Chapter 69 Surgical Management of Intracerebral Hemorrhage
Epidemiology
Intracerebral hemorrhage (ICH), or hemorrhage within the brain parenchyma, occurs with an incidence estimated to range from 15 to 35 cases per 100,000 people per year. The incidence is up to twice that of subarachnoid hemorrhage by some estimates. Each year, approximately 37,000 to 52,000 people in the United States have an ICH. The rate is expected to double during the next 50 years as a result of the increasing age of the population and changes in racial demographics. A 1993 report found that only 38% of patients affected with ICH survive the first year,1 while a 2009 report found improvement up to 51% for 3-year survival in ICH patients.2
Six risk factors for ICH have been identified—age, male sex, race, hypertension, high alcohol intake, and low serum cholesterol. Regarding other possible risks, current or past smoking and diabetes mellitus are weak risk factors, if at all.3 The incidence of ICH increases significantly after age 55 and doubles with each decade of age until the age of 80, at which point the incidence increases 25-fold each decade.4 ICH is more common in men than women. ICH also affects blacks and Japanese more than whites. During the 20-year period covered by the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study, the incidence of ICH among blacks was 50 per 100,000, a little over twice the incidence among whites.5 It has been hypothesized that hypertension and factors leading to limited access to health care result in the higher incidence of ICH within the African-American community. The higher incidence of ICH in Japan has been attributed to a higher incidence of hypertension in Japanese populations and diets leading to low serum cholesterol, another risk factor for ICH. The reversibility of the dietary factor may lead to reductions in ICH seen when Japanese people emigrate to the United States, while their persistent hypertension may explain why their rates never drop to the same level as whites even after they emigrate to the United States.
There have been 11 case-controlled studies on hypertension and risk of ICH, with all showing a positive association between hypertension and ICH. Hypertension is classified as high normal (systolic 130–139 or diastolic 85–89), stage I hypertension (systolic 140–159 or diastolic 90–99), stage II hypertension (systolic 160–179 or diastolic 100–109), or stage III hypertension (systolic > 180 or diastolic > 110). Suh et al. found a relative risk of 2.2 for high normal, 5.3 for stage I hypertension, 10.4 for stage II hypertension, and 33 for stage III hypertension.6 Iribarren et al. found for each one standard-deviation increase in systolic blood pressure (18 mm Hg in men; 19 mm Hg in women) a relative risk of 1.14 in men and 1.17 in women.7 Leppala et al. found a relative risk of 2.20 for systolic blood pressure 140 to 159 mm Hg and 3.78 for systolic blood pressure greater than 160 mm Hg compared with systolic blood pressure less than 139 mm Hg.8 The correlation between blood pressure and ICH also leads to diurnal and seasonal variations in the onset of ICH. In general, ICH onset is usually during activity and rarely during sleep, which may be related to elevated blood pressure or increased cerebral blood flow. One study covering a decade of ICH cases in a Japanese city found that men 69 years of age and younger had a bimodal distribution of ICH-onset time, with an initial peak between 8:00 a.m. and 10:00 a.m., and a second, lower peak between 6:00 and 8:00 p.m. Men 70 years of age or older and women of all ages exhibited only a single evening peak, between 6:00 and 10:00 p.m.9 Men exhibited peak ICH in winter and a trough in summer, while women had no seasonal patterns.9 The incidence of ICH correlates with the daily times of blood pressure peaks in the sexes, and the ability of the autonomic nervous system to raise blood pressure during the winter may particularly affect men because they tend to work outdoors more often.
Alcohol consumption is a risk factor in both the short term and long term. During the 24 hours preceding an ICH, moderate alcohol consumption (41 to 120 g of ethanol, where one standard drink averages 12 g of ethanol) causes a 4.6 relative risk of ICH, while heavy alcohol consumption (>120 g of ethanol) causes an 11.3 relative risk of ICH. During the week preceding ICH, low (1–150 g of ethanol), moderate (151–300 g of ethanol), and heavy (>300 g of ethanol) alcohol consumption carry relative risks of 2.0, 4.3, and 6.5, respectively.10 ICH in patients with high ethanol consumption tends to be lobar.11 Ethanol promotes ICH by impairing coagulation and by directly affecting the integrity of cerebral vessels.11
A counterintuitive finding has been the identification of low serum cholesterol as a risk factor for ICH. Iribarren et al. found that for each one standard deviation increase in serum cholesterol (1.45 mmol/L in men and 1.24 mmol/L in women), there was a relative risk reduction of 0.84 in men and 0.92 in women.7 One potential mechanism may be that patients with low serum cholesterol may exhibit reduced consumption of animal products, and such patients will have reduced concentrations of arachidonic acid in their cell membranes.12 Arachidonic acid is a vital structural component of the cell membranes of vascular endothelium and its metabolites are involved in regulation of vascular tone and repair of injured vascular endothelium.12 Defects in this pathway may increase the risk of ICH. However, hypercholesterolemia is a proven risk factor for morbidities such as myocardial infarction that are far more common than ICH and should therefore be avoided. Furthermore, patients taking cholesterol lowering statin drugs before experiencing an ICH have been shown to have lower hematoma volumes, although the difference has not been shown to impact clinical outcome after ICH.13
Etiology
In primary ICH, the hemorrhage arises from vessels damaged by chronic hypertension or amyloid angiopathy. Chronic hypertension causes degenerative changes in the walls of small penetrating arteries originating from the anterior, middle, or posterior cerebral arteries. These changes reduce vessel compliance and increase the likelihood of spontaneous rupture. Patients with chronic hypertension incur an annual risk of recurrent ICH of 2%, but this risk can be reduced by treatment of hypertension.14 In 1868, Charcot and Bouchard attributed ICH to rupture at points of dilation in the walls of small arterioles that they called microaneurysms.15 These microaneurysms were later found to be subadventitial hemorrhages or extravascular clots resulting from endothelial damage by the hematoma. Electron-microscopy studies have since suggested that most ICH occurs at or near the bifurcation of affected arteries, where prominent degeneration of the media presumably caused by chronic hypertension can be seen.
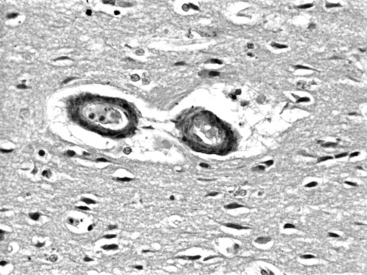
In amyloid angiopathy, β-amyloid protein, an acellular eosinophilic material, is deposited within the media of small- and medium-sized arteries in the cerebral cortex and leptomeninges, which causes primary ICH in the white matter of the cerebral lobes, particularly the parietal and occipital areas, in persons older than 70 years of age who exhibit no evidence of systemic amyloidosis. These patients face an annual risk of recurrent hemorrhage of 10.5%.16 Cerebral amyloid angiopathy is present in the brains of 50% of people over the age of 70; however, most do not experience ICH. Amyloid angiopathy may be associated with genetic factors including the apolipoprotein E allele and may be more prevalent in patients with Down’s syndrome. O’Donnell et al. reported that the presence of the ε2 or ε4 alleles of the apolipoprotein E gene was associated with a tripling of the risk of recurrent ICH among survivors of primary lobar ICH attributable to amyloid angiopathy.16 Among patients with lobar ICH, those with the apoE ε4 allele typically have their first ICH more than 5 years earlier than noncarriers (average age of 73 versus 79),17 and experience a statistically independent decrease in survival.18 Although they are distinct diseases, there is some overlap between amyloid angiopathy and Alzheimer’s disease, in that the amyloid in amyloid angiopathy is identical to that found in the senile plaques of Alzheimer’s disease and apolipoprotein-ε4 is associated with both the parenchymal plaque amyloid seen in Alzheimer’s disease and the deposits of β-amyloid protein in cerebral vessel walls seen in amyloid angiopathy. Cerebral amyloid angiopathy may increase the risk of ICH by potentiating plasminogen, a finding that may be of some relevance to patients receiving tissue plasminogen activator (t-PA) to treat myocardial infarcts or cerebrovascular accidents. Amyloid angiopathy can be diagnosed suggestively on the basis of radiologic findings such as hemosiderin deposits from small cortical and subcortical petechial hemorrhages on gradient-echo magnetic resonance imaging (MRI). Histologic findings include deposits of acellular eosinophilic material in the media of vessels in the hematoma or in noninvolved brain (Fig. 69-1). Perivascular microglia, thickened vessel walls, vessel dilatation, and microaneurysms are also seen in the vessels of patients with cerebral amyloid angiopathy. After staining with Congo red, the amyloid in the media of vessel walls exhibits apple-green birefringence under polarized light. A definitive diagnosis can be made on the basis of all three of the following findings: lobar, cortical, or corticosubcortical ICH; severe cerebral amyloid angiopathy on histopathologic exam; and absence of another diagnostic lesion. A probable diagnosis with supporting pathologic evidence occurs with all three of the following findings: lobar, cortical, or corticosubcortical ICH; some degree of vascular amyloid deposition on histopathologic exam; and absence of another diagnostic lesion. Probable amyloid angiopathy without pathologic evidence occurs with all 3 of the following findings: age over 60 years; a history of multiple hemorrhages in the lobar, cortical, or subcortical regions; and absence of another cause of hemorrhage. A diagnosis of possible amyloid angiopathy occurs with age over 60 combined with either a single lobar, cortical, or corticosubcortical hemorrhage without another cause or multiple hemorrhages with a possible but not a definitive cause.
Secondary ICH is far less common than primary ICH but because the etiologies of secondary ICH include tumors and vascular malformations that will need surgical intervention, or coagulopathies that need to be immediately corrected, attention must always be paid to secondary ICH as a possibility with any ICH. Tumors that produce ICH are usually malignant metastases. Hemorrhage is present in 3% to 14% of metastases and is most commonly seen in metastases from renal cell carcinoma, choriocarcinoma, melanoma, and renal cell carcinoma, with hemorrhage occurring in 70%, 50%, 40%, and 25% of the brain metastases from these respective primaries.19 However, bronchogenic carcinoma represents the most common source of hemorrhagic cerebral metastases because, although only 9% of metastatic bronchogenic carcinomas undergo hemorrhage, it is a much more common metastasis than the other four tumor types. When ICH appears on an initial CT scan, the presence of nonhemorrhagic necrotic or hypodense tissue and pronounced surrounding vasogenic edema are radiologic clues to the underlying neoplasm and warrant an MRI with gadolinium to look for tumor. Vascular malformations that can give rise to secondary ICH are usually arteriovenous malformations (AVMs), with 81% of hemorrhages from AVMs having a significant intraparenchymal component. Cavernous malformations also tend to cause hemorrhage with a significant intraparenchymal component, but only represent 10% of central nervous system vascular malformations. The diagnosis is strongly suggested by finding a mixed signal core indicative of old hemorrhage and a T2 dark rim on an MRI. ICH is unusual from aneurysmal rupture, which usually causes subarachnoid hemorrhage. Aneurysms that become adherent to the brain surface due to fibrosis from inflammation or previous hemorrhage can sometimes produce ICH rather than subarachnoid hemorrhage when they rupture. Oral anticoagulant therapy is a known source of secondary ICH. The relative risk of ICH during oral anticoagulant therapy increases more than 10-fold in patients over the age of 50.20 Bleeding is more protracted and hematomas larger in patients treated with anticoagulants than in those with spontaneous ICH.21 The management of these patients requires rapid reversal of their coagulopathy. Vitamin K provides long-term reversal and stabilization of the international normalized ratio (INR), while fresh frozen plasma (FFP) provides faster reversal, although one study showed that 24 hours after administering 1000 ml of FFP, patients on coumadin with ICH dropped their INR from 3.35 to 1.40.22 Slightly less than one third of these patients experienced radiographic hematoma enlargement within 24 hours of their initial CT scan,22 suggesting that the time it takes FFP to reverse a coagulopathy may be too slow, particularly in elderly patients who cannot tolerate rapid administration of volume. A suggested alternative is prothrombin complex concentrate (PCC), which can counteract the effects of warfarin as early as 10 minutes in much smaller volumes than FFP.22
Pathophysiology
The presence of hematoma initiates edema and neuronal damage in the surrounding parenchyma. Animal models of ICH have identified three phases of perihematoma edema—immediate (within 24 hours), intermediate (24 hours to 5 days), and late onset (from 5 days to several weeks after ICH). Immediate edema occurs within the first 24 hours and can often be seen at a histologic, but not radiographic level. This initial edema develops secondary to osmotically active plasma proteins accumulating in the extravascular space.23 The blood–brain barrier is intact at this point, so the proteins most likely arise from the hematoma. After the initial hemorrhage, the clotting cascade activates thrombin, which disrupts the blood–brain barrier and activates the complement cascade, leading to lysis of red blood cells and other bystander cells. Vasogenic edema and cytotoxic edema subsequently follow owing to the disruption of the blood–brain barrier, the failure of the sodium pump, and the death of neurons.24 This represents the intermediate edema seen at 24 hours to 5 days. This intermediate edema is noticeable radiographically and histologically. Red blood cell lysis releases hemoglobin and leads to formation of free radicals, which account for the late onset edema. The role of the coagulation cascade in intermediate perihematoma edema may explain why ICH related to thrombolysis or coagulopathy causes less perihematoma edema than spontaneous ICH.
Studies in animals and humans have refuted the notion that cerebral ischemia in areas of ICH occurs due to mechanical compression by the hematoma, and have suggested that secondary mediators may cause the delayed development of neuronal injury adjacent to a hematoma.23 It is currently thought that blood products mediate most secondary processes initiated after ICH.25 Recent evidence has suggested the presence of apoptosis or programmed cell death in neurons adjacent to ICH associated with nuclear factor-kB expression in neuronal nuclei.26 Other studies have suggested that heme derived from erythrocytes extravasated during ICH is degraded into bilirubin and bilirubin oxidation products, which activate microglia, which secrete cytokines that recruit leukocytes into the brain, which contribute to the injury process.27
Presentation by Location
Intracerebral hemorrhage into the cerebral white matter includes ICH into the occipital, temporal, frontal, and parietal lobes, including ICH arising from the cortex and subcortical white matter, as opposed to ICH of deep structures such as the basal ganglia, thalamus, and infratentorial structures. Frontal lobe ICH causes frontal headache with contralateral hemiparesis, usually in the arm with mild leg and facial weakness. Parietal lobe ICH causes contralateral hemisensory deficit with mild hemiparesis. Occipital lobe ICH causes ipsilateral eye pain and contralateral homonymous hemianopsia, with some sparing of the superior quadrant. Temporal lobe ICH can be asymptomatic on the nondominant side, but, on the dominant side, produces fluent dysphasia with poor auditory comprehension but relatively good repetition. Lobar ICH is more likely to be associated with structural abnormalities such as AVMs or tumors than deep hemorrhages. Lobar ICH is also more common in patients with alcohol consumption. In one study, significant independent risk factors for lobar ICH included the presence of an apolipoprotein E ε2 or ε4 allele, frequent alcohol use, prior stroke, and first-degree relative with ICH, while significant independent risk factors for nonlobar ICH were hypertension, prior stroke, and first-degree relative with ICH,28 suggesting different etiologies for lobar ICH than the other locations of ICH. Lobar ICH may also have a more benign outcome than basal ganglia and thalamic ICH.
In putaminal ICH, 62% of patients experience smooth gradual deterioration with only 30% exhibiting their maximal deficit at the onset. In some studies, the 30-day mortality rate has been 50%.29 The clinical presentation of putaminal hemorrhage may vary from relatively minor pure motor hemiparesis to profound weakness, sensory loss, eye deviation, hemianopsia, aphasia, and depressed level of consciousness. Headache is a presenting symptom in only 14% of putaminal ICHs. In putaminal ICH, intraventricular extension portends a poor prognosis, because the hematoma must be quite large to track through the internal capsule and reach the ventricle.
Patients with supratentorial ICH involving the putamen, caudate, or thalamus have contralateral sensory-motor deficits of varying severity due to involvement of the internal capsule. Higher-level cortical dysfunction, including neglect, gaze deviation, hemianopsia, and, for dominant hemisphere lesions, aphasia, can occur as a result of disruption of connecting fibers in the subcortical white matter and functional suppression of overlying cortex, known as diaschisis.30
Any deep, large hematoma can extend into the ventricles causing intraventricular hemorrhage (IVH). Common non-specific initial symptoms include headache and vomiting due to increased intracranial pressure and meningismus resulting from blood in the ventricles. As any hematoma becomes larger, patients will exhibit a decreased level of consciousness due to increased intracranial pressure and direct compression or distortion of the thalamic and brain-stem reticular activating system. Small, deep lesions can occasionally impair consciousness due to decreased central benzodiazepine receptor binding on cortical neurons.31
Cerebellar ICH can cause patients’ level of consciousness to progress from impaired to comatose due to direct compression of the brain stem, without any associated hemiparesis, unlike supratentorial ICH. Cerebellar ICH can present with the abrupt onset of vertigo, headache, vomiting, and inability to walk without any associated hemiparesis. Cranial nerve palsies are common, particularly an abducens palsy or a peripheral facial palsy. In one study, at least two of the three characteristic clinical signs—appendicular ataxia, ipsilateral gaze palsy, and peripheral facial palsy—were present in 73% of the cases of cerebellar ICH.32
Evaluation
Initial evaluation is typically through a CT scan, which is rapid and easily demonstrates ICH as high-density material within the brain parenchyma. Although mass effect on adjacent brain is common, the tendency for the hemorrhage to dissect through brain tissue often results in less mass effect than would be anticipated from the size of the clot. Clot volume can be estimated by computer programs that allow one to outline the hematoma on each slice and then model the hematoma in three dimensions and estimate a volume, or it can be approximated using the established practice of the modified ellipsoid volume, (A × B × C)/2, where A, B, and C are the diameters of the clot in each of three orthogonal dimensions, with one of the dimensions, C, being superoinferior such that C is equal to the number of slices with hematoma × slice thickness.33 Clot volume carries significant prognostic significance. One study demonstrated a much steeper dependence of mortality on clot volume for deep ICH than lobar ICH, consistent with the fact that deep areas of the brain are less able to accommodate large volumes. Hematomas were divided into small (≤30 cm3), medium (30–60 cm3), and large (≥60 cm3). The 30-day mortalities for small, medium, and large hematomas were 23%, 60%, and 71% for lobar ICH, compared to 7%, 64%, and 93% for deep ICH.34 The overall 30-day mortality was 39% for lobar ICH and 48% for deep ICH. Hematoma volume also correlates with risk of rehemorrhage, with one retrospective study showing that 39% of ICH patients who experienced rehemorrhage had initial clot volumes greater than or equal to 25 cm3, compared to only 23% of ICH patients who did not experience rehemorrhage.35
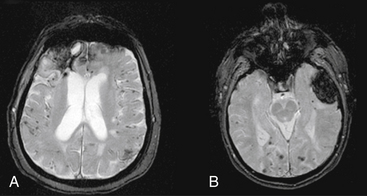
Magnetic resonance imaging is not the initial study of choice, as it is more time consuming, makes it difficult to access a patient who may acutely deteriorate, and does not show blood well within the first few hours. MRI may, however, may be useful later once a patient stabilizes to identify cerebral amyloid angiopathy, cavernous malformations, or underlying tumors. Gradient-echo MRI is the most useful modality for identifying ICH of various ages. Gradient-echo MRI increases the amount of signal dropout from deposits of iron representing residual blood products as a result of past hemorrhage. This increases the potential for detecting two findings that are typical of patients with cerebral amyloid angiopathy: (1) small prior punctuate petechial hemorrhages; and (2) previous lobar ICH, as manifested by a dark hemosiderin ring around an area of lobar encephalomalacia (Fig. 69-2). MRI has improved to the point that gradient echo can now detect ICH as early as 2.5 hours with 99.5% sensitivity.36 However, the specificity of MRI is limited in the hyperacute stage.37 Overall, MRI remains a secondary study compared to CT, and is most useful when a CT scan has findings listed above that suggest an underlying lesion such as a tumor or cavernous malformation. In these cases, MRI with gadolinium can be used to search for enhancing areas consistent with tumor; MR spectroscopy can be used to identify areas with high choline peaks, an inverted lactate peak, and absence of creatinine and N-acetyl-aspartate peaks; or T2 MRI can show a central mixed density core suggesting old hemorrhages surrounded by a hypodense rim.
Cerebral aniography is used to identify arteriovenous malformations (AVMs) and aneurysms in patients with ICH. In a prospective study in which 206 ICH cases were investigated with CT and angiography, anigoraphic yield was significantly higher in patients (1) at or below the age of 45, and (2) without preexisting hypertension.38 Analysis of angiographic yield of different sites of hemorrhage taken together with these two factors showed that (1) lobar ICH had a 10% angiographic yield in patients older than 45 with preexisting hypertension; (2) putaminal, thalamic, and posterior fossa hemorrhages in patients of all ages with preexisting hypertension had a 0% angiographic yield; (3) lobar ICH had a 65% angiographic yield in normotensive patients younger than 45; (4) putaminal, thalamic, and posterior fossa hemorrhages in normotensive patients younger than 45 had a 48% angiographic yield; and (5) putaminal, thalamic, and posterior fossa hemorrhages in normotensive patients older than 45 had a 7% angiographic yield. Isolated intraventricular hemorrhage (IVH) patients had 63% yield in the older and 67% yield in the younger groups. Taken together, these findings led the authors to recommend DSA in ICH patients except those older than 45 years who also have preexisting hypertension and thalamic, putaminal, or posterior fossa hemorrhages. More recently, the safer and more rapid technique of three-dimensional (3D) CTA has begun to replace DSA. CTA involves a head CT with thin 2.5-mm axial cuts occurring 5 seconds after administering a 45 mL contrast bolus at 7 cc/sec. Imaging software programs, which reformat the axial cuts and subtract out all but the contrast and adjacent brain tissue, are used to generate 3D images of the cerebral circulation. Preliminary studies have shown that CTA is 95% as sensitive and just as specific as DSA in the detection of cerebral aneurysms,39 a gap that is expected to close as CTA technology improves.
Acute Rehemorrhage
Although it was initially believed that ICH was largely a monophasic event that stopped quickly as a result of clotting and tamponade by the surrounding regions, a number of investigators have shown that rehemorrhage is common, with one study of 627 ICH patients showing a 14.0% rehemorrhage rate within 24 hours of admission.40 Brott et al. found that the hematoma expanded in 26% of ICH patients within 1 hour of the initial CT scan and in another 12% within 20 hours.41 Expansion has been attributed to continued bleeding from the primary source and to the mechanical disruption of surrounding vessels from compression by the hematoma. Acute hypertension after the initial ICH, a local coagulation deficit, or both may be associated with expansion of hematoma.35
Factors associated with rehemorrhage in the initial 24-hour period include: (1) a previous history of brain infarction, (2) liver disease, (3) uncontrolled diabetes, (4) systolic blood pressure on admission greater than 195, (5) a history of alcohol abuse, (6) coagulation abnormalities, (7) a hematoma larger than 25 cm3 on the initial CT scan, (8) irregular hematoma shape because irregularly shaped hematomas seem to indicate active bleeding from multiple sources, (9) a large peripheral white cell count, and (10) elevated body temperature on admission.40 CT angiography performed within 12 hours of the ICH revealing extravasation of contrast predicts rehemorrhage on a 24-hour post ICH CT scan with 60% specificity and 100% sensitivity.42
Systolic blood pressure control using an arterial line and intravenous drips of antihypertensives like nitroprusside remains the primary medical intervention designed to prevent rehemorrhage. Recommendations written by a panel of experts commissioned by the American Heart Association (AHA) in 1999 are to maintain the mean arterial blood pressure below 130 mm Hg (systolic BP < 180 and/or diastolic BP < 105) in patients with a history of chronic hypertension,43 but therapeutic trials are needed. In patients with elevated ICP documented by an ICP monitor, cerebral perfusion pressure should be kept above 70 mm Hg.
Medical Management
Whether ICH is best managed medically or with a combination of surgery and medicines remains a subject of considerable debate. Regardless of which therapy is chosen, the goal is improvement in function, an objective that is based on the concept of a penumbra around ICH. There is now mounting evidence that there is a penumbra of functionally impaired, but potentially reversible, neuronal injury surrounding a hematoma. Adjacent brain tissue is displaced and compressed by extravasated blood. Animal models have shown that compression causes edema, ischemia, and hemorrhagic necrosis at the margin of the clot.44 The volume of this penumbra may exceed the volume of the ICH several-fold. SPECT studies have confirmed that the penumbra tissue exhibits reversible ischemia.
Mannitol has been shown to improve mortality in ICH patients, primarily by acting as an osmotic agent that lowers intracranial pressure caused by the hematoma.45 Because of issues of mannitol failure and rebound increases in ICP, new osmolar agents have been investigated, including hypertonic saline, which can be given in 30-ml boluses of 23.4% saline as needed for refractory intracranial pressure documented via an ICP monitor. Such treatments can lower ICP, with the effect lasting 15 hours in our hands. Randomized studies are needed for these alternative osmolar agents.
External ventricular drains (EVDs) are placed for ICH cases with associated IVH that has led to an obstructive hydrocephalus. Unfortunately, in the presence of a large amount of IVH, the EVD will fill with blood, which will clot and occlude the EVD frequently. For these cases, we administer 5 mg of intraventricular tissue plasminogen activator (tPA) twice a day for 5 days, in a fashion similar to previous reports.46 After each administration, the EVD is clamped for 30 minutes if the intracranial pressure (ICP) transduced by the EVD does not elevate, in order to prevent the tPA from leaking out of the ventricles. We have experienced no complications from the use of intraventricular tPA in treating EVDs occluded with blood products, although it is not our policy to use this therapy in the presence of an unsecured vascular lesion.
Convulsive seizures occur in 5% to 10% of patients with supratentorial ICH.47 Because seizures increase intracranial pressure, prophylactic antiepileptic drugs (AEDs) have traditionally been given to patients with lobar ICH, the group of ICH patients with the largest seizure risk.43 However, seizures have been shown to not be associated with poor outcome after ICH47 and a recent retrospective analysis of 295 ICH patients found that AEDs were initiated on 8% of patients without documented seizure and that initiation of AEDs was robustly associated with poor outcome even after adjusting for other known predictors of outcome after ICH.48 The mechanism by which AEDs impact outcome is unclear, but it may be related to sedation or cardiovascular side effects of AEDs. A randomized trial may therefore be warranted to determine whether prophylactic AEDs are indicated for lobar ICH patients, or if AED use is only appropriate when ICH patients have had a seizure but not as a prophylactic measure.
Fever of 37.5°C or higher is seen in over 90% of supratentorial ICH patients.49 The incidence, duration, and magnitude of fever are even greater in patients with ventricular hemorrhage.49 The duration of fever is associated with poor long-term outcome.49 Therefore, we aggressively treat fever with acetaminophen and cooling blankets, targeting a temperature of ≤37.5°C.
Hyperglycemia in nondiabetic patients and worsening of baseline blood sugars in diabetic patients both occur after ICH.50 Regardless of whether there is pre-existing diabetes, hyperglycemia is a predictor of poor outcome after supratentorial ICH.50 We utilize intravenous insulin infusions titrated to maintain blood glucose between 80 and 110 mg/dl, rather than treating high blood glucose levels with subcutaneous insulin based on a sliding scale. Although this type of strict blood sugar control has been shown to reduce mortality, particularly due to septic end organ failure, in surgical intensive care units,51 a study focusing on the outcomes associated with strict blood sugar control in neurosurgical patients is still needed.
In patients with normal coagulation, the most feasible hemostatic agents for ultraearly ICH therapy include the antifibrinolytic amino acids aminocaproic acid and tranexamic acid, aprotinin, and activated recombinant factor VII. The amino acids have a risk of cerebral ischemia, while aprotinin can cause hypersensitivity reactions and arterial or venous thrombosis, so attention in ICH patients has focused on factor VII. Factor VII, of which only 1% circulates in the active form, forms a complex with exposed tissue factor in the subendothelial layer of a damaged vessel wall, activating the hemostatic mechanism locally to form a hemostatic plug. In the randomized, phase-III fVIIa for Acute Hemorrhagic Stroke Treatment (FAST) trial, 841 ICH patients were randomized to receive placebo or intravenous recombinant activated factor VII administered within 4 hours of the onset of symptoms. Recombinant, activated factor VII reduced the growth in ICH volume at 24 hours from 26% in the placebo group to 11% in the group receiving the higher of two factor VII doses studied, but there was no significant difference among the groups in mortality or in the proportion of patients with poor clinical outcomes.52
There has been interest in neuroprotectant medications to protect the at-risk penumbra surrounding the ICH. A randomized phase III clinical trial of NXY-059, a free radical–trapping neuroprotectant, studied 607 patients and found that, while NXY-059 was associated with slightly less hematoma growth than placebo, the drug had no effect on long-term mortality or disability.53 Neuroprotectants that have shown promise in the laboratory whose benefit remains to be proven clinically include the GABA antagonist muscimol and the NMDA receptor antagonists MK801 and D-(E)-4-(3-phodphonoprop-2-enyl)-piperazine-2-carboxylic acid (D-CPP-ene), which have been shown to increase tolerance of larger hematomas, reduce edema 24 hours after ICH, and protect adjacent white matter in animal models.54
There are other medical agents that some have suggested for ICH but have not proven to be beneficial. A randomized study following 93 patients with ICH showed no statistical difference in neurologic outcome in patients who received decadron, with the group receiving decadron exhibiting 11 times more complications than the control group, including hyperglycemia, septicemia, and gastrointestinal bleeding.55
Medical versus Surgical Management
There have been 10 randomized prospective clinical trials comparing medical versus surgical management of ICH. The number of patients enrolled in these trials has ranged from 20 to 1033. The results are summarized in Table 69-1. Three trials showed somewhat better outcomes with medical treatment, with none achieving statistical significance in long-term outcomes, while seven trials showed somewhat better outcomes with surgical treatment, with two achieving statistical significance in long-term outcomes (Table 69-1). The largest trial by far was the International Surgical Trial in Intracerebral Hemorrhage (STICH), the results of which were released in January 2005.56 The STICH trial showed that surgical intervention within 24 hours of randomization offered no benefit in survival or prognosis-based indices for patients with lobar, basal ganglia, or thalamic ICH measuring greater than 2 cm in diameter and a Glasgow Coma Score (GCS) of 5 or more.56 Analysis of subgroups defined by age, GCS, ICH laterality, ICH location, ICH volume, distance from cortical surface, method of evacuation, motor and speech deficits, anticoagulation treatment, and country found that the only subgroups to show heterogeneity of outcome were those defined by distance of the ICH from the cortical surface, with a favorable outcome from early surgery more likely if the hematoma was 1 cm or less from the cortical surface (absolute benefit 8%; p = 0.02).56 Of note, of patients randomized to initial conservative treatment, 26% went on to require surgery a few days after randomization due to clinical deterioration, and the outcome for this group was poorer than for patients who had early surgery or who were maintained on conservative management.56 Thus the STICH trial suggests that a significant benefit for early operative intervention existed only for patients with superficial hematomas. These findings will be confirmed in the recently commenced STICH II trial, which will prospectively test the benefits of surgery in lobar ICH patients with hematomas extending to within 1 cm of the cortical surface without intraventricular extension. For deeper ICH, until factors are identified that can predict which patients will go on to experience clinical deterioration, the results of the STICH trial suggest that operative intervention should be reserved for patients who experience clinical deterioration, at which time surgery will improve outcomes relative to not operating but will unfortunately not restore outcomes to what they would have been had the deterioration not occurred.
Table 69-1 Summary of Prospective Randomized Controlled Trials Comparing Surgery to Medical Treatment for Intracerebral Hemorrhage
Overall, considerable variability remains in ICH management among physicians worldwide. Recently reported operation rates for spontaneous supratentorial ICH spanned a wide range, including 2% in Hungary, 20% in the United States, 50% in Germany and Japan, and 74% in Lithuania.57
The basal ganglia is the most common site of ICH, representing 60% of hypertensive ICH, and basal ganglia ICH is associated with a 50% mortality rate.29 The majority of these basal ganglia hemorrhages occur in the putamen. In an early study by Kanaya and Kuuoda, patients who received medical treatment for basal ganglia hemorrhages less than 30 ml in volume fared better than those treated surgically.58 A follow-up study by Tan et al. randomized 34 patients with basal ganglia hematomas greater in volume than 30 ml into surgical and nonsurgical treatment groups. No difference in outcome was reported at 1-year follow-up.59 Although stereotactic and endoscopic techniques minimize surgically morbidity from basal ganglia hematoma evacuation, Auer et al. failed to demonstrate any benefit from stereotactically guided endoscopic basal ganglia hematoma evacuation.60 While recent studies61–63 have shown that stereotactic aspiration of basal ganglia hematomas can aspirate over 80% of the hematoma, this volume reduction has not proven to be of benefit, perhaps due to the ability of residual blood products to trigger persistent and consequential perihematoma edema.
In the end, complete surgical clot evacuation through open craniotomy may prove beneficial if the proper patient group is selected and the surgery is performed by a group experienced in the surgical approach to the basal ganglia. A study by Kaya et al. 2 years after the study by Tan et al. compared conservative medical treatment to open craniotomy for putaminal hematomas greater than 30 mL volume.61 The surgical group had 34% mortality at 6 months, compared to 63.1% in the medically treated group. When subdivided by initial neurologic grade, patients in stupor or semicoma without herniation signs fared better with nonsurgical treatment, while patients in semicoma with herniation signs fared better with surgical treatment. The more favorable results for surgery obtained by Kaya et al. compared to Tan et al. may reflect improved ability to evacuate basal ganglia hematomas safely through open craniotomies during the 2 years that transpired between the studies. Figure 69-3 illustrates results when we used an algorithm similar to that followed by Kaya et al. in the management of two different basal ganglia hematomas.
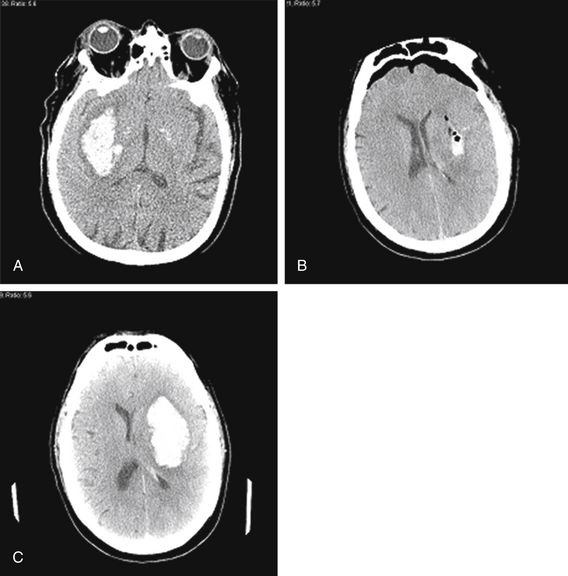
FIGURE 69-3 Management of hypertensive basal ganglia hemorrhages based on size of clot and other radiographic and clinical findings. Kaya et al.61 suggest that patients with basal ganglia hematomas larger than 30 cm3 who exhibit diminished mental status and radiographic signs of herniation fare better with surgical rather than medical treatment. A and B, Sixty-two-year-old male with a history of hypertension presented with right hemiparesis and aphasia. CT scan (A) identified a left basal ganglia hemorrhage measuring 6 cm (AP) × 3 cm (LR) × 8 cuts (4 cm SI) = 144 cm3, causing 9-mm midline shift, and effacement of the cisterns. We went to the operating room and evacuated the hematoma through a left frontal craniotomy, eliminating much of the midline shift and herniation (B), consistent with recommendations of Kaya et al.61 Unfortunately, the patient succumbed to an aspiration pneumonia 45 days after his surgery. (C) Sixty-nine-year-old male with a history of hypertension presented with left arm weakness but intact mental status. CT scan (C) identified a right basal ganglia hemorrhage measuring 5 cm (AP) × 2 cm (LR) × 7 cuts (3.5 cm SI) = 35 cm3 causing 2-mm midline shift with no cisternal effacement. He was managed conservatively, consistent with recommendations of Kaya et al.,61 and remained awake and conversant with stable left arm weakness.
Thalamic ICH is almost always managed medically with external ventricular drain placement when third ventricular outlet obstruction is present. Anatomically, the thalamus is difficult to access safely. There is a high risk of causing neurologic deficits from the parietal lobe, internal capsule, or transcallosal transventricular dissection when approaching thalamic hematomas. Regardless of whether the approach is open craniotomy, stereotactic, or endoscopic, the risk of damaging adjacent functional thalamic tissue often precludes a surgical approach. The only study comparing surgical versus medical treatment for thalamic ICH showed no benefit to endoscopic evacuation compared to medical treatment.60
Most patients with pontine hematoma are managed conservatively due to the difficulty in achieving safe surgical access to the brain stem and the morbidity associated with the brain-stem manipulation that is required for hematoma evacuation. The mortality from pontine ICH is estimated to be around 18% during hospitalization64 and 69% at 1 year.65 Most mortality is associated with hypertensive pontine ICH and large paramedian pontine ICH, with pontine ICH from cavernous malformation and lateral tegmental pontine ICH having a better outcome.64,65 Uncontrolled case series have documented successful stereotactic aspiration of pontine hematomas, but the effect on outcomes remains uncertain.
Cerebellar ICH is unique in that the posterior fossa is unable to tolerate large changes in volume without causing significant brain-stem compression, which can lead to rapid and often fatal deterioration. A second source of morbidity and mortality in these patients is hydrocephalus when the hematoma compresses the fourth ventricle. Although the use of external ventricular drainage would seem a reasonable, less invasive means of addressing the hydrocephalus, there is concern about upward cerebellar herniation and rostral brain-stem compression if surgical decompression of the posterior fossa is not performed simultaneously. Cohen et al. studied 37 patients with cerebellar ICH and found that patients with hematomas less than 3 cm in maximal diameter had 100% good outcomes, compared to 57% of these patients who underwent surgery.66 On the other hand, patients with hematomas larger than 3 cm in maximal diameter who were not operated on immediately had a good outcome in only 33% of cases, compared to a good outcome in 50% of surgical patients with hematomas of this size.66 Other studies have confirmed 3 cm in diameter as the recommended threshold for surgical intervention for cerebellar hematomas (Fig. 69-4), with an external ventricular drain placed if hydrocephalus is present.
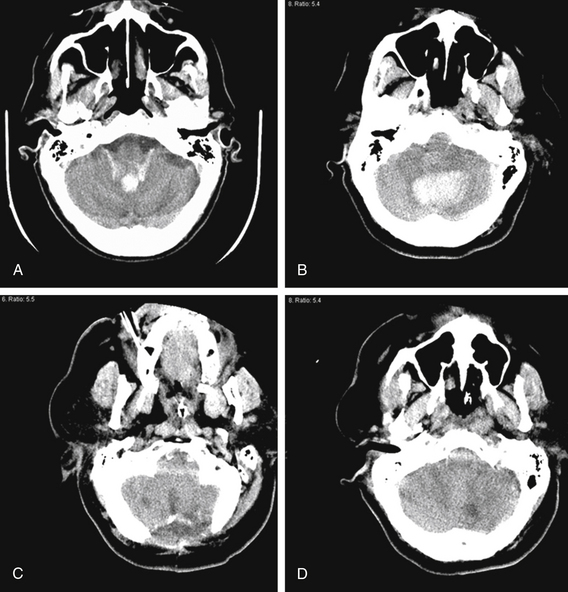
FIGURE 69-4 Management of cerebellar ICH depends on clinical status of patient and size of blood clot. A, A 75-year-old man with a history of hypertension presented with headache, nausea, vomiting, and syncope. Head CT identified a 1.7 cm (AP) × 1.5 cm (LR) × 2 cuts (1 cm SI) vermian cerebellar hemorrhage extending into the fourth ventricle, causing moderate hydrocephalus. Because of his intact mental status and because the hematoma was less than 3 cm in maximal diameter, he was managed medically with an admission to the intensive care unit and an external ventricular drain placed for the hydrocephalus. The hydrocephalus subsequently resolved, the drain was discontinued, and he was discharged from the hospital 10 days later. B to D, A 65-year-old woman with a history of hypertension collapsed. She was unresponsive to painful stimuli with intact brain-stem reflexes in the emergency room. Head CT (B) identified a 4.6 cm (AP) × 3.1 cm (LR) × 6 cuts (3 cm SI) cerebellar hematoma centered in the dentate nucleus, but favoring the left over the right cerebellar hemisphere, causing moderate hydrocephalus. Because of her clinical condition, she was brought emergently to the operating room, where a suboccipital craniectomy (C) and cerebellar hematoma evacuation (D) were performed. Unfortunately, she did not regain neurologic function and care was withdrawn by her family 1 week later. These two cases illustrate the finding documented before that 3 cm in maximal diameter is the cutoff after which cerebellar hematomas are likely to impair mental status and will likely require immediate surgery in order to have any chance for clinical improvement.66
Regarding the timing of surgery, Morgenstern et al. demonstrated improved neurologic function regardless of hematoma location if ICH patients were operated on within 12 hours of the onset of symptoms.67 In a follow-up study evaluating ultraearly surgery at less than 4 hours after the onset of symptoms, they found an increased rehemorrhage rate associated with increased mortality, with rebleeding occurring in 40% of the patients operated on within 4 hours compared to 12% of the patients operated on at 12 hours.68 These results suggest that the optimum timing for surgical clot evacuation may be within the 4- to 12-hour window after symptoms begin.
As a result of the paucity of data, guidelines for ICH management written in 1999 by a panel of experts commissioned by the American Heart Association (AHA) were largely limited to grade C recommendations based on case series and nonrandomized cohort studies.43 The only grade A or B recommendations (based on randomized trials) were to use head CT for ICH diagnosis; to avoid corticosteroid therapy for ICH; to avoid surgical evacuation in patients with a Glascow Coma Scale of 4 or less, or ICH volume below 10 cm3; and to consider surgical evacuation in young patients with moderate or large lobar hemorrhages who are clinically deteriorating. Surgical evacuation was recommended for the following three categories of patients: (1) patients with cerebellar hemorrhages greater than 3 cm in diameter who have brain-stem compression and hydrocephalus or who are neurologically deteriorating (grade C recommendation); (2) ICH associated with a structural lesion such as an AVM or tumor that is surgically accessible (grade C recommendation); and (3) young patients with moderate or large lobar ICH who are clinically deteriorating (grade B recommendation). More recently, in 2006 and 2007, the American Stroke Association (ASA) Stroke Council69 and the European Stroke Organization (EUSI)70 released guidelines in which they did not recommend routine evacuation of supratentorial ICH by craniotomy within 96 hours of ictus. Both guidelines recommend surgery for patients presenting with lobar ICH within 1 cm of the surface, particularly for those with good neurologic status who are deteriorating clinically. Guidelines acknowledge that operative removal within 12 hours, particularly with minimally invasive methods, has the most evidence for beneficial effect and could be considered for deep ICH causing mass effect. Guidelines also noted that very early craniotomy might be associated with an increased risk of recurrent ICH. The degree of neurologic deterioration must also be considered before recommending surgery, as one study of lobar ICH evacuated in patients with a mental status of stupor or worse or midline shift greater than 1 cm with cisternal obliteration showed that 22% of operative patients regained independence afterward, 22% remained severely disabled, and 56% died.71 All patients who had absent papillary, corneal, or oculocephalic reflexes combined with extensor posturing died, indicating a level of severity beyond salvage surgically.
Specific Surgical Techniques
Craniotomy
Lobar hemorrhages are evacuated using craniotomies and corticectomies centered over the hematoma, with sparing of eloquent tissue. The head should be positioned so that the trajectory to the clot is as vertical as possible. Self-retaining brain retractors are attached to the Mayfield fixation device. After the corticectomy, superficial hematomas can be accessed by using a combination of bipolar cautery and small suction tips until the hematoma cavity is entered. Once in the hematoma, tumor forceps or a pituitary biter can be used to remove solid portions of the hematoma. Semisolid portions of the hematoma can be removed using suction tips. Hemostasis can then be achieved with a combination of topical hemostatic agents such as Avitene (microfibrillar collagen, Alcon, Humacao, PR), FloSeal (gelatin matrix thrombin sealant, Baxter Healthcare, Deerfield, IL), hydrogen peroxide–soaked cotton balls, thrombin-soaked Gelfoam (gelatin sponge, Baxter), and Surgicel (oxidized cellulose, Ethicon, Somerville, NJ). The systolic blood pressure can be raised 10 to 20 points before closure to identify any potential bleeding sources. The mass effect is typically relieved after hematoma removal, and the bone flap can be replaced. For deeper lobar hematomas, intraoperative ultrasound can be used for hematoma localization and to verify complete removal of the hematoma. Ultrasound can also be used to guide placement of a ventriculostomy catheter into the hematoma cavity. Self-retaining retractors can then be placed alongside the ventriculostomy and a 1-cm corticectomy can be opened down to the clot by following the ventriculostomy catheter, thereby minimizing damage to nearby cortex.
Thalamic and pontine hemorrhages are not evacuated with open craniotomies, due to the amount of intact cerebral tissue put at risk during such a procedure. The stereotactic or endoscopic procedures described below have been somewhat successful in evacuating pontine hemorrhages, but a benefit has yet to be shown in a large randomized trial.
Stereotactic Aspiration
Benes et al. first reported the use of stereotactic hematoma drainage in 1965, with limited success.72 With improvement in techniques, including administration of fibrinolytics, the success rate has improved. Although no randomized, prospective, controlled studies have compared stereotactic aspiration with craniotomy and conservative therapy, studies show favorable outcomes, especially with deep-seated lesions such as those in the basal ganglia or pons. Use of a stereotactic frame or the endoscopy and frameless stereotaxy approaches described below are less invasive procedures than open craniotomies, but still require that the patient be intubated if not already so, because ICH patients often have too tenuous a mental status to tolerate the sedation and lack of airway access associated with awake stereotactic procedures. Honda et al. retrospectively compared stereotactic aspiration and medical therapy for thalamic hemorrhages.73 Patients with hematomas smaller than 2.5 cm in diameter had significantly higher activities of daily living (ADL) scores with aspiration. In another study, 71 ICH patients were randomized into a group that received stereotactic aspiration and administration of urokinase and another group that received systemic medical management only.63 Although stereotactic aspiration reduced hematoma volume by 18 ml over 7 days, compared to 7 ml of reduction in the control group, no difference in morbidity or mortality was noted at 180 days.63 The volume reduction achieved by stereotactic aspiration may be less beneficial than the more complete hematoma evacuation achieved by a craniotomy because residual hematoma can still cause considerable edema and mass effect. To make hematomas more amenable to stereotactic aspiration, various techniques have been incorporated into the stereotactic hematoma drainage, including (1) repeated injection of urokinase or recombinant tissue plasminogen activator into the hematoma to liquefy the clot and render it amenable to subsequent aspiration, and (2) equipment aimed at physical fragmentation of the clot like systems based on the Archimedes screw,74 devices using high-pressure fluid irrigation,75 ultrasonic aspirators,76 or the Nucleotome probe.77 The lack of direct visualization and the risk of rebleeding may still limit this technique to patients whose neurologic condition warrants drainage rather than medical treatment alone but whose hemorrhages are deep and whose overall medical condition precludes open craniotomy, especially during the hyperacute phase of hemorrhage. The stereotactic treatment of intracerebral hematoma by means of a plasminogen activator (SICHPA) multicenter randomized trial was published in 2003 and showed a slight but statistically insignificant benefit on survival and independence of stereotactic hematoma removal after liquefaction by means of plasminogen activator.63 These encouraging results have led to the ongoing minimally invasive surgery plus tissue plasminogen activator for intracerebral hemorrhage evacuation (MISTIE) trial, which will study different doses of recombinant tissue plasminogen activator to better refine the methodology and will hopefully enroll enough patients to achieve a result with statistical significance.78
Endoscopy
Endoscopy represents another minimally invasive means of ICH draining, which, unlike stereotaxy, is not limited to the chronic stage of ICH. In a recent technical note, four pontine hematomas were removed using a 1.7-mm fiberscope placed through a burr hole 3 cm from the midline at the bregma.79 After entering the third ventricle through the foramen of Monro, patients with acute hydrocephalus were treated by forming a third ventriculostomy, followed by slight dilation of the aqueduct of Sylvius. The guide tube was advanced into pontine areas found to have yellow discoloration. Hematoma was evacuated by repeated rinsing with physiologic saline. Hemostasis was achieved using a KTP laser. An external ventricular drain was left in place, and flushed with 6000 U of urokinase 3 hours after surgery. The urokinase must be administered because only liquid blood products could be washed out of the pons using the endoscope, and areas of clot had to be treated with intraventricular urokinase. The procedure typically took 1 hour. Advantages of endoscopic treatment over stereotaxy and open craniotomy were rapidity of the procedure and ability to treat associated hydrocephalus. Ability to anticoagulate is an additional advantage over stereotaxy.
Frameless Stereotaxy and Intraoperative MRI
Tyler and Mandbybur reported on 10 patients harboring intracerebral hematomas who were treated by frameless stereotactic means without fiducial markers.80 Using an intraoperative MRI scanner, these patients underwent frameless stereotactic evacuation of 70% to 90% of each clot, with no complications or rehemorrhages. All patients showed some improvement. Similarly, Bernays et al. reported complete evacuation in 62% of their 13 ICH patients treated with a specially designed artifact-free aspiration cannula using intraoperative MRI.81 No rebleeding was demonstrated and neurologic function improved in 11 of 12 patients.
Long-Term Outcome
Recently, Becker et al. pointed out that the most important variable predicting poor outcome in ICH patients is the level of provided medical support. Perception of futility of aggressive therapy leads to early withdrawal of medical support, which is less likely in ICH patients who are surgically treated.82 It may be worth studying if ICH patients have better outcomes in high volume centers that frequently treat ICH patients medically and surgically, a phenomenon demonstrated in the operative management of unruptured aneurysms.83
Reported 30-day mortality for lobar, basal ganglia, thalamic, pontine, and cerebellar ICH have been 13%, 50%, 23%, 13%, and 16%, respectively.84,85 Lobar ICH has consistently been shown to have the lowest mortality and best long-term outcomes, and basal ganglia ICH the worst. One of the sources of debate in long-term post-ICH management remains when it is safe to resume anticoagulation. In one study, epidemiologic data from the medical literature was used to generate a Markov-state transition decision model, in which effectiveness of therapy was measured in quality-adjusted life-years.86 The authors found that, for patients with prior lobar ICH, withholding anticoagulation therapy indefinitely led to the best outcome regardless of the indication for anticoagulation, leading to an improvement of life expectancy by 1.9 quality-adjusted life-years. On the other hand, patients with deep interhemispheric ICH, because of a lower risk of recurrent ICH due to the lack of amyloid angiopathy in this group, should never receive anticoagulation for nonvalvular atrial fibrillation, but should resume anticoagulation with aspirin when the risk of thromboembolic event is moderately higher and with coumadin when the risk of thromboembolic events is in the highest range. Both the ASA Stroke Council69 and the EUSI guidelines70 recommend that warfarin anticoagulation can be resumed in patients with a high risk of thromboembolism (artificial heart valve) 7 to 14 days after the onset of ICH.87
The overall long-term recurrence rate of ICH has been estimated to be 2.4% per year, and is 3.8-fold higher after lobar ICH caused by cerebral amyloid angiopathy (Fig. 69-5) than it is with hypertensive deep ICH.88 Factors that are positive predictors of recurrent hemorrhage include age greater than 65 years and male sex.89 Use of anticoagulation after ICH triples the risk of recurrent hemorrhage.89
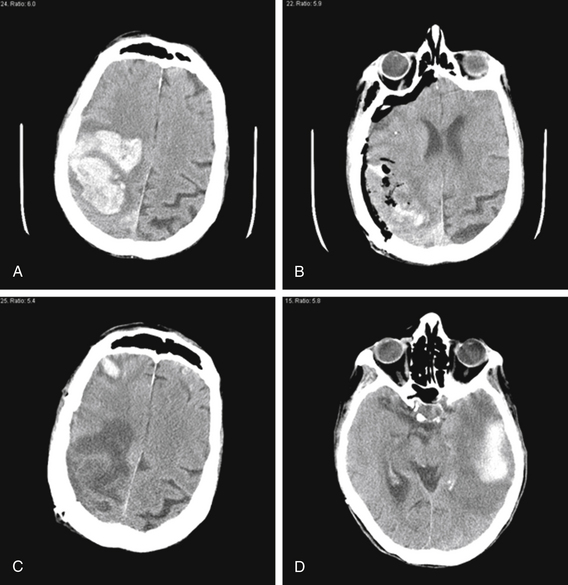
FIGURE 69-5 Amyloid angiopathy causes high risk of recurrent ICH. A 77-year-old woman presented to our institution with left hemiparesis. CT scan (A) showed 7 cm (AP) × 6 cm (LR) × 11 cuts (5.5 cm SI) = 231 cm3 right frontoparietal hematoma with surrounding edema, causing 8 mm of midline shift. She was brought to the operating room, where a right frontoparietal craniotomy enabled nearly complete hematoma evacuation and resolution of midline shift (B). Pathologic analysis of the clot confirmed the diagnosis of amyloid angiopathy. She was discharged from the hospital with some improvement in left-sided strength. Unfortunately, 1 month later, she was found unresponsive. CT scan showed encephalomalacia in the area of the evacuated right frontoparietal hematoma and two new hematomas—a 1.8 cm (AP) × 0.7 cm (LR) × 3 cuts (1.5 cm SI) = 2 cm3 right frontal hematoma (C) and a 4.9 cm (AP) × 1.9 cm (LR) × 7 cuts (3.5 cm SI) = 33 cm3 left temporal hematoma (D). She was managed medically because her history of prior ICH and the bilateral nature of her most recent ICH meant that surgery would have a high morbidity. She remained unresponsive, and was eventually discharged to a nursing home after a tracheostomy and gastrotomy tube were placed.
Conclusion
Clinical evidence suggests the importance of achieving three goals in managing ICH patients: stop further bleeding, remove hematoma when appropriate, and controlling cerebral perfusion pressure. While several agents have been shown to slightly reduce hematoma expansion, none of these have shown clinical benefit. And the STICH trial raised questions about the role of early operative intervention in ICH patients. Because the outcome after ICH is still extremely poor, ongoing trials will be needed to identify agents capable of exerting a more powerful effect on preventing further bleeding or protecting the at-risk penumbra in a manner that leads to improved outcomes. Another key area of research will be determining which subset of ICH patients benefit from early operative intervention, an issue that will be addressed for lobar ICH patients during the upcoming STICH II trial.
Cheng X.C., Wu J.S., Zhou X.P., et al. The randomized multicentric prospective controlled trial in the standardized treatment of hypertensive intracerebral hematomas: the comparison of surgical therapeutic outcomes with conservative therapy. Chin J Clin Neurosci. 2001;9:365-368.
Hattori N., Katayama Y., Maya Y., Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomized study. J Neurosurg. 2004;101:417-420.
Kaya R.A., Turkmenoglu O., Ziyal I.M., et al. The effects on prognosis of surgical treatment of hypertensive putaminal hematomas through transsylvian transinsular approach. Surg Neurol. 2003;59:176-183. discussion 183
Mayer S.A., Brun N.C., Begtrup K., et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127-2137.
Mendelow A.D., Gregson B.A., Fernandes H.M., et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387-397.
Morgan T., Zuccarello M., Narayan R., et al. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008;105:147-151.
Morgenstern L.B., Frankowski R.F., Shedden P., et al. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359-1363.
Salvati M., Cervoni L., Raco A., Delfini R. Spontaneous cerebellar hemorrhage: clinical remarks on 50 cases. Surg Neurol. 2001;55:156-161. discussion 161
Tan S.H., Ng P.Y., Yeo T.T., et al. Hypertensive basal ganglia hemorrhage: a prospective study comparing surgical and nonsurgical management. Surg Neurol. 2001;56:287-292. discussion 292-293
Teernstra O.P., Evers S.M., Lodder J., et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke. 2003;34:968-974.
Zuccarello M., Brott T., Derex L., et al. Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. 1999;30:1833-1839.
1. Dennis M.S., Burn J.P., Sandercock P.A., et al. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796-800.
2. Zia E., Engstrom G., Svensson P.J., et al. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40:3567-3573.
3. Ariesen M.J., Claus S.P., Rinkel G.J., Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060-2065.
4. Giroud M., Gras P., Chadan N., et al. Cerebral haemorrhage in a French prospective population study. J Neurol Neurosurg Psychiatry. 1991;54:595-598.
5. Qureshi A.I., Giles W.H., Croft J.B. Racial differences in the incidence of intracerebral hemorrhage: effects of blood pressure and education. Neurology. 1999;52:1617-1621.
6. Suh I., Jee S.H., Kim H.C., et al. Low serum cholesterol and haemorrhagic stroke in men: Korea Medical Insurance Corporation Study. Lancet. 2001;357:922-925.
7. Iribarren C., Jacobs D.R., Sadler M., et al. Low total serum cholesterol and intracerebral hemorrhagic stroke: is the association confined to elderly men? The Kaiser Permanente Medical Care Program. Stroke. 1996;27:1993-1998.
8. Leppala J.M., Virtamo J., Fogelholm R., et al. Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke. 1999;30:2535-2540.
9. Inagawa T. Diurnal and seasonal variations in the onset of primary intracerebral hemorrhage in individuals living in Izumo City. Japan. J Neurosurg. 2003;98:326-336.
10. Juvela S., Hillbom M., Palomaki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke. 1995;26:1558-1564.
11. Monforte R., Estruch R., Graus F., et al. High ethanol consumption as risk factor for intracerebral hemorrhage in young and middle-aged people. Stroke. 1990;21:1529-1532.
12. Golfetto I., Min Y., Wang Y., et al. Serum cholesterol and haemorrhagic stroke. Lancet. 2001;358:508. author reply 508
13. Eichel R., Khoury S.T., Ben-Hur T., et al. Prior use of statins and outcome in patients with intracerebral haemorrhage. Eur J Neurol. 2010;17:78-83.
14. Furlan A.J., Whisnant J.P., Elveback L.R. The decreasing incidence of primary intracerebral hemorrhage: a population study. Ann Neurol. 1979;5:367-373.
15. Fisher C.M. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536-550.
16. O’Donnell H.C., Rosand J., Knudsen K.A., et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240-245.
17. Greenberg S.M., Rebeck G.W., Vonsattel J.P., et al. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254-259.
18. McCarron M.O., Weir C.J., Muir K.W., et al. Effect of apolipoprotein E genotype on in-hospital mortality following intracerebral haemorrhage. Acta Neurol Scand. 2003;107:106-109.
19. Weisberg L.A. Hemorrhagic primary intracranial neoplasms: clinical-computed tomographic correlations. Comput Radiol. 1986;10:131-136.
20. Wintzen A.R., de Jonge H., Loeliger E.A., Bots G.T. The risk of intracerebral hemorrhage during oral anticoagulant treatment: a population study. Ann Neurol. 1984;16:553-558.
21. Franke C.L., de Jonge J., van Swieten J.C., et al. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21:726-730.
22. Yasaka M., Minematsu K., Naritomi H., et al. Predisposing factors for enlargement of intracerebral hemorrhage in patients treated with warfarin. Thromb Haemost. 2003;89:278-283.
23. Zazulia A.R., Diringer M.N., Derdeyn C.P., Powers W.J. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167-1173.
24. Wagner K.R., Xi G., Hua Y., et al. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88:1058-1065.
25. Lee K.R., Kawai N., Kim S., et al. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood–brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272-278.
26. Hickenbottom S.L., Grotta J.C., Strong R., et al. Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke. 1999;30:2472-2477. discussion 2477-2478
27. Loftspring M.C., Hansen C., Clark J.F. A novel brain injury mechanism after intracerebral hemorrhage: the interaction between heme products and the immune system. Med Hypotheses. 2010;74(1):63-66.
28. Woo D., Sauerbeck L.R., Kissela B.M., et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190-1195.
29. Little K.M., Alexander M.J. Medical versus surgical therapy for spontaneous intracranial hemorrhage. Neurosurg Clin North Am. 2002;13:339-347.
30. Tanaka A., Yoshinaga S., Nakayama Y., et al. Cerebral blood flow and clinical outcome in patients with thalamic hemorrhages: a comparison with putaminal hemorrhages. J Neurol Sci. 1996;144:191-197.
31. Hatazawa J., Shimosegawa E., Satoh T., et al. Central benzodiazepine receptor distribution after subcortical hemorrhage evaluated by means of [123I]iomazenil and SPECT. Stroke. 1995;26:2267-2271.
32. Ott K.H., Kase C.S., Ojemann R.G., Mohr J.P. Cerebellar hemorrhage: diagnosis and treatment. A review of 56 cases. Arch Neurol. 1974;31:160-167.
33. Kothari R.U., Brott T., Broderick J.P., et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304-1305.
34. Broderick J.P., Brott T.G., Duldner J.E., et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987-993.
35. Kazui S., Minematsu K., Yamamoto H., et al. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370-2375.
36. Schellinger P.D., Jansen O., Fiebach J.B., et al. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke. 1999;30:765-768.
37. El-Koussy M., Guzman R., Bassetti C., et al. CT and MRI in acute hemorrhagic stroke. Cerebrovasc Dis. 2000;10:480-482.
38. Zhu X.L., Chan M.S., Poon W.S. Spontaneous intracranial hemorrhage: which patients need diagnostic cerebral angiography? A prospective study of 206 cases and review of the literature. Stroke. 1997;28:1406-1409.
39. Dehdashti A.R., Rufenacht D.A., Delavelle J., et al. Therapeutic decision and management of aneurysmal subarachnoid haemorrhage based on computed tomographic angiography. Br J Neurosurg. 2003;17:46-53.
40. Fujii Y., Takeuchi S., Sasaki O., et al. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160-1166.
41. Brott T., Broderick J., Kothari R., et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1-5.
42. Murai Y., Takagi R., Ikeda Y., et al. Three-dimensional computerized tomography angiography in patients with hyperacute intracerebral hemorrhage. J Neurosurg. 1999;91:424-431.
43. Broderick J.P., Adams H.P.Jr., Barsan W., et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905-915.
44. Bullock R., Mendelow A.D., Teasdale G.M., Graham D.I. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: Description of technique, ICP changes and neuropathological findings. Neurol Res. 1984;6:184-188.
45. Duff T.A., Ayeni S., Levin A.B., Javid M. Nonsurgical management of spontaneous intracerebral hematoma. Neurosurgery. 1981;9:387-393.
46. Goh K.Y., Poon W.S. Recombinant tissue plasminogen activator for the treatment of spontaneous adult intraventricular hemorrhage. Surg Neurol. 1998;50:526-531. discussion 531-532
47. Passero S., Rocchi R., Rossi S., et al. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175-1180.
48. Messe S.R., Sansing L.H., Cucchiara B.L., et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care. 2009;11:38-44.
49. Schwarz S., Hafner K., Aschoff A., Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354-361.
50. Passero S., Ciacci G., Ulivelli M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology. 2003;61:1351-1356.
51. Van den Berghe G., Wilmer A., Milants I., et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55:3151-3159.
52. Mayer S.A., Brun N.C., Begtrup K., et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127-2137.
53. Lyden P.D., Shuaib A., Lees K.R., et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke. 2007;38:2262-2269.
54. Mendelow A.D. Mechanisms of ischemic brain damage with intracerebral hemorrhage. Stroke. 1993;24:I115-I117. discussion I118-I119
55. Poungvarin N., Bhoopat W., Viriyavejakul A., et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316:1229-1233.
56. Mendelow A.D., Gregson B.A., Fernandes H.M., et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387-397.
57. Mendelow A.D. International surgical trial in intracerebral hemorrhage: 668 patients randomized. J Neurosurg. 2002;96:186A-187A.
58. Kanaya H., Kuuoda K. Development in neurosurgical approaches to hypertensive intracerebral hemorrhage in Japan. In: Kaufman H.H., editor. Intracerebral Hematomas. New York: Raven Press; 1992:197-210.
59. Tan S.H., Ng P.Y., Yeo T.T., et al. Hypertensive basal ganglia hemorrhage: a prospective study comparing surgical and nonsurgical management. Surg Neurol. 2001;56:287-292. discussion 292-293
60. Auer L.M., Deinsberger W., Niederkorn K., et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70:530-535.
61. Kaya R.A., Turkmenoglu O., Ziyal I.M., et al. The effects on prognosis of surgical treatment of hypertensive putaminal hematomas through transsylvian transinsular approach. Surg Neurol. 2003;59:176-183. discussion 183
62. Marquardt G., Wolff R., Seifert V. Multiple target aspiration technique for subacute stereotactic aspiration of hematomas within the basal ganglia. Surg Neurol. 2003;60:8-13. discussion 13-14
63. Teernstra O.P., Evers S.M., Lodder J., et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke. 2003;34:968-974.
64. Rabinstein A.A., Tisch S.H., McClelland R.L., Wijdicks E.F. Cause is the main predictor of outcome in patients with pontine hemorrhage. Cerebrovasc Dis. 2004;17:66-71.
65. Dziewas R., Kremer M., Ludemann P., et al. The prognostic impact of clinical and CT parameters in patients with pontine hemorrhage. Cerebrovasc Dis. 2003;16:224-229.
66. Cohen Z.R., Ram Z., Knoller N., et al. Management and outcome of non-traumatic cerebellar haemorrhage. Cerebrovasc Dis. 2002;14:207-213.
67. Morgenstern L.B., Frankowski R.F., Shedden P., et al. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359-1363.
68. Morgenstern L.B., Demchuk A.M., Kim D.H., et al. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56:1294-1299.
69. Broderick J., Connolly S., Feldmann E., et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116:e391-e413.
70. Steiner T., Kaste M., Forsting M., et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis. 2006;22:294-316.
71. Rabinstein A.A., Atkinson J.L., Wijdicks E.F. Emergency craniotomy in patients worsening due to expanded cerebral hematoma: to what purpose? Neurology. 2002;58:1367-1372.
72. Benes V., Vladyka V., Zverina E. Sterotaxic evacuation of typical brain haemorrhage. Acta Neurochir (Wien). 1965;13:419-426.
73. Honda E., Hayashi T., Shimamoto H., et al. [A comparison between stereotaxic operation and conservative therapy for thalamic hemorrhage.]. No Shinkei Geka. 1988;16:665-670.
74. Nguyen J.P., Decq P., Brugieres P., et al. A technique for stereotactic aspiration of deep intracerebral hematomas under computed tomographic control using a new device. Neurosurgery. 1992;31:330-334. discussion 334-335
75. Mukai H., Yamashita J., Kitamura A., Ito H. Stereotactic aqua-stream and aspirator in the treatment of intracerebral hematoma. An experimental study. Stereotact Funct Neurosurg. 1991;57:221-227.
76. Hondo H., Uno M., Sasaki K., et al. Computed tomography controlled aspiration surgery for hypertensive intracerebral hemorrhage. Experience of more than 400 cases. Stereotact Funct Neurosurg. 1990;54-55:432-437.
77. Kaufman H.H. Treatment of deep spontaneous intracerebral hematomas. A review. Stroke. 1993;24:I101-I106. discussion I107-I108
78. Morgan T., Zuccarello M., Narayan R., et al. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008;105:147-151.
79. Takimoto H., Iwaisako K., Kubo S., et al. Transaqueductal aspiration of pontine hemorrhage with the aid of a neuroendoscope. Technical note. J Neurosurg. 2003;98:917-919.
80. Tyler D., Mandybur G. Interventional MRI-guided stereotactic aspiration of acute/subacute intracerebral hematomas. Stereotact Funct Neurosurg. 1999;72:129-135.
81. Bernays R.L., Kollias S.S., Romanowski B., et al. Near–real-time guidance using intraoperative magnetic resonance imaging for radical evacuation of hypertensive hematomas in the basal ganglia. Neurosurgery. 2000;47:1081-1089. discussion 1089-1090
82. Becker K.J., Baxter A.B., Cohen W.A., et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766-772.
83. Barker F.G.2nd, Amin-Hanjani S., Butler W.E., et al. In-hospital mortality and morbidity after surgical treatment of unruptured intracranial aneurysms in the United States, 1996-2000: the effect of hospital and surgeon volume. Neurosurgery. 2003;52:995-1007. discussion 1007-1009
84. Cheung R.T., Zou L.Y. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 2003;34:1717-1722.
85. Salvati M., Cervoni L., Raco A., Delfini R. Spontaneous cerebellar hemorrhage: clinical remarks on 50 cases. Surg Neurol. 2001;55:156-161. discussion 161
86. Eckman M.H., Rosand J., Knudsen K.A., et al. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34:1710-1716.
87. Butler A.C., Tait R.C. Restarting anticoagulation in prosthetic heart valve patients after intracranial haemorrhage: a 2-year follow-up. Br J Haematol. 1998;103:1064-1066.
88. Hill M.D., Silver F.L., Austin P.C., Tu J.V. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-127.
89. Vermeer S.E., Algra A., Franke C.L., et al. Long-term prognosis after recovery from primary intracerebral hemorrhage. Neurology. 2002;59:205-209.
90. McKissock W., Richardson A., Taylor J. Primary intracerebral haemorrhage: a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;278:221-226.
91. Juvela S., Heiskanen O., Poranen A., et al. The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg. 1989;70:755-758.
92. Batjer H.H., Reisch J.S., Allen B.C., et al. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990;47:1103-1106.
93. Zuccarello M., Brott T., Derex L., et al. Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. 1999;30:1833-1839.
94. Cheng X.C., Wu J.S., Zhou X.P. The randomized multicentric prospective controlled trial in the standardized treatment of hypertensive intracerebral hematomas: the comparison of surgical therapeutic outcomes with conservative therapy. Chin J Clin Neurosci. 2001;9:365-368.
95. Hattori N., Katayama Y., Maya Y., Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomized study. J Neurosurg. 2004;101:417-420.