Chapter 36 Surgical Management of Sphenoid Wing Meningiomas
Sphenoid wing meningiomas (SWMs) constitute about 14% to 20% of intracranial meningiomas.1 Although they originate from arachnoid cells, they are usually attached to dural thickening or folding, where they receive their blood supply. Infiltration of the adjacent bone is not unusual; neither is growth around or inside the cranial base foramina.2 Most SWMs are relatively easy to remove; however, they are sometimes challenging, especially when they invade the cavernous sinus, internal carotid artery (ICA), and visual pathway. In these cases, total excision is extremely difficult, resulting in high morbidity and a high rate of regrowth or recurrence.3
Anatomic Observations
The designation SWM refers to tumors that originate in any part of the bony crest formed by wings (lesser and greater) of the sphenoid bone, which represents the boundary between the anterior and the middle cranial floor.4 This anatomic portion is also known as the sphenoid ridge, where the lesser wing constitutes its internal two thirds and the greater wing its external third. The lesser wing is the most complex area because of its relationship with the orbit, ICA, cavernous sinus, optic foramen, superior orbital fissure, sylvian fissure, middle cerebral artery (MCA), tip of the temporal lobe, and basal surface of the frontal lobe. In contrast, the greater wing is located in the external portion and is in relation to the frontal and temporal lobes (predominantly with their opercular areas) in its intracranial surface and with the orbit and zygomatic fossa in its extracranial surface, where it serves as insertion to the temporal muscle. It also forms the pterion when it articulates with the frontal squama, temporal, and parietal bones.4
Classification
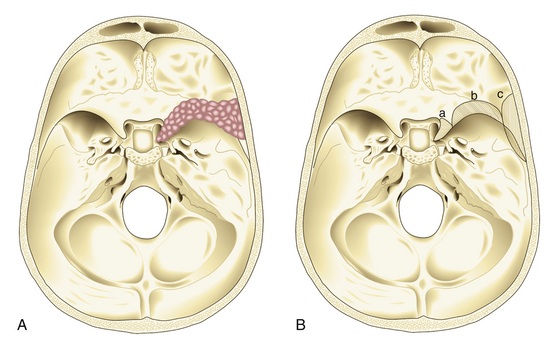
In 1938, Cushing and Eisenhardt classified SWMs into two main varieties: en plaque and globoid (Fig. 36-1).
En-plaque Meningiomas
Also known as spheno-orbital meningiomas or hyperostotic meningiomas of the sphenoid wing, en-plaque meningiomas refer to tumors with a carpet-like dural growth, which are associated with a reactive hyperostosis that, in most cases, is marked and principally responsible for clinical manifestations5,6 (Fig. 36-1A). Sphenoid wing hyperostosis has been reported as high as 42% of all meningiomas in this area.7,8 Due to its extensive bone involvement, differential diagnosis should include fibrous dysplasia, osteoma, osteoblastic metastasis, Paget’s disease, hyperostosis frontalis interna, erythroid hyperplasia, and sarcoidosis.6,9,10 It frequently extends posteriorly toward the cavernous sinus and anteriorly toward the orbital apex, where it causes proptosis and oculomotor deficits, the primary clinical manifestations of these lesions.5 Hyperostosis in meningiomas was initially described by Brissaud and Lereboullet in 1903.11 Theories regarding the cause of hyperostosis include vascular disturbances, irritation of bone without actual invasion, previous trauma, bone production by tumor cells, or osteoblastic stimulation of normal bone. Currently, the most widely accepted theory is that this bone growth is actually bone invasion by tumor cells.3,11,12
Globoid Meningiomas
Globoid meningiomas have traditionally been classified into three groups: (1) deep, inner, or clinoidal; (2) middle or alar; and (3) lateral, outer, or pterional (Fig. 36-1B). Middle or alar meningiomas have radiologic characteristics similar to lateral or pterional meningiomas. Surgical resection and clinical results of both types are almost identical. For this reason, some authors suggest that globoid meningiomas of the sphenoid wing can be classified into only two groups: deep, inner, or clinoidal and lateral, outer, or pterional, discharging the middle or alar variety.13 Deep, inner, or clinoidal meningiomas represent the most complex variety of these tumors, whose resection, in most cases, implies increased morbidity and a high rate of recurrence.14–17 In this region, the cavernous sinus is the most critical area; therefore, clinoidal meningiomas are subclassified into two groups: tumors without extradural growth and tumors with extradural growth into the cavernous sinus.13,18
There is a third and debatable variety of SWMs. These are tumors that grow within the diploë without an epidural, subdural, or subcutaneous component and are referred to as intraosseous or intradiploic meningiomas.3,6 The origin of these tumors in the skull base is controversial. Arachnoid cells have been described, following the vessels and nerves in bone foramina or trapped within the sutures. However, some authors19 doubt the existence of these tumors, proposing that they are really a variety of en-plaque meningiomas in which the dural component of the tumor is not well identified on preoperative imaging studies, mostly considering that many of these tumors were described before the era of magnetic resonance imaging (MRI).
Clinical Course
The most common clinical sign of en-plaque meningiomas is proptosis, which usually is slowly evolving, unilateral, nonpulsatile, and irreducible.11 Possible causes of this sign are hyperostosis of the orbital walls, periorbital tumor invasion, intraorbital tumor, and venous stasis caused by compression of the ophthalmic veins. Related symptoms include headache, orbital pain, visual deficit, ptosis, diplopia, ectropion, conjunctivitis, corneal ulceration, and scleral hemorrhages. Unilateral loss of vision is rare and is caused by a narrowing of the optic foramen.
In regard to clinoidal meningiomas, clinical manifestations are dominated by visual field problems presenting virtually any pattern. On the other hand, visual acuity deficits are only somewhat variable and are reported in between 39% and 92% of patients.1,7 When these tumors invade the cavernous sinus, the most common symptoms are oculomotor deficit (especially on cranial nerve VI) and facial hypoesthesia.
Diagnosis
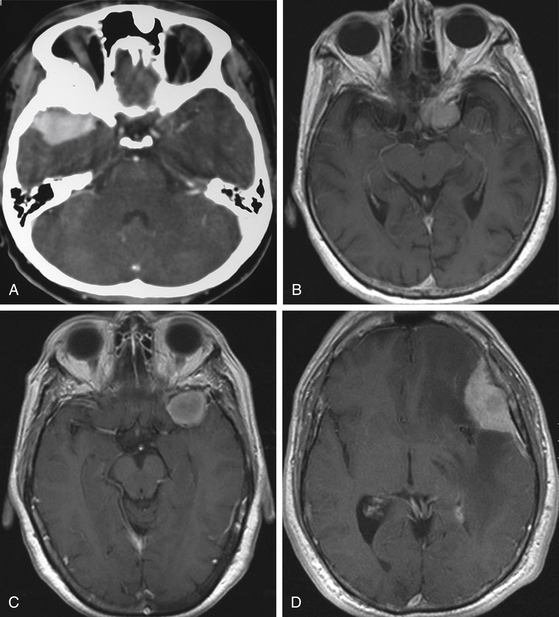
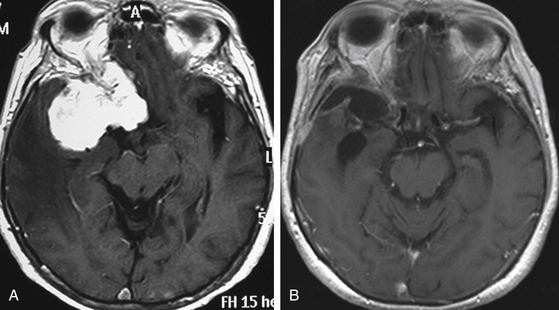
Although clinical manifestations may be strongly suggestive of the diagnosis, especially in spheno-orbital meningiomas, imaging studies are indispensable to establish the diagnosis and to propose the best therapeutic option (Fig. 36-2).
Computed Tomography
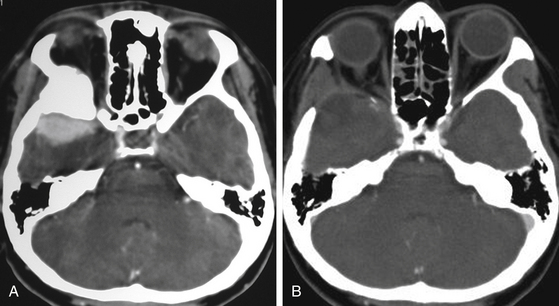
Computed tomography (CT) is predominantly useful in en-plaque meningiomas because it provides more precise information regarding the extent of bone invasion, also allowing correct planning for reconstruction (Fig. 36-2A). The dural component of these tumors is typically found as an isodense image with contrast enhancement, being contiguous with the surrounding dura mater.11 However, it is not always possible to observe this due to its carpet-like appearance. Also, it may be occulted by the high hyperdensity of the contiguous bone growth. There is no correlation between size of the hyperostosis and size of the meningeal tumor. In fact, small dural components are frequently found associated with large bone masses. A bone window algorithm is mandatory in terms of obtaining better definition of bone thickening. Performing axial and coronal projections, as well as three-dimensional (3D) reconstruction, also facilitates both complete resection and reconstruction. The most common locations of hyperostosis of en-plaque meningiomas are, in order of frequency, the lesser wing of the sphenoid bone, the greater wing of the sphenoid, the roof of the orbit, the inferior orbital fissure, the infratemporal fossa, and the orbital rim.
Globoid tumors appear on CT as well-defined isodense lesions that present an intense and homogeneous contrast enhancement. Even though they are usually slow-growing masses, it is not uncommon to find perilesional edema invading the temporal lobe and centrum semiovale, which is attributed to compression or occlusion in the superficial middle cerebral vein or sphenoparietal sinus; there is no direct relationship between the grade of edema and the size of the tumor. Clinoidal meningiomas usually show hyperostosis of the anterior clinoid process (ACP), causing narrowing of the optic canal and the superior orbital fissure.1 CT has some limitations when these tumors infiltrate the cavernous sinus; however, computed angiotomography (CAT) may help by providing more precise information about blood supply and vascular relations of the tumor.
Magnetic Resonance Imaging
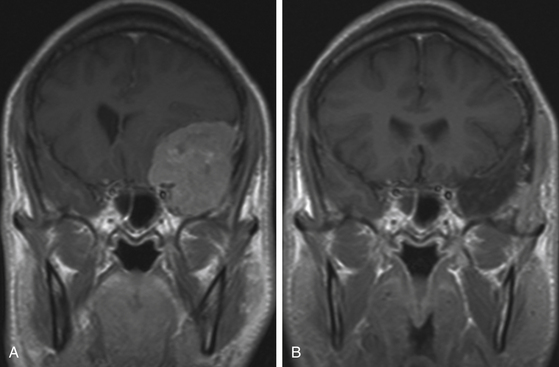
MRI is more useful in globoid meningiomas (Fig. 36-2B to D) and for the correct evaluation of the dural component of en-plaque meningiomas; however, this study’s principal limitation is the osseous tissue. Globoid meningiomas show different appearances on MRI. When their vascularity is not so marked, they usually present as a homogeneous isointense lesion in both T1- and T2-weighted images;20 on the other hand, when they are highly vascularized (angioblastic meningiomas), multiple hypointense images (“empty signals”) can be seen in the interior of the tumor. Gadolinium enhancement is usually intense and uniform, and a T2-weighted image is particularly useful in demonstrating perilesional edema.
Angiography
Considering the clear image of the vascular anatomy obtained with CAT and MRA, particularly with 3D reconstructions, angiography is indicated only in special SWM cases. Selective catheterization provides specific information about the blood supply of the tumor1 and allows the possibility of preoperative embolization. Another indication for angiography is for performing carotid occlusion tests, especially in tumors invading the cavernous sinus and when arterial bypass is planned.
Treatment
Surgical management is, without doubt, the best therapeutic option for SWMs. This treatment is indicated considering the following factors: size of the lesion, presence of signs or symptoms, patient’s condition, changes in the adjacent cerebral tissue (edema) on imaging studies, and surgeon’s experience. In general, surgery is indicated in all patients who are in good health and have a tumor size greater than 2.5 cm. The goal of surgery in all cases should be radical excision of the tumor, which means resection of the lesion, along with the dural implant (1-cm margin) and all hyperostotic bone. This objective is accomplished in the large majority of SWMs except in some spheno-orbital and clinoidal meningiomas, particularly when they present invasion to the cavernous sinus. For these cases, most authors recommend excising the tumor, dura mater, and infiltrated bone on extracavernous areas but leaving the intracavernous portion for another adjuvant treatment, such as radiosurgery, because even in experienced hands, oculomotor morbidity is extremely high after a direct approach to this region.1,2,4,18,21
Positioning
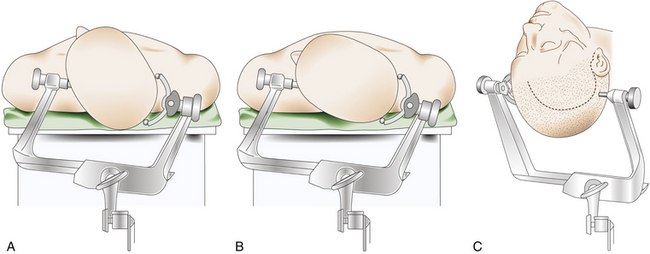
The patient is placed in the supine decubitus position with the head fixed in a three-pin headholder with slight extension and rotated toward the contralateral side of the tumor (Fig. 36-3). There are certain variations in this head rotation, depending on the exact location of the meningioma: for clinoidal tumors a lesser rotation (between 30 and 40 degrees) is preferred, whereas for alar and pterional lesions a major rotation (between 40 and 50 degrees) is indicated2 (Fig. 36-3A and B).
Skin Incision
Most SWMs can be operated on through a frontotemporal (pterional) curvilinear skin incision starting at the root of the zygomatic arch, just 5 mm in front of the tragus, which runs vertically upward. Once it passes the ear, it is curved rostrally and superiorly toward the ipsilateral frontal region until it reaches the midline or beyond, always keeping behind the hairline (Fig. 36-3C). The midportion of the incision can be extended backward, especially in cases of pterional meningiomas with large infiltration of the pterion. If an orbitozygomatic (OZ) approach is required, it is necessary to extend the incision vertically down to the level of the ear lobe.
Dissection of Epicranial Planes
Beginning this dissection in the preauricular area of the incision is recommended for facilitating early identification of the superficial temporal artery, which must be preserved to maintain vascularity of the myocutaneous flap.11 In this region, this vessel usually has a posterior branch that has to be coagulated and cut to allow anterior displacement of the main trunk, along with the skin flap. Dissection continues until the temporal fascia is identified in the entire area of the skin incision. It is important not to perform a wide separation between the temporal fascia and the skin flap because, in this manner, injury to the frontotemporal branch of the facial nerve is unavoidable. There are two forms to achieve the preservation of this branch of the facial nerve. The first consists of making the cut in a single plane from skin to bone, including the cut in the skin, temporal fascia, and muscle fibers. In this way, a single fasciomyocutaneous flap is created, which is detached from the bone and displaced forward. This is a simple and safe form to preserve facial function; however, it presents some problems. First, the fasciomyocutaneous flap created in this way is bulky and sometimes interferes with the surgeon’s deep vision, especially when dealing with clinoidal tumors. Second, particularly in pterional meningiomas, there are some cases that present tumor infiltration to the temporal muscle that is not seen (and therefore not removed) because the muscle remains attached to the galea. For these reasons, there is another way to accomplish preservation of the facial branch, which consists of sectioning of the two layers (superficial and deep) of the temporal fascia in the same direction as the skin incision. In this way, the skin flap and the two layers of the temporal fascia (with the fat pad and the branch of the facial nerve lying between them) are separated from the temporal muscle fibers and reflected anteriorly.11 Once this is completed, detachment and separation of the temporalis muscle is done. It is recommended to perform this dissection in a retrograde direction (from downward to upward) to preserve the epicranial surface of the temporal muscle and reduce its atrophy.22 In this way, two epicranial planes are created: one composed by the skin and temporal fascia (fasciocutaneous flap) and the other formed by the temporal muscle alone (muscle flap). In this manner, the two flaps can be displaced in slightly different directions: the fasciocutaneous flap forward and the muscle flap downward, which facilitates exposure.11
Pterional
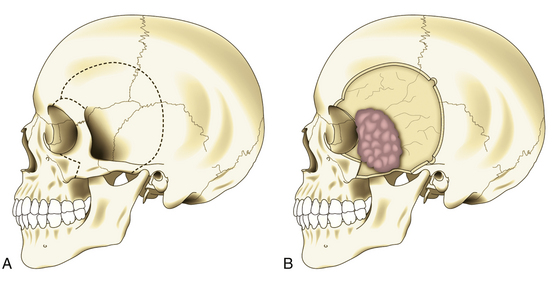
In pterional tumors, hyperostosis is usually seen immediately in the pterion once the temporal muscle is detached. Craniotomy is then planned around the bone infiltration, forming a bone flap of about 5 cm around it; if the hyperostosis is not seen, a standard pterional craniotomy is performed (Fig. 36-4A). To raise and remove the bone flap, it is often necessary to section the tumor, leaving in situ the dural infiltration (Fig. 36-4B). This step must be done carefully because of risk of profuse bleeding that may be seen in some cases. Resection of the hyperostosis is done in the bone flap, being certain to remove at least a 1-cm free margin. The meningeal portion of the tumor is then removed, also being certain to include at least a 1-cm free margin of healthy dura mater2 (Fig. 36-4C). However, in some cases of predominantly osseous tumors, its division is not possible during craniotomy. Under this condition, a craniectomy is then preferred, removing the bony tumor from the onset by using a high-speed drill until exposing the dural implant.
Alar
In alar tumors, osseous infiltration is usually not seen from the beginning; therefore, a frontotemporal craniotomy is performed with standard dimensions of a 6- to 8-cm diameter centered on the pterion. Craniotomy is followed by extradural resection of the lesser wing of the sphenoid bone. This step is sometimes troublesome because of bleeding, which may be profuse, generated when performing dural detachment.14,15 Bone removal is continued until complete exposure of the superior orbital fissure and base of the ACP. The dura mater is then opened following a curvilinear frontotemporal incision, reflecting the dural flap forward. Next, splitting of the sylvian fissure is done to gently separate the frontal and temporal lobes to expose the tumor. If the position is correct and cerebrospinal fluid (CSF) evacuation is adequate, it is usually not necessary to place automatic retractors. In these cases, “en bloc” resection is only possible in small tumors; therefore, initial debulking is preferred in the majority of these cases, leaving deeper portions and dural implant for the end of the procedure.
Clinoidal
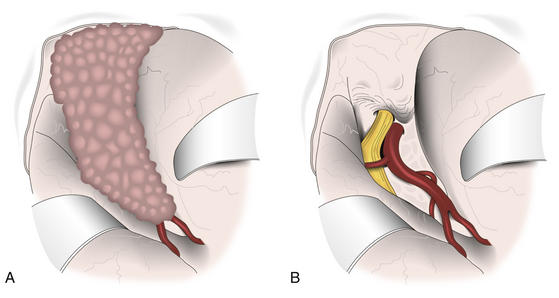
In clinoidal tumors, a frontotemporal or standard pterional craniotomy is performed, as previously mentioned. Bone management is begun extradurally with resection of the sphenoid ridge from the pterion to the base of the ACP. The superior orbital fissure is also completely opened;14 if there is an orbital component of the tumor, the posterolateral wall of the orbit is also removed.14,16 In almost all clinoidal tumors, it is also necessary to perform an anterior clinoidectomy using a high-speed drill, being careful to irrigate profusely to avoid damage to the optic nerve due to heat generated by the drilling. This anterior clinoidectomy can also be performed by holding the ACP with a rongeur and applying a gentle “wiggle and jiggle” movement of the surgeon’s wrist.23 This maneuver may be difficult in cases with marked hyperostosis. Drilling is continued until the optic canal is completely unroofed, being careful not to open the sphenoid or ethmoid sinus when working in the superomedial portion of this channel. The curvilinear dural incision is the same as described earlier, and if the tumor is invading the optic nerve, it is recommended to make another incision following bisection of the dural flap toward the optic sheath and extending across the falciform ligament to the annulus of Zinn. This allows initial identification and free mobilization of the optic nerve before tumor removal.15,16 Dural opening is followed by a wide splitting of the arachnoid membrane on the sylvian fissure and the free drainage of CSF (Fig. 36-5). Retractors are placed on the frontal and temporal lobes, more to protect their cortical surfaces than to actively retract them, and the lateral portion of the tumor is then identified (Fig. 36-5A). Initially, the dural implants in the frontal and temporal regions are coagulated, which reduces vascular supply of the tumor and facilitates its resection. The distal branches of the MCA and, if possible, the main trunk of this artery are then identified, focusing special attention on early identification and preservation of lenticulostriate branches. Dissection is followed in a distal to proximal direction, which in a large tumor necessarily implies an initial debulking. Arterial dissection is continued proximally to identify the carotid bifurcation and anterior cerebral, posterior communicating, and anterior choroidal arteries, reaching the ICA. The optic nerve is next referred intradurally and released from the tumor. Once macroscopic resection of the tumor is completed, extirpation of the dural implant is done, including margins as previously described (Fig. 36-5B). However, this is extremely difficult to achieve in the vicinity of the ACP.
Resection of intracavernous meningiomas deserves a special description. Due to its complexity, it is discussed in detail elsewhere in this book. This involves manipulation of the ICA and oculomotor nerves, as well as the first and second branches of the trigeminal nerve.21 In these tumors, it is mandatory to have initial proximal control of the ICA, preferably in its petrous portion, which also serves as anatomic orientation. To do this, it is necessary to combine pterional craniotomy with an OZ approach. It is recommended to begin tumor resection extradurally by finely peeling the lateral wall of the cavernous sinus. If necessary, it can be continued intradurally, entering through the different anatomic triangles. However, as has been previously mentioned, gross total resection is almost impossible when the tumor invades this region.
En Plaque
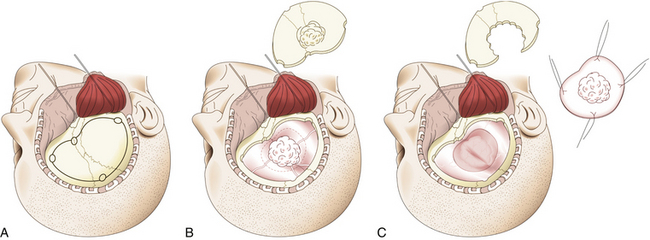
In en-plaque tumors, it is easier to expose the entire hyperostosis if pterional craniotomy is combined with an OZ osteotomy, particularly when the lesion extends into the inferior orbital fissure, infratemporal fossa, or orbit (Fig. 36-6). This osteotomy is performed with a reciprocating saw, beginning the cut in the orbital rim at the level of the supraorbital notch or slightly medial to this. The cut is directed backward over the orbital roof to a distance of about 1.5 to 2 cm, at which point the direction is changed and continues laterally to reach the inferior orbital fissure. The bone cut is then continued on the orbital surface from the inferior orbital fissure toward the malar bone. The cut on this bone is completed on its extracranial surface, making an inverted V shape to facilitate relocation. Finally, a diagonal cut is made at the root of the zygomatic arch (Fig. 36-6A). Once the OZ bar is extracted, it is easy to observe the totality of the hyperostosis from a lateral perspective and the drilling begins (Fig. 36-6B). Drilling should be systematic and must include all bony structures of the region invaded by hyperostosis. Occasionally, due to extensive bone invasion, opening the ethmoid and sphenoid sinuses is unavoidable; in such cases, these sinuses should be occluded with fat and fibrin adhesive. Once excision of the osseous component of the tumor is finalized, the intradural portion and, finally, all infiltrated dura are resected, attempting to extend this resection beyond the area of dural enhancement seen on imaging studies. This step is facilitated with the use of neuronavigation systems.
Reconstruction and Closure
Considering that in all SWMs it is necessary to resect a free dural margin, closure of the dura mater necessarily implies application of a graft. For this purpose, local tissue can be used, such as aponeurotic galea, pericranium, or temporal fascia. Distant tissues such as fascia lata or abdominal fascia can also be used, as can synthetic and biologic materials, but with a slightly higher risk of infection. Watertight closure is mandatory and can be reinforced with the use of fibrin sealants. There is an ample variety of options for reconstruction of the pterional defect. Autologous materials such as split calvarial bone graft or ribs can be used, as well as synthetic materials such as methylmethacrylate and titanium, which have the advantage of offering a better cosmetic result. Reconstruction of the orbital walls is controversial. The only incontrovertible point regarding this issue is when the floor, the orbital rim, or both are removed; in these cases, all authors agree that reconstruction is required due to the high risk of orbital ptosis, postoperative diplopia, or cosmetic defect. The controversy focuses mainly on reconstruction of the superior and lateral walls of the orbit. Some authors mention that reconstruction is unnecessary when the roof is removed, as well as the greater and lesser wings of the sphenoid bone;6 however, other authors have reported an increased risk of pulsatile enophthalmos or cosmetic deficit if this reconstruction is not performed.5,11,24
Complications and Results
Although surgery for an SWM has a high margin of safety, because in the majority of cases it is possible to get excellent surgical results (Figs. 36-7 to 36-9), there are some complications reported that must be considered. There is a risk of postoperative hematoma, especially epidural, due to the wide dural detachment done in some cases and the spaces created by resection of large bone formations. CSF leakage is another risk, which is secondary to wide resection of the dural implant. Possibility of seizures should also be considered because SWMs sometimes grow near potentially epileptogenic cortical areas. Finally, cosmetic results may be a problem after inadequate reconstruction. More serious complications are indeed rare. They are primarily associated with a lesion on functional areas of the brain, especially when they occur on the dominant side and are related to excessive manipulation of brain parenchyma, poor use of retractors, or vascular lesion in the MCA or its branches.
Risk of infection is also present, especially when prosthetic materials are used for reconstruction or when frontal, ethmoid, or sphenoid sinuses are inadvertently opened. Finally, mortality after surgery for an SWM is very rare and, when this happens, is usually secondary to a concomitant preexisting problem.
In general, the short- and midterm follow-up results after SWM resection are excellent. In the majority of cases, gross total resection is accomplished with minimal morbidity. However, the critical point is in long-term follow-up because of the high risk of recurrence, which is inversely proportional to the degree of tumor resection.11,18,19,25 Factors most commonly reported to be associated with incomplete resection of these tumors are extent of bone invasion, undervaluation of the dural component, and invasion of adjacent neurovascular structures. In addition, biologic behavior of the tumor must be considered because if anaplasia is demonstrated or if there is a high proliferative index or complex karyotype, recurrence risk significantly increases. In general, 10-year recurrence has been estimated to be between 9% and 15%, which increases to 19% in a 20-year follow-up. However, a 35% to 50% index of recurrence has been reported when associated with orbital extension.6
Abdel-Aziz K.M., Froelich S.C., Dagnew E., et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery. 2004;54:1375-1384.
Bassiouni H., Asgari S., Sandalcioglu E., et al. Anterior clinoidal meningiomas: functional outcome after microsurgical resection in a consecutive series of 106 patients. J Neurosurg. 2009;111:1078-1090.
Basso A., Carrizo A.G., Duma C.. Sphenoid ridge meningiomas, Schmidek H.H., editor. Operative Neurosurgical Techniques. Indications, Methods and Results, 4th ed, Philadelphia: WB Saunders, 2000. pp. 316-324
Bikmaz K., Mrak R., Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107:905-912.
Brotchi J., Pirotte B. Sphenoid wing meningiomas. In: Sekhar L.N., Fessler R.G. Atlas of Neurosurgical Techniques. Brain. New York: Thieme, 2006. pp. 623-632
Carvi y., Nievas M.N. Volume assessment of intracranial large meningiomas and considerations about their microsurgical and clinical management. Neurol Res. 2007;29:787-797.
Chang D.J. The “no-drill” technique of anterior clinoidectomy: a cranial base approach to the paraclinoid and parasellar region. Neurosurgery 64 (suppl 3). 2009:ONS96-ONS106.
Cunha e Sa M., Quest D.Q. Sphenoid wing meningiomas. In: Apuzzo M.L.J., editor. Brain Surgery. Complication Avoidance and Management. New York: Churchill Livingstone, 1993. pp. 248-268
Goel A., Gupta S., Desai K. New grading system to predict resectability of anterior clinoid meningiomas. Neurol Med Chir (Tokyo). 2000;40:610-617.
Guinto G., Abello J., Félix I., et al. Lesions confined to the sphenoid ridge. Differential diagnosis and surgical treatment. Skull Base Surg. 1997;7:115-121.
Honeybul S., Neil-Dwyer G., Lang D.A., et al. Sphenoid wing meningiomas en plaque: a clinical review. Acta Neurochir (Wien). 2001;143:749-758.
Kim K.S., Rogers L.F., Goldblatt D. CT features of hyperostosing meningiomas en plaque. AJR Am J Roentgenol. 1987;149:1017-1023.
Leake D., Gunnlaugsson C., Urban J., et al. Reconstruction after resection of sphenoid wing meningiomas. Arch Facial Plast Surg. 2005;7:99-103.
Lee J.H., Jeun S.S., Evans J., et al. Surgical management of clinoidal meningiomas. Neurosurgery. 2001;48:1012-1021.
Lee J.H., Sade B., Park B.J. A surgical technique for the removal of clinoidal meningiomas. Neurosurgery. 2006;59(1 Suppl 1):ONS108-ONS114.
Nakamura M., Roser F., Vorkapic P., et al. Medial sphenoid wing meningiomas: clinical outcome and recurrent rate. Neurosurgery. 2006;58:626-639.
Nakasu S., Nakasu Y., Nakajima M., et al. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999;90:455-462.
Oikawa S., Mizuno M., Muraoka S., et al. Retrograde dissection of the temporalis muscle preventing muscle atrophy for pterional craniotomy. J Neurosurg. 1996;84:297-299.
Pamir M.N., Belirgen M., Özduman K., et al. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases. Acta Neurochir (Wien). 2008;150:625-636.
Pieper D.R., Al-Mefty O., Hanada Y., et al. Hyperostosis associated with meningiomas of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-747.
Ringel F., Cedzich C., Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60(4 Suppl 2):ONS214-ONS222.
Russell S.M., Benjamin V. Medial sphenoid ridge meningiomas: classification, microsurgical anatomy, operative nuances, and long-term surgical outcome in 35 consecutive patients. Neurosurgery. 2008;62(3 Suppl 1):38-50.
Schick U., Bleyen J., Bani A., et al. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006;104:208-214.
Shrivastava R.K., Sen C., Costantino P.D., et al. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103:491-497.
Tobias S., Kim C.H., Kosmorsky G., et al. Management of surgical clinoidal meningiomas. Neurosurg Focus. 2003;14:e5.
1. Basso A., Carrizo A.G., Duma C.. Sphenoid ridge meningiomas, Schmidek H.H., editor. Operative Neurosurgical Techniques. Indications, methods and results, 4th ed., Philadelphia: WB Saunders, 2000. pp. 316-324
2. Cunha e Sa M., Quest D.Q. Sphenoid wing meningiomas. In: Apuzzo M.L.J., editor. Brain Surgery. Complication Avoidance and Management. New York: Churchill Livingstone, 1993. pp. 248-268
3. Guinto G., Abello J., Félix I., et al. Lesions confined to the sphenoid ridge. Differential diagnosis and surgical treatment. Skull Base Surg. 1997;7:115-121.
4. Brotchi J., Pirotte B. Sphenoid wing meningiomas. In: Sekhar L.N., Fessler R.G. Atlas of Neurosurgical Techniques. Brain. New York: Thieme, 2006. pp. 623-632
5. Shrivastava R.K., Sen C., Costantino P.D., et al. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103:491-497.
6. Schick U., Bleyen J., Bani A., et al. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006;104:208-214.
7. Bassiouni H., Asgari S., Sandalcioglu E., et al. Anterior clinoidal meningiomas: functional outcome after microsurgical resection in a consecutive series of 106 patients. J Neurosurg. 2009;111:1078-1090.
8. Goel A., Gupta S., Desai K. New grading system to predict resectability of anterior clinoid meningiomas. Neurol Med Chir (Tokyo). 2000;40:610-617.
9. Honeybul S., Neil-Dwyer G., Lang D.A., et al. Sphenoid wing meningiomas en plaque: a clinical review. Acta Neurochir (Wien). 2001;143:749-758.
10. Kim K.S., Rogers L.F., Goldblatt D. CT features of hyperostosing meningiomas en plaque. AJR Am J Roentgenol. 1987;149:1017-1023.
11. Bikmaz K., Mrak R., Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107:905-912.
12. Pieper D.R., Al-Mefty O., Hanada Y., et al. Hyperostosis associated with meningiomas of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-747.
13. Russell S.M., Benjamin V. Medial sphenoid ridge meningiomas: classification, microsurgical anatomy, operative nuances, and long-term surgical outcome in 35 consecutive patients. Neurosurgery. 2008;62(3 Suppl 1):38-50.
14. Tobias S., Kim C.H., Kosmorsky G., et al. Management of surgical clinoidal meningiomas. Neurosurg Focus. 2003;14:e5.
15. Lee J.H., Sade B., Park B.J. A surgical technique for the removal of clinoidal meningiomas. Neurosurgery. 2006;59(1 Suppl 1):ONS108-ONS114.
16. Lee J.H., Jeun S.S., Evans J., et al. Surgical management of clinoidal meningiomas. Neurosurgery. 2001;48:1012-1021.
17. Pamir M.N., Belirgen M., Özduman K., et al. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases. Acta Neurochir (Wien). 2008;150:625-636.
18. Nakamura M., Roser F., Vorkapic P., et al. Medial sphenoid wing meningiomas: clinical outcome and recurrent rate. Neurosurgery. 2006;58:626-639.
19. Ringel F., Cedzich C., Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60(4 Suppl 2):ONS214-ONS222.
20. Carvi y., Nievas M.N. Volume assessment of intracranial large meningiomas and considerations about their microsurgical and clinical management. Neurol Res. 2007;29:787-797.
21. Abdel-Aziz K.M., Froelich S.C., Dagnew E., et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery. 2004;54:1375-1384.
22. Oikawa S., Mizuno M., Muraoka S., et al. Retrograde dissection of the temporalis muscle preventing muscle atrophy for pterional craniotomy. J Neurosurg. 1996;84:297-299.
23. Chang D.J. The “no-drill” technique of anterior clinoidectomy: a cranial base approach to the paraclinoid and parasellar region. Neurosurgery. 2009;64(suppl 3):ONS96-ONS106.
24. Leake D., Gunnlaugsson C., Urban J., et al. Reconstruction after resection of sphenoid wing meningiomas. Arch Facial Plast Surg. 2005;7:99-103.
25. Nakasu S., Nakasu Y., Nakajima M., et al. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999;90:455-462.