Chapter 140 Surgical Management of Major Skull Defects and Potential Complications
In the present era, a decompressive craniectomy is a neurosurgical procedure that describes the temporary removal of a large piece of the skull to alleviate high intracranial pressure (ICP) that can be caused by a variety of neurosurgical disorders (Fig. 140-1). In the trauma setting, two main techniques relieve high ICP: removal of the frontotemporal–parietal–occipital bone over one or both cranial hemispheres and removal of bilateral frontal bone as first described by Kjellberg and Prieto in 1971.1–3 In cases of an ischemic infarct, most craniectomies performed are removal of the frontotemporal–parietal–occipital bone over one hemisphere, and in the case of a posterior circulation infarct, a suboccipital craniectomy is generally performed. Other skull defects may result from operations in which infection or malignancies extend into the skull. The timing of the cranioplasty operation depends on the origin of the skull defect.
Historically, it has generally been recommended that cranioplasty be performed at least 3 months after initial craniectomy because this period allows sufficient time for postoperative edema to resolve and possibly reduces the rate of infection and hydrocephalus.30 However, surgeons may encounter difficulties during dissection for cranioplasty due to severe adhesion, and many surgeons advocate early cranioplasty within a month.4,5 Before the autologous bone flap can be replaced, a variety of clinical endpoints have to be established. These include a well-healed scalp with no local, systemic, or cranial infections; resolution of elevated ICP; a stable neurologic condition; and resolution of the herniation of the brain underlying the bone defect. There are several reasons reported in the literature as to why early cranioplasty helps neurologic function, one of them being the restoration of normal CSF flow dynamics.6 Communicating hydrocephalus is a common problem following a decompressive hemicraniectomy, and many authors propose early cranioplasty to prevent the requirement for permanent CSF diversion.5 Reports have revealed that CSF pressure was higher in a model with a large skull defect versus no defect,7,34 and others have shown that early cranioplasty, along with a programmable shunt, has led to reduced number of surgeries, improved patient outcomes, and reduced complications.6
In addition to hydrocephalus, the “syndrome of the trephined” is a phenomenon that improves after cranioplasty. Clinically, patients have a “sunken head” appearance on the side of the cranial defect. In 1945, Gardner described this syndrome characterized by headaches, dizziness, irritability, epilepsy, discomfort, and psychiatric symptoms occurring in patients with large cranial defects. He was the first to describe an improvement in the neurologic function of some patients undergoing cranioplasty with tantalum.8 This array of nonspecific symptoms is believed to be related to alteration of CSF dynamics, the atmosphere pressure transmitted through the unsupported scalp, and cerebral blood flow changes.9,10 Current reports characterize changes in the CSF flow dynamics, as well as increases in venous outflow.10 The sinking skin flap would transmit the atmospheric pressure to the underlying brain tissue, which would result in a low cerebral blood flow. Studies show that low cerebral blood flow increased and reached normal levels after cranioplasty.11,12
Infection following craniotomy is another situation in which the autologous bone flap is removed, and delayed cranioplasty is indicated once the infection has cleared. Reports have shown successful operative sterilization and débridement leading to avoidance of cranioplasty and preservation of the autologous bone flap.13–15 However, many situations necessitate discarding the bone flap if it has been devitalized by the infection or if the patient has an extensive scalp and brain infection. There is no clear standard time for cranioplasty after bone flap infection; intervals of 6 to 12 weeks, 6 months,16,17 and more than 12 months have been advocated.18 Assessment of the soft tissues is crucial for the timing of cranioplasty after infection. Logically, all wounds must be well healed and the scalp must be elastic enough to allow closure without excessive tension.
Bone flaps are usually placed either sterilely into a freezer or subcutaneously in the patient’s abdominal fat until time for cranioplasty. Some reports show almost identical bioactivity of the bone flap compared to the remaining intact skull and a lower reoperation rate when the bone was placed subcutaneously.19 A logical disadvantage of storing cranioplasty subcutaneously is the morbidity of an abdominal incision; alternatively, storing the flap in a freezer at −20°C or colder has been shown to be effective as a preservation method.20,21 Some reports state a large disadvantage of frozen autologous bone flap is the higher rate of resorption compared to other synthetic materials, especially in the pediatric population.22,23 Autologous bone flap infection is not uncommon; therefore, careful attention must be undertaken during storage of the flap after craniectomy. The bone flap must be cultured prior to cranioplasty; any bacterial contamination of the flap is a contraindication for reinsertion into the patient.
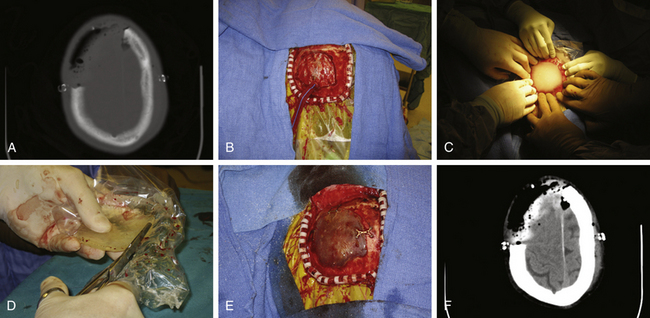
Perhaps the most common synthetic material used today is MMA (Fig. 140-2). MMA is a polymerized ester of acrylic acid that comes in a powdered form. It is relatively inexpensive when compared to other synthetic materials. When ready to use, it is mixed with a liquid monomer, benzoyl peroxide. An exothermic reaction takes place, and the MMA forms a supple substance with consistency like paste. It then cools to form a semitranslucent material that is comparable to human bone in strength. Before the final cooling process, MMA is malleable, and it can be molded to fit any skull defect. This becomes especially useful when the skull defect is a technically challenging reconstruction. This prosthesis is at a higher risk of infection than autologous bone, because it is not biologically viable and a layer of fibrous tissue grows around the allograft to which bacteria may adhere.24 Propionibacterium acnes and Staphylococcus aureus are the most common bacteria involved in postoperative craniotomy bone flap infections.25
Other disadvantages of MMA include burns during the exothermic reaction that can reach temperatures up to 70°C.26 This risk can be minimized with continuous irrigation with saline until the MMA is cooled. A few types of MMA are commercially available. We are most familiar with Aneuroplastic, a product made by Codman & Shurtleff that has a fast setting time of 3 to 5 minutes. Orthopedic surgeons also commonly use Surgical Simplex by Stryker Howmedica, which is a bone cement made of a poly-MMA and barium sulfate mixer. A potential benefit of Surgical Simplex is a lower exothermic reaction temperature.
An alternative is a unique carbonated calcium phosphate cement with physical characteristics similar to those of the mineral phase of bone. It exists as a powder and forms a malleable substance when mixed with liquid sodium phosphate. The most commonly used calcium phosphate material is hydroxyapatite.27 Covalent has a hydroxyapatite product by the brand name Neobone, and Synthes makes a similar compound by the brand name Norian.28,29 Norian is most soluble at low pH, which in theory aids in resorption and replacement by natural bone.28 Most authors propose the use of calcium phosphate bone cement for small cranial defects as an onlay over bone or titanium mesh. Unlike MMA, hydroxyapatite promotes bone growth, thus serving as a platform for growth of new bone. For this reason, it is often used for bony defects in the pediatric population.28,29
Titanium mesh is another commonly used material in fixing cranial defects. It can be used alone or in conjunction with other synthetic materials. Titanium is a noncorrosive, nonferromagnetic material that does not cause an inflammatory reaction and is highly biocompatible. Most of the consensus in the literature proposes that titanium is a good cranioplasty option with low infection rates and satisfactory cosmesis.30,31
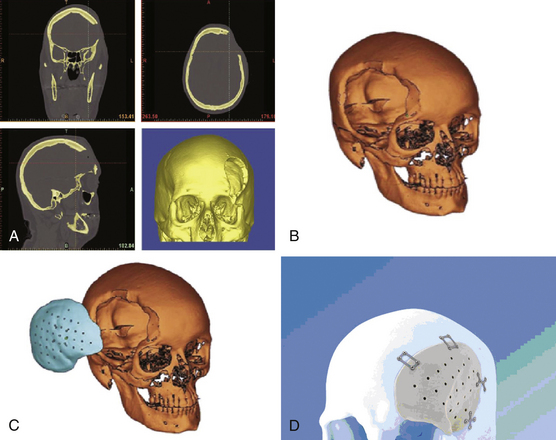
For complex cranioplasty remodeling, some neurosurgeons utilize computer-generated implants from CT reconstructions of the patient’s skull defect (Fig. 140-3). In the past 10 to 15 years, CT-guided stereolithography, using liquid ultraviolet (UV) photopolymer resin and a UV laser to form anatomic models by CT, has become increasing in popularity. Patient-customized models can be developed using this technology, such as MMA prostheses, titanium plates, and hydroxyapatite implants. The disadvantage of this premade prosthesis is the price, estimated to be as much at $4000.32,33 There are many potential advantages of custom models, including anatomic alignment with near-perfect cosmetic results and a relatively straightforward fixation process of the implant to the native skull as compared to other methods of cranioplasty.
Cranioplasty complications can vary and include autologous bone resorption, infection, fluid collections, hematomas, and hydrocephalus. Placing titanium mesh under the MMA plate can increase the strength of the construct. There is a possibility of bone resorption with the autologous bone flap, which occurs if the flap is devitalized or is not in contact with the native bone edge for vascularization and nutrients. This complication increases if the soft tissue and scar tissue are not adequately removed from the native bone edge. As discussed previously, the risks factors for infection include systemic or local infections, communication between the cranioplasty site and the facial sinuses, devascularized scalp such as from multiple prior procedures, and inadequate time interval between craniectomy and cranioplasty.4,6 Frequently, cranioplasty improves CSF flow dynamics and restores normal ICP relationships within the skull.5 In traumatic brain injury or subarachnoid hemorrhage, because of the loss of brain parenchyma, CSF obstruction and hydrocephalus may not become evident until after cranioplasty. Generally, CSF shunting procedures are performed during the time of cranioplasty or after the patient’s neurologic exam has stabilized after cranioplasty.10,11 CSF shunting is usually not performed prior to cranioplasty because of the possibility that depressed scalp defect would increase the technical difficulty of cranioplasty, which may lead to further complications such as hematomas.6,10
Akins P.T., Guppy K.H. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9:269-276.
Auguste K.I., McDermott M.W. Salvage of infected craniotomy bone flaps with the wash-in, wash-out indwelling antibiotic irrigation system. Technical note and case series of 12 patients. J Neurosurg. 2006;105:640-644.
Baumeister S., Peek A., Friedman A., et al. Management of postneurosurgical bone flap loss caused by infection. Plastic Reconstr Surg. 2008;122:195-208.
Blum K.S., Schneider S.J., Rosenthal A.D. Methyl methacrylate cranioplasty in children: Long-term results. Pediatr Neurosurg. 1997;26:33-35.
Bruce J.N., Bruce S.S. Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg. 2003;98:1203-1207.
Carvi y Nievas M.N., Hollerhage H.G. Early combined cranioplasty and programmable shunt in patients with skull bone defects and CSF–circulation disorders. Neurol Res. 2006;28:139-144.
Delgado-Lopez P.D., Martin-Velasco V., Castilla-Diez J.M., et al. Preservation of bone flap after craniotomy infection. Neurocirugia. 2009;20:124-131.
Dujovny M., Fernandez P., Alperin N., et al. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997;19:311-316.
Elwatidy S. Bifrontal decompressive craniotomy for malignant brain edema. Saudi Med J. 2006;27:1547-1553.
Gardner W.J. Closure of defects of the skull with tantalum. Surg Gynecol Obstet. 1945;80:303-312.
Gasparini G., Boniello R., Moro A., et al. Cranial reshaping using methyl methacrylate: technical note. J Craniofac Surg. 2009;20:184-190.
Gilardino M.S., Cabiling D.S., Barlett S.P. Long-term follow-up experience with carbonated calcium phosphate cement (Norian) for cranioplasty in children and adults. Plast Reconstr Surg. 2009;123:983-994.
Grossman N., Shemesh-Jan H.S., Merkin V., et al. Deep-freeze preservation of cranial bones for future cranioplasty: nine years of experience in Soroka University Medical Center. Cell Tissue Bank. 2007;8:243-246.
Isago T., Nozaki M., Kikuchi Y., et al. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288-292.
Iwama T., Yamada J., Imai S., et al. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52:591-596.
Kjellberg R.N., Prieto A.Jr. Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34:488-493.
Liang W., Xiaofeng Y., Weiguo L., et al. Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg. 2007;18:526-532.
Malis L.I. Titanium mesh and acrylic cranioplasty. Neurosurgery. 1989;25:351-355.
Marbacher S., Andres R.H., Fathi A.R., Fandino J. Primary reconstruction of open depressed skull fractures with titanium mesh. J Craniofac Surg. 2008;19:490-495.
Miyake H., Ohta T., Tanaka H. A new technique for cranioplasty with L-shaped titanium plates and combination ceramic implants composed of hydroxyapatite and tricalcium phosphate (Ceratite). Neurosurgery. 2000;46:414-418.
Movassaghi K., Ver Halen J., Ganchi P., et al. Cranioplasty with subcutaneously preserved autologous bone grafts. Plast Reconstr Surg. 2006;117:202-206.
Nisbet M., Briggs S., Ellis-Pegler R., et al. Propionibacterium acnes: an under-appreciated cause of post-neurosurgical infection. J Antimicrob Chemother. 2007;60:1097-1103.
Polin R.S., Shaffrey M.E., Bogaev C.A., et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84-92.
Vignes J.R., Jeelani N.O., Dautheribes M., et al. Cranioplasty for repair of a large bone defect in a growing skull fracture in children. J Craniomaxillofac Surg. 2007;35:185-188.
Waziri A., Fusco D., Mayer S.A., et al. Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery. 2007;61:489-493.
Winder J., Cooke R.S., Gray J., et al. Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J Med Eng Technol. 1999;23:26-28.
1. Elwatidy S. Bifrontal decompressive craniotomy for malignant brain edema. Saudi Med J. 2006;27:1547-1553.
2. Kjellberg R.N., Prieto A.Jr. Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34:488-493.
3. Polin R.S., Shaffrey M.E., Bogaev C.A., et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84-92.
4. Liang W., Xiaofeng Y., Weiguo L., et al. Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg. 2007;18:526-532.
5. Waziri A., Fusco D., Mayer S.A., et al. Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery. 2007;61:489-493.
6. Carvi y Nievas M.N., Hollerhage H.G. Early combined cranioplasty and programmable shunt in patients with skull bone defects and CSF–circulation disorders. Neurol Res. 2006;28:139-144.
7. Langfitt T.W. Increased intracranial pressure. Clin Neurosurg. 1968;16:436-471.
8. Gardner W.J. Closure of defects of the skull with tantalum. Surg Gynecol Obstet. 1945;80:303-312.
9. Akins P.T., Guppy K.H. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9:269-276.
10. Dujovny M., Fernandez P., Alperin N., et al. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997;19:311-316.
11. Erdogan E., Duz B., Kocaoglu M., et al. The effect of cranioplasty on cerebral hemodynamics: evaluation with transcranial Doppler sonography. Neurol India. 2003;51:479-481.
12. Isago T., Nozaki M., Kikuchi Y., et al. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288-292.
13. Auguste K.I., McDermott M.W. Salvage of infected craniotomy bone flaps with the wash-in, wash-out indwelling antibiotic irrigation system. Technical note and case series of 12 patients. J Neurosurg. 2006;105:640-644.
14. Bruce J.N., Bruce S.S. Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg. 2003;98:1203-1207.
15. Delgado-Lopez P.D., Martin-Velasco V., Castilla-Diez J.M., et al. Preservation of bone flap after craniotomy infection. Neurocirugia. 2009;20:124-131.
16. Eppley B.L., Kilgo M., Coleman J.J.3rd. Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: indications, surgical technique, and long-term follow-up. Plast Reconstr Surg. 2002;109:864-871.
17. Lee C., Antonyshyn O.M., Forrest C.R. Cranioplasty: indications, technique, and early results of autogenous split skull cranial vault reconstruction. J Craniomaxillofac Surg. 1995;23:133-142.
18. Baumeister S., Peek A., Friedman A., et al. Management of postneurosurgical bone flap loss caused by infection. Plastic Reconstr Surg. 2008;122:195-208.
19. Movassaghi K., Ver Halen J., Ganchi P., et al. Cranioplasty with subcutaneously preserved autologous bone grafts. Plast Reconstr Surg. 2006;117:202-206.
20. Iwama T., Yamada J., Imai S., et al. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52:591-596.
21. Grossman N., Shemesh-Jan H.S., Merkin V., et al. Deep-freeze preservation of cranial bones for future cranioplasty: nine years of experience in Soroka University Medical Center. Cell Tissue Bank. 2007;8:243-246.
22. Grant G.A., Jolley M., Ellenbogen R.G., et al. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004;100:163-168.
23. Vignes J.R., Jeelani N.O., Dautheribes M., et al. Cranioplasty for repair of a large bone defect in a growing skull fracture in children. J Craniomaxillofac Surg. 2007;35:185-188.
24. Blum K.S., Schneider S.J., Rosenthal A.D. Methyl methacrylate cranioplasty in children: long-term results. Pediatr Neurosurg. 1997;26:33-35.
25. Nisbet M., Briggs S., Ellis-Pegler R., et al. Propionibacterium acnes: an under-appreciated cause of post-neurosurgical infection. J Antimicrob Chemother. 2007;60:1097-1103.
26. Gasparini G., Boniello R., Moro A., et al. Cranial reshaping using methyl methacrylate: technical note. J Craniofac Surg. 2009;20:184-190.
27. Miyake H., Ohta T., Tanaka H. A new technique for cranioplasty with L-shaped titanium plates and combination ceramic implants composed of hydroxyapatite and tricalcium phosphate (Ceratite). Neurosurgery. 2000;46:414-418.
28. Baker S.B., Weinzweig J., Kirschner R.E., Bartlett S.P. Applications of a new carbonated calcium phosphate bone cement: early experience in pediatric and adult craniofacial reconstruction. Plast Reconstr Surg. 2002;109:1789-1796.
29. Gilardino M.S., Cabiling D.S., Barlett S.P. Long-term follow-up experience with carbonated calcium phosphate cement (Norian) for cranioplasty in children and adults. Plast Reconstr Surg. 2009;123:983-994.
30. Malis L.I. Titanium mesh and acrylic cranioplasty. Neurosurgery. 1989;25:351-355.
31. Marbacher S., Andres R.H., Fathi A.R., Fandino J. Primary reconstruction of open depressed skull fractures with titanium mesh. J Craniofac Surg. 2008;19:490-495.
32. Chim H., Schantz J.T. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast Reconstr Surg. 2005;116:1726-1741.
33. Winder J., Cooke R.S., Gray J., et al. Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J Med Eng Technol. 1999;23:26-28.
34. Winkler P.A., Stummer W., Linke R., et al. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93:53-61.