Chapter 186 Surgical Management of Intramedullary Spinal Cord Tumors in Adults
The neurosurgical literature on intramedullary spinal cord tumors (IMSCTs) contains many case reports and few large series, even for tumors of glial origin, which are the most numerous.1–15 As a matter of fact, these lesions are relatively rare and occur in any age group. Indeed, IMSCTs account for 2% to 4% of all central nervous system tumors in adults and 15% of all primary intradural tumors in adults.16–18 Ependymomas are the most common tumors in adults and astrocytomas are the most common in children.11,19 Hemangioblastomas and cavernomas represent special entities and require specific strategies.20,21 Other tumors of nonglial origin are still more exceptional. It is well known that IMSCTs have no typical clinical presentation.
At present, magnetic resonance imaging (MRI) is the best and, in most cases, the only examination to perform in investigating these cases. Although MRI can be a highly accurate diagnostic tool, it does not always provide accurate differentiation between ependymomas and astrocytomas. Evoked potentials, both sensory and motor, are now standard intraoperative monitoring tools used during the surgery of these lesions.22–24 The ultrasonic aspirator is now used routinely and provides significant assistance.
Anatomy
The spinal meninges differ from those of the brain owing to the presence of a thicker pia mater attached to the inner dural surface by the dentate ligaments. The medial border of each is adherent to the lateral column, all along the spinal cord. The lateral border of each ligament is free, with the exception of the areas adjacent to the roots. These are thick serrations whose apices are attached to the dura between the overlying and underlying root sheaths. The arachnoid consists of a dense impermeable superficial layer adjoining the dura and of fenestrated dorsal septa that run from the superficial layer of the arachnoid to the pial surface of the spinal cord. That is why the cord is strengthened by the meninges without interference with the free circulation of the cerebrospinal fluid in the subarachoid space. The spinal dura encloses the spinal cord and the cauda equina from the foramen magnum to the sacrum. The diameter of the dural tube is smaller than that of the spinal canal but is much larger than that of the spinal cord. The dura, which forms a cylindrical sheath, is separated from the spinal canal by the epidural space, containing fat and the epidural venous plexuses. That is why the spinal cord is protected by the meninges and the cerebrospinal fluid and can be slightly mobilized within the spinal canal.
Incidence, Types, and Prognosis
Tumors of Glial Origin
The group of tumors of glial origin is by far mainly represented by ependymomas and astrocytomas.20,25 Others, such as oligodendriogliomas, as rare.26
Ependymomas
Spinal cord ependymomas (Fig. 186-1) constitute the most common IMSCTs in adult patients. They can reach a considerable size because they are slow-growing processes. The majority of spinal cord ependymomas have a good prognosis because most of the time they can be removed completely and are grade II according to the World Health Organization (WHO) classification. In the literature, the rate of complete removal varies from 69% to 97%.3,15,27 The functional prognosis is particularly satisfying as far as patients who are operated on when their preoperative condition are still good. Indeed, according to the literature, 48% to 75% are stabilized after the procedure, 10% and 40% improve, and between 9% and 15% deteriorate.3,11,15,28,29 This highlights the need for prompt surgery in case of neurologic deterioration.3,11,15,28,29 The 5- and 10-year survival rates reach respectively between 83% to 96% and 80% to 91%.11,15,27,29–31 Incomplete resection is considered the most important factor predicting recurrence.3,32,33
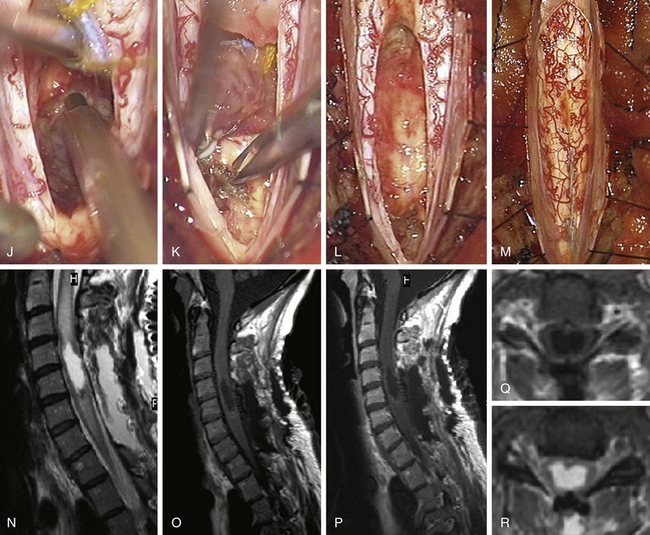
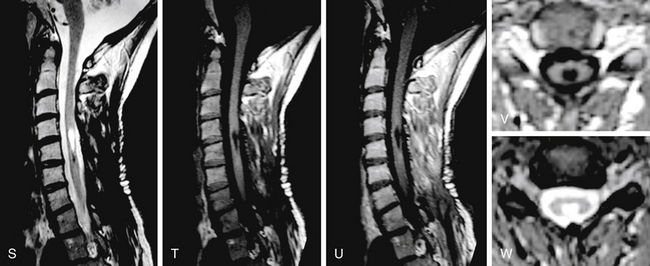
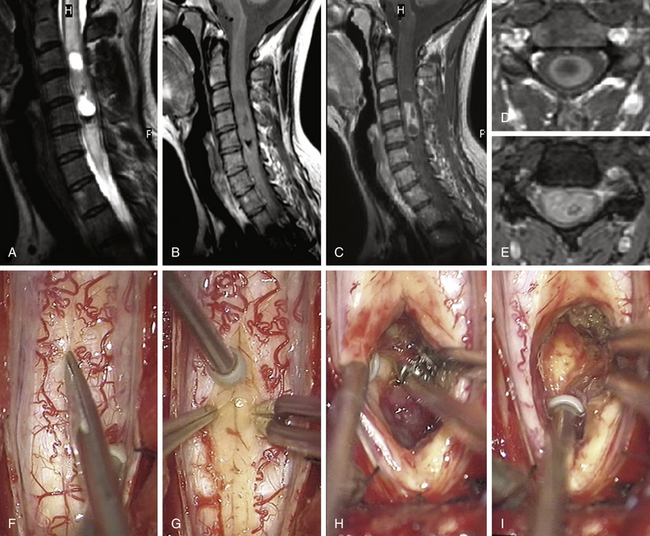
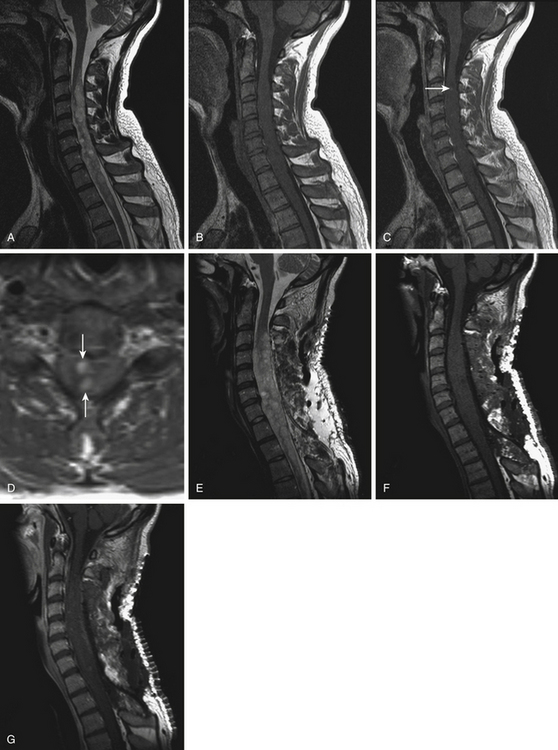
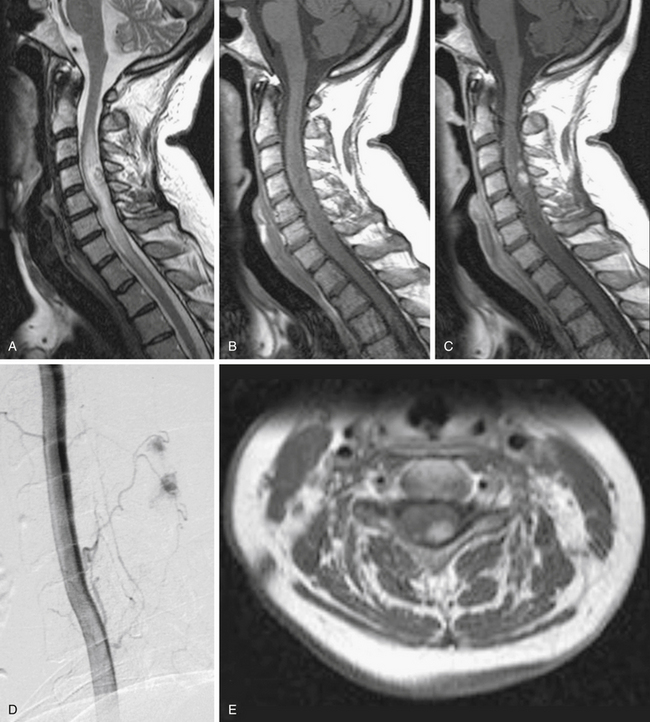
FIGURE 186-1 Grade II cervical ependymoma. A to E, Preoperative magnetic resonance images (MRIs). A, Sagittal T2-weighted image. B, Sagittal T1-weighted image. C, Sagittal contrast-enhanced T1-weighted image. D, Axial contrast-enhanced T1-weighted image. E, Axial T2-weighted image. F to M, Perioperative views. F, Division of the pia mater above the medial sulcus. G, Midline approach with posterior columns separated like a book.Figure 186-1, cont’dH to J, The tumor is progressively resected by dissection of the tumor–spinal cord interface and debulking with an ultrasonic aspirator. K, Great care must be taken when reaching its anterior aspect to prevent any damage to the anterior spinal artery, which is often close to the lesion. L, View after complete resection of the lesion. M, The spinal cord has been closed. N to W, Early (N to R) and 9-month (S to W) postoperative MRIs comparable to A to E. Used with permission from Hôpital Erasme, Université Libre de Bruxelles.
Astrocytomas
Spinal cord astrocytomas (Fig. 186-2) represent more than 80% of the IMSCTs in children,11 and in adults astrocytomas is the second most common type of tumor.11 The fraction of pilocytic astrocytomas varies greatly in large studies, from more than 30% to more than 60%.11,15 The percentage of anaplastic lesions or glioblastomas is higher in adults (25% to 30%) than in children (10% to 17%).1,5,8,11,34–36 Unfortunately, unlike with ependymomas, radical resection is most of the time not possible at surgery because by nature grades II to IV tumors display an infiltrative behaviour. Surgery consists then in removing as much tumor as possible or, in some circumstances, in performing only biopsy or decompression with duraplasty. The reported rates of complete and subtotal removal vary respectively between 11% to 31% and 21% to 62%.11,15,37 Pilocytic astrocytomas and the surgeon’s experience are factors associated with higher rates of complete resection.11,15 In low-grade astrocytomas the 5- and 10-year survival rates rise about 77% to 82%, but this rate drops to 27% and 14% for malignant ones.11,38 Low spinal level (conus), malignant grade, and adult age are considered important factors in relation with higher tumor recurrence rate.11 The amount of resection has not been found in relation with the progression-free survival or the overall survival in many series when tumor debulking has been carried out,6,11,16,35,38,39 whereas it was for others in low-grade astrocytomas.5
Gangliogliomas
Gangliogliomas are very uncommon lesions mainly reported as case reports and a few series.10,40 These tumors develop mainly in children and young adults.10 In a large series of 56 patients, complete or subtotal resection has been obtained in 82% and 18% of the cases with 5-year actuarial survival rate of 88% and progression-free survival rate of 67%.10
Outcome Factors
The major determinant of long-term patient survival is the histologic composition of the tumor. Indeed, the 5-year progression-free survival rate drops from 78% for low-grade gliomas down to 30% in high-grade gliomas.5
A longitudinal database (Surveillance, Epidemiology, and End Results [SEER] database) encompassing 26% of the U.S. population has been published including 1814 patients suffering from a spinal cord glioma.13 In this study, age, histology, and grade were defined as significant predictors of outcome.13
Tumors of Nonglial Origin
Within the group of tumors of nonglial origin, hemangioblastomas and cavernomas are the most common. These are benign vascular tumors, being characteristically well delineated and sometimes multifocal. Other lesions such as metastasis, epidermoids, dermoid cysts, lipomas, intramedullary schwannomas, primitive neuroectodermal tumors (PNET), and teratomas can also be enumerated.11,25,39 Besides these rare tumors, we have also encountered lymphomas and neuroglial cysts.
Hemangioblastomas
Hemangioblastomas (Figs. 186-3 and 186-4) represent almost 2% to 15% of IMSCTs in some series.11,15,21,24,41,42 These highly vascular lesions consist of two main components: large vacuolated stromal cells, which have been identified as the neoplastic cell of origin, and a rich capillary network.43 Hemangioblastomas are often observed as an encapsulated lesion abutting the pia, especially at the posterior or posterolateral aspect of the spinal cord nearby the dorsal root entry zone, but they may be also observed anteriorly or, rarely, are purely intramedullary.11,21,42 Radicular arteries or anterior branches provide the blood supply to these lesions.44 Associated cyst and syrinx, present in 80% to 90% of cases, and edema can be responsible for significant neurologic morbidity.21
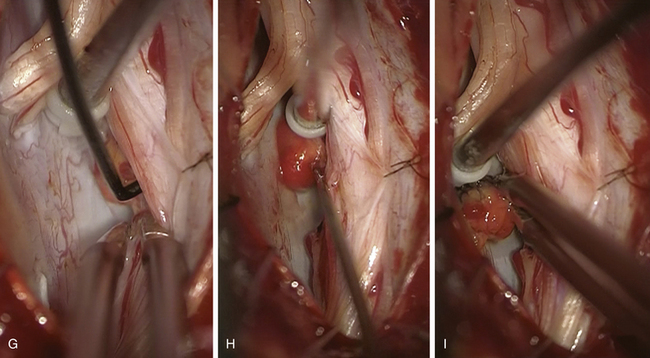
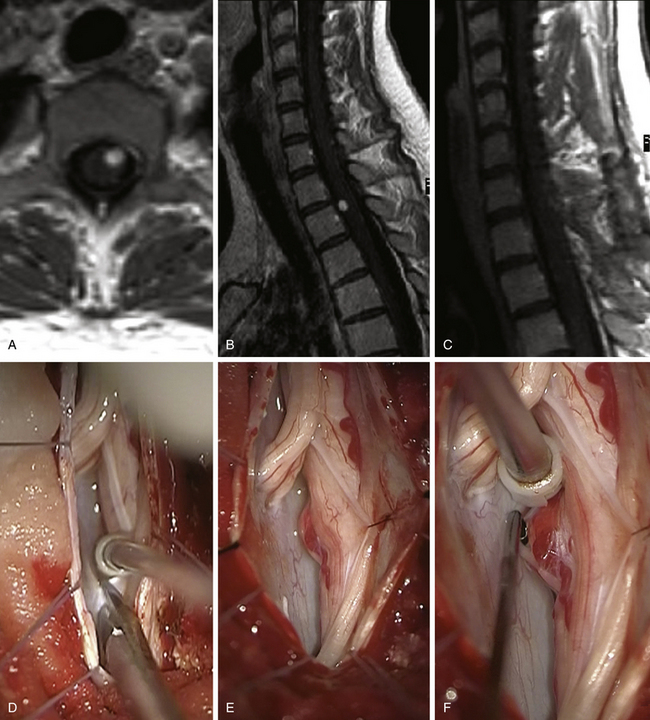
FIGURE 186-3 Left anterior cervical hemangioblastoma. A and B, Preoperative contrast-enhanced magnetic resonance image (MRI) in axial and sagittal planes. C, Postoperative control after complete resection. D to I, Perioperative views. D, Division of the dentate ligament. E, Tracting the dentate ligament allows rotating the spinal cord and exposing the lesion on its anterior aspect. F, A nerve rootlet is dissected.Figure 186-3, cont’dG and H. The lesion is dissected en bloc. I, The final step consists in the coagulation of the draining vein. Used with permission from Hôpital Erasme, Université Libre de Bruxelles.
The treatment of choice is en bloc surgical resection, achieving excellent neurological outcome. Not all patients require surgery: patients with incidental asymptomatic solitary lesions may simply be followed.21 Surgery is indicated as soon as symptoms develop or sequential MRI demonstrates tumor or cyst growth.11 The timing of surgery remains nevertheless a matter of debate for patients with von Hippel-Lindau disease and multiple lesions.42 Some authors advocate operating on asymptomatic patients if radiologic progression is observed, before significant neurologic deficits occur.42 The follow-up of patients with von Hippel-Lindau disease must be carried out yearly by spinal and brain MRI and also for associated disease such as pheochromocytomas and for renal and pancreatic cancers.11,45
Cavernomas
Intramedullary cavernomas (Fig. 186-5) accounted for 3% to 16% of IMSCTs in large series.11,15,24,46 Cavernous malformations are well-delineated lesions composed of closely packed, capillary-like vessels, without intervening brain or spinal tissue.46 Their clinical presentation may be variable: Stepwise neurologic deterioration explained by repeated hemorrhages, slow progressive deterioration induced by small hemorrhages and increasing gliosis, or acute onset with rapid or gradual deterioration due to major bleeding.46–51 Checking the entire central nervous system is recommended by some authors when an intraspinal cavernoma is discovered.46 In fact as many as 40% of patients with a spinal cavernoma harbor a coexisting intracranial lesion.52
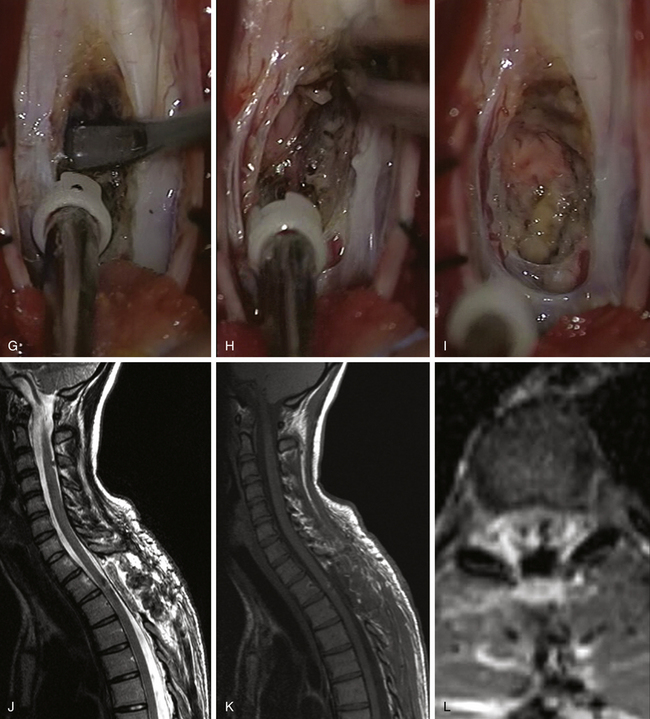
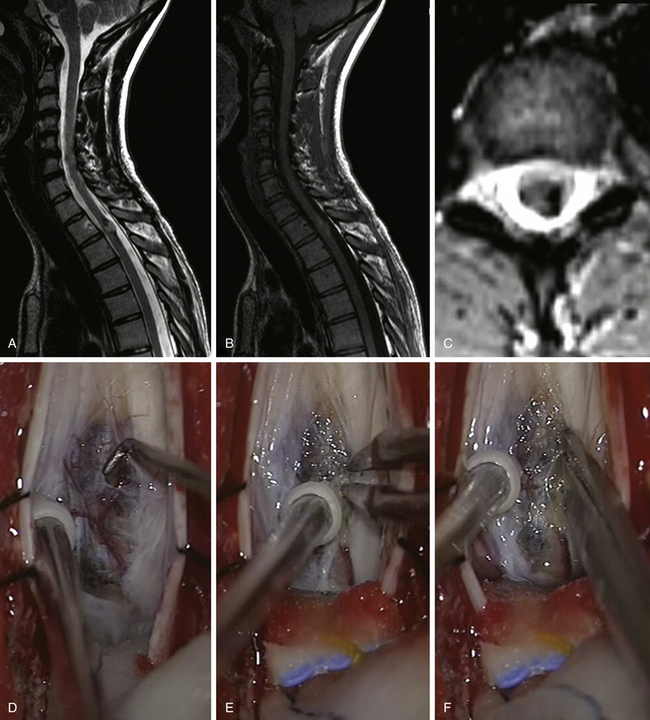
FIGURE 186-5 Thoracic intramedullary spinal cord cavernoma. A to C: Preoperative magnetic resonance image (MRI). A, Sagittal T2-weighted image. B, Sagittal T1-weighted image. C, Axial T2-weighted image. D to I: Perioperative views. D, The lesion is rapidly identified when opening the pia mater.E to H, En bloc removal is obtained by dissecting the cavernoma spinal cord interface. Figure 186-5, cont’dI, View after complete resection of the lesion. The hemosiderin ring has been left in place. J to L, Early postoperative MRI comparable to A to C. Used with permission from Hôpital Erasme, Université Libre de Bruxelles.
In our opinion, removal of intramedullary cavernomas is indicated in two conditions: if the lesion is symptomatic or if the lesion is easily accessible, meaning located posteriorly and abutting the pial surface, even in asymptomatic patients. The strategy is controversial for asymptomatic patients,47,48 but other authors defend the same philosophy.11,53 The annual rate of hemorrhage must be taken into account: It ranges in the literature from 1.4% to 4.5% in cases of symptomatic lesions but rises even to 66% in case of previous bleeding.51,54 Most lesions can be totally removed, and postoperatively most patients are satisfied by clinical improvement. A better postoperative outcome can be obtained if symptoms last for less than 3 years.51
Pseudotumors
Demyelinating diseases can present like pseudotumors, with histologic features identical to those encountered in the brain. Sarcoidosis has also been described as a primary spinal cord lesion. Spinal cord involvement is rare, occurring in less than 1% of patients with sarcoidosis.55 For this reason, any isolated spinal cord intramedullary lesion that enhances diffusely and is not associated with a mass effect should be rigorously investigated for inflammatory disease.
Associated Pathologies
Most IMSCTs develop sporadically; only a minority are associated with genetic diseases. Hemangioblastomas are a component of von Hippel-Lindau disease; ependymomas and astrocytomas are elements of neurofibromatosis types 1 and 2.11,21,42,56,57 Overall, IMSCTs are noted in these genetic disorders in respectively 20% to 40% and 19% of the cases.21,58 Hamartomas may be accompanied by a different kind of spinal dysraphism such as dermal sinus associated with dermoid cysts.11,59–62
Clinical Considerations
MRI is the diagnostic study of choice in the investigation of spinal cord tumors. However, it is optimal when the images can be correlated with previously obtained clinical data. The therapeutic decision depends on the patient’s age and on the context and the state of the patient’s clinical functioning at the time of operation, which is graded using the McCormick classification, taking into account both sensory and motor deficits.1,63
On a clinical point of view, demyelinating pathologies can be distinguishing from IMSCTs by the fact that intermittent symptom regression is never noted in spinal cord tumors.11
Imaging
MRI is nowadays the diagnostic modality of choice in management of IMSCTs. It is the most effective and sensitive technique for the detection of an intramedullary spinal cord lesion.1,25 Any tumoral infiltration produces spinal cord enlargement. Conversely, an enlarged spinal cord is not necessarily caused by a neoplastic process, particularly when enlargement is limited to one or two vertebral segments. The interpretation of the images allows the clinician to know the location and the extension of the lesion, to distinguish between cystic and solid tumoral components, and to propose a histologic diagnostic formulation. However, apart from lesions with specific appearances such as hemangioblastomas, lipomas, or pseudotumors like dermoid cysts, epidermoid cysts, and cavernomas, there are few tumor-specific MRI characteristics.
Ependymomas are observed typically at the center of the spinal cord because they arise from ependymal cells lining the central canal.64–66 On MRI, their signal can be variable on T1-weighted images and hypersignal on T2-weighted images. Typically, ependymomas appear as well-circumscribed lesions and enhance vividly and homogeneously after gadolinium injection.25 Four patterns of contrast-enhancement have been determined by Miyazawa:67 homogeneous pattern, heterogeneous pattern, heterogeneous with cyst wall enhancement, and enhancing nodule on cyst wall. Associated satellite cysts are found in 60% of the cases.11,25 On T2 sequences, it is interesting to look for the caplike appearance, corresponding to hyposignals at both poles of the solid part of the tumor. Although this finding is not pathognomonic, it was observed in our experience in 30% of ependymomas and 12% of hemangioblastomas. We have not observed this sign in astrocytomas. It has been correlated histologically and intraoperatively with areas of chronic hemorrhage with hemosiderin deposits.25
Unlike ependymomas, intramedullary spinal cord astrocytomas can be strongly suspected when the tumoral image is eccentric, when the contrast medium enhancement is heterogeneous, and especially if the tumor image has poorly defined borders. On MRI, astrocytomas appear most of the time hypointense on T1-weighted images and hyperintense on T2-weighted images.25 This last sequence is advocated to determine the exact extension of an astrocytoma whose borders are not well demarcated owing to its infiltrative nature. A cystic component is depicted in 27% to 42% 11,25 of the cases and associated satellites and hydromyelia in about half of the cases.25 Grade II astrocytomas are not enhancing after gadolinium administration.25 Conversely, pilocytic astrocytomas enhance intensely, as do high-grade lesions that appear more heterogeneous with necroticocystic areas.25 Gradient echo T2-weighted imaging is a useful sequence for determining hemorrhagic zones within the lesion.25 However, as in the case of ependymomas, there is no MRI-pathognomonic appearance in astrocytomas. Insofar as the only possibility of cure results from gross total resection at the first operation, it appears that all IMSCTs with appropriate imaging appearance should be considered to be ependymomas until proved otherwise.
In contrast to glial tumors, MRI has a high degree of accuracy with nonglial tumors. The nodule of a hemangioblastoma appears classically iso- to hypointense on T1-weighted images and iso- to hyperintense on T2-weighted images.25 Brisk enhancement is evident after gadolinium injection. Proton density imaging and T2-weighted imaging are appropriate for detailing enlarged feeding arteries, rich tumoral vascular network, and dilated draining veins.25 An associated cyst has been found in 88% of the cases in large series.11 Angio MRI using contrast-enhanced bolus three-dimensional T1-weighted sequences can identify some feeding arteries and large draining veins. Angiography is considered only when preoperative embolization is required to reduce the hemorrhagic risk during operation and should be performed immediately prior to surgery.
Cavernous malformations have a characteristic appearance on MRI; the lesion is composed of mixed signal intensities, surrounded by a hemosiderin hypointense ring. Completing the preoperative workup by a brain MRI is advocated because associated lesions are often noted and reinforce the diagnosis.25
Differential Diagnosis
Several pathologies must be known in order to avoid unnecessary surgery. Ruling out an intramedullary tumor and determining the diagnosis is sometimes possible based on crucial information given by circumstances, associated conditions, and timing of development. Indeed, intramedullary bacterial, fungal, or parasitic abscesses are unusual in the absence of systemic disease and, unlike IMSCTs, a rapid clinical deterioration is observed.68 An abrupt onset can also lead one to suspect a medulla infarction.69
Multiple sclerosis can mimic an intramedullary tumor, especially active lesions that enhance on MRI.39 In the case of any doubt, one has to screen the complete central nervous system for other lesions by MRI. If no other lesion is discovered, the diagnosis of demyelinating or inflammatory disease cannot be excluded definitively because some patients present with a solitary lesion; on the other hand, detecting other lesions can help in defining the diagnosis.11
Cerebrospinal fluid sampling looking for oligoclonal bands and serum markers can also be relevant.39 If doubt persists, a control MRI has to be planned a few weeks or months later. Indeed, the signal characteristics of the lesion on MRI can change in the case of demyelinating or inflammatory disease, but this delay is too short to be associated with changes in the case of a neoplastic process.11
Spinal cord edema and rimlike contrast enhancement can also be encountered in cases of subacute necrotizing myelopathy. In this pathology, the spinal cord atrophies over time.68 The same evolution can also appear following post-traumatic intramedullary hypersignal that initially can appear to be a tumor. In a study of patients operated on for neoplasic-like lesions, reported by Lee and colleagues,70 the most consistent characteristic found for differentiating non-neoplastic lesions from neoplastic ones was the absence of or minimal spinal cord expansion on preoperative MRI.70
Intraoperative Electrophysiologic Monitoring
Intraoperative monitoring of evoked potentials is a standard monitoring tool for surgical treatment of spinal cord lesions. Monitoring of sensory-evoked and motor-evoked potentials (SEPs and MEPs) is used to assess the functional integrity of the spinal cord during surgical procedures.22 The perioperative analysis of evoked potentials recording provides invaluable information about the functional status of the spinal cord and shows the abnormalities of the sensory and motor pathways, transient or not, that are used to guide the extent of surgical removal of the tumor. At the end, the monitoring allows one to predict the occurrence of postoperative neurologic deficit. Carrying out intraoperative electrophysiologic monitoring is technically difficult but useful during the operative procedure. This technique requires strict collaboration with the neurosurgeon and the electrophysiology team.
One limitation of SEP monitoring is that the evoked responses may be absent or attenuated preoperatively and cannot be monitored intraoperatively. The evoked potentials may be lost during the procedure, but it is essential to know if the abnormalities are transient or permanent. Dorsal column injury is currently detected during the operation. Irreversible abnormalities predict postoperative sensory deficits. Conversely, the absence of dorsal column conduction changes is a good indicator. However, motor function may be damaged without changes in the SEP intraoperative recording. That is why monitoring both SEPs and MEPs is useful every time these waveforms are monitorable. The same limitation concerns MEP monitoring: patients with severe preoperative motor deficit cannot be monitored. Changes caused by nonsurgical factors are easily recognized by the anesthesiologist. Changes observed during the procedure have to be understood insofar as warning to the surgeon, which allows a change in the surgical approach before the motor pathways are injured. However, in patients with a monitorable preoperative curve of MEPs, a reduction of amplitude that remains until the end of the procedure can predict a postoperative transient motor deficit.
Operative Procedure
General anesthesia is managed taking into account the requirements of intraoperative neurophysiologic monitoring.1,22,71 The surgical procedure is performed while the SEPs and MEPs are recorded. Halogenated volatile anesthetics should be avoided because they modify the SEPs. General anesthesia is established with intravenous opioids and the continuous administration of propofol without bolusing drugs during the operative procedure. MEPs cannot be monitored under complete neuromuscular blockade. Therefore, short-acting muscle relaxants are used to facilitate intubation and to avoid potentially dangerous movements by the patient. These drugs are not routinely readministered or are kept at a minimum, allowing MEPs to be monitored throughout an often lengthy surgical procedure.
Ependymomas and Astrocytomas
In our practice, the midline surgical approach is used except when the lesion is located in one dorsal column and is apparent on the surface without any cortical mantle or in the rare case of an exophytic tumor. We do not incise the tissue and we disagree with those authors who recommend laser myelotomy. We prefer to open the spinal cord by spreading the dorsal columns apart with microscissors and microdissectors and then carefully retracting them with warm saline-moistened cottonoids. The surgical field is extended over the entire length of the solid portion of the tumor and continued to expose the rostral and caudal cysts, if these are present. The opening of the spinal cord must allow exposure of the poles of the lesion and the cyst walls. Pial sutures improve the surgical exposure and reduce the severity of repeated trauma due to dissection. This can be accomplished using a fine suture without any tension to hold the median pia mater and dura mater close together.
Hemangioblastomas and Cavernomas
In rare cases in which the lesion is found entirely inside the spinal cord, it has to be accessed through the midline, as described earlier. But, most of the time, hemangioblastomas and cavernomas develop essentially superficially. The lesion may then be observed directly abutting the posterior or posterolateral surface of the spinal cord after dura opening and can therefore be straightforwardly approached.72 Whenever one is present, we always take advantage of a huge and tense syringomyelic cyst by gently withdrawing the fluid with a 22-gauge needle. This maneuver collapses the spinal cord and facilitates the access to the solid tumoral nodule.73
In the case of huge hemangioblastomas, the surgeon must be particularly cautious to avoid any coagulation of the draining vein at the beginning of the procedure. Indeed, interrupting this venous outflow will induce hyperpressure inside the malformation and provoke uncontrollable tumoral bleeding. If the lesion is located on the anterolateral aspect of the spinal cord, its access is possible by dividing one or two dentate ligament attachments and gently rotating the spinal cord with 6-0 silk sutures applied on the ligaments. In this way, most anterolateral lesions may be discovered through the transparent pia mater.20,74 In hemangioblastomas, biopsy or tumor debulking is never performed in order to prevent massive bleeding that will compromise the surgical procedure.
Under microscopic magnification, tumoral limits have to be found. An area with few vessels represents our starting dissection point. The cleavage plane remains distinct as long as bleeding does not interfere with the dissection. Slight tumor retraction can be obtained by gentle coagulation on its surface to facilitate detachment of the lesion from the cord. This maneuver must be completed with nonadherent bipolar forceps or under fluid washing. Repeated coagulations and divisions of vascular connections allow a progressive devascularization until the draining vein is reached. It is only at the end of the procedure that this vein can be interrupted safely. In fact, during this devascularization process, the draining vein appears more and more blue, reflecting the suppression of arterial feeders, as in the surgical treatment of arteriovenous malformations. In the case of huge cystic cavities adjacent to hemangioblastomas, removal of the tumor nodule with opening of the cyst is definitively sufficient without any need for shunting.20
As with hemangioblastomas, cavernomas should be removed most of the time en bloc. Nevertheless, in rare circumstances, reducing the volume of large cavernomas with the ultrasonic aspirator can be necessary. Then, the lesion is shrunk by gentle coagulation on its surface to decrease the pressure on the dissection plane. In some huge cavernomas, it might even be mandatory to use strategies combining different approaches (posterior and anterolateral) in the same surgical procedure.
Outcome
Although our results for the surgery of IMSCTs have been published previously,1,20,71 we reiterate our main conclusions here.
The evaluation of surgical results with IMSCTs requires analysis of postoperative functional outcome related to the patient’s functional preoperative state. This evaluation must take into account both motor and sensory functions, as proposed by McCormick in a neurologic grading scale, which is useful for evaluating patients before and after surgery.1
• In preoperative grade I patients, 95% were unchanged, and 5% worsened.
• In preoperative grade II patients, 7% improved, 86% were unchanged, and 7% worsened.
• In preoperative grade III patients, 28% improved, 53% were unchanged, and 19% worsened.
• In preoperative grade IV patients, none except one child improved. Most remained unchanged.
If spinal deformity is one of the earliest symptoms leading to the discovery of an intramedullary tumor, particularly in children, postoperative spinal deformities present before surgery are not exacerbated by surgery.5,75 The sometimes extensive laminectomies required for the surgical treatment of intramedullary tumors may be responsible for severe, but fortunately rather rare, disorders of spinal stability. Four factors are responsible for these severe postoperative deformities: young age, the presence of preoperative spinal deformity, laminectomy involving at least six vertebrae (especially if it includes C2), and malignant neoplasm or adjunctive radiotherapy, or both. In the great majority of cases, postoperative spinal deformations such as increased lordosis or scoliosis or, most often, kyphosis or kyphoscoliosis of varying degrees of severity, are spontaneously stabilized within a few months.
Postoperative Strategy
We are definitively convinced that there is absolutely no indication for radiation therapy in benign IMSCTs even after incomplete removal, recurrence, or progression. Our personal opinion is based on a follow-up longer than 5 years in 193 patients surgically treated either for a low-grade ependymoma or a low-grade astrocytoma, without adjunctive radiotherapy. In 90.2% of 122 ependymomas, we were able to resect the lesion completely. The rate of complete removal drops in astrocytomas, but 40.8% of 71 were nonetheless resected completely as ependymomas. When residual tumor is known to remain and is confirmed on postoperative imaging, we recommend repeating MRI evaluation at more frequent intervals during the first postoperative years. We only observed two recurrent ependymomas after 18 and 19 years and three recurrent astrocytomas after, respectively 5, 6, and 7 years in those completely removed tumors. In fact, even low-grade astrocytomas partially removed have a very indolent evolution. Seventeen remain stable in spite of partial removal; a few show a slow MRI evolution without any clinical consequences.
We only recommend, as have others,76 radiation therapy in malignant gliomas. In the rare case of malignant ependymoma, postoperative radiotherapy may be performed because palliation is based more on compassion than on proof of efficacy. For patients who presented with a malignant astrocytoma, the results also remain poor. Irrespective of the treatments, repeat surgery, radiotherapy, or chemotherapy, the disease is usually fatal within 1 to 3 years.
Balériaux D., Gultasli N. Intradural spinal tumors. In: Van Goethem J.W.M., van den Hauwe L., Parizel P.M. Spinal Imaging. Berlin: Springer-Verlag; 2007:417-460.
Brotchi J. Intrinsic spinal cord tumor resection. Neurosurgery. 2002;50:1059-1063.
Brotchi J., Bruneau M., Lefranc F., et al. Surgery of intraspinal cord tumors. Clin Neurosurg. 2006;53:209-216.
Brotchi J., Fischer G. Spinal cord ependymomas. Neurosurg Focus. 1998;4:e2.
Chang U.K., Choe W.J., Chung S.K., et al. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57:133-139.
Constantini S., Miller D.C., Allen J.C., et al. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93:183-193.
Cooper P.R. Outcome after operative treatment of intramedullary spinal cord tumors in adults: intermediate and long-term results in 51 patients. Neurosurgery. 1989;25:855-859.
Ferrante L., Mastronardi L., Celli P., et al. Intramedullary spinal cord ependymomas—a study of 45 cases with long-term follow-up. Acta Neurochir (Wien). 1992;119:74-79.
Fisher G., Brotchi J. Intramedullary Spinal Cord Tumors. New York: Thieme; 1996.
Garces-Ambrossi G.L., McGirt M.J., Mehta V.A., et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11:591-599.
Guidetti B., Mercuri S., Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54:323-330.
Harrop J.S., Ganju A., Groff M., et al. Primary intramedullary tumors of the spinal cord. Spine. 2009;34:S69-S77.
Houten J.K., Cooper P.R. Spinal cord astrocytomas: presentation, management and outcome. J Neurooncol. 2000;47:219-224.
Hsu F.P.K., Clatterbuck R.E., Kim L.J., Spetzler R.F. Intramedullary spinal cord cavernous malformations. Oper TechNeurosurg. 2003;6:32-40.
Jallo G.I., Freed D., Epstein F.J. Spinal cord gangliogliomas: a review of 56 patients. J Neurooncol. 2004;68:71-77.
Jallo G.I., Freed D., Zareck M., et al. Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus. 2006;21:e10.
Klekamp J., Samii M. Intramedullary tumors. In: Klekamp J., Samii M. Surgery of Spinal Tumors. Berlin: Springer-Verlag; 2007:20-131.
Lefranc F., Brotchi J. Surgical strategy in spinal cord hemangioblastomas. Operative Techniques in Neurosurgery. 2003;6:24-31.
McCormick P.C., Torres R., Post K.D., et al. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523-532.
Milano M.T., Johnson M.D., Sul J., et al. Primary spinal cord glioma: a surveillance, epidemiology, and end results database study. J Neurooncol. 2010;98(1):83-92.
Raco A., Esposito V., Lenzi J., et al. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56:972-981. discussion 972-981
Schwartz T.H., McCormick P.C. Intramedullary ependymomas: clinical presentation, surgical treatment strategies and prognosis. J Neurooncol. 2000;47:211-218.
Schwartz T.H., McCormick P.C. Intramedullary spinal cord tumors. Special issue. J Neurooncol. 2000;47:187-317.
Van Velthoven V., Reinacher P.C., Klisch J., et al. Treatment of intramedullary hemangioblastomas, with special attention to von Hippel-Lindau disease. Neurosurgery. 2003;53:1306-1313. discussion 1313-1314
Zevgaridis D., Medele R.J., Hamburger C., et al. Cavernous haemangiomas of the spinal cord. A review of 117 cases. Acta Neurochir (Wien). 1999;141:237-245.
1. Fisher G., Brotchi J. Intramedullary Spinal Cord Tumors. New York: Thieme; 1996.
2. Schwartz T.H., McCormick P.C. Intramedullary spinal cord tumors. Special issue. J Neurooncol. 2000;47:187-317.
3. Chang U.K., Choe W.J., Chung S.K., et al. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57:133-139.
4. Abdel-Wahab M., Etuk B., Palermo J., et al. Spinal cord gliomas: a multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys. 2006;64:1060-1071.
5. Constantini S., Miller D.C., Allen J.C., et al. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93:183-193.
6. Cooper P.R. Outcome after operative treatment of intramedullary spinal cord tumors in adults: intermediate and long-term results in 51 patients. Neurosurgery. 1989;25:855-859.
7. Garces-Ambrossi G.L., McGirt M.J., Mehta V.A., et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11:591-599.
8. Guidetti B., Mercuri S., Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54:323-330.
9. Harrop J.S., Ganju A., Groff M., et al. Primary intramedullary tumors of the spinal cord. Spine. 2009;34:S69-S77.
10. Jallo G.I., Freed D., Epstein F.J. Spinal cord gangliogliomas: a review of 56 patients. J Neurooncol. 2004;68:71-77.
11. Klekamp J., Samii M. Intramedullary tumors. In: Klekamp J., Samii M. Surgery of Spinal Tumors. Berlin: Springer-Verlag; 2007:20-131.
12. McGirt M.J., Goldstein I.M., Chaichana K.L., et al. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery. 2008;63:55-60. discussion 60-61
13. Milano M.T., Johnson M.D., Sul J., et al. Primary spinal cord glioma: a surveillance, epidemiology, and end results database study. J Neurooncol. 2010;98(1):83-92.
14. Minehan K.J., Brown P.D., Scheithauer B.W., et al. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 2009;73:727-733.
15. Raco A., Esposito V., Lenzi J., et al. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56:972-981. discussion 972-981
16. Stein B.M., McCormick P.C. Intramedullary neoplasms and vascular malformations. Clin Neurosurg. 1992;39:361-387.
17. Osborn A.G. Tumors, cysts and tumorlike lesions of the spine and spinal cord. In: Osborn A.G., editor. Diagnostic Neuroradiology. Saint Louis: Mosby; 1994:876-918.
18. Cooper P.R., Epstein F. Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 1985;63:492-499.
19. Constantini S., Epstein F. Pediatric intraspinal tumors. In: Choux M., Di Rocco R.C., Hockley A., Walker M. Pediatric Neurosurgery. London: Churchill-Livingstone; 1999:601-613.
20. Brotchi J. Intrinsic spinal cord tumor resection. Neurosurgery. 2002;50:1059-1063.
21. Mandigo C.E., Ogden A.T., Angevine P.D., et al. Operative management of spinal hemangioblastoma. Neurosurgery. 2009;65:1166-1177.
22. Kothbauer F.K. Intraoperative neurophysiologic monitoring for intramedullary spinal cord tumor surgery. Operative Techniques in Neurosurgery. 2003;6:2-68.
23. Morota N., Deletis V., Constantini S., et al. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery. 1997;41:1327-1336.
24. Matsuyama Y., Sakai Y., Katayama Y., et al. Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection. J Neurosurg Spine. 2009;10:404-413.
25. Balériaux D., Gultasli N. Intradural spinal tumors. In: Van Goethem J.W.M., van den Hauwe L., Parizel P.M. Spinal Imaging. Berlin: Springer-Verlag; 2007:417-460.
26. Guppy K.H., Akins P.T., Moes G.S., et al. Spinal cord oligodendroglioma with 1p and 19q deletions presenting with cerebral oligodendrogliomatosis. J Neurosurg Spine. 2009;10:557-563.
27. Ferrante L., Mastronardi L., Celli P., et al. Intramedullary spinal cord ependymomas—a study of 45 cases with long-term follow-up. Acta Neurochir (Wien). 1992;119:74-79.
28. Brotchi J., Fischer G. Spinal cord ependymomas. Neurosurg Focus. 1998;4:e2.
29. Lonjon M., Goh K.Y., Epstein F.J. Intramedullary spinal cord ependymomas in children: treatment, results and follow-up. Pediatr Neurosurg. 1998;29:178-183.
30. Shirato H., Kamada T., Hida K., et al. The role of radiotherapy in the management of spinal cord glioma. Int J Radiat Oncol Biol Phys. 1995;33:323-328.
31. Sgouros S., Malluci C.L., Jackowski A. Spinal ependymomas—the value of postoperative radiotherapy for residual disease control. Br J Neurosurg. 1996;10:559-566.
32. Vijayakumar S., Estes M., Hardy R.W.Jr., et al. Ependymoma of the spinal cord and cauda equina: a review. Cleve Clin J Med. 1988;55:163-170.
33. Rawlings C.E.3rd, Giangaspero F., Burger P.C., et al. Ependymomas: a clinicopathologic study. Surg Neurol. 1988;29:271-281.
34. Epstein F.J., Farmer J.P. Pediatric spinal cord tumor surgery. Neurosurg Clin N Am. 1990;1:569-590.
35. Houten J.K., Cooper P.R. Spinal cord astrocytomas: presentation, management and outcome. J Neurooncol. 2000;47:219-224.
36. Innocenzi G., Raco A., Cantore G., et al. Intramedullary astrocytomas and ependymomas in the pediatric age group: a retrospective study. Childs Nerv Syst. 1996;12:776-780.
37. Kim M.S., Chung C.K., Choe G., et al. Intramedullary spinal cord astrocytoma in adults: postoperative outcome. J Neurooncol. 2001;52:85-94.
38. Jallo G.I., Danish S., Velasquez L., et al. Intramedullary low-grade astrocytomas: long-term outcome following radical surgery. J Neurooncol. 2001;53:61-66.
39. McCormick P.C., Stein B.M. Intramedullary tumors in adults. Neurosurg Clin N Am. 1990;1:609-630.
40. Park C.K., Chung C.K., Choe G.Y., et al. Intramedullary spinal cord ganglioglioma: a report of five cases. Acta Neurochir (Wien). 2000;142:547-552.
41. Murota T., Symon L. Surgical management of hemangioblastoma of the spinal cord: a report of 18 cases. Neurosurgery. 1989;25:699-707. discussion 708
42. Van Velthoven V., Reinacher P.C., Klisch J., et al. Treatment of intramedullary hemangioblastomas, with special attention to von Hippel-Lindau disease. Neurosurgery. 2003;53:1306-1313. discussion 1313-1314
43. Böhling T.P.K., Haltia M.J., Alitalo K., Neumann H.P.H. Von Hippel-Lindau disease and capillary haemangioblastoma. In: Kleihues P.C.W., editor. Tumours of the Nervous System. Pathology and Genetics. Lyon: IARCPress; 2000:223-226.
44. Hurth M. [Intraspinal hemangioblastomas.]. Neurochirurgie. 1975;21(Suppl 1):1-136.
45. Michels V.V. Investigative studies in von Hippel-Lindau disease. Neurofibromatosis. 1988;1:159-163.
46. Jallo G.I., Freed D., Zareck M., et al. Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus. 2006;21:e10.
47. Cantore G., Delfini R., Cervoni L., et al. Intramedullary cavernous angiomas of the spinal cord: report of six cases. Surg Neurol. 1995;43:448-451. discussion 451-452
48. Cristante L., Hermann H.D. Radical excision of intramedullary cavernous angiomas. Neurosurgery. 1998;43:424-430. discussion 430-431
49. Deutsch H., Jallo G.I., Faktorovich A., et al. Spinal intramedullary cavernoma: clinical presentation and surgical outcome. J Neurosurg. 2000;93:65-70.
50. Ogilvy C.S., Louis D.N., Ojemann R.G. Intramedullary cavernous angiomas of the spinal cord: clinical presentation, pathological features, and surgical management. Neurosurgery. 1992;31:219-229. discussion 229-230
51. Zevgaridis D., Medele R.J., Hamburger C., et al. Cavernous haemangiomas of the spinal cord. A review of 117 cases. Acta Neurochir (Wien). 1999;141:237-245.
52. Cohen-Gadol A.A., Jacob J.T., Edwards D.A., et al. Coexistence of intracranial and spinal cavernous malformations: a study of prevalence and natural history. J Neurosurg. 2006;104:376-381.
53. Ojemann R.G., Crowell R.M., Ogilvy C.S. Management of cranial and spinal cavernous angiomas (honored guest lecture). Clin Neurosurg. 1993;40:98-123.
54. Sandalcioglu I.E., Wiedemayer H., Gasser T., et al. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26:253-256.
55. Varron L., Broussolle C., Candessanche J.P., et al. Spinal cord sarcoidosis: report of seven cases. Eur J Neurol. 2009;16:289-296.
56. Lee M., Rezai A.R., Freed D., et al. Intramedullary spinal cord tumors in neurofibromatosis. Neurosurgery. 1996;38:32-37.
57. Schwartz T.H., McCormick P.C. Intramedullary ependymomas: clinical presentation, surgical treatment strategies and prognosis. J Neurooncol. 2000;47:211-218.
58. Parsa A.T., Fiore A.J., McCormick P.C., et al. Genetic basis of intramedullary spinal cord tumors and therapeutic implications. J Neurooncol. 2000;47:239-251.
59. Barkovich A.J., Edwards M., Cogen P.H. MR evaluation of spinal dermal sinus tracts in children. AJNR Am J Neuroradiol. 1991;12:123-129.
60. Gok A., Bayram M., Coskun Y., et al. Unusual malformations in occult spinal dysraphism. Turk J Pediatr. 1995;37:391-397.
61. Shen W.C., Chiou T.L., Lin T.Y. Dermal sinus with dermoid cyst in the upper cervical spine: case note. Neuroradiology. 2000;42:51-53.
62. Peter J.C., Sinclair-Smith C., de Villiers J.C. Midline dermal sinuses and cysts and their relationship to the central nervous system. Eur J Pediatr Surg. 1991;1:73-79.
63. McCormick P.C., Torres R., Post K.D., et al. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523-532.
64. Fine M.J., Kricheff II, Freed D., et al. Spinal cord ependymomas: MR imaging features. Radiology. 1995;197:655-658.
65. Parizel P.M., Baleriaux D., Rodesch G., et al. Gd-DTPA-enhanced MR imaging of spinal tumors. AJR Am J Roentgenol. 1989;152:1087-1096.
66. Sun B., Wang C., Wang J., et al. MRI features of intramedullary spinal cord ependymomas. J Neuroimaging. 2003;13:346-351.
67. Miyazawa N., Hida K., Iwasaki Y., et al. MRI at 1.5 T of intramedullary ependymoma and classification of pattern of contrast enhancement. Neuroradiology. 2000;42:828-832.
68. Schwartz T.H., McCormick P.C. Non-neoplastic intramedullary pathology. Diagnostic dilemna: to Bx or not to Bx. J Neurooncol. 2000;47:283-292.
69. Andrews B.T., Kwei U., Greco C., et al. Infarct of the conus medullaris simulating a spinal cord tumor: case report. Surg Neurol. 1991;35:139-142.
70. Lee M., Epstein F.J., Rezai A.R., et al. Nonneoplastic intramedullary spinal cord lesions mimicking tumors. Neurosurgery. 1998;43:788-794. discussion 794-795
71. Brotchi J., Bruneau M., Lefranc F., et al. Surgery of intraspinal cord tumors. Clin Neurosurg. 2006;53:209-216.
72. Roonprapunt C., Silvera V.M., Setton A., et al. Surgical management of isolated hemangioblastomas of the spinal cord. Neurosurgery. 2001;49:321-327. discussion 327-328
73. Lefranc F., Brotchi J. Surgical strategy in spinal cord hemangioblastomas. Operative Techniques in Neurosurgery. 2003;6:24-31.
74. Hsu F.P.K., Clatterbuck R.E., Kim L.J., Spetzler R.F. Intramedullary spinal cord cavernous malformations. Oper TechNeurosurg. 2003;6:32-40.
75. Houten J.K., Weiner H.L. Pediatric intramedullary spinal cord tumors: special considerations. J Neurooncol. 2000;47:225-230.
76. Benes V.3rd, Barsa P., Benes V.Jr., et al. Prognostic factors in intramedullary astrocytomas: a literature review. Eur Spine J. 2009;18:1397-1422.
77. Resche F., Moisan J.P., Mantoura J., et al. Haemangioblastoma, haemangioblastomatosis, and von Hippel-Lindau disease. Adv Tech Stand Neurosurg. 1993;20:197-304.