Chapter 73 Surgical Management of Posterior Communicating, Anterior Choroidal, Carotid Bifurcation Aneurysms
Approximately 25% of all intracranial aneurysms are PComA aneurysms, making them the second most common overall after anterior communicating artery aneurysms. ICA bifurcation aneurysms account for about 4% to 15% of all intracranial aneurysms; those at the AChA are even more uncommon and comprise less than 5% of such aneurysms.1,2 Although the incidence of intracranial aneurysms in the pediatric population is relatively low, the ICA termination is the most common location of intracranial aneurysms in this patient group.
The first described aneurysm clipping was of a PComA aneurysm by Dandy in 1938 at the Johns Hopkins Hospital. The patient presented with a complete third cranial nerve palsy and periorbital headaches. Dandy described complete obliteration of the aneurysm neck and preservation of the parent vessel with clip application. The patient recovered uneventfully with complete resolution of the third nerve palsy 7 months later.3,4
Embryology of the ICA
Fetal Posterior Cerebral Artery
The caudal division of the ICA continues to grow into the posterior cerebral artery (PCA) by 8 weeks, and the PComA regresses as the vertebrobasilar system develops. The caudal divisions also supply the stems of the PCAs. By 8 weeks, the PCAs may be identified as the posterior communication of the PComAs. If the embryonic PComA fails to regress, the dominant blood supply to the occipital lobes comes from the ICA via the fetal PCA instead of the vertebrobasilar system.5 This occurs in approximately 20% to 30% of cases. A fetal PCA is defined as a variant of the PComA with the same caliber as the P2 segment of the PCA and is coupled with a hypoplastic P1 segment. In this configuration of the circle of Willis, the primary supply to the PCA territory is derived from the fetal PCA; therefore, clipping or coiling of PComA aneurysms must be achieved without jeopardizing flow to this artery.
Diagnosis, Preoperative Planning, and Patient Selection
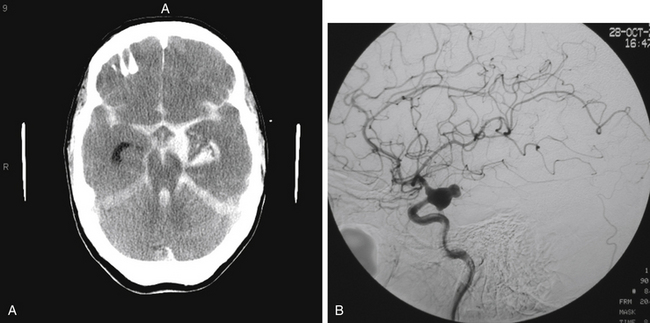
The most common presentation of PComA, AChA, and ICA bifurcation aneurysms is SAH (Fig. 73-1A). While those presenting with SAH are considered for treatment on an emergency basis, preferably within 24 hours of presentation, to minimize the risk of rebleeding, those patients who are found to have symptomatic, unruptured aneurysms should also be considered for urgent treatment. The decision of whether to treat incidental unruptured aneurysms entails consideration of aneurysm- and patient-related factors, such as family history and prior history of aneurysmal SAH. Similarly, the complex choice of either microsurgical or endovascular therapy for any ruptured or unruptured aneurysm rests on multiple factors, such as the patient’s age and medical condition; aneurysm size, location, and morphology; and operator experience. As greater understanding of the natural history continues, technological advances evolve, centralization of care to specialized centers with multidisciplinary capabilities expands, and refined microsurgical and endovascular techniques develop, an aneurysm-specific treatment strategy may be optimally tailored to the individual patient.
Presentation with symptoms caused by mass effect or hemorrhage other than SAH is also possible for these aneurysms. An enlarging unruptured or ruptured PComA aneurysm may also present with a partial or complete, non-pupil–sparing, third nerve palsy. The pupil dilates due to mechanical compression of the parasympathetic fibers that course on the outer surface of the oculomotor nerve. Since relief of the compressive effect caused by the aneurysm is readily accomplished with clipping, improvement in diplopia and ptosis is more often associated with clipping but remains possible with coiling. In univariate analysis,6 the only variable that showed significant association with complete improvement of oculomotor nerve function following microsurgical clipping was severity of the third nerve palsy at admission. In this report, all patients with a partial third nerve palsy regained full oculomotor nerve function. Only 40% of patients with complete third nerve palsy demonstrated complete recovery. Furthermore, there is no statistically significant association between early surgery and improvement of the third nerve function.6 Recovery of third nerve function after coiling has also been documented in case reports.3
Patients presenting with ruptured aneurysms at the PComA (Fig. 73-1B) and AChA (Fig. 73-2) locations may have, in addition to SAH, an associated temporal intracerebral hemorrhage (ICH) or an acute subdural hematoma for which evacuation may be accomplished, along with craniotomy for aneurysm clipping. Ruptured AChA aneurysms often are associated with intraventricular hemorrhage (IVH) and hydrocephalus. Occasionally, a ruptured ICA termination aneurysm manifests with a basal ganglia ICH, with or without IVH.
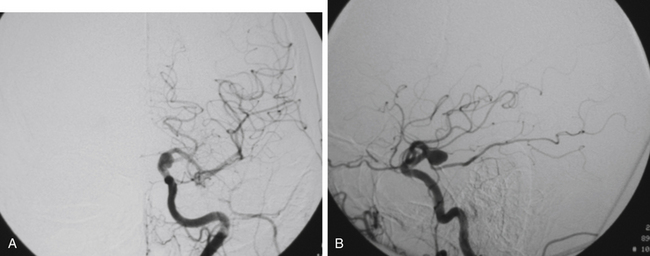
FIGURE 73-2 Left ICA injection on a DSA with an AChA aneurysm: anterior–posterior (A) and lateral (B) views.
In addition, presentation with ischemic symptoms or stroke due to emboli from the aneurysm occurs in about 3.3% of patients. Although more frequently associated with anterior circulation aneurysms, ischemic events in the distal vascular territory attributable to the aneurysm can arise from aneurysms of all sizes, even small aneurysms.7
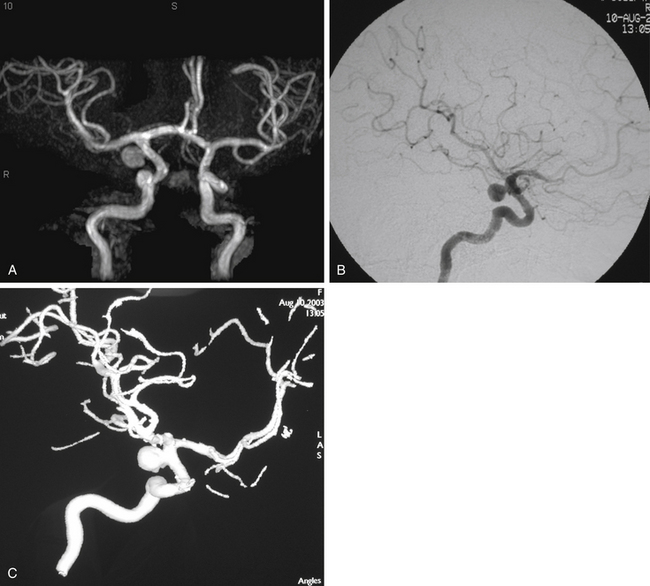
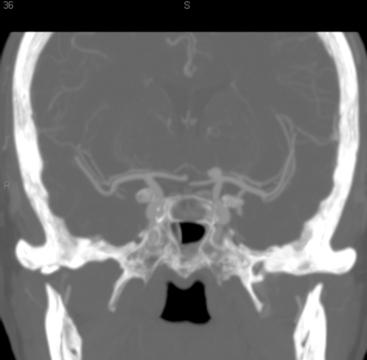
In patients presenting acutely with suspected SAH, computed tomography (CT) scanning remains the initial imaging modality for diagnosis. If the clinical suspicion remains high despite the absence of SAH on head CT, then lumbar puncture is warranted due to its greater sensitivity in detecting SAH. Computed tomography angiography (CTA) has gained increasing utility for aneurysm diagnosis and preoperative planning. Due to the wide availability of magnetic resonance angiography (MRA) and CTA, these noninvasive imaging studies are often the initial screening modality that establishes the diagnosis of an incidentally discovered, unruptured aneurysm (Fig. 73-3A).
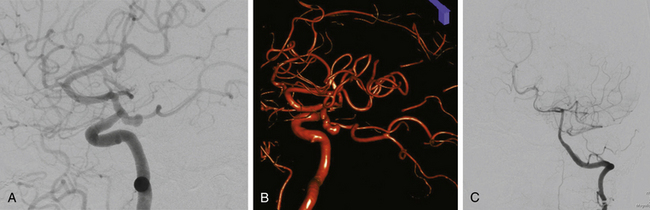
The gold standard for diagnosis and preoperative planning remains the digital subtraction angiogram (DSA) (Fig. 73-3B), as this modality allows selective arterial injections, rotational views, three-dimensional (3D) reconstructions (Fig. 73-3C), and cross-compression testing, if necessary. When assessing the vasculature during the preoperative planning of PComA aneurysm treatment, a fetal PCA (Fig. 73-4A and B) is defined by absence of filling of the PCA or lack of visualization of the P1 segment with the vertebral artery injection (Fig. 73-4C).
In preoperative planning for ICA bifurcation aneurysms, the absence or presence of cross-filling of the ipsilateral ACA across the anterior communicating artery from the contralateral A1 is important to assess with a cross-compression test during the diagnostic angiogram. This maneuver entails compression of the ipsilateral ICA during contralateral ICA injection to determine whether the ipsilateral ACA territory can be supplied by the contralateral ICA. The presence of cross-filling implies that compromise of the ipsilateral A1 during clip reconstruction of ICA bifurcation aneurysms may be compensated for by filling of the ipsilateral A2 via the contralateral A1 across the anterior communicating artery, rather than via the ipsilateral A1.
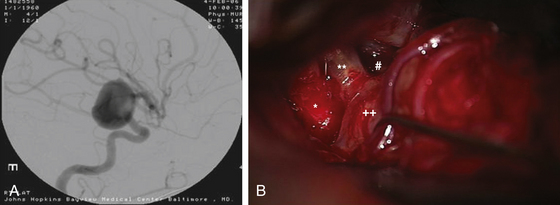
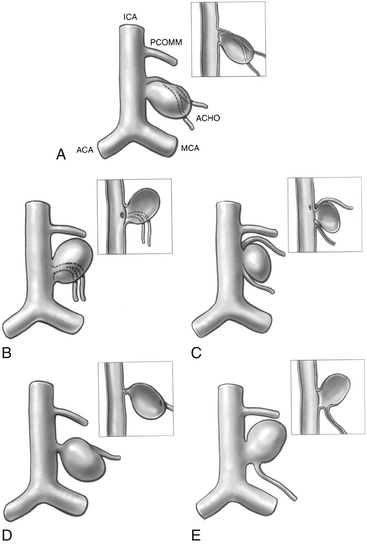
Anterior circulation aneurysms located at the posterior communicating segment (Fig. 73-5), anterior choroidal segment, and terminal ICA bifurcation are typically favorable for microsurgical clipping. Posterior communicating and anterior choroidal aneurysms are carotid wall aneurysms that frequently include the origin of the branch artery at the aneurysm base (Fig. 73-6A). Meticulous identification of the branch artery adjacent to the proximal neck of the aneurysm allows for clip application across the neck to be accomplished with simultaneous preservation of the origin of the branch artery and minimal brain retraction (Fig. 73-6B to E).
The ICA termination aneurysms that may be relatively more favorable for endovascular therapy than microsurgical clipping are those with heavily calcified walls or a primary posterior projection.8 CT or CTA (Fig. 73-7) may be useful in treatment planning to determine the presence of calcification in the aneurysm walls. Using clip blades to gather the neck of a thick-walled aneurysm in the presence of severe atherosclerosis and calcification may risk hemodynamically significant stenosis of the proximal A1 or M1 segment at its origin from the terminal carotid bifurcation. A strategy of placing the clips more distally on the neck to avoid constriction of the origins of the A1 and M1 segments may be employed. Endovascular therapy may be preferable for those ICA termination aneurysms that project posteriorly. Since the perforators originate from the distal ICA at its posterior wall, clipping of posteriorly projecting ICA termination aneurysm may be treacherous due to poor visualization of the perforating arteries.
Anatomic and Clinical Considerations
Posterior Communicating Segment
The communicating segment of the ICA courses between the optic and the oculomotor nerves to the anterior perforated substance at the medial section of the sylvian fissure.2 Angiographically, this segment extends from the origin of the PComA to the origin of the AChA. The PComA arises from the posteromedial or posterior surface of the ICA; courses medially and inferiorly, superior and medial to the oculomotor nerve, through the membrane of Liliequist; and joins the PCA at the junction of the P1 and P2 segments. Multiple, variable perforating arteries arise from the PComA, namely, the anterior thalamic perforators, which are at risk of compromise during aneurysm clipping.
PComA aneurysms are most commonly ICA aneurysms that arise from the ICA and incorporate the origin of the PComA into its neck, rather than arising directly from the PComA. PComA aneurysms are considered relatively straightforward aneurysms for clipping, because their location is very proximal on the supraclinoid ICA. They comprise approximately 25% of all ruptured aneurysms.9 The direction of the dome projection has significant implications for presentation and microsurgical treatment. Yasargil has described a classification system based on the direction of the dome.10 When the dome projects anterolaterally, the origin of the PComA is likely to be obscured from view by the aneurysm. In addition, the aneurysm dome may adhere to the anterior clinoid process. This situation requires cautious dissection to mobilize the dome and visualize the origin of the PComA during clip placement. A superolateral dome is occasionally encountered in which the dome points toward the medial sphenoid ridge. Rupture of these aneurysms is likely associated with presentation with a subdural hematoma. Rare cases of a contralateral frontal ICH from PComA aneurysm rupture have been reported in the literature.11 Aneurysms with a posterolateral superior projection point into the medial temporal lobe and are often associated with intraparenchymal hemorrhage or even hemorrhage into the temporal horn of the lateral ventricle. PComA aneurysms with a posterolateral dome projection may present with a dilated pupil and oculomotor paresis due to compression of the third cranial nerve. A posterolateral inferior fundus may penetrate the membrane of Liliequist with projection into the interpeduncular fossa. Comprising approximately 5% of all PComA aneurysms, the variant of a “true PComA aneurysm” refers to the configuration in which the neck of the aneurysm originates entirely from the PComA.9,12–14 Essential for operative planning is review of the angiogram to determine whether the PCA is supplied by the posterior circulation and fills from the vertebral artery injection or is fetal in configuration and fills from the ICA injection. In the 4% to 25%3,9,15,16 of patients who have a fetal PCA, the P1 segment of PCA is hypoplastic, and the PCA arises directly from the PComA. PComA aneurysms associated with a fetal PCA must be reconstructed either with clips or with coils in a manner that preserves the patency of the ICA and fetal PCA vessels. In contrast, a diminutive PComA may be sacrificed if necessary.
The origin of the PComA is usually found proximal and medial to the proximal neck of the aneurysm. PComA aneurysms typically project laterally and posteriorly; they rarely project medially.2 The arachnoid of the sylvian fissure should be divided appropriately to reveal the medial border of the temporal lobe and to appreciate the PComA origin and the course and number of AChA vessels. The relationship between the dome of the aneurysm and the medial temporal lobe should not be disturbed before the arachnoid has been dissected adequately and proximal control of the ICA has been achieved. The dome of the aneurysm may adhere to the edge of the tentorium. If the dome is attached to the medial temporal lobe, temporal lobe retractors should be avoided or used cautiously. The two most common causes of intraoperative rupture are premature medial retraction of the supraclinoid ICA and retraction of the temporal lobe while the aneurysm dome remains adherent to the temporal lobe. In general, temporal lobe retraction should be avoided in ruptured cases.
In rare instances, a PComA aneurysm may be partially obscured by the anterior clinoid process.17,18 In this situation, adequate proximal control of the ICA and visualization of the proximal neck of the aneurysm may necessitate partial removal of the anterior clinoid process, resection of the anterior petroclinoid fold, or exposure of the ICA in the neck. A predictor of the need for clinoidectomy is a short distance between the tip of the anterior clinoid process and the proximal neck of the aneurysm assessed using CTA.
Adequate dissection of the sylvian fissure facilitates identification and preservation of the AChA. This artery may travel medial to the aneurysm and adhere to the dome of the aneurysm at its cisternal segment, making it necessary to separate the vessel from the distal neck of the aneurysm. The AChA should be preserved in all instances. The AChA supplies the optic chiasm, optic tract, lateral geniculate nucleus, uncus and amygdala, middle third of the cerebral peduncle, caudate, globus pallidus, genu and posterior limb of internal capsule, anterior hippocampus and dentate gyrus, fornix, pulvinar, and choroid plexus.19
Exposure of the contralateral PComA and choroidal segment can be accomplished either through the interoptic space or through the contralateral opticocarotid space. Aneurysms at these locations are the most difficult aneurysms to expose via a contralateral approach. Boundaries of the triangular contralateral opticocarotid space are the lateral aspect of the contralateral optic nerve, chiasm, and tract; the medial aspect of the contralateral ICA; and the inferior aspect of the precommunicating segment of the contralateral ACA.20 Since these aneurysms frequently project posterolaterally, the neck region and origins of the PComA and AChA may be partially obscured by the contralateral ICA and optic apparatus. Optic chiasm position can interfere with visualization.
Internal Carotid Artery
Rarely, internal carotid anterior or posterior wall aneurysms occur in communicating or choroidal segments of the ICA. These aneurysms are not associated with an arterial branch of the ICA. Anterior wall aneurysms are traditionally known as dorsal wall ICA aneurysms. These dorsal wall ICA aneurysms are often broad based and may be particularly treacherous if they harbor blister-type, thin walls. Wrap clipping has been reported in patients with a ruptured blister-type aneurysm of the supraclinoid ICA using materials such as a Silastic sheet coated with Dacron mesh, collagen-impregnated Dacron knitted fabric, and polytetrafluoroethylene. Encircling clips may be required for clip reconstruction. Clip-wrap techniques for treatment of fusiform and unclippable aneurysms is safe, and it can be associated with a low rate of acute or delayed postoperative complications.21
Anterior Choroidal Segment
The choroidal segment of the ICA starts from the origin of the AChA and terminates at the ICA bifurcation. The AChA is the first branch from the supraclinoid ICA that is distal and lateral to the origin of the PComA. The cisternal segment of the AChA courses lateral initially and then posteriorly, following the optic tract and supplying the mesial temporal structures. Its main trunk then continues posteriorly, inferior to the optic tract, to enter the choroidal fissure. The choroidal segment supplies the choroid plexus of the temporal horn. The size of the artery is variable, from 0.5 to 2 mm, and duplication occurs in as many as 30% of the normal population.22 In cases of duplicated AChA (Fig. 73-6C), each branch requires meticulous preservation, since occlusion may lead to contralateral hemiparesis, hemianopia, and hemisensory deficit. Adequate opening of the sylvian fissure facilitates vessel preservation by revealing the course of the AChA. In some cases, the AChA travels medial to the PComA aneurysm, making it necessary to separate the vessel from the neck of the aneurysm before clipping. Clear identification of the origin of the AChA should be made in all cases of PComA and AChA aneurysms. Compromise of the AChA may be inadvertent if the clip blades are placed on the distal neck of a wide-necked or large PComA aneurysm if the AChA origin is nearby and not first dissected away from the distal neck.
Operative repair of AChA aneurysms carries a risk of postoperative stroke as high as 16%, and the risk of stroke is associated with the anatomy of the aneurysm. Typically, the neck of an AChA aneurysm is relatively wider than the caliber of the AChA vessel, and compensatory clip placement more distally on the neck to preserve the origin of the AChA at the aneurysm base may be needed. Although judicious preservation of the AChA during aneurysm clipping is essential, many postoperative AChA-territory strokes occur several hours after surgery; these are thought to be related to delayed thrombosis of this small vessel. Although Buck and Cooper,23 as well as others,24,25 reported during the mid-20th century that the AChA might be safely occluded, contemporary experience suggests that AChA occlusion carries an associated risk of cerebral infarction and neurologic morbidity. In 1956, Diller et al. described 16 patients who underwent AChA occlusion for the treatment of Parkinson’s disease. Tremor and rigidity improved in 10 of those patients, 3 remained the same, 1 died, 1 became hemiplegic, and 1 became hemiparetic.24 It can be concluded that preservation of the AChA is a goal in optimizing outcomes after aneurysm treatment.
Carotid Bifurcation
Carotid bifurcation aneurysms are located at the terminal bifurcation of the ICA where it splits into the A1 segment of the ACA and the M1 segment of the MCA. Carotid bifurcation aneurysms comprise 4% to 15% of aneurysms. The ICA bifurcation is typically the highest point of the circle of Willis. The apex of the ICA bifurcation, similar to the MCA bifurcation or the apex of the basilar artery, is under particular hemodynamic stress and high wall shear stress. Some ICA bifurcation aneurysms arise preferentially from the A1 and others from the M1 portion of the ICA termination. It is important to identify this asymmetry and direction of dome projection in the planning of clip reconstruction. Most commonly, they project superiorly (medially), impinging on the orbitofrontal gyrus or olfactory tract, with the dome projecting along the A1 segment. Others project posteriorly, occupying the sylvian cistern and accompanying the MCA. It is crucial to identify the anatomy of the perforating vessels surrounding the neck of the aneurysm. The most critical perforators are the medial lenticulostriate arteries from the posterior aspect of A1 and M1 segments, and from the ICA bifurcation that course through the anterior perforated substance to the basal ganglia. Since these lenticulostriate vessels originate posteriorly, clipping of anteriorly projecting ICA bifurcation aneurysms is relatively more favorable than clipping of those that project posteriorly and thereby obscure the view of these perforators. Other vessels that often require identification and preservation are deep branches from the PComA and AChA and the recurrent artery of Heubner from the ACA.
Ruptured ICA bifurcation aneurysms typically present with primarily ICH and minimal SAH.26 Isolated cranial nerve abnormalities are rare, but symptoms related to mass effect can arise from giant lesions. ICA bifurcation aneurysms tend to be larger than those detected at the PComA and AChA locations. The morbidity of carotid bifurcation aneurysms is higher than that of PComA or MCA aneurysms. Due to the relatively longer natural history of carotid bifurcation aneurysms before detection, there is a tendency for these to have calcification in the aneurysm walls at the time of presentation. Aneurysmal calcification can be detected with a CT scan. Calcium deposition may make clip obliteration challenging because of the rigidity of the neck of aneurysm. For this reason, endovascular therapy is the preferred strategy to treat those aneurysms with calcified walls.
Operative Procedure and Complication Avoidance
POSTERIOR COMMUNICATING ARTERY Aneurysms
The opticocarotid triangle is completely opened to expose the medial aspect of the ICA. Dissection continues on the anterior–superior surface of the supraclinoid ICA until proximal control is established. Arachnoid dissection proceeds with entry into the sylvian cistern at the region of the frontal operculum. Wide opening of the vertical portion of the sylvian fissure in a lateral to medial direction diminishes the need for vigorous frontal lobe retraction and offers an expansive corridor for dissection of the basal cisterns. Coagulation of the temporopolar veins draining into the sphenoparietal sinus untethers the anterior temporal lobe. Retraction of the anteromedial temporal lobe is not typically required; it should be avoided in all ruptured cases and whenever the dome points laterally.
Relative to microsurgical treatment of aneurysms in other locations, neurologic morbidity from PComA aneurysms is the lowest. Wirth and co-workers report an operative morbidity of 5% compared to that associated with other anterior circulation aneurysms: MCA of 8%, ICA termination of 12%, and anterior communicating artery of 16%.9,27
ANTERIOR CHOROIDAL ARTERY Aneurysms
AChA aneurysms may be approached subfrontally utilizing a method of microdissection similar to that for PComA aneurysms. Early proximal control on the supraclinoid ICA is readily established via this approach. Excessive temporal lobe retraction is best avoided due to the potential avulsion of the aneurysm dome that frequently adheres to the mesial temporal lobe. Identification of the origin of the AChA is critical. About one third of cases have a duplicated AChA and rarely a triplicated AChA.28 If there is a duplicated AChA, the aneurysm typically arises from the largest branch. Preservation of all AChA vessels and choroidal perforators is critical to ensure a good outcome. An AChA infarct may result in hemiparesis, hemianopia, and hemisensory deficit,23–25 but a range of deficits is possible after AChA occlusion due to the variability in collateral supply to the supplied structures.
Microsurgical treatment of AChA aneurysms is associated with a risk of significant ischemic complications.1,29 Due to the relatively small lumen of the AChA compared to the aneurysm neck, variations in the branches, and perforators of the AChA, an ischemic complication may occur even after blood flow had been confirmed after clip placement. Meticulous dissection and microsurgical technique are essential.
Carotid Bifurcation Aneurysms
In addition to the prerequisite proximal control of the ICA, identification of both the M1 and the A1 segments originating from the base of the aneurysm is essential. Extensive arachnoid dissection of the carotid, optic, and sylvian cisterns to mobilize the supraclinoid ICA and the A1 and M1 segments is needed. Opening of the proximal third of the sylvian fissure is usually adequate for the majority of carotid bifurcation aneurysms. Large or giant carotid bifurcation aneurysms require a more extensive opening of the sylvian fissure. After the superficial arachnoid of the sylvian fissure is divided and access to the sylvian cistern is gained, the dissection proceeds proximally by gently spreading the fissure from the inside out. Small crossing veins may be cauterized and divided if necessary to allow frontal lobe retraction and decrease tension on the sylvian veins.30,31 Minimal and cautious retraction of the frontal lobe is prudent, because the aneurysm dome is usually embedded in and adherent to the subfrontal cortex. Following identification of the proximal portion of the MCA (M1 segment), dissection proceeds along its lateral surface to identify the anterolateral wall of the ICA. Further medial dissection reveals the A1 segment. With carotid bifurcation aneurysms that project anteriorly, the A1 segment may be hidden behind the aneurysm dome. As the dissection of the ICA, M1, A1, and aneurysm neck proceeds, the objective is to identify and preserve all branches and perforators in the region of the carotid bifurcation so that their location and course are clear before applying temporary or permanent clips.
The final focus of dissection centers on identification and preservation of the ICA bifurcation perforators.19 In small carotid bifurcation aneurysms, these perforators are usually free from the aneurysm base, but they may adhere to the dome of large or giant aneurysms. The dome of the carotid bifurcation aneurysms is typically buried in the orbitobasal frontal lobe, so cautious frontal lobe retraction should be utilized during the dissection. A limited resection of the frontobasal cortex may be necessary for adequate visualization in the situation of cerebral edema after acute SAH or when the carotid bifurcation aneurysm is embedded in the frontal lobe.
Al-Yamani M., Wallace M.C.. Intracranial internal carotid artery aneurysms, Winn H.R., editor. Youman’s Neurological Surgery II, 5th Edition, Saunders, 2004. pp. 1915-1921
Chang D.J. The “no-drill” technique of anterior clinoidectomy: a cranial base approach to the paraclinoid and parasellar region. Neurosurgery. 2009;64:96-105. discussion 105-106
Chittiboina P., Cuellar H., Ballenilla F., et al. Two cases of ruptured cerebral aneurysms presenting with contralateral hematomas. Emerg Radiol. 2011;18:39-42.
Dandy W.E. Intracranial aneurysm of the internal carotid artery: cured by operation. Ann Surg. 1938;107:654-659.
Dean B.L., Wallace R.C., Zabramski J.M., et al. Incidence of superficial sylvian vein compromise and postoperative effects on CT imaging after surgical clipping of middle cerebral artery aneurysms. AJNR Am J Neuroradiol. 2005;26:2019-2026.
Diller L., Laszewski Z., Riklan M. Effects of anterior choroidal artery occlusion and of chemopallidectomy on the tremor and rigidity of Parkinson’s disease: an independent appraisal. J Am Geriatr Soc. 1956;4:1246-1248.
Figueiredo E.G., Foroni L., Monaco B.A., et al. The clip-wrap technique in the treatment of intracranial unclippable aneurysms. Arq Neuropsiquiatr. 2010;68:115-118.
Friedman J.A., Pichelmann M.A., Piepgras D.G., et al. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 2001;94:565-572.
Golshani K., Ferrell A., Zomorodi A., et al. A review of the management of posterior communicating artery aneurysms in the modern era. Surg Neurol Int. 2010;1:88.
Hammers R., Hacein-Bey L., Origitano T.C. Anomalous medial origin of the anterior choroidal artery with associated aneurysm. J Neurol Sci. 2009;287:250-252.
Heros R.C. Microneurosurgical management of anterior choroidal artery aneurysms. World Neurosurg. 2010;73:459-460.
Horikoshi T., Akiyama I., Yamagata Z., et al. Magnetic resonance angiographic evidence of sex-linked variations in the circle of Willis and the occurrence of cerebral aneurysms. J Neurosurg. 2002;96:697-703.
Javalkar V, Cardenas R, Nanda A. Recovery of third nerve palsy following surgical clipping of posterior communicating artery aneurysms. World Neurosurg. 73:353–356.
Jongen J.C., Franke C.L., Ramos L.M., et al. Direction of flow in posterior communicating artery on magnetic resonance angiography in patients with occipital lobe infarcts. Stroke. 2004;35:104-108.
Lehecka M., Dashti R., Romani R., et al. Microneurosurgical management of internal carotid artery bifurcation aneurysms. Surg Neurol. 2009;71:649-667.
Liu X., Rinkel G.J. Aneurysmal and clinical characteristics as risk factors for intracerebral haematoma from aneurysmal rupture. J Neurol. 2011;258(5):862-865.
Macdonald R.L. Vascular Neurosurgery. New York: Thieme; 2009.
Muneda K., Yoshizu H., Terada H. [True posterior communicating artery aneurysm]. No Shinkei Geka. 2001;29:163-168.
Park S.K., Shin Y.S., Lim Y.C., et al. Preoperative predictive value of the necessity for anterior clinoidectomy in posterior communicating artery aneurysm clipping. Neurosurgery. 2009;65:281-285. discussion 285-286
Pyysalo L.M., Keski-Nisula L.H., Niskakangas T.T., et al. Long-term follow-up study of endovascularly treated intracranial aneurysms. Interv Neuroradiol. 2010;16:231-239.
Qureshi A.I., Mohammad Y., Yahia A.M., et al. Ischemic events associated with unruptured intracranial aneurysms: multicenter clinical study and review of the literature. Neurosurgery. 2000;46:282-289. discussion 289-290
Tobenas-Dujardin A.C., Duparc F., Ali N., et al. Embryology of the internal carotid artery dural crossing: apropos of a continuous series of 48 specimens. Surg Radiol Anat. 2005;27:495-501.
Uz A., Erbil K.M., Esmer A.F. The origin and relations of the anterior choroidal artery: an anatomical study. Folia Morphol (Warsz). 2005;64:269-272.
Wen H.T., Rhoton A.L.Jr., de Oliveira E., et al. Microsurgical anatomy of the temporal lobe: part 2—sylvian fissure region and its clinical application. Neurosurgery. 2009;65:1-35. discussion 36
Yasargil M.G. Carotid-posterior communicating aneurysms. In: Microneurosurgery II: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. New York: Thieme; 1994. pp. 71-73
1. Friedman J.A., Pichelmann M.A., Piepgras D.G., et al. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 2001;94:565-572.
2. Al-Yamani M., Wallace M.C.. Intracranial internal carotid artery aneurysms, Winn H.R., editor. Youman’s Neurological Surgery II, 5th Edition, Saunders, 2004. pp. 1915-1921
3. Dandy W.E. Intracranial aneurysm of the internal carotid artery: cured by operation. Ann Surg. 1938;107:654-659.
4. Kretzer R.M., Coon A.L., Tamargo R.J., Walter E. Dandy’s contributions to vascular neurosurgery. J Neurosurg. 2010;112:1182-1191.
5. Tobenas-Dujardin A.C., Duparc F., Ali N., et al. Embryology of the internal carotid artery dural crossing: apropos of a continuous series of 48 specimens. Surg Radiol Anat. 2005;27:495-501.
6. Javalkar V., Cardenas R., Nanda A. Recovery of third nerve palsy following surgical clipping of posterior communicating artery aneurysms. World Neurosurg. 2010;73:353-356.
7. Qureshi A.I., Mohammad Y., Yahia A.M., et al. Ischemic events associated with unruptured intracranial aneurysms: multicenter clinical study and review of the literature. Neurosurgery. 2000;46:282-289. discussion 289-290
8. Pyysalo L.M., Keski-Nisula L.H., Niskakangas T.T., et al. Long-term follow-up study of endovascularly treated intracranial aneurysms. Interv Neuroradiol. 2010;16:231-239.
9. Golshani K., Ferrell A., Zomorodi A., et al. A review of the management of posterior communicating artery aneurysms in the modern era. Surg Neurol Int. 2010;1:88.
10. Yasargil M.G. Carotid-posterior communicating aneurysms. In: Microneurosurgery II: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. New York: Thieme; 1994. pp. 71-73
11. Chittiboina P., Cuellar H., Ballenilla F., et al. Two cases of ruptured cerebral aneurysms presenting with contralateral hematomas. Emerg Radiol. 2010;18:39-42.
12. Alexander T.D., Macdonald R.L., Weir B., et al. Intraoperative angiography in cerebral aneurysm surgery: a prospective study of 100 craniotomies. Neurosurgery. 1996;39:10-17. discussion 17-18
13. Krayenbuhl H.A., Yasargil M.G., Flamm E.S., et al. Microsurgical treatment of intracranial saccular aneurysms. J Neurosurg. 1972;37:678-686.
14. Muneda K., Yoshizu H., Terada H. [True posterior communicating artery aneurysm]. No Shinkei Geka. 2001;29:163-168.
15. Horikoshi T., Akiyama I., Yamagata Z., et al. Magnetic resonance angiographic evidence of sex-linked variations in the circle of Willis and the occurrence of cerebral aneurysms. J Neurosurg. 2002;96:697-703.
16. Jongen J.C., Franke C.L., Ramos L.M., et al. Direction of flow in posterior communicating artery on magnetic resonance angiography in patients with occipital lobe infarcts. Stroke. 2004;35:104-108.
17. Park S.K., Shin Y.S., Lim Y.C., et al. Preoperative predictive value of the necessity for anterior clinoidectomy in posterior communicating artery aneurysm clipping. Neurosurgery. 2009;65:281-285. discussion 285-286
18. Chang D.J. The “no-drill” technique of anterior clinoidectomy: a cranial base approach to the paraclinoid and parasellar region. Neurosurgery. 2009;64:96-105. discussion 105-106
19. Lehecka M., Dashti R., Romani R., et al. Microneurosurgical management of internal carotid artery bifurcation aneurysms. Surg Neurol. 2009;71:649-667.
20. Macdonald R.L. Vascular Neurosurgery. New York: Thieme; 2009.
21. Figueiredo E.G., Foroni L., Monaco B.A., et al. The clip-wrap technique in the treatment of intracranial unclippable aneurysms. Arq Neuropsiquiatr. 2010;68:115-118.
22. Uz A., Erbil K.M., Esmer A.F. The origin and relations of the anterior choroidal artery: an anatomical study. Folia Morphol (Warsz). 2005;64:269-272.
23. Buck J.F., Cooper I.S. Speech problems in parkinsonian patients undergoing anterior choroidal artery occlusion or chemopallidectomy. J Am Geriatr Soc. 1956;4:1285-1290.
24. Diller L., Laszewski Z., Riklan M. Effects of anterior choroidal artery occlusion and of chemopallidectomy on the tremor and rigidity of Parkinson’s disease: an independent appraisal. J Am Geriatr Soc. 1956;4:1246-1248.
25. Rand R.W., Brown W.J., Stern W.E. Surgical occlusion of anterior choroidal arteries in parkinsonism; clinical and neuropathologic findings. Neurology. 1956;6:390-401.
26. Liu X., Rinkel G.J. Aneurysmal and clinical characteristics as risk factors for intracerebral haematoma from aneurysmal rupture. J Neurol. 2011;258(5):862-865.
27. Wirth F.P., Laws E.R.Jr., Piepgras D., et al. Surgical treatment of incidental intracranial aneurysms. Neurosurgery. 1983;12:507-511.
28. Hammers R., Hacein-Bey L., Origitano T.C. Anomalous medial origin of the anterior choroidal artery with associated aneurysm. J Neurol Sci. 2009;287:250-252.
29. Heros R.C. Microneurosurgical management of anterior choroidal artery aneurysms. World Neurosurg. 2010;73:459-460.
30. Dean B.L., Wallace R.C., Zabramski J.M., et al. Incidence of superficial sylvian vein compromise and postoperative effects on CT imaging after surgical clipping of middle cerebral artery aneurysms. AJNR Am J Neuroradiol. 2005;26:2019-2026.
31. Wen H.T., Rhoton A.L.Jr., de Oliveira E., et al. Microsurgical anatomy of the temporal lobe: part 2—sylvian fissure region and its clinical application. Neurosurgery. 2009;65:1-35. discussion 36