouples CBF and CMRO2 in a dose-dependent manner, thereby producing cerebral vasodilation and a decline in CMRO2.31 Enflurane, like halothane, is a cerebral metabolic depressant but has weaker vasodilatory properties. Because of its potential effects on ICP and the risk of inducing seizures, high concentrations of enflurane should be avoided in neurosurgical patients. Sevoflurane and desflurane have cerebrovascular effects similar to those of enflurane and should also be avoided. Isoflurane is not as lipid soluble as other volatile agents. Because of its depressive effect on cerebral metabolism, isoflurane may be neuroprotective and is a more desirable anesthetic for neurosurgical procedures among the inhaled anesthetic agents. Isoflurane also produces a moderate increase in CBF and a pronounced beneficial decrease in cerebral metabolism.
Intravenous anesthetics also cause a decrease in CBF and CMRO2 because of their depressant effect on cerebral metabolism. In recent years, propofol has become the “mainstay” of most anesthetic and sedation regimens for patients with TBI. Propofol’s metabolic effect on cerebral metabolism is similar to that of barbiturates and it may decrease ICP.32 This drug is very useful in neurosurgical patients for sedation and as an anesthetic, provided that hypotension is prevented. Barbiturates (thiopental, pentobarbital) produce a potent dose-dependent reduction in CBF, CMRO2, and ICP, as well as a burst suppression pattern on electroencephalography (at about a 40% decrease in CBF). Barbiturates also attenuate the cerebral vasodilation produced by volatile anesthetics.33 Etomidate has metabolic effects similar to those of barbiturates, and both may be used for “cerebral protection” and to control brain swelling intraoperatively after TBI.34 Benzodiazepine derivatives (e.g., midazolam) are also useful as induction or supplemental drugs during anesthesia in neurosurgical patients with TBI. A newer agent currently under intense scrutiny for use in neurosurgery is dexmedetomidine. It is an α2-agonist that has been promoted as a sedative that does not cause respiratory depression. Although its full utility in neuroanesthetic practice is still being investigated, it is primarily used in operative settings requiring patient interaction and very rapid onset/offset times (e.g., functional cases and awake craniotomies). At the current time, its role in TBI appears to best be suited to the intensive care unit in patients requiring mild sedation yet frequent neurological assessment. More studies are needed to establish the cost-effectiveness and utility of dexmedetomidine versus older agents, such as propofol.
Narcotic analgesics (morphine, fentanyl, sufentanil) have modest depressive effects on CBF and CMRO2, and in most circumstances they have only a minimal effect on ICP. They should generally be avoided or used in small amounts during anesthesia for acute central nervous system trauma because of their long duration of action and the availability of other agents.35
The use of nondepolarizing muscle relaxants (tubocurarine, pancuronium, atracurium, vecuronium) changes CBF and ICP only minimally if respiration is well controlled. If succinylcholine is used, a small dose of a nondepolarizing muscle relaxant should be given before its administration to prevent a rise in ICP with fasciculation.36
Ventriculostomy
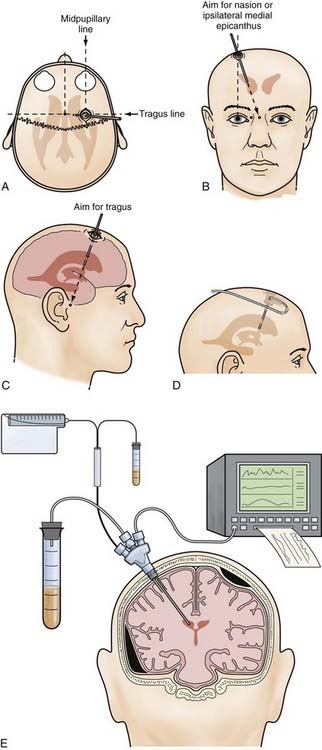
After shaving the scalp and sterile surgical preparation, the patient’s head is placed supine. A small incision (<3 cm) is made in the midpupillary line, 4 cm from the midline and 2 cm in front of the coronal suture on the right side (Fig. 335-6A). The incision is carried down to the underlying bone, and the periosteum is elevated. If the coronal suture cannot be felt easily under the scalp, a distance of approximately 10 cm is measured upward and posteriorly from the superior orbital margin in the midpupillary plane. Next, a twist drill is used to make an opening in the skull perpendicular to the plane of the skull. The twist drill hole is cleaned of any bony fragments with a small curet, and the underlying dura is pierced with a blunt stylet. A ventriculostomy catheter is placed with use of the standard landmarks while keeping the trajectory of the cannula in the coronal plane of the coronal suture and aiming at the medial canthus of the ipsilateral eye (Fig. 335-6B and C). A distinct “popping” sensation is usually felt as one reaches the frontal horn of the lateral ventricle. This should occur when the tip of the ventricular catheter is 7 to 10 cm from the surface of the skull, which usually means that it is at the opening of the foramen of Monro of the ipsilateral frontal horn. Return of CSF should be apparent; if not, another pass should be made. The catheter should not be advanced more than 10 cm from the skull because of the risk for damage to the diencephalic structures. The ventriculostomy catheter is tunneled for a few centimeters in the subgaleal space (Fig. 335-6D) and brought out through a separate stab incision. It is then secured firmly to the skin with sutures and connected to the pressure transducer system.
For the placement of intraparenchymal monitoring devices, the twist drill opening is made as described earlier. After opening the dura with a blunt stylet, the transducer tip is placed directly in the brain parenchyma at a distance of 2 to 3 cm. The catheter is then tunneled under the skin. A fixation bolt may be useful for these devices (Fig. 335-6E).
Before leaving the operating room, a ventriculostomy catheter should be placed in all patients with an initial GCS score of 8 or less because raised ICP requiring treatment develops in at least 80% of these patients.12,13,37 It may also be placed under direct vision before closure of the bone flap.
Exploratory Bur Holes
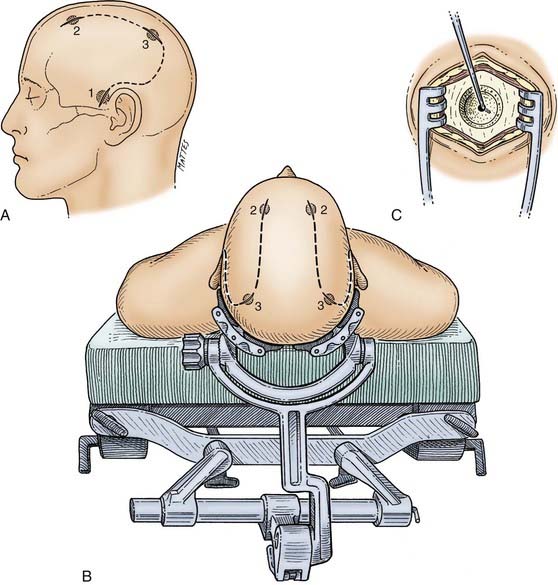
The patient is placed supine with the head preferably in a head holder, which also gives wide access to both sides (Fig. 335-7B). The whole head is shaved, prepared, and draped to allow access to the frontal, parietal, and temporal areas. The bur holes are typically placed on the side of localizing neurological findings—ipsilateral to a dilated pupil, contralateral to the most abnormal motor response, and ipsilateral to a skull fracture. It must be stressed that none of these signs are absolute and that if no hematoma is found on the suspected side, the other side should be explored in all cases. Initially, a bur hole is placed in the temporal region 2.5 cm above the zygomatic arch (see Fig. 335-7A). After diagnosis of either an acute subdural or extradural hematoma or no hematoma, two additional bur holes can be placed appropriately in the parietal and frontal regions. The skin incisions must be made in such a manner that if a formal craniotomy is required, they can be joined to form the skin flap (see Fig. 335-7A).
General Considerations for Supratentorial Hematomas
Craniotomy Technique
Scalp Incision
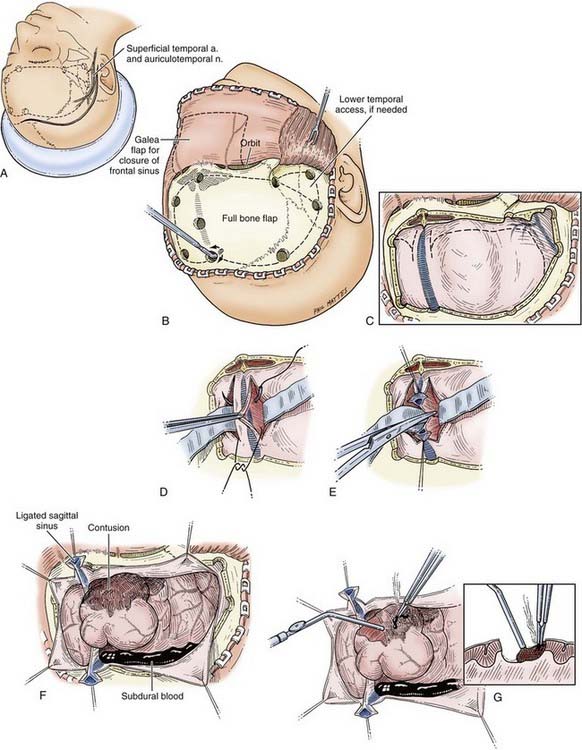
The superficial temporal artery should be palpated and marked and the vertical limb of the incision placed between the artery and the tragus. The skin incision is started at the zygomatic arch and then curved backward to the parietal eminence and upward above the auricle to reach 2 cm from the midline. It is then carried forward to the frontal region and curved across the midline just behind the hairline (Fig. 335-8A). In balding patients, the frontal part of the incision may be carried into the forehead, but it should be closed with 5-0 nylon suture for a good cosmetic result. The incision can be made with a scalpel, although use of a needle-tipped (Colorado) Bovie may minimize scalp bleeding. Hemostasis of the skin margins is achieved with Raney clips or electrocautery. Then, using a Bovie cutting diathermy device, an incision is made in the superficial temporal fascia and in the temporalis muscle down to the bone, close to the margin of the skin opening. The myocutaneous flap is then reflected inferiorly and secured. Ideally, the margins of the bony craniotomy should be approximately 15 by 12 to 15 cm in size.
Rapid Temporal Decompression
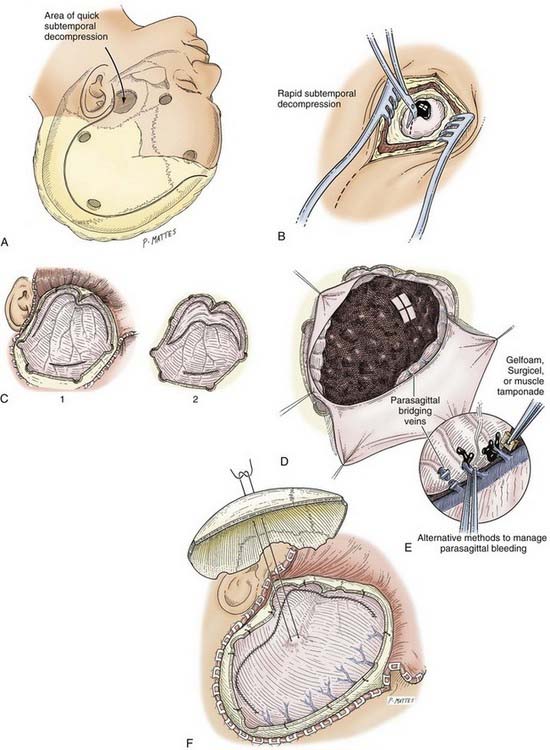
In a patient who has herniated or whose condition is deteriorating from an ICH, the temporal end of the incision is opened rapidly, for 3 to 4 cm only, and a bur hole is made. This opening is then rapidly extended with Leksell rongeurs to form a limited craniectomy about 3 cm in diameter (see Fig. 335-8A). If the hematoma is in the subdural space, the dura is opened, and the underlying hematoma is promptly evacuated (Fig. 335-8B). This procedure helps reduce ICP as early as possible and allows the craniotomy to then be completed more slowly, with better hemostasis.
Bone Flap
Multiple bur holes (five to seven) are placed in the parietal and frontal regions (see Fig. 335-8A) with an ACRA-CUT bit for the Midas Rex or Anspach drill systems (or an 8-mm cutting bur). The bur holes are then enlarged with a Kerrison bone punch and undermined to allow a Penfield No. 3 dissector to slide between the dura and the inner aspect of the bone flap for gentle separation of the dura from the skull. Special care is needed at this stage near the sagittal sinus. If the dura is torn during this step, it is desirable to drill extra bur holes to prevent inadvertent lacerations of the dura by the craniotome. Joining the bur holes with a craniotome completes the craniotomy opening. The bone flap is fashioned so that its medial margin is at least 1.5 to 2 cm from the midline. If greater medial exposure is needed, it can be carried out more safely with Leksell rongeurs under direct vision. Further exposure of the middle fossa is obtained with the use of Leksell rongeurs to remove parts of the lateral sphenoid wing and the temporal bone in piecemeal fashion, as low as needed, for temporal tip access. The first goal after removing the bone flap is decompression of any mass lesions that may be present.
Dural Opening
Any dural opening should be carefully planned to avoid injury to the underlying brain tissue or venous sinuses, facilitate closure, and in the case of TBI, deal with intraoperative swelling. Commonly described techniques include a C-shaped durotomy based on the sagittal sinus, a cruciate opening, and a “basal” or “reversed U-shaped” durotomy. We favor “basal durotomy” (Fig. 335-8C) because it is a technique that is particularly useful in dealing with massive intraoperative swelling.38 With this technique, a No. 15 scalpel blade is used to start the dural incision at the frontobasal eminence of the frontal lobe anterior to the pterion. A second durotomy is begun over the lowest part of the temporal lobe so that the temporal tip may be removed, if major brain swelling ensues, after any subdural clots are removed. The dura should not be cut near the midline to avoid damage to the parasagittal bridging veins. Instead, the dural flap should be based along the sinus. The incision is carried low across the middle meningeal artery toward the temporal lobe to fashion the reversed U-shaped dural opening. Care is taken to protect the underlying brain tissue with a cottonoid pad if indicated. This durotomy technique permits good access to the basal frontal and temporal lobes and prevents parasagittal herniation of the frontal lobe and occlusion of bridging veins against the dural edge. Next, smaller slit incisions may be made circumferentially around the craniotomy (although not parasagittally) to grant additional access for removing the hematoma (i.e., over the convexities). Once the hematoma is removed, the surgeon may decide to connect and complete the full extent of the durotomy, depending on the amount of swelling present or how much visualization is needed to adequately address the surgical goals.
Epidural Hematoma
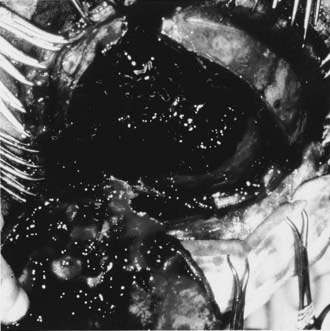
In 1990, Pickard and coworkers showed that surgical management of posttraumatic EDH is one of the most “cost-effective” of all surgical procedures in terms of quality of life and years preserved.39 EDH is estimated to complicate 2.7% to 4% of TBIs, with a mortality rate of approximately 10%. However, EDH does not always occur in isolation. Associated ICHs are found in 30% to 50% of patients with surgically evacuated EDHs.20 EDHs are located primarily in the temporal (see Fig. 335-4) and temporoparietal regions and are usually caused by tears in the anterior or posterior divisions of the middle meningeal artery with an associated linear fracture of the skull. Bleeding can also originate from the middle meningeal vein, the diploic veins, or the venous sinuses. One study reported an arterial source of bleeding in only 36% of adults and 18% of children.40 The deformation of the skull is probably responsible for initiating the process of dural stripping. As the hematoma enlarges, the dura is progressively stripped from the inner table. The blood in the epidural space is usually clotted (Fig. 335-9). Sometimes, depressed skull fractures overlying the major venous sinuses are associated with EDHs. Posterior fossa EDHs, which often extend above and below the tentorium, account for approximately 5% to 10% of these lesions.41,42 EDH is one of the most urgent neurosurgical emergencies because it is commonly associated with rapidly evolving brain compression, and prompt evacuation is indicated.
When assessing the need for surgery in patients with EDH, there are some subtle details regarding EDH that differ from the general population of TBI patients. It has already been mentioned that EDHs in temporal locations should have a lower threshold for surgery. Additionally, although it is generally understood that increasing age correlates with poor outcome in TBI, that trend is not as pronounced in patients with EDH. In fact, the GCS score at initial encounter has proved to be a better predictor of outcome.43–45
Operative Technique for Epidural Hematoma*
Operative technique for epidural hematoma.
Operative technique for acute subdural hematoma.
Operative technique for intraparenchymal hemorrhage and contusion.
The preoperative checklist is carried out as described earlier, and the patient is positioned as shown in Figure 335-5. The patient is given prophylactic antibiotics within 60 minutes of skin incision. To maximize intracranial relaxation, the patient may be given mannitol and mildly hyperventilated (PaCO2 ≈30 mm Hg). However, if no associated intradural lesion is present and the EDH is not very large, these steps should be avoided to maximize the capacity of the brain to fill the epidural space and occlude the hematoma cavity after removal. Historically, a large “trauma flap” (frontotemporoparietal or a large pterional craniotomy) was advocated for EDH, as well as for SDH. However, with better localization by CT and earlier detection of smaller EDHs, it is becoming routine to perform a more limited “slash” incision with a smaller two– to four–bur hole focused craniotomy for the evacuation of aEDHs (Fig. 335-10). Using the preoperative CT scan as a guide, the incision is planned over the epicenter of the EDH to provide complete exposure of the hematoma such that the margins are approximately 5 mm less than the diameter of the EDH to allow optimal dural “hitching” to the bony edges of the craniotomy. The slash incision, which typically runs vertically, should be fashioned so that it can be extended to the larger “trauma flap” if necessary (e.g., if intradural bleeding were to develop with reperfusion of the brain after removal of the EDH). If neurological deterioration has been rapid or herniation is present, an initial bur hole is placed over the thickest part of the clot as seen on CT, and the clot is promptly removed to reduce ICP.
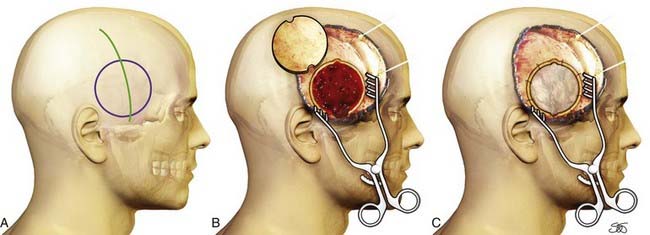
FIGURE 335-10 A–C, Acute epidural hematoma. A smaller “slash” incision is used for a more focused craniotomy.
The incision is made with a scalpel or a Colorado needle-tipped Bovie and taken directly down to the bone. After the skin incision, hemostasis is obtained with the help of Raney clips or electrocautery. The periosteum is then rapidly stripped to fully expose the cranium in the region of the hematoma. A circular or ellipsoid craniotomy is customarily fashioned and started by using a high-speed drill and an ACRA-CUT perforator or an acorn bit to create two to four bur holes at the opposing poles of the EDH. More bur holes can be created as necessary depending on the size or location of the clot. With such a vertical incision, removal of the hematoma may be achieved within about 5 minutes of beginning the incision. Although an aEDH by definition strips the dura from the inner table of the skull, correct placement of the craniotomy is crucial to optimally occlude the epidural space and coagulate the bleeding points on the dura, usually the middle meningeal artery. A Kerrison punch and a curved curet can be used to enlarge and undercut the bur holes to allow a Penfield No. 3 dissector to slide into a plane between the dura and the inner aspect of the skull. A cutting craniotome is then used to complete the craniotomy, with the size of the opening based on measurements taken from the CT scan and made large enough to encompass the clot in the craniotomy. The blood, which is usually clotted, is removed with the help of suction, irrigation, and cup forceps (see Fig. 335-9). The bleeding source, typically a branch of the middle meningeal artery, can generally be controlled with bipolar diathermy. Occasionally, the main trunk of the middle meningeal artery is bleeding because of a fracture involving the petrous bone. In these cases, adequate low temporal exposure is facilitated by the vertical incision advocated here. The foramen spinosum can be packed with bone wax or a combination of bone wax and Surgicel. After evacuation of the hematoma, meticulous hemostasis is obtained in the epidural space with Surgicel and Gelfoam, and bone wax is applied to the bone edges. Dural tack-up sutures are placed at no more than 2.5-cm intervals in a circumferential fashion with 4-0 silk and the use of bone drill holes (similar to that shown in Fig. 335-8F). Central dural tack-up sutures are also placed in the center of the bone flap to obliterate the central dead space under the bone flap (see Fig. 335-8F). The bone flap is then replaced, preferably with a miniplate system. Bur hole covers should always be placed over the frontal bur holes for the best cosmetic result. A 3.5-mm vacuum drain (e.g., medium Hemovac) is placed under the galeal layer. The superficial temporal fascia and then the galea are approximated with 2-0 Dexon or similar suture. Skin staples are placed on the skin margins, the wound is dressed, and a head bandage is placed.
Occasionally, the craniotomy will need to be enlarged to expose the bleeding point. In such cases, both the incision and the craniotomy can be expanded to a more traditional frontotemporoparietal craniotomy. Recently, several case reports have advocated nonsurgical management of smaller aEDHs detected early in conscious patients. Emergency angiography has demonstrated bleeding points and traumatic tears in the middle meningeal artery, which can be embolized with coils or Onyx.46 It remains to be demonstrated whether such endovascular approaches are safe, cost-effective, or worthwhile. Within the entire spectrum of surgery, removal of an EDH in unconscious patients has been shown to be the single most cost-effective operation39 if carried out rapidly before secondary ischemic damage is induced because of the high ICP.
Acute Subdural Hematoma
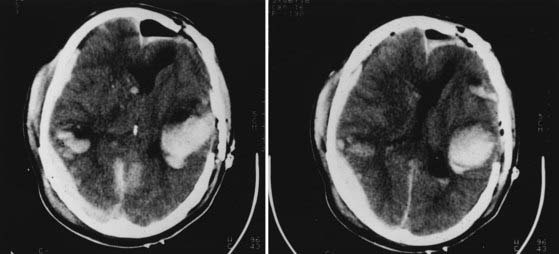
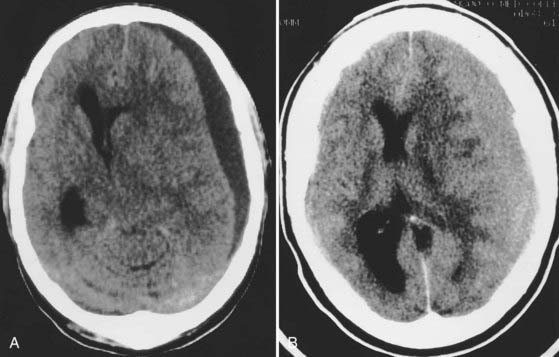
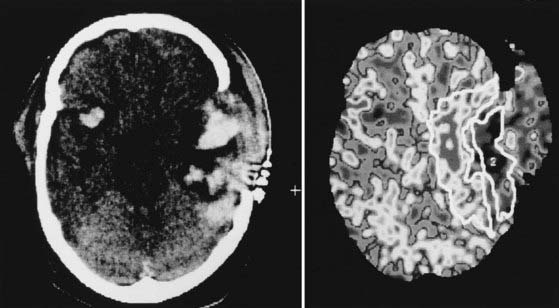
Despite major advances in modern head injury management, patients in whom aSDH develops have a worse prognosis than any other group of head-injured patients. Mortality has been reported to be between 50% and 60% in various series and between 57% and 68% in patients initially seen in coma.47–50 aSDHs are found in 21% of patients with severe TBI and in 11% of those with any degree of TBI.20 They are most commonly caused by motor vehicle collisions in younger patients and by falls in elderly populations. aSDHs are due to bleeding from contusions, lacerated cortex, torn bridging veins, or a torn cortical blood vessel. As is true for most ICHs after TBI, aSDH does not usually occur in isolation. Approximately 50% of patients (70% to 75% if the GCS score is less than 10) have associated intracranial lesions, including contusions, hematomas, or cortical lacerations, with the majority occurring in the frontal and temporal lobes (Fig. 335-11). Therefore, surgical planning should consider the need to contend with concomitant lesions or parenchymal injuries. Important predictors of outcome include age, admission GCS score, pupillary responsiveness, ICP, and the presence of hypotension and hypoxia. Age is the most significant factor in the prediction of outcome. In a retrospective study, 34 patients older than 65 years were compared with a similarly treated younger patient population (33 patients aged 18 to 40 years); mortality rates were more than 4 times higher in the older patients.51 Recently, it has also been shown that the primary underlying brain injury is as important as the subdural clot itself in dictating outcome, thus emphasizing the need for postoperative control of ICP.52,53
aSDHs are frequently an important and potentially lethal complication of anticoagulant therapy.54 They are usually large and can be part of a coexistent chronic SDH (Fig. 335-12). In a study of 68 consecutive patients with warfarin-related intracranial hemorrhages, Mathiesen and associates reported that traumatic hematomas were found in 26 patients.54 The hematomas were subdural in 23 patients (acute in 10 and chronic in 13), epidural in 1, and intraparenchymal and associated with contusions in 2. Mortality was 79% in the group with traumatic intracranial hemorrhages. However, lower mortality rates of 48% and 16% have been reported after warfarin-related intracranial hemorrhages.55,56
Operative Technique*
After it is decided to evacuate an aSDH, the emergency preoperative work-up and positioning of the patient are as described earlier. A large frontotemporoparietal craniotomy is generally required to adequately remove an aSDH and to address the underlying parenchymal injuries. As with all trauma craniotomies, it is customary to takes measures to maximize intracranial relaxation. Such measures include the administration of mannitol and the institution of mild hyperventilation. The patient is also given prophylactic antibiotics within 60 minutes of incision. After a generous dural opening (see Fig. 335-8C), the clot is carefully removed with irrigation, suction, and cup forceps. The subdural space should be widely inspected for additional hematoma, bleeding, and surface contusions. Intracerebral hematomas underlying an aSDH are sometimes located on the surface and can be surgically removed through a limited corticotomy. Achieving and maintaining hemostasis at the operative site is the most difficult and most important aspect of trauma neurosurgery. Frequently, these patients are coagulopathic, with thrombocytopenia making hemostasis especially difficult to maintain.57 Bleeding points on the cortical surface are cauterized with bipolar diathermy. Sometimes, two surgeons with two bipolar instruments are optimal to do this. Occasionally, a bridging vein is clearly avulsed and can be cauterized with bipolar diathermy (see Fig. 335-8E). When the bleeding is coming directly from the sinus wall, however, the use of bipolar diathermy is contraindicated because it may enlarge the opening. In these situations, it is best to use Gelfoam, Surgicel, or Avitene and gentle tamponade with a cottonoid pad to stop the oozing. A muscle “patch” about 15 by 15 mm helps in these situations. It is vital that pressure in the sagittal sinus be decreased by placing the patient in a 10- to 15-degree head-up posture and ensuring that there is no jugular compression. Gentle irrigation and suction are applied to wash any additional clots from under the dural margins. Care must be taken during these maneuvers to prevent any additional bleeding.
If there are large areas of focal brain contusion, they should be removed with gentle suction and the use of bipolar diathermy, as demonstrated in Figure 335-15G. If diffuse oozing is persistent throughout evacuation of the hematoma, the anesthesiologist should be asked to send blood for a fresh set of coagulation studies (PT, PTT, platelet count, and INR), and a simple in vitro clotting time is determined. Five to 10 units of FFP, rFVIIa, or up to 5 to 10 packs of platelet concentrate may be needed. After the clot is evacuated and hemostasis is obtained, closure of the dura is considered. Dural peripheral tack-up sutures are first placed as described for EDHs, and usually, a dural augmentation procedure (duraplasty) is performed. After aSDH, brain swelling is almost inevitable, and in general, dural closure should be avoided. Duraplasty may be followed by routine closure of the craniotomy with miniplates and bur hole covers. However, in more than 80% of patients, whenever brain swelling is anticipated (Fig. 335-13), a dural substitute is placed loosely over the swollen brain and sewn to the dural margins. In these situations, the bone flap is not replaced. The patient is then treated medically for raised ICP. The bone flap can be replaced in 6 weeks to 2 months. If there is any evidence of brain swelling and the patient’s preoperative GCS score was 8 or lower, a ventriculostomy catheter is placed either ipsilateral or contralateral to the craniotomy site before closure.
Intraparenchymal Hemorrhage and Contusion
Many patients (13% to 35%) with severe TBI have traumatic parenchymal mass lesions; however, only about 20% of trauma craniotomies are performed primarily for space-occupying contusional intracerebral hematomas. Associated SDHs and EDHs are often encountered. Intracerebral hematomas and contusions generally do not require surgical evacuation unless there is a significant mass effect or intracranial hypertension. They resorb in 4 to 6 weeks by macrophage phagocytosis and gliosis. The majority of intraparenchymal lesions occur in the temporal and frontal lobes as a consequence of the brain impacting against the skull, especially the sphenoid ridges. Parietal and occipital lobe hematomas are much less common and are usually due to direct impact, as in assaults.58–61
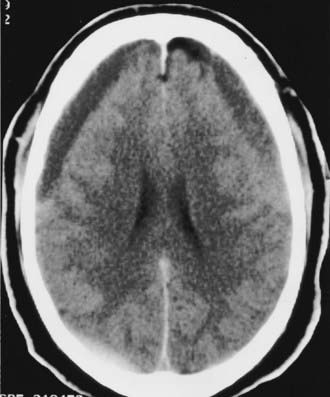
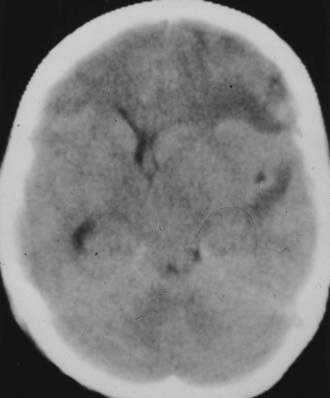
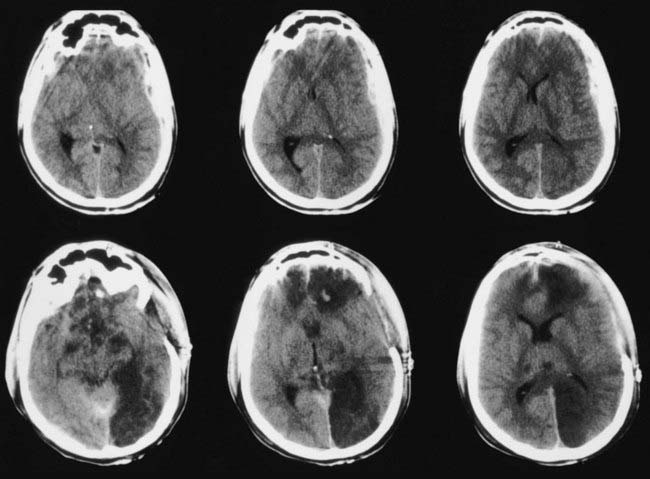
A significant challenge in the management of parenchymal hemorrhages is their propensity for enlargement. At least 25% of patients with intracranial mass lesions show clinical or radiologic worsening in the first 2 to 3 days after injury, usually as a result of enlarging contusions and associated edema. Enlargement of contusions and increased perilesional edema are the norm rather than the exception, as shown in Figure 335-14. Accordingly, CT should be repeated at least daily over the first 3 days after injury unless ICP is being monitored and remains low.
In one study, ICP monitoring failed to predict a rise in ICP in 17% of patients with low ICP initially.62 Five of the 30 patients who had ICP monitors suddenly deteriorated or died 6 to 11 days after injury, with features of high ICP demonstrated clinically or at autopsy.
In a patient with worsening neurological status, intractable ICP, or a mass effect on CT secondary to an intracerebral hematoma or contusion, a bifrontal craniotomy must sometimes be performed. This procedure provides a large area of decompression for patients with diffuse swelling. In addition, the underlying contused and hemorrhagic tissue can more readily be visualized and removed if necessary. This type of bifrontal decompressive craniectomy can also be performed over areas of maximal hemispheric swelling. Decompressive bifrontal craniectomy for intractable ICP in the absence of mass lesions is controversial; however, two recent studies showed better outcomes when the procedure was performed before irreversible ischemic brain damage resulted.63,64 Two major ongoing international randomized trials to evaluate the effect of decompressive craniectomy on outcomes are nearing completion in 2009 (the RESCUEicp trial at www.rescueicp.com and the DECRA trial at www.clinicaltrials.gov, identifier NCT001559987).
Operative Technique*
The preoperative work-up and positioning of the patient are as described earlier. To maximize intracranial relaxation, the patient is given mannitol and mildly hyperventilated. Prophylactic antibiotics are also administered before the procedure. For a bifrontal craniotomy, the patient is placed supine on the operating table with the head resting on a foam pad or “horseshoe” Mayfield headrest (Fig. 335-15). A bicoronal incision is marked out behind the hairline, starting low at the level of the zygoma (see Fig. 335-15A). In the temporal region, the incision is first carried down to the galea. Above the superior temporal line, the incision is carried down to bone. Raney clips are then applied to the skin margins for hemostasis. A Bovie monopolar cutting diathermy device is used to incise the superficial temporal fascia and the temporalis muscle while taking special care to avoid possible injury to the facial nerve by remaining above the zygoma at the inferior end of the incision. Using a combination of periosteal elevators and monopolar diathermy to separate the periosteum from the skull, a myocutaneous flap is reflected as low as possible anterior to the supraorbital margins and the sphenoid wing in the temporal region. The scalp flap is then pulled down and secured. Using a high-speed drill, multiple bur holes are placed in the temporal and frontal regions and two parasagittally, about 1.5 cm apart, on either side of the sagittal sinus near the plane of the coronal suture (Fig. 335-15B). A Kerrison punch and a curved curet are then used to enlarge the bur holes and undermine the inferior aspect to allow a Penfield No. 3 dissector to separate the dura from the inner aspect of the skull (see Fig. 335-15B). The dura is gently and carefully stripped over the midline sagittal sinus. After this step, a cutting craniotome blade is used to perform the craniotomy cuts, again keeping as low as possible in the frontal and temporal areas. The last cut of the craniotomy should be the one carried over the midline sagittal sinus region to minimize bleeding. The midline low frontal bur hole is best made 10 mm above the nasion. We recommend using a high-speed 8-mm cutting bur rather than an ACRA-CUT perforator to allow the bur hole to be enlarged to gain low frontal access near the olfactory groove and crista galli. The frontal air sinus will be exenterated and its ostia plugged afterward. The superior sagittal sinus must be freed completely from the crista galli to the cephalad bur hole, and extra bur holes may be needed. Bone wax is applied to the bone margins, and strips of Surgicel or Gelfoam are placed around the craniotomy margins and over any small bleeding points from the sagittal sinus. Dural tack-up 4-0 silk sutures are placed around the margins of the craniotomy site. Bilateral dural openings are created with the flaps based toward the sagittal sinus (Fig. 335-15C). Care is taken during the dural opening to protect the underlying brain tissue and parasagittal draining veins with cottonoid pads. If bifrontal contusions or an aSDH is present or if frontobasal access is required, the most proximal superior sagittal sinus is double-ligated and cut as close to the crista galli as possible, where it is usually small (Fig. 335-15D to F). Using bipolar diathermy, the pia mater and superficial vessels are cauterized over the cortical area with the most damage, swelling, and contusion. The pia mater is opened with a No. 15 blade, and a subpial plane is developed, if possible, to minimize bleeding. With gentle suction and bipolar diathermy, the contused and hemorrhagic areas are removed (Fig. 335-15G). Gentle retraction of the surrounding brain tissue adjacent to the cavity is performed with a handheld malleable retractor. If significant brain swelling is present, a right frontal or temporal “polectomy” can be carried out to minimize the pressure effects of postoperative brain swelling. However, no more than 3 to 4 cm of the frontal lobe and 5 to 6 cm of the right temporal lobe should be removed. On the left side, only contused, amorphous tissue should be removed. The areas of the frontal lobe most prone to hemorrhagic contusions are the inferior orbital aspects of the gyrus rectus and inferior frontal gyrus. In the temporal regions, it is the tips of the lobe. After adequate decompression, hemostasis is achieved in the surgical bed with Surgicel, or gentle tamponade can be maintained with cotton balls soaked in saline or half-strength hydrogen peroxide, which is an effective hemostatic adjunct. The dura is then closed in watertight fashion with 4-0 silk sutures, especially over the subfrontal region behind the frontal air sinus. If there is significant brain swelling, the dura should be left open and a dural patch graft sutured to the margins of the dural edges. In this situation, the bone flap should also be left out. If the bone flap is to be replaced, central tack-up sutures are placed in the dura and a miniplate system is used to secure the flap to the skull. Bur hole covers are placed over the frontal bur hole openings to improve the cosmetic appearance. The wound is then closed as described earlier. A ventriculostomy catheter is usually placed before closure if brain swelling is present or anticipated or if the patient is in coma.
Posterior Fossa Hematoma
Diagnosis
The posterior fossa is an uncommon site for traumatic ICHs, but this location may account for 3% to 5% of hematomas seen on CT. Clinical progression of the hematoma is usually silent and slow, but deterioration because of obstructive hydrocephalus and brainstem compression can be sudden and rapidly fatal if not treated promptly. Clues to an impending crisis include sudden increases in blood pressure and changes in respiratory pattern. Pupillary reflexes, increases in ICP, or altered sensorium may not be present with a posterior fossa hematoma and cannot be counted on to indicate impending herniation. Posterior fossa hematomas are usually associated with occipital skull fractures. The majority of patients in whom posterior fossa lesions develop have sustained direct occipital trauma, and signs of occipital trauma are evident in more than 80% of patients and include scalp abrasions, lacerations, hematomas, and open fractures. An EDH is the most common posterior fossa lesion that requires surgery (Fig. 335-16), followed by SDH and intracerebellar hematoma. Infratentorial EDH is most often due to lateral sinus injury, but injury to venous sinuses deep at the skull base may be more frequent than commonly realized.63 Occipital fractures extend into the petrous bones in more than a third of patients and result in significant permanent hearing loss (mostly sensorineural) in 20% of patients and facial nerve injury in 9%.65
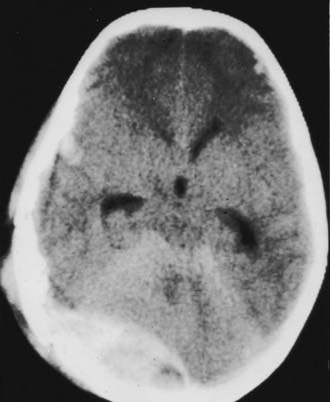
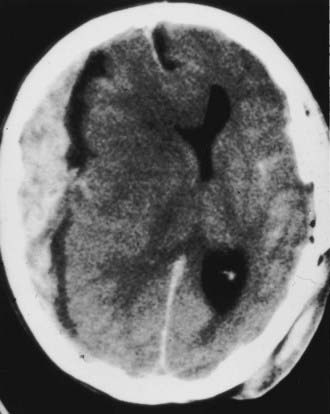
FIGURE 335-16 Axial computed tomographic scan showing a posterior fossa extradural hematoma with early dilation of the ventricular system.
When preparing a patient with a posterior fossa hematoma for emergency craniotomy, the most important goals are avoiding hypotension and hypoxia, treating intracranial hypertension, and assessing and treating coagulopathy. Seizure prophylaxis is indicated in most patients as well because of coexistent supratentorial injuries. A ventriculostomy catheter is always placed first for drainage of CSF and reduction of ICP in patients with posttraumatic surgical lesions. To avoid the rare possibility of upward herniation after placement of the ventriculostomy, some authorities advise slower CSF drainage, but as soon as the posterior fossa is decompressed, this risk is eliminated.66
Operative Technique
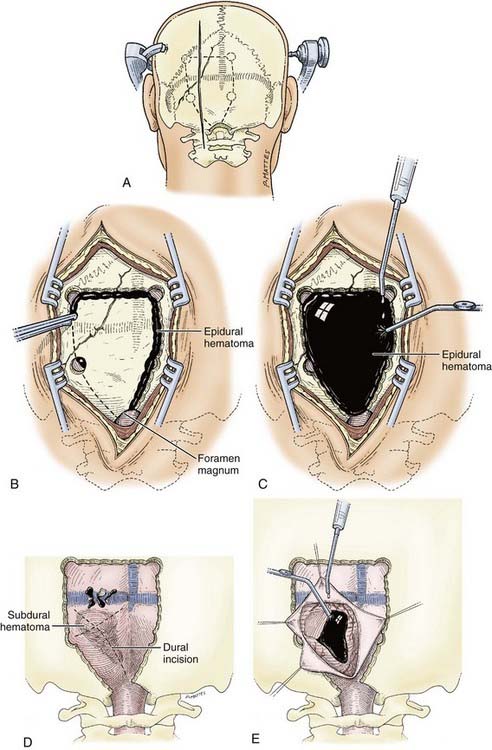
The patient is taken to the operating room as urgently as possible in view of the risk for obstructive hydrocephalus. To maximize intracranial relaxation, the patient is given mannitol and hyperventilated to a PaCO2 of approximately 25 to 30 mm Hg initially. Prophylactic antibiotics are also administered before the procedure. Ventriculostomy is mandatory before surgery for any posterior fossa traumatic lesion. The patient’s head is fixed in a Mayfield three-point fixation device (Fig. 335-17A). After turning the patient to the prone position, the head of the bed is raised so that the patient’s head is slightly above the level of the heart. The head is then flexed anteriorly at the occipitocervical junction to optimally expose the occipital region. Particular care must be taken to assess and document the absence of cervical fractures because the customary cervical collar must be removed in such patients. A midline incision is carried out and the occipital bone is exposed widely and bilaterally. Using a high-speed 8-mm bur, multiple bur holes are placed below the transverse sinus (Fig. 335-17B). If an extradural hematoma is present, the dura will already be separated from the underlying bone by the clot, and the craniotomy may then be completed with a craniotome (Fig. 335-17C). Because the dura is already stripped from the inner table of the bone, posterior fossa EDHs are amenable to a craniotomy rather than the usual craniectomy. However, careful bone removal over the venous sinuses is necessary, and this should be performed last. Separation of the underlying bone from the sinuses is achieved with the help of a Penfield No. 3 dissector. The extradural hematoma is then gently removed with the help of cup forceps and suction. Any bleeding over the dura should be meticulously controlled, especially if it arises from the venous sinuses. A combination of Surgicel, Gelfoam, and Avitene and sometimes a “muscle patch” can be used to stop venous sinus bleeding. After hemostasis over the dural surface is achieved, multiple dural 4-0 silk tack-up sutures are placed along the craniotomy margin at 20-mm intervals and centrally through the middle of the bone flap. The bone flap is then secured with cranial miniplates. The wound is closed in not less than four layers to the muscle and subcutaneous tissues with 2-0 Dexon. Stainless steel staples are used for skin closure. A vacuum drain system is always advisable.
When a posterior fossa SDH is large and hence necessitates surgery, major bleeding should be anticipated. Large-bore lines should be placed, and crossmatched blood should be available in the operating room. The initial bur holes over the cerebellar convexities should be widened and the exposure converted to a large craniectomy with the help of a high-speed bur to thin the bone and completed with Leksell rongeurs as quickly as possible. Bone removal should include the rim of the foramen magnum inferiorly and up to the edge of the transverse sinus superiorly and laterally as far as the digastric groove to provide wide decompression. This will allow identification and control of bleeding from the sinus or from torn bridging veins over the superior cerebellar surface. Initially, a small dural opening is created to aspirate part of the underlying clot and rapidly reduce pressure in the posterior fossa. After decompression, the dura is opened in a cruciate manner for improved exposure (Fig. 335-17D). The hematoma is then removed with the help of gentle suction (Fig. 335-17E). If the underlying cerebellar surface appears to be contused, it is gently aspirated and hemostasis is established with bipolar diathermy. A dural substitute graft is always used with a wide decompression. The posterior arch of the atlas can also be removed as part of the bony decompression.
Decompressive Craniectomy
The role of decompressive craniectomy in TBI and in the control of intracranial hypertension remains a matter of debate.67,68 Studies have evaluated its role in the control of intracranial hypertension, and earlier results have been inconclusive.69–71 Nonetheless, decompressive craniectomy is now a commonly used tactic in many centers specializing in the management of TBI. Making definitive conclusions on the issue difficult is the fact that there have been no well-designed randomized controlled trials exploring the efficacy of decompressive craniectomy in adults after TBI. Currently, two multicenter randomized controlled trials are under way that may help determine the role of decompressive craniectomy (the RESCUEicp trial at www.rescueicp.com and the DECRA trial at www.clinicaltrials.gov, identifier NCT001559987).
Early decompressive craniectomy, as opposed to its use as a post-herniation salvage procedure, may improve outcomes.72 Moreover, the specific postoperative management after craniectomy may play an important role in dictating the success of the procedure. Modern management strategies targeted at preventing excessive hypertension are crucial in preventing the reactive edema that has been reported by some authors after decompressive craniectomy.73
Operative Technique
The decompressive craniectomy must always be extensive. Aarabi and associates advocated for an approximately 15-cm craniectomy (the mean anteroposterior diameter), along with expansive duraplasty, as the optimal size threshold for the procedure.72 The detailed operative technique has been discussed in previous sections of this chapter. When unilateral decompression is necessary, a frontotemporoparietal craniectomy is most commonly used. The technique is comparable to that previously described for evacuation of aSDH. It is vital to decompress the floor of the temporal fossa by inferior craniectomy and to adequately extend the craniectomy posteriorly and inferiorly. In the case of bilateral frontal swelling, one can perform a bifrontal craniectomy as described earlier. Alternatively, bilateral frontotemporoparietal craniectomies can be performed and may be done in serial fashion if high ICP persists after the first procedure. Once the bone is removed, the dura must be opened widely as well. Opening the dura has been shown to improve the reduction in ICP from 30% (dura left intact) to 85% (dura opened).74,75 Dural closure can be done by either sewing in a dural patch or laying a dural substitute over the open dural incision. After adequate exposure is obtained, residual hematomas and necrotic tissue can be removed to create more space for viable brain tissue. The removed bone flap should be wrapped in antibiotic-soaked gauze, sealed in a sterile bag, and stored in a subzero refrigerator. Alternatively, in “austere environments” or when transfer of the patient is anticipated, some surgeons place the bone flap in the subcutaneous layer of the abdomen.
In many instances, intractable ICP develops after a craniotomy has already been performed for the treatment of an intracranial lesion. In such cases, management can be as simple as reopening the previous incision and removing the bone flap. However, in most cases it is advisable to enlarge the original craniotomy. This may be achieved by a “T-off” from the original incision, starting at the most superior and posterior portion of the existing incision and gently curving toward the inion.76 The craniotomy can then be expanded effectively.
Depresssed Skull Fracture
Operative Technique
To maximize intracranial relaxation, the patient may need mannitol and mild hyperventilation. Prophylactic antibiotics are also administered at least an hour before the procedure. The patient is placed on the operating table with the head resting on a foam pad or horseshoe head holder and elevated above the level of the heart. If the scalp is lacerated, the incision can be extended to include the laceration in a “lazy S” fashion; a linear incision may also be appropriate. The incision should be planned carefully so that it exposes the entire fracture adequately and includes excision of any ragged, devitalized scalp tissue or contamination (Fig. 335-18A). A bur hole is placed at the edge of the fracture, and the underlying dura is exposed (Fig. 335-18B). A Leksell rongeur or Kerrison punch is used to remove bone around the fracture to free the underlying fragments (Fig. 335-18C and D). Besides being depressed, the fragments often tend to slide under the edge of the fracture margins. These fragments are carefully elevated with a Penfield No. 3 dissector and removed while taking care to prevent any fragments from being driven down into the brain. During this process, care must be taken to not apply any downward pressure on the underlying dura and brain. Frequently, a dural tear with an underlying cerebral contusion is present. Once all the bone fragments are taken off the defect, if clean and the fracture recent, they may be fitted and held together with miniplates. Alternatively, for clean, recent, smaller compound fractures, a primary titanium mesh cranioplasty may be considered. The underlying dura is inspected carefully for any tears, which are sutured primarily or grafted. If an underlying SDH or intracerebral hematoma is present, the dura is opened widely (Fig. 335-18E), and the underlying hematoma is gently evacuated with the help of suction and bipolar diathermy. Careful hemostasis is obtained with bipolar diathermy and Surgicel (Fig. 335-18F). The dural edges are then approximated in watertight fashion with 4-0 silk suture. Sometimes the underlying dura is lacerated and may have to be replaced with a synthetic dural substitute such as Duraguard (bovine pericardium) (Fig. 335-18G). The fractured bone fragments held together with miniplates are then placed over the defect and secured to the edges of the bone with a miniplate and screw system. The scalp layers are closed as described earlier. When the wound is heavily contaminated or more than 24 hours old, bone fragments should not be placed primarily. Delayed cranioplasty 1 to 2 months later can be performed with titanium mesh, acrylic, or a titanium sheet prosthesis.
Compound Frontal Air Sinus Injury
In patients with compound frontal air sinus injuries, careful attention must be paid to fractures involving both the anterior and posterior walls. Nondepressed fractures through only the posterior wall of the frontal sinus do not generally warrant surgical repair other than closure of the scalp. When the force is significant enough to penetrate the anterior and posterior tables, the probability of delayed infection is very high.77 Such fractures necessitate intracranial exploration and repair. This should be done within a few days of injury, especially with penetration by a sharp object. Wooden objects are notorious for delayed sepsis, even years after apparently trivial injuries. This approach is also appropriate for delayed anterior fossa CSF leaks.
Operative Technique
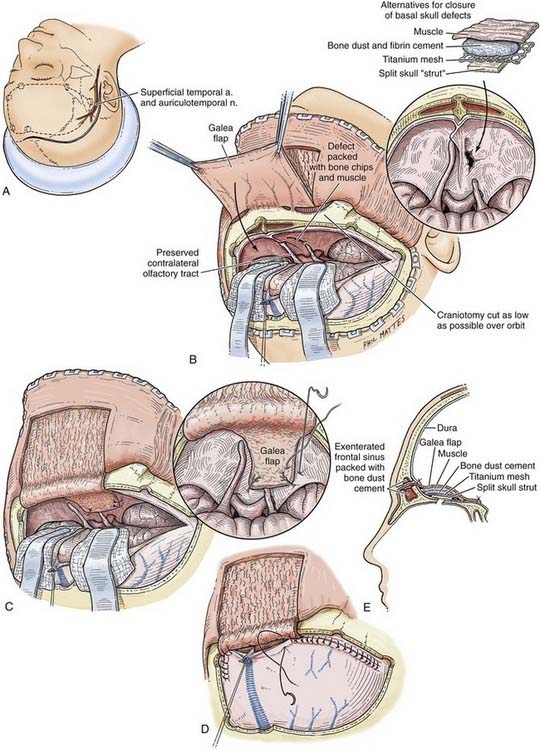
Repair of the fracture requires a bicoronal craniotomy, as described previously. To minimize retraction on the frontal lobes, in the majority of patients it is prudent to induce transient hyperventilation to a PaCO2 of 25 to 30 mm Hg and administer an intravenous bolus of mannitol, 1 g/kg, at the onset of surgery. The patient is placed supine on the operating table with the head immobilized in a three-pin Mayfield head holder and held in extension (Fig. 335-19A). A bicoronal scalp flap is marked out about 15 mm behind the hairline (see Fig. 335-19A). The coronal flap is elevated to the supraorbital rim and the zygomatic arches, as described previously. A vascularized galeal flap based on the reflected scalp margin is created for placement over the sinus defect (Fig. 335-19B). Multiple bur holes are then made, including holes over the sagittal sinus in the midline (see Fig. 335-15B), to safely reflect the underlying dura with the help of a Penfield No. 3 instrument and curved curets. The craniotomy is then completed as usual by joining the bur holes with a craniotome. Stripping out the mucosa then exenterates the entire frontal air sinus. If there are any depressed fragments of the anterior or posterior sinus wall, they are elevated or removed. The sinus mucosa is best removed with curets and cup forceps, as well as cauterized with monopolar diathermy, to avoid future mucocele formation.78 The ostia are occluded with a muscle plug (temporalis) glued into position with fibrin tissue sealant. The dura is opened as shown in Figure 335-19B, and the underlying brain is débrided of any necrotic, contused tissue. A vascularized pericranial flap is then placed over the cranialized frontal sinus (see Fig. 335-19B and C). If fractures of the floor of the frontal fossa are seen, including the ethmoid bones, further reinforcement can be placed over the defect. Repairs can be augmented with a dural substitute patch held in position with fibrin glue. Large defects may require malleable titanium mesh reconstruction of the anterior fossa floor fixed in place with small screws (see Fig. 335-19E and Chapter 339). Alternatively, a piece of inner table taken from the craniotomy can be used. A paste made of bone dust, kept at the time of the craniotomy, and combined with fibrin glue to seal the leak in the base of the skull may be all that is needed. The fragments of the anterior table of the frontal sinus are repaired with miniplates, and the craniotomy flap is replaced and fixed to the adjacent bone with miniplates and screws. The pericranial flap is closed in layers and a vacuum drain used. For all patients with a documented CSF fistula or dural laceration underlying the frontal sinus, a 3- to 5-day period of lumbar or ventricular drainage is instituted.79
Depressed Skull Fracture over a Venous Sinus
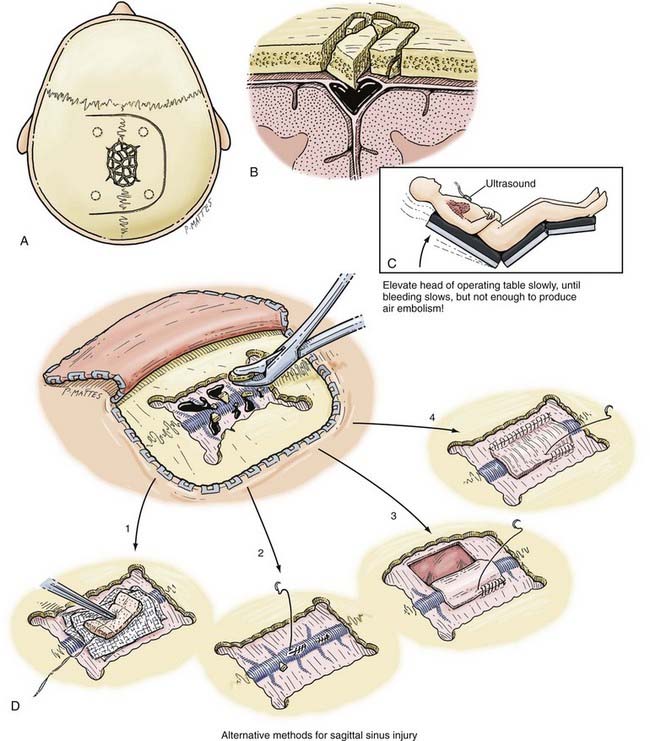
A depressed skull fracture that is closed, is not exerting a mass effect, and does not require repair for cosmetic reasons should not be elevated if overlying a venous sinus. The choice of treatment depends on the neurological condition of the patient, the site of venous sinus involvement, and the degree of venous flow compromise. If occlusion of a major sinus results in raised ICP with deteriorating neurological status, urgent fracture elevation and sinus repair with restoration of blood flow is required.80 Kapp and associates, in a report of 78 Vietnam War casualties injured by missiles, ligated the anterior 50% of the superior sagittal sinus in 27 patients, with only one death (4%).81 Injuries to the posterior half of the sagittal sinus were associated with a 24% mortality rate. Principles concerning involvement of the transverse sinuses are more difficult to define because their contribution to venous drainage of the supratentorial and infratentorial spaces is variable and bilateral. Determination of transverse sinus dominance is critical because many individuals have significant asymmetry, with the right side being dominant. If the superior sagittal sinus drains unilaterally into the right transverse sinus, ligation at this site would be disastrous. Unless urgently addressed, occlusion of the torcular or a dominant transverse or sigmoid sinus will result in the rapid development of sustained intracranial hypertension and frequently death. If, however, the superior sagittal sinus drains bilaterally or through the uninvolved sinus, ligation may be safe. Preoperative angiography or magnetic resonance angiography should be used whenever possible to assist in making this determination.
Operative Technique
In any operative procedure involving the sinuses, the personnel and materials should be in place to deal with potentially severe hemorrhage. The most important aspect of such procedures is to ensure that the patient’s head is elevated above the level of the heart on the operating table so that bleeding and the risk for air embolism are minimized (Fig. 335-20C). The head of the table should be easily maneuverable. In this way, venous pressure can be lowered to decrease hemorrhage and raised to prevent air embolism. A gentle Valsalva maneuver may be helpful to judge this. All precautions should be taken to prevent venous obstruction in the neck by avoiding extremes of flexion and rotation of the head on the shoulders. A right atrial catheter should always be inserted for aspiration should an air embolism occur, and an esophageal stethoscope should be used by the anesthesiologist.82 At least 2 to 4 units of packed cells should be available in the operating room.
A curvilinear or large horseshoe incision is made over the involved sinus to maximize the exposure (see Fig. 335-20A and B). To decompress the sinus from any depressed bone fragments, sufficient bone is removed around the margins of the sinus with Leksell rongeurs (see Fig. 335-20D). This allows proximal and distal control of the sinus. Long (≈25 mm) aneurysm clips or vascular “bulldog” clamps are kept ready to temporarily occlude the sinus and parasagittal veins if necessary, and a variety of Weck clips should be available to occlude the sinus if necessary. The sinus can be directly sutured with continuous suture (see Fig. 335-20D). An onlay graft using fascia lata can also be used (see Fig. 335-20D). If temporary distal venous sinus occlusion is going to be carried out, it is advisable to measure ICP during occlusion. If the pressure rises more than 20 mm Hg, mannitol should be administered and hyperventilation increased until venous flow is rapidly restored. After successful repair of the venous sinus, hemostasis is achieved, and the depressed bone fragments can be replaced with a miniplate system.
Chronic Subdural Hematoma
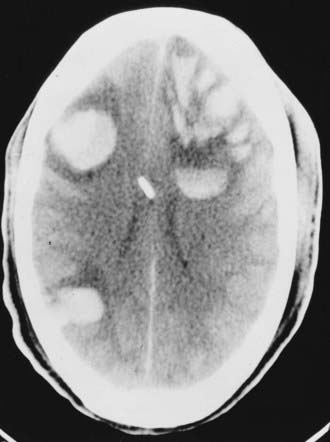
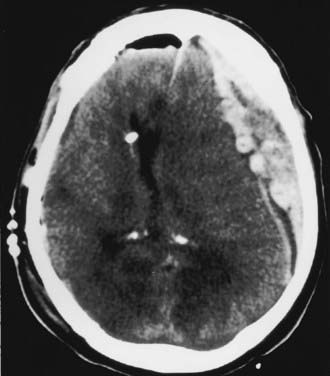
With chronic SDHs, the trauma is minimal or even unrecognized in a third of cases (see also Chapter 37).83 An SDH is considered chronic when it is discovered 3 weeks or more after the initiating injury. Posttraumatic subdural fluid collections are reported to occur in about 6% of head-injured patients, and chronic SDHs develop in 5% of patients with persistent subdural fluid collections.84,85 Many patients are elderly, with cerebral atrophy being the most important predisposing factor. This explains the amazingly high mortality rates of 15% to 20% reported in some series.83,86 The lesions are bilateral in 15% to 20% of patients (Fig. 335-21). The symptoms and signs of chronic SDH are variable and nonspecific. The classic manifestation in patients with posttraumatic chronic SDH includes two phases. The first phase is the traumatic event, which is usually minor. After its dissipation and a silent period of variable length, the second phase begins with the onset of delayed clinical manifestations that usually consist of headaches, decline in mental function, and motor deficits. Coagulopathy and anticoagulant therapy may accelerate these symptoms in many cases.
Chronic SDHs can be of differing densities on CT and can also result in a significant mass effect requiring urgent evacuation (Fig. 335-22). The mechanisms for the early development and enlargement of chronic SDHs are not yet fully understood. In a recent review of more than 800 acute SDHs, including those treated nonoperatively, hematomas less than 10 mm in maximal thickness always resolved spontaneously, whereas those greater than 10 mm thick evolved into chronic SDHs.18 Rupture of the bridging veins as they cross the subdural space from the cortex to the sagittal sinus may form the basis of the lesion, but tearing of small arterial adhesions may also be a factor. Various theories have been put forward to explain enlargement of the subdural collection once it forms, including an osmotic theory with fluid passing from CSF to the hematoma because of a difference in oncotic pressure. More plausible is interplay between fibrinolysis and coagulation as the body attempts to break down and absorb the hematoma. These conditions produce an environment marked by fragility of the neocapillaries in the outer membranes with subsequent microhemorrhages, accumulation of fluid, and excessive fibrinolytic activity in the outer membrane.87–95 Weir and Gordon found that subdural fluid contained low fibrinogen and high fibrin degradation product concentrations in 25 cases of chronic SDH.96 These products are known to have anticoagulant effects and could inhibit platelet aggregation.
Bur Hole Drainage
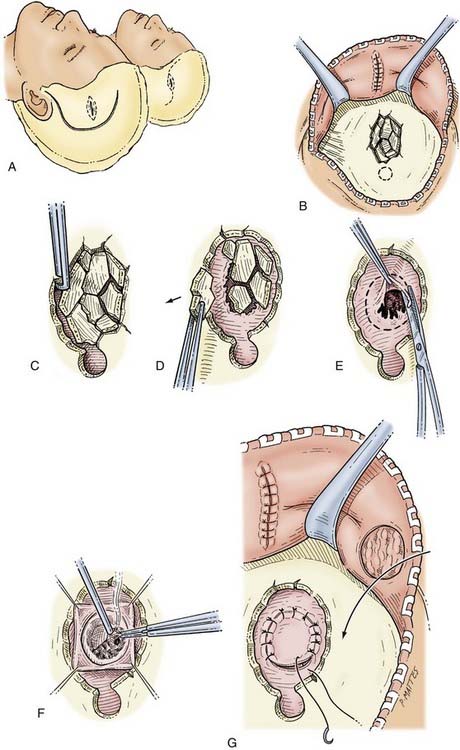
Bur holes are the most widely used method for the drainage of chronic SDHs. Many studies have shown this procedure to be efficacious, but the rate of recurrence requiring a second operation is 2% to 18.5%.97–100
In patients who are not agitated, especially those who are very frail with multiple medical problems, the bur hole drainage procedure can be carried out under local anesthesia induced with 1% lidocaine (Xylocaine). The patient is positioned with the head in a subdural head holder (see Fig. 335-7B). The bur holes are usually placed over the thickest part of the hematoma as seen on CT. The incisions in the scalp are made so that they can be incorporated into a craniotomy flap if required (see Fig. 335-7A). After making the skin incision and separating the underlying periosteum, a single bur hole is placed. The underlying dura is coagulated with diathermy, and a cruciate opening is made in the dura to release the subdural fluid. One of the keys to a successful operation is proper identification and subsequent opening of all the neomembranes formed by a chronic SDH. This can be a challenge, especially with well-organized SDHs, which can have several septated membranes. If this is not done, the brain may not return to its proper anatomic position because it remains tethered by the membrane, and the SDH may recur. Thorough evaluation of the preoperative CT scan with careful scrutiny of the visualized brain surface can help prevent this complication. The second bur hole is then made, the dura is opened, and a soft Silastic irrigation catheter (e.g., a Becker ventriculostomy catheter) is guided over a Penfield No. 3 dissector into the subdural space. Care must be taken to be sure that the catheter is not directed into a sulcus and thus into the surprisingly soft underlying atrophic brain tissue. This is best achieved by using the curve of a Penfield No. 3 dissector to direct the catheter subdurally. The subdural fluid collection is then gently washed out with at least 400 mL of warm normal saline. The drainage catheters are then tunneled under the skin and secured. A recent randomized study has documented an advantage with the use of drains through bur holes in patients with chronic SDHs. Meticulous hemostasis is obtained at the dural margins, and the wound is closed in layers with 2-0 Dexon in the galea and staples at the skin margins. The subdural catheters are then connected to closed drainage bags without suction and kept at the level of the head to facilitate drainage. The patient is usually kept flat in bed for 24 to 48 hours.
Twist Drill Drainage
The advantage of this procedure is that it can be done at the bedside quickly in frail, medically unfit, or very elderly individuals, but recurrences are more common. Several studies have shown twist drill drainage to be a safe and efficacious procedure.86,101,102 Under local anesthesia, a single twist drill hole is made over the most prominent part of the subdural collection based on CT scans. A ventriculostomy catheter is then guided carefully into the subdural space and tunneled several centimeters away from the twist drill site. The risks associated with intracranial passage of a catheter are higher with twist drill drainage. The catheter is then connected to a closed drainage bag. Initially, the fluid is drained slowly for about 30 minutes to 1 hour.
Shunting
A subdural hematoma can be drained into the peritoneum, a procedure that is usually carried out in infants and children and rarely in adults with multiple recurrences.103 Complications of this procedure include poor reexpansion of the cerebral hemisphere, seizures, acute bleeding, and pneumocephalus.
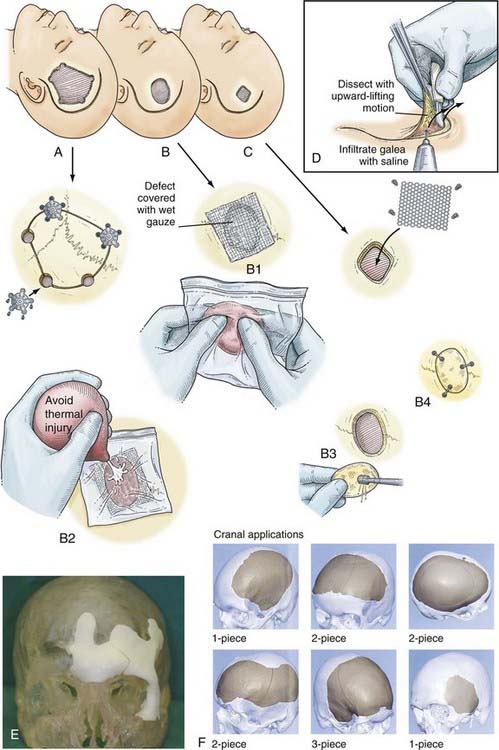
Cranioplasty
In modern practice, the most common indication for cranioplasty is after decompressive craniectomy for raised ICP (see also Chapter 332). It is usually carried out 6 to 8 weeks after the initial procedure, assuming that the patient has recovered from the initial injury and hydrocephalus or brain swelling is not present. In the interim, the patient is protected in a “hockey helmet” against any direct injury to the cranial defect.
Usually, the autologous bone flap, frozen after the initial surgery, is used and provides good cosmetic results. The bone flap remains sterile in a −70°C freezer for many months. Autoclaving of the bone before use has been advocated (e.g., if contaminated by a compound scalp wound before cranioplasty); however, this can reduce the viability of the graft. Others have advocated storing the bone flap in the patient’s own abdominal subcutaneous tissues to preserve better bone viability.104 Complications associated with abdominal preservation of bone flaps include bone resorption (5% to 10% of cases) as a result of hypovascular bone necrosis and sepsis of the flap. Materials such as methyl methacrylate and titanium mesh can be used as substitutes when the bone is heavily comminuted or contaminated.105,106 For large, cosmetically important defects, the use of casts, stereolithographic models, and CT-based “computer-assisted design” reconstruction technology can improve the appearance of the defect, but this is much more expensive and requires several months for fabrication (Fig. 335-23E and F).
Operative Technique
The patient is placed supine with the head on a horseshoe head holder, turned to the opposite side, and elevated above the level of the heart. To maximize intracranial relaxation, the patient is given mannitol (a Foley catheter must be in place) and mildly hyperventilated. Prophylactic antibiotics are also administered at least an hour before the procedure. The previous craniotomy incision is reopened. Dissection in the subgaleal plane is carried out and may be facilitated by first infiltrating the space with saline to open the planes and lifting the galea from the underlying dura. Dissection is done with an upward lifting motion of the skin margins (see Fig. 335-23D). The back of the blade or the scalpel blade handle may be used. The entire flap is then dissected off the underlying dura. The bony margins of the craniotomy opening are exposed with a periosteal elevator and curved curet while protecting the underlying dura, which can be friable. Meticulous hemostasis is obtained with the help of monopolar and bipolar diathermy to prevent postcranioplasty hematomas, which are common (3% to 10%) and predispose to infection. If the patient’s own bone flap is used, a miniplate system is used to fix it to the margins of the defect. Bur hole covers are placed over the frontal holes for improved cosmesis (see Fig. 335-23A). If methyl methacrylate is used, great care must be taken because the polymerized state is reached with the generation of an exothermic reaction. Thus, the curing cement may cause devastating thermal injury to the brain if it is allowed to cure in contact with the cortex. Therefore, it is best to place the material in a plastic bag and mold it to the underlying defect with the use of copious amounts of cold normal saline (see Fig. 335-23B); it must be removed from the patient for the final hardening stages to avoid any thermal injury to the underlying structures. This process can be carried out over a piece of wet gauze to protect the brain. The molded methyl methacrylate is then removed, and the outer surfaces are smoothed with a high-speed drill. It is then fixed to the skull margins with a miniplate system. Alternatively, a precurved sheet of titanium mesh can be used after trimming the edges to fit the craniotomy defect (see Fig. 335-23C). It too can be fixed to the bone edges with miniplate screws while allowing at least a 2-cm overlap of the defect, and placement of screws at the outer edge provides the best cosmetic curvature. A subgaleal drain is inserted and the flap is closed with 2-0 Dexon in the galea and staples at the skin margins. Central dural “tack-up sutures” are advisable.
Complications
Bleeding
Arterial bleeding during the craniotomy can generally be stopped after the source has been visualized, although it may be necessary to extend the craniotomy by nibbling away more bone. Rarely, profuse bleeding may arise from a laceration of the carotid artery at the base of the skull. Packing with muscle may be temporarily successful in controlling this bleeding, but emergency angiography and intra-arterial balloon occlusion are generally needed. Management of arterial injuries is discussed in greater detail elsewhere in this volume (see Chapter 336, Chapter 352, and Chapter 382). Increasing the head-up tilt can usually control venous bleeding from the sinus. If this is not successful, the possibility of raised intrathoracic pressure because of pneumothorax or increased intra-abdominal pressure should be considered.
Skull Base Fracture and Cerebrospinal Leaks
This topic is reviewed extensively in Chapter 338 of this volume.
Coagulopathy Associated with Traumatic Brain Injury
TBIs are often complicated by coagulopathy and can offer significant challenges to the surgeon. A recent study reported TBI-related coagulopathy in 36% of patients with severe TBI and multiple injuries and in 34% of patients with isolated severe TBI.107 Coagulopathic states can make timely surgical intervention unfeasible, and they can generate dangerous postoperative environments fraught with the potential for delayed hemorrhages or enlargement of intracranial lesions. Talving and colleagues cited an approximate 10-fold increase in mortality rates in patients with TBI coagulopathy.107 Head trauma is an independent risk factor for disseminated intravascular coagulation, possibly because the brain is rich in thromboplastin (also called tissue factor), which is released into the systemic circulation with trauma.108,109 Moreover, patients with TBI are very often victims of polytrauma, and the subsequent resuscitation efforts can produce a dilutional coagulopathy. As mentioned previously, administration of rFVIIa may allow very rapid correction of TBI-related coagulopathy and has been shown to decrease the time until intervention in this population of patients.27 A recent small, randomized controlled trial demonstrated the ability to safely administer the drug, even at relatively high concentrations, in hemodynamically unstable polytrauma patients.110 The rFVIIa ICH Study Group recently published the results of a dose escalation clinical trial in which it was demonstrated that administration of rFVIIa can attenuate progression of the hematoma in the setting of traumatic contusions and ICHs.111
Acute/Intraoperative Brain Swelling
Fulminate brain swelling is one of the most dreaded complications in neurosurgery and represents a pathophysiologic entity that is not yet fully understood (Fig. 335-24).112–114 Different components, such as vasogenic and cytotoxic edema, reactive hyperemia, and vascular engorgement, are probably the main factors.48,115 It has been reported in up to a fifth of patients with aSDH (Fig. 335-25).113 When fulminant brain swelling occurs intraoperatively, the position of the endotracheal tube should be reassessed and arterial blood gas analysis performed. PaO2 should be maintained above 100 mm Hg and transient hyperventilation to a PaCO2 of approximately 25 mm Hg begun until closure is achieved. The head of the operating table should be elevated and rotation of the patient’s head and neck minimized. Marked arterial hypertension should be prevented because these patients do not autoregulate their cerebral vasculature. That said, even modest hypotension must be avoided because drops in blood pressure typically result in exacerbation of edema and significant increases in ICP.116 Thus, a mean arterial blood pressure of about 70 to 80 mm Hg should be maintained.117,118 As these maneuvers are carried out, further narcotic sedation, muscle relaxants, and mannitol should be given and a ventriculostomy attempted for drainage of CSF. The possibility of occult bleeding from an evolving ipsilateral intracerebral hematoma or a contralateral surface hematoma must be excluded. Intraoperative ultrasonography, if available, should be performed, and contralateral bur holes can be placed.119 In patients who remain refractory to these measures, hypothermia or pentobarbital coma can be initiated with a 10-mg/kg intravenous bolus given over a period of 20 to 30 minutes. Alternatively, the short-acting general anesthetic agents etomidate and propofol can be used to effectively reduce ICP. When hypotension is a concern, a short course of etomidate is preferable over propofol or pentobarbital because the latter two agents are more likely to significantly lower blood pressure. The use of cerebral protectants for managing refractory intracranial hypertension is discussed further in Chapter 334. Closure can usually be achieved by removing a large bone flap, enlarging the bony defect, and performing a large duraplasty. It is often necessary to resect part of the temporal or frontal lobe to achieve closure in these circumstances and undermine the scalp widely. After surgery, CT should be performed urgently and may occasionally show a new clot in a remote site.
Postcraniotomy Hematoma
ICHs develop under the previous craniotomy after approximately 3% to 7% of craniotomies for TBI (Fig. 335-26).58,107,112,120 However, ICHs remote from the operative site have also been reported (Fig. 335-27). Of 850 patients who underwent craniotomy for evacuation of a traumatic intracranial mass, second hematomas developed in 59 (6.9%) at the initial operative site and required another operation.121 Of these, 39 patients had EDHs, 12 had intracerebral hematomas, and 8 had mixed EDHs and SDHs.
Reaccumulation rates ranged from 7.8% in a series of extradural hematomas reported about 30 years previously by Lobato and colleagues to 61% of aSDHs drained by bur holes.113 In the older neurosurgical literature, higher rates of recurrence of aSDH were almost always due to the use of bur holes instead of craniotomy for evacuation. Bur holes did not allow adequate visualization of the source of bleeding or complete removal of the hematoma.58,61,112,114 Delayed intracerebral hematomas may be a result of the hypoxic, hypotensive, and coagulation defects seen in these patients.58,107,112
Aarabi B, Hesdorffer DC, Ahn ES, et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469-479.
Alves OL, Bullock R. “Basal durotomy” to prevent massive intraoperative traumatic brain swelling. Acta Neurochir (Wien). 2003;145:583-586.
Andrews BT, Chiles BW, Olsen WL, et al. The effect of intracerebral hematoma location on the risk of brain-stem compression and on clinical outcome. J Neurosurg. 1988;69:518-522.
Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):1-106.
Bullock MR, Chesnut R, Ghajar J, et al. Guidelines for the surgical management of traumatic brain injury author group: acknowledgments. Neurosurgery. 2006;58(3):S2.
Bullock R, Golek J, Blake G. Traumatic intracerebral hematoma—which patients should undergo surgical evacuation? CT scan features and ICP monitoring as a basis for decision making. Surg Neurol. 1989;33:181-187.
Chesnut RM, Ghajar J, Maas AI, et al. Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma. 2000;17:557-565.
Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993;33:67-72.
Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304-1305.
Labadie EL, Glover D. Local alterations of hemostatic-fibrinolytic mechanisms in reforming subdural hematomas. Neurology. 1975;25:669-675.
Marshall LF, Gautille T, Klauber MR, et al. The outcome of severe closed head injury. J Neurosurg. 1991;75(suppl):S28-S36.
Mathew P, Oluoch-Olunya DL, Condon BR, et al. Acute subdural hematoma in the conscious patient: outcome with initial non-operative management. Acta Neurochir (Wien). 1993;121:100-108.
Narayan RK, Maas AI, Marshall LF, et al. Recombinant factor VIIA in traumatic intracerebral hemorrhage: results of a dose-escalation clinical trial. for the rFVIIa Traumatic ICH Study Group. Neurosurgery. 2008;62;:776-786.
Pickard JD, Bailey S, Sanderson H, et al. Steps towards cost-benefit analysis of regional neurosurgical care. BMJ. 1990;301:629-635.
Reilly P, Bullock R. Head Injury: Pathophysiology and Management. London: Hodder Arnold; 2005.
Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;1:CD003983.
Sprick C, Bettag M, Bock WJ. Delayed traumatic intracranial hematomas: clinical study of seven years. Neurosurg Rev. 1989;12(suppl 1):228-230.
Stein DM, Dutton RP, Kramer ME, et al. Recombinant factor VIIa: decreasing time to intervention in coagulopathic patients with severe traumatic brain injury. J Trauma. 2008;64:620-627.
Stein SC, Young GS, Talucci RC, et al. Delayed brain injury after head trauma: significance of coagulopathy. Neurosurgery. 1992;30:160-165.
Talving P, Benfield R, Hadjizacharia P, et al. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma. 2009;66:55-61.
Valadka AB, Robertson CS. Surgery of cerebral trauma and associated critical care. Neurosurgery. 2007;61(1 suppl):203-220.
1 Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-378.
2 Finkelstein EA, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. New York: Oxford University Press; 2006.
3 Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602-615.
4 Gennarelli TA, Champion HR, Sacco WJ, et al. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29:1193-1202.
5 Pal J, Brown R, Fleiszer D. The value of the Glasgow Coma Scale and Injury Severity Score: predicting outcome in multiple trauma patients with head injury. J Trauma. 1989;29:746-748.
6 Vollmer DG, Torner JC, Jane JA. Age and outcome following traumatic coma: why do older patients fare worse? J Neurosurg. 1991;75:S37-S49.
7 Eisenberg H, Jane JA, Marmarou A, et al. The Traumatic Coma Data Bank: design methods and baseline characteristics. J Neurosurg. 1991;75:S8-S13.
8 Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954-957.
9 Reilly P, Bullock R. Head Injury: Pathophysiology and Management. London: Hodder Arnold; 2005.
10 Kuroda Y, Inglis FM, Miller JD, et al. Transient glucose hypermetabolism after acute subdural hematoma in the rat. J Neurosurg. 1992;76:471-477.
11 Gopinath SP, Cormio M, Ziegler J, et al. Intraoperative jugular desaturation during surgery for traumatic intracranial hematomas. Anesth Analg. 1996;83:1014-1021.
12 Albericho AM, Ward JD, Choi SC, et al. Outcome after severe head injury, relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. 1987;67:648-656.
13 Marmarou A, Andersin RL, Ward JD, et al. NINDS Traumatic Coma Data Bank: Intracranial pressure monitoring methodology. J Neurosurg. 1991;75:S21-S27.
14 Narayan RK, Kishore PRS, Becker DP, et al. Intracranial pressure: to monitor or not to monitor? J Neurosurg. 1982;56:650-659.
15 Miller JD, Becker DP, Ward JD, et al. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503-516.
16 Foundation BT. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):1-106.
17 Bullock R, Teasdale G. Head injuries—surgical management of traumatic intracerebral hematomas. In: Braakman R, editor. Handbook of Clinical Neurology. Amsterdam: Elsevier; 1990:249-298.
18 Mathew P, Oluoch-Olunya DL, Condon BR, et al. Acute subdural hematoma in the conscious patient: outcome with initial non-operative management. Acta Neurochir (Wien). 1993;121:100-108.
19 Galbraith S, Teasdale G. Predicting the need for operation in the patient with an occult traumatic intracranial hematoma. J Neurosurg. 1981;55:75-81.
20 Bullock MR, Chesnut R, Ghajar J, et al. Guidelines for the surgical management of traumatic brain injury author group: Acknowledgments. Neurosurgery. 2006;58(3):S2.
21 Andrews BT, Chiles BW, Olsen WL, et al. The effect of intracerebral hematoma location on the risk of brain-stem compression and on clinical outcome. J Neurosurg. 1988;69:518-522.
22 Marshall LF, Barba D, Toole BM, et al. The oval pupil: clinical significance and relationship to intracranial hypertension. J Neurosurg. 1983;58:566-568.
23 Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304-1305.
24 Chesnut RM, Ghajar J, Maas AI, et al. Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma. 2000;17:557-565.
25 Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777-785.
26 Bartal C, Freedman J, Bowman K, et al. Coagulopathic patients with traumatic intracranial bleeding: defining the role of recombinant factor VIIa. J Trauma. 2007;63:725-732.
27 Stein DM, Dutton RP, Kramer ME, et al. Recombinant factor VIIa: decreasing time to intervention in coagulopathic patients with severe traumatic brain injury. J Trauma. 2008;64:620-627.
28 Zhang J, Groff RF, Chen XH, et al. Hemostatic and neuroprotective effects of human recombinant activated factor VII therapy after traumatic brain injury in pigs. Exp Neurol. 2008;210:645-655.
29 Lam A, Mayberg T. Use of nitrous oxide in neuroanesthesia: why bother? J Neurosurg Anesthesiol. 1992;4:290-294.
30 Boarini DJ, Kassell NF, Coester H, et al. Comparison of systemic and cerebrovascular effects of isoflurane and halothane. Neurosurgery. 1984;15:400-409.
31 Eintrei C, Leszniewski W, Carlsson C. Local application of 133-xenon for measurement of regional cerebral blood flow (rCBF) during halothane, enflurane, and isoflurane anesthesia in humans. Anesthesiology. 1985;63:391-394.
32 Pinaud M, Lelausque JN, Chetanneau A, et al. Effects of propofol on cerebral hemodynamics and metabolism in patients with brain trauma. Anesthesiology. 1990;73:404-409.
33 Kassell NF, Hitchon PW, Gerk MK, et al. Alterations in cerebral blood flow, oxygen metabolism, and electrical activity produced by high dose sodium thiopental. Neurosurgery. 1980;7:598-603.
34 Dearden NM, McDowell DG. Comparison of etomidate and althesin in the reduction of increased intracranial pressure after head injury. Br J Anaesth. 1985;57:361-368.
35 Albanese J, Viviand X, Potie F, et al. Sufentanil, fentanyl, and alfentanil in head trauma patients: a study on cerebral hemodynamics. Crit Care Med. 1999;27:407-411.
36 Minton MD, Grosslight K, Stirt JA, et al. Increases in intracranial pressure from succinylcholine: prevention by prior nondepolarizing blockade. Anesthesiology. 1986;65:165-169.
37 Miller JD, Dearden NM, Piper IR, et al. Control of intracranial pressure in patients with severe head injury. J Neurotrauma. 1992;9(Suppl 1):S317-S326.
38 Alves OL, Bullock R. “Basal durotomy” to prevent massive intraoperative traumatic brain swelling. Acta Neurochir (Wien). 2003;145:583-586.
39 Pickard JD, Bailey S, Sanderson H, et al. Steps towards cost-benefit analysis of regional neurosurgical care. BMJ. 1990;301:629-635.
40 Mohanty A, Kolluri VR, Subbakrishna DK, et al. Prognosis of extradural haematomas in children. Pediatr Neurosurg. 1995;23:57-63.
41 Holzschuh M, Schuknect B. Traumatic epidural hematomas of the posterior fossa: 20 new cases and a review of the literature since 1961. Br J Neurosurg. 1989;3:171-180.
42 Bozbuga M, Izgi N, Polat G, et al. Posterior fossa epidural hematomas: observations on a series of 73 cases. Neurosurg Rev. 1999;22:34-40.
43 Kuday C, Uzan M, Hanci M. Statistical analysis of the factors affecting the outcome of extradural haematomas: 115 cases. Acta Neurochir (Wien). 1994;131:203-206.
44 Lee EJ, Hung YC, Wang LC, et al. Factors influencing the functional outcome of patients with acute epidural hematomas: analysis of 200 patients undergoing surgery. J Trauma. 1998;45:946-952.
45 Uzan M, Yentür E, Hanci M, et al. Is it possible to recover from uncal herniation? Analysis of 71 head injured cases. J Neurosurg Sci. 1998;42:89-94.
46 Lv XL, Li YX, Liu AH, et al. A complex cavernous sinus dural arteriovenous fistula secondary to covered stent placement for a traumatic carotid artery-cavernous sinus fistula: case report. J Neurosurg. 2008;108:588-590.
47 Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: mortality, morbidity and operative timing. J Neurosurg. 1991;74:212-218.
48 Becker DP. Acute subdural hematomas. In: Vigourox RP, editor. Neurotraumatology. Vienna: Springer-Verlag; 1986:51-95.
49 Miller JD, Butterworth JF, Gudeman SK, et al. Further experience in the management of severe head injury. J Neurosurg. 1981;54:289-299.
50 Marshall LF, Gautille T, Klauber MR, et al. The outcome of severe closed head injury. J Neurosurg. 1991;75(Suppl):S28-S36.
51 Howard MAIII, Gross AS, Dacey RGJr, et al. Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg. 1989;71:858-863.
52 Massaro F, Lanotte M, Faccani G, et al. One hundred and twenty-seven cases of acute subdural hematoma operated upon: correlation between CT findings and outcome. Acta Neurochir (Wien). 1996;138:185-191.
53 Ko RK, Akdemir H, Oktem IS, et al. Acute subdural hematoma: outcome and outcome prediction. Neurosurg Rev. 1997;20:239-244.
54 Mathiesen T, Benediktsdottir K, Johnson H, et al. Intracranial traumatic and non-traumatic hemorrhagic complications of warfarin treatment. Acta Neurol Scand. 1995;91:208-214.
55 Wintzen AR, Tijssen JGP. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39:69-72.
56 Mattle H, Kohler S, Huber P, et al. Anticoagulation-related intracranial extracerebral hemorrhage. J Neurol Neurosurg Psychiatry. 1989;52:829-837.
57 Resnick DK, Marion DW, Darby JM. The effect of hypothermia on the incidence of delayed traumatic intracerebral hemorrhage. Neurosurgery. 1994;34:252-255.
58 Gudeman SK, Kishore PR, Miller JD, et al. The genesis and significance of delayed traumatic intracerebral hematoma. Neurosurgery. 1979;5:309-313.
59 Rivano C, Borzone M, Carta F, et al. Traumatic intracerebral hematomas: 72 cases surgically treated. J Neurosurg Sci. 1980;24:77-84.
60 Jamieson KG, Yelland JDN. Traumatic intracerebral hematoma: report of 63 surgically treated cases. J Neurosurg. 1972;37:528-532.
61 Sprick C, Bettag M, Bock WJ. Delayed traumatic intracranial hematomas: clinical study of seven years. Neurosurg Rev. 1989;12(Suppl 1):228-230.
62 Bullock R, Golek J, Blake G. Traumatic intracerebral hematoma—which patients should undergo surgical evacuation? CT scan features and ICP monitoring as a basis for decision making. Surg Neurol. 1989;33:181-187.
63 Guerra WK-W, Gaab MR, Dietz H, et al. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187-196.
64 Polin RS, Shaffrey ME, Bogaev CA, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84-94.
65 Young HA, Schmidek HH. Complications accompanying occipital skull fracture. J Trauma. 1982;22:914-920.
66 Cuneo RA, Caronna JJ, Pitts L, et al. Upward transtentorial herniation: seven cases and a review of the literature. Arch Neurol. 1979;36:618-623.
67 Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;1:CD003983.
68 Schirmer CM, Ackil AA, Malek AM. Decompressive craniectomy. Neurocrit Care. 2008;8:456-470.
69 Guerra WK, Gaab MR, Dietz H, et al. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187-196.
70 Stein SC, Young GS, Talucci RC, et al. Delayed brain injury after head trauma: significance of coagulopathy. Neurosurgery. 1992;30:160-165.
71 Taylor A, Butt W, Rosenfeld J, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154-162.
72 Aarabi B, Hesdorffer DC, Ahn ES, et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469-479.
73 Hatashita S, Koike J, Sonokawa T, et al. Cerebral edema associated with craniectomy and arterial hypertension. Stroke. 1985;16:661-668.
74 Jourdan C, Convert J, Mottolese C, et al. [Evaluation of the clinical benefit of decompression hemicraniectomy in intracranial hypertension not controlled by medical treatment.]. Neurochirurgie. 1993;39:304-310.
75 Reithmeier T, Löhr M, Pakos P, et al. Relevance of ICP and PtiO2 for indication and timing of decompressive craniectomy in patients with malignant brain edema. Acta Neurochir (Wien). 2005;147:947-951.
76 Valadka AB, Robertson CS. Surgery of cerebral trauma and associated critical care. Neurosurgery. 2007;61(1 Suppl):203-220.
77 Sataloff RT, Sariego J, Myers DL, et al. Surgical management of the frontal sinus. Neurosurgery. 1984;15:593-596.
78 Donald PJ, Bernstein L. Compound frontal sinus injuries with intracranial penetration. Laryngoscope. 1978;88:225-231.
79 Cooper PR. Depressed skull fracture. In: Apuzzo MLJ, editor. Brain Surgery Complication Avoidance and Management. New York: Churchill Livingstone; 1993:1273-1282.
80 Kapp JP, Gielchinsky I. Management of combat wounds of the dural venous sinuses. Surgery. 1972;71:913-917.
81 Kapp JP, Gielchinsky I, Deardourff SL. Operative techniques for management of lesions involving the dural venous sinuses. Surg Neurol. 1977;7:330-342.
82 Albin MS, Carroll RG, Maroon JC. Clinical considerations concerning detection of venous air embolism. Neurosurgery. 1978;3:380-384.
83 Cameron M. Chronic subdural hematoma: a review of 114 cases. J Neurol Neurosurg Psychiatry. 1987;41:834.
84 French BN, Cobb CA, Corkill G, et al. Delayed evolution of post-traumatic subdural hygroma. Surg Neurol. 1978;9:145-148.
85 Ohno K, Suzuki R, Masaoka H, et al. Chronic subdural hematoma preceded by persistent traumatic subdural fluid collection. J Neurol Neurosurg Psychiatry. 1987;50:1694-1697.
86 Hubschmann OR. Twist drill craniostomy in the treatment of chronic and subacute subdural hematomas in severely ill and elderly patients. Neurosurgery. 1980;6:233-236.
87 Gjerris F, Sorensen S. Colloid osmotic and hydrostatic pressures in chronic subdural hematomas. Acta Neurochir (Wien). 1980;54:53-60.
88 Rabe EF, Young GF, Dodge PR. The distribution and fate of subdurally instilled human serum albumin in infants with subdural collections of fluid. Neurology. 1964;14:1020-1028.
89 Weir B. The osmolality of subdural hematoma fluid. J Neurosurg. 1971;34:528-533.
90 Weir B. Oncotic pressure of subdural fluids. J Neurosurg. 1980;53:512-515.
91 Friede RL. Incidence and distribution of neomembranes of dura mater. J Neurol Neurosurg Psychiatry. 1971;34:439-446.
92 Friede RL, Schachenmayr W. The origin of subdural neomembranes. II. Fine structure of neomembranes. Am J Pathol. 1978;92:69-78.
93 Labadie EL, Glover D. Local alterations of hemostatic-fibrinolytic mechanisms in reforming subdural hematomas. Neurology. 1975;25:669-675.
94 Ito H, Yamamoto S, Komai T, et al. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976;45:26-31.
95 Ito H, Komai T, Yamamoto S. Fibrinolytic enzymes in the lining walls of chronic subdural hematoma. J Neurosurg. 1978;48:197-200.
96 Weir B, Gordon P. Factors affecting coagulation: fibrinolysis in chronic subdural fluid collections. J Neurosurg. 1983;58:242-246.
97 Kotwica Z, Brzezinski J. Chronic subdural hematoma treated by burr holes and closed drainage: personal experience in 131 patients. Br J Neurosurg. 1991;5:461-465.
98 Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993;33:67-72.
99 Matsumoto K, Akagi K, Abekura M, et al. Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage. Neurol Res. 1999;21:277-280.
100 Smely C, Madlinger A, Scheremet R. Chronic subdural hematoma—a comparison of two different treatment modalities. Acta Neurochir (Wien). 1997;139:818-825.
101 Carlton CK, Saunders R. Twist drill craniotomy and closed system drainage of chronic and subacute subdural hematomas. Neurosurgery. 1983;13:153-159.
102 Tabaddor K, Shulman K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46:220-226.
103 Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637-645.
104 Kreider GN. Repair of cranial defect by a new method. JAMA. 1920;64:1024.
105 Malis LI. Titanium mesh and acrylic cranioplasty. Neurosurgery. 1989;25:351-355.
106 Prolo DJ, Hara H, Ichinose Y. The use of bone grafts and alloplastic materials in cranioplasty. Clin Orthop Relat Res. 1991;268:270-278.
107 Talving P, Benfield R, Hadjizacharia P, et al. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma. 2009;66:55-61.
108 Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J. Pathol. 1989;134:1087-1097.
109 Goodnight SH, Kenoyer G, Rapaport SI, et al. Defibrination after brain-tissue destruction: a serious complication of head injury. N Engl J Med. 1974;290:1043-1047.
110 Kluger Y, Riou B, Rossaint R, et al. Safety of rFVIIa in hemodynamically unstable polytrauma patients with traumatic brain injury: post hoc analysis of 30 patients from a prospective, randomized, placebo-controlled, double-blind clinical trial. Crit Care. 2007;11(4):R85.
111 Narayan RK, Maas AI, Marshall LF, et al. Recombinant factor VIIA in traumatic intracerebral hemorrhage: results of a dose-escalation clinical trial. Neurosurgery. 2008;62:776-786.
112 Ninchoji T, Emara K, Shimoyama I, et al. Traumatic intracerebral hematomas of delayed onset. Acta Neurochir (Wien). 1984;71:69-90.
113 Lobato RD, Rivas JJ, Cordobes F, et al. Acute epidural hematoma: an analysis of factors influencing the outcome of patients undergoing surgery in coma. J Neurosurg. 1988;68:48-57.
114 Tindall GT, Payne NS, O’Brien MS. Complications of surgery for subdural hematoma. Clin Neurosurg. 1976;23:465-482.
115 Kobrine AJ, Timmons E, Rajjoub JK, et al. Demonstration of massive traumatic brain swelling within 20 minutes after injury. J Neurosurg. 1977;46:256-258.
116 Bouma GJ, Muizelaar JP, Bandoh K, et al. Blood pressure and intracranial pressure-volume dynamics in severe head injury: relationships with cerebral blood flow. J Neurosurg. 1992;77:15-19.
117 Rosner MJ. Pathophysiology and management of increased intracranial pressure. In: Andrews BT, editor. Neurosurgical Intensive Care. New York: McGraw-Hill; 1993:57-112.
118 Andrews BT, Bederson JB, Pitts LH. Use of intraoperative ultrasonography to improve the diagnostic accuracy of exploratory burr holes in patients with traumatic tentorial herniation. Neurosurgery. 1989;24:345-347.
119 Stone JL, Rifai MHS, Sugar O, et al. Subdural hematomas. I. Acute subdural hematoma: progress in definition, clinical pathology, and therapy. Surg Neurol. 1983;19:216-231.
120 Bullock R, Hanemann CO, Murray L, et al. Recurrent hematomas following craniotomy for traumatic intracranial mass. J Neurosurg. 1990;72:9-14.
121 Richards T, Hoff T. Factors affecting survival from acute subdural hematoma. Surgery. 1974;75:253-258.