Chapter 82 Surgical Management of Infratentorial Arteriovenous Malformations
Introduction
Brain stem and cerebellar arteriovenous malformations (AVMs) are rare vascular lesions that represent only a small fraction of all intracranial AVMs. Whereas brain stem AVMs are less likely to be appropriate for surgery, given their involvement of vital neural structures,1 cerebellar AVMs are often treated surgically.2–6 Olivecrona in 1932 performed the first successful resection of an infratentorial (cerebellar) AVM.6,7 Current treatment algorithms for brain stem and cerebellar AVMs involve a multidisciplinary approach, where surgical resection, stereotactic radiosurgery (SRS), and endovascular embolization are used alone or in combination.8–12
Anatomy and Classification
Infratentorial AVMs consist of lesions located in the brain stem and the cerebellum. Given their location in the posterior fossa, AVMs in these locations are usually discussed together, despite their marked differences in clinical presentation, pathophysiology, and treatment options.13 In this chapter we discuss them separately.
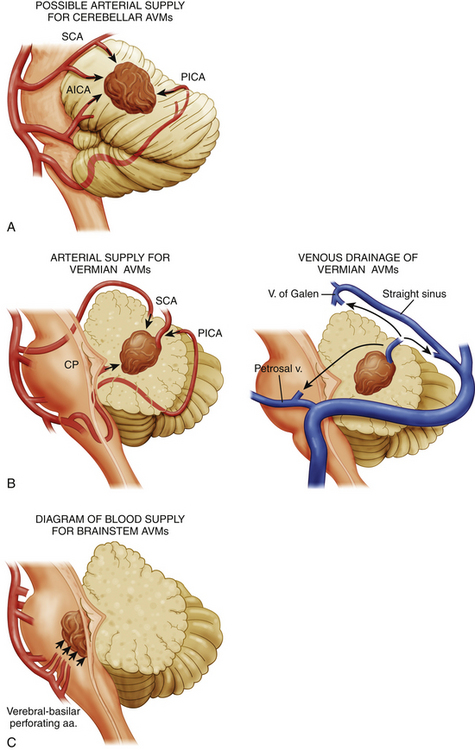
Brain stem AVMs encompass lesions in the midbrain, pons, and medulla. Angiography demonstrates the main arterial territories supplying the AVMs and facilitates their classification into three broad categories: (1) midbrain AVMs, supplied primarily by the superior cerebellar artery (SCA); (2) pontine AVMs, supplied primarily by the anterior inferior cerebellar artery (AICA); and (3) medullary AVMs, supplied primarily by the posterior inferior cerebellar artery (PICA) (Fig. 82-1).
Brain stem AVMs can then be subclassified according to the depth of their location into either superficial pial or parenchymal lesions. Pial brain stem AVMs are typically found in the anterolateral pons and are typically supplied by branches of an enlarged AICA, with venous drainage into the pontine-Galenic and petrosal systems,14 the mesencephalic tectum, or the floor of the fourth ventricle. Parenchymal brain stem AVMs have variable locations within the brain stem and typically extend into the cerebellar peduncles. The most important difference between these two groups, however, lies on their potential resectability. Whereas superficial pial AVMs may be resectable—given early visualization and control of their superficial blood supply, as well as limited manipulation of critical structures—parenchymal AVMs have an arterial supply primarily consisting of perforating arteries, and they often require an approach through critical nuclei and fiber tracts.14,15
Cerebellar AVMs can be anatomically classified into hemispheric, vermian, and tonsillar lesions. Each of these locations has a characteristic arterial supply and a unique relationship to the fourth ventricle. Cerebellar hemispheric AVMs are typically supplied by SCA, AICA, and PICA branches and rarely involve the fourth ventricular ependyma. Vermian AVMs are most often supplied by the SCA and PICA and can extend into the fourth ventricular roof. Tonsilar AVMs are supplied by PICA branches and often project dorsally, away from the fourth ventricle (Fig. 82-1).
Although the Spetzler and Martin AVM classification16 has been widely used and validated in supratentorial AVMs, this grading system, which takes into consideration size of the nidus, pattern of venous drainage, and location in eloquent areas, has been partially validated in infratentorial AVMs in only a few studies.2,8,10
Epidemiology
The annual incidence of brain AVMs has been estimated to be approximately 0.82 to 1.8 cases per 100,000 individuals.17–19 The prevalence of AVMs is more difficult to ascertain, given that many are asymptomatic. In a total of 17 studies discussing the epidemiology of AVMs,18–34 11 were population studies18–22,24,25,29,32–34 and 6 were autopsy studies.23,26–28,30,31 These studies were conducted in centers across the United States,19,23,24,30,32–34 Europe (Austria, Finland, Scotland, and Sweden),20,25–28 the Caribbean Islands,18 and Australia.21 In these studies, the prevalence of AVMs ranged from 5 to 613 cases per 100,000 individuals. We then calculate the mean prevalence of AVMs to be 0.3% based on 284 cases per 100,000 individuals, with a 95% confidence interval (CI) of 108 to 461 cases per 100,000 individuals.
Infratentorial AVMs represent at most 20% of all intracranial AVMs.9,14,35–37 However, because many of these malformations remain asymptomatic, a true estimate of the prevalence of posterior fossa AVMs has not been formulated. Cerebellar AVMs constitute 12% to 16% of all intracranial AVMs and are four times more frequent that brain stem AVMs, which represent only 3% to 4% of all intracranial AVMs.14,38
Although patients with all infratentorial AVMs present at a mean age of 42 years,39 those with brain stem AVMs present earlier, with their first symptoms occurring at a mean age of 32 years (range 9-65 years).9 Patients diagnosed later in life with infratentorial AVMs tend to present with a hemorrhagic event.39
Although gender predominance has not been demonstrated in infratentorial AVMs, some studies report a slight female predominance.9,12,39 Males with brain stem AVMs have a slightly higher risk of hemorrhage as opposed to females.39
Natural History
Infratentorial AVMs are rare entities that, in contrast to their supratentorial equivalents, present more commonly with hemorrhage (1.99 odds ratio, 1.07-3.69 95% CI).39 The size of the AVM is a controversial risk factor for the first bleeding episode, since different analyses have yielded contradictory results.40 Nevertheless, patients with small infratentorial AVMs may present more frequently with hemorrhage than those with large AVMs, given that small malformations otherwise remain asymptomatic.40 Each episode of hemorrhage is accompanied by a 30% morbidity and a 10% mortality. Morbidity due to bleeding of posterior fossa AVMs is high given their critical location. Patients with posterior fossa AVMs have a high mortality associated with the first hemorrhagic episode (66.7%). In some studies, however, authors have found only a 27% mortality from the initial bleeding episode.36 Subsequent hemorrhagic episodes often result in devastating neurologic deficits and have a mortality of 35.7%.8,13 Risk factors for hemorrhage identified for all brain AVMs—but not necessarily for infratentorial AVMs—include young age, large size of the malformation, deep venous drainage, associated aneurysms, and infratentorial location.1,40,41
The annual risk of hemorrhage of infratentorial AVMs appears to be higher than that of all AVMs and ranges from 8.4% to 13%, but it is higher in patients with a prior hemorrhage.2,8,39 After the first hemorrhagic episode and during the first 5 years after the same, infratentorial AVMs tend to hemorrhage repeatedly at a rate of 11.6% annually.40 After this 5-year period, infratentorial AVMs carry an inherent risk of subsequent hemorrhage of 7.5% at 5 years and of 5% at 10 years.42,43 Prospective studies have found that infratentorial AVMs have a higher annual rupture rate after the initial hemorrhage as compared to that of supratentorial AVMs.40,41,44 Other studies have found an overall annual risk for subsequent hemorrhage of 8.4%, which increases to 9.4% in patients who initially presented with a hemorrhage.8 The presence of associated aneurysms is another risk factor for poor outcome and subsequent hemorrhagic episodes. Hemodynamic factors that increase pressure gradients in the AVM, such as deep venous drainage, may also predispose patients to repeated hemorrhages.35 Differences regarding the site of venous drainage or the location and degree of venous stenosis also affect bleeding rates of AVMs.45,46
Clinical Presentation
Patients with infratentorial AVMs can present with headaches, neurologic deficits, or pain.38 Hemorrhage appears to be the most frequent clinical presentation and is more common in patients with small infratentorial AVMs (less than 2 cm in size).47 Hemorrhagic episodes can be related to associated aneurysms on the feeding arteries of these lesions.1 In patients with fourth ventricular hemorrhages, initial symptoms include those of obstructive hydrocephalus.
Less often, patients may present with progressive neurologic deficits but without hemorrhage. Under these circumstances, patients can present with a diverse array of symptoms, including headache, cranial nerve deficits, ataxia, dizziness, or hemiparesis. Although rare, infratentorial AVMs can cause mass effect and compression of important structures, especially in the confined space of the posterior fossa. While cerebellar compression may result in ataxia, dysmetria, and nystagmus, compression of the brain stem can affect different cranial nerve nuclei or traversing nerve fibers and may even present as trigeminal or glossopharyngeal neuralgia or as hemifacial spasm. Compression of the motor and sensory tracts may lead to hemiparesis and hemihypoalgesia.12,15,48
Preoperative Considerations
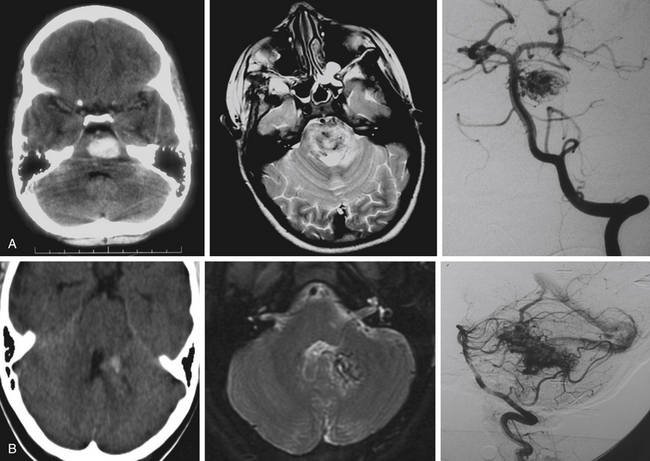
The three imaging studies needed to fully evaluate posterior fossa AVMs and plan their treatment are computed tomography (CT), magnetic resonance imaging (MRI), and digital subtraction angiography (DSA) (Fig. 82-3). CT scan best detects blood intraparenchymally, intraventricularly, or in the subarachnoid space, and is useful in identifying calcifications within the AVM. MRI reveals in detail the anatomic location of the AVM and its relationship to the surrounding structures. DSA provides critical information about feeding arteries and draining veins, as well as the structure of the nidus (i.e., compact vs. diffuse). All three studies are essential in the evaluation of an AVM.
CT scans are most useful in the initial evaluation of AVMs and in emergency settings in which the patient can quickly deteriorate as a result of intraparenchymal or intraventricular hemorrhage with hydrocephalus. CT scans detect approximately 95% of AVMs when contrast is used and about 84% when contrast is not used.48 Hyperdense lesions are seen either intraparenchymally (i.e., after hemorrhage in the subarachnoid space), or intraventricularly.48 Computed tomography angiography (CTA) may be useful in the initial evaluation of arterial supply and venous drainage of these lesions, and it may show enlarged arteries and draining veins, but CTA is not a substitute for DSA.49
MRI shows the anatomic relationship of the AVM to surrounding neural and vascular structures, either localizes the lesion entirely within the brain stem parenchyma or shows its pial representation, and can assist in determining previous bleeding episodes through hemosiderin-based sequences.50 In T2 sequences, flow voids are evident and a hyperintense signal may be noted surrounding the lesion. Fluid-attenuated inversion recovery images show a hyperintense signal surrounding the AVM due to gliosis. Magnetic resonance angiography and venography can be misleading in AVMs because of the intermediate flow characteristics of the AVM vasculature (which is partially arterial and partially venous) and because turbulence and artifact from bony obstructions often encountered in the posterior fossa.49
The critical study for the diagnosis and evaluation of infratentorial AVMs is DSA with selective catheterization, which defines the arterial supply of the AVM, its venous drainage, and the size of the nidus.49 In approximately 10% of posterior fossa AVMs, DSA shows a significant dural arterial component that originates from external carotid artery branches.51 Also of critical importance are the presence of associated aneurysms and the visualization of en passage feeding arteries. These arteries do not connect directly to the malformation but go through and give branches to it, and they must be identified early on, since these vessels also supply critical structures in the brain stem.3
In preparation for surgery, size reduction of infratentorial AVMs may be possible using either endovascular embolization or SRS. The vascular supply to brain stem AVMs, however, makes endovascular therapies dangerous—particularly in parenchymal brain stem lesions, as perfusion through perforating arteries is frequently encountered and manipulation with a microcatheter and displacement of embolization materials can result in ischemia and stroke. The mortality and morbidity associated with embolization of brain stem AVMs can be high. In a series of cerebral AVMs treated with embolization, the authors found a mortality rate of 1.3% and a rate of moderate and severe complications of approximately 20%.52
SRS is often used in surgically inaccessible brain stem AVMs and has been shown to reduce the risk of hemorrhage.10 In addition, SRS can be useful as a preoperative adjunct prior to microsurgical resection and in some series has minimized the need for embolization, decreased operative time, shortened hospital stay, and lowered morbidity.53
Timing of Surgery
Timing of surgery should be individualized by taking into consideration the general medical condition of the patient, age, and neurologic status, but in general we postpone surgery in patients with hemorrhagic presentations. Delaying surgery allows the patient to recover from the initial hemorrhage, facilitates hematoma organization or resolution, and allows for the evolution of dissection planes between the hematoma and the surrounding parenchyma.3 In general, life-threatening hemorrhages and hematomas in the brain stem and cerebellum should be evacuated emergently to decrease mass effect and prevent acute hydrocephalus. In cases of acute intervention, it is often advisable to avoid the AVM nidus and to postpone surgical or radiologic treatment for at least 6 to 8 weeks.14 A second angiogram should be obtained 6 to 8 weeks after the initial hemorrhagic episode and prior to microsurgical resection, since infratentorial AVMs may change overtime and even thrombose. Furthermore, the initial hematoma can prevent full visualization of compressed portions of the AVM.
Surgical Approaches
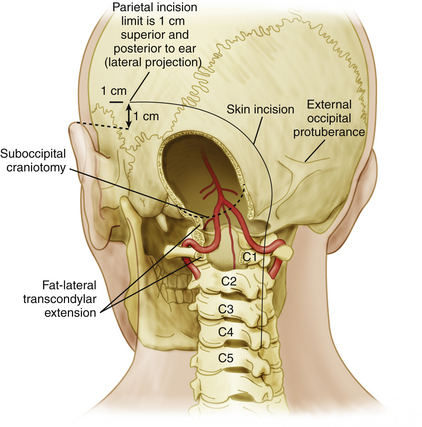
A large craniectomy and dural opening are important to gain full access to the vascular malformation within the limited space afforded by the posterior fossa. The craniectomy should expose the venous sinuses involved in drainage of the AVM. While access to cerebellar AVMs is easily obtained via midline, lateral, or combined suboccipital craniectomies, access to brain stem AVMs can be achieved through a variety of more complex corridors, depending on the location of the AVM. For instance, in ventrolateral pontine lesions, a lateral retrosigmoid exposure may not provide sufficient exposure and a far-lateral transcondylar approach may be required (Figure 82-2). The latter facilitates early visualization of the parent arterial feeders. Access to AVMs in the dorsal brain stem is dictated by their craniocaudal location and can be obtained via transvermian or telovelar exposures.
Craniectomy
A large suboccipital craniectomy should always be performed to allow exposure of the entire lesion, since complete visualization of feeding arteries, draining veins, and involved transverse or sigmoid sinuses is critical prior to resection of the nidus.6 Careful dissection of the dura from the periosteal surface prior to elevation of the flap (if a craniotomy as opposed to a craniectomy is performed) is important to avoid injury to draining veins, since emissary veins and tributaries into the transverse, sigmoid, or petrosal sinuses or to the jugular bulb can be encountered. For lesions located in the midline along the cerebellar vermis, a large midline exposure is adequate; for lesions located in the lateral aspect of the cerebellum and brain stem, including the cerebellopontine angle, a lateral exposure via a suboccipital retrosigmoid approach or a far-lateral transcondylar approach (Fig. 82-2) can provide adequate visualization and if needed can be extended laterally to access the cerebellopontine angle cistern14 and the ventrolateral brain stem. Removal of the lip of the foramen magnum is usually helpful.
Intraoperative Monitoring
Brain stem auditory evoked potentials and somatosensory evoked potentials are used routinely during the resection of infratentorial AVMs. Electrophysiologic monitoring has proved useful in determining the extent of the resection of brain stem AVMs.54,55
Intraoperative angiography before conclusion of the case is essential to confirm obliteration of the AVM.14 If residual malformation is identified, the resection can be completed and angiography repeated. The presence of a residual malformation can increase the risk for postoperative complications.6
Lesion Resection
Early identification and interruption of the arterial supply as close to the nidus as possible and preservation of the draining veins until the final stages of the resection are critical. Resection of the AVM follows a standard technique of circumferential dissection. Feeding arteries and draining veins have aberrant morphology and are invariably arterialized, which often makes differentiation between arteries and veins challenging. For this reason, feeding arteries should be occluded preliminarily using temporary aneurysm clips to observe the response of the AVM. Darkening of the blood in the AVM and decreased turgidity are favorable signs. An increase in AVM pulsation and turgidity indicate that the temporarily occluded vessel may be a draining vein. The dissection proceeds circumferentially from superficial to deep until all arterial vessels are ligated. Small hemostatic clips are preferred over aneurysm clips for permanent arterial ligation due to their lower profile and size. The disadvantage of hemostatic clips is that they are difficult to remove once applied. Special attention should be given to small vascular loops that are encountered in the periphery of the AVM. These loops that emerge from the AVM wall and reenter the lesion are almost always shunting vessels or draining venules that should be preserved until the arterial supply has been completely obliterated. Cumulative interruption of many of these loops can be equivalent to premature interruption of a major draining vein. After complete dissection and devascularization of the AVM, draining veins are ligated and divided and the remainder of the lesion is dissected from the surrounding parenchyma.
Although we do not typically use intraoperative ultrasound, some authors advocate its use to guide the resection of parenchymal AVMs and report that it can reduce operative time and decrease blood loss.56,57
Postoperative Management
Postoperatively, patients are admitted to the neurosurgical intensive care unit and monitored closely for signs of acute intracranial hypertension due to postoperative hemorrhage or thrombosis of the venous system. Surveillance of arterial pressure and maintenance of normotension or hypotension in the first 48 to 72 hours of the postoperative period may protect against bleeding in the surgical bed. It may also be necessary to implement measures to avoid obstructive hydrocephalus secondary to postoperative cerebellar edema, such as ventriculostomy and corticosteroids. If intraoperative electrophysiologic monitoring suggests possible injury of cranial nerves, affecting swallowing reflex or corneal sensation, measures may be taken to avoid further complications (i.e., tracheostomy, gastrostomy, temporary tarsorrhaphy, moisture chambers, and meticulous eye care). Intraoperative angiography may be complemented by postoperative angiography if a residual vascular malformation is suspected in the intraoperative angiogram.3
Complications
Intraoperatively, complications such as hemorrhage, edema, and ischemia can occur due to compromise of draining veins or injury to an en passage vessel. Disturbance of the normal venous drainage of the cerebellum can result in significant edema. Cerebellar and brain stem edema can also result from inadequate patient ventilation or obstructive hydrocephalus. Disturbance of arteriovenous shunts within the malformation during the resection can result in edema and hemorrhage of the parenchyma surrounding the AVM, but loss of autoregulation of the cerebral vasculature and obstruction of the venous drainage are also contributing factors.58–61 Inadvertent occlusion or delayed thrombosis of normal veins during resection can similarly be problematic. Postoperatively, patients can develop a range of problems, including cranial neuropathies, ataxia and dysmetria secondary to cerebellar and peduncular injury, hemiplegia from corticospinal tract injury, and proprioceptive loss from injury to medial lemniscal fibers. Brain stem ischemia and strokes can also present after surgery due to lack of collateral vessels.1,8,9,12,50
Alternative Treatments
Stereotactic aspiration of the hematoma performed acutely to relieve mass effect from hemorrhage has been described.62,63 This technique, however, has the risk of injury to vital brain stem structures as precise targeting and planning of the needle trajectory may be hindered by evolving edema and artifact from the acute hemorrhage. Furthermore, if the malformation vasculature is injured a bleeding episode may ensue, and hematoma evacuation would be suboptimal.6,64
Endovascular Therapy
As discussed previously, endovascular embolization of brain stem AVMs may be useful before surgical resection to decrease flow to the nidus and should target large vessels that are difficult to reach during the initial stages of the procedure, such as the ventricular branches of AICA and SCA. When the feeding arteries arise from PICA, embolization of large infratentorial malformations is often feasible. Proximal embolization of these vessels should be avoided to circumvent the possibility of cerebellar infarction, more so when the vessels are readily accessible during the surgical procedure. Complications due to embolization, such as brain stem and cerebellar infarcts and hemorrhage, can be significant and should be considered before utilizing this treatment method.65 Combined surgical and endovascular treatment should be considered in superficial AVMs of the brain stem and cerebellum, since these may be safely resected.8
Stereotactic Radiosurgery
Radiosurgery has evolved as the main treatment modality of brain stem AVMs and AVMs of the deep cerebellar nuclei. Parenchymal brain stem AVMs should be treated with radiosurgery alone. Radiosurgery has been extensively used to treat brain stem AVMs, with an obliteration rate of 73% at 3 years after treatment.5 Complications due to radiosurgery have been seen in approximately 10% of patients in previous studies.8 These complications include radiation necrosis of the brain stem and cerebellum, leading to cranial nerve palsies and hemiparesis.
Conclusions
Surgical treatment of infratentorial AVMs is challenging and carries significant risks. Cerebellar and brain stem AVMs have distinct clinical and pathophysiologic features, and they require different therapeutic approaches. While cerebellar AVMs are often resectable, brain stem AVMs are approached selectively based on their location, as pial malformations may be amenable for surgical resection but parenchymal malformations are rarely resected and instead are treated with SRS. Diagnostic evaluation requires CT and MRI scans and DSA. Microsurgical resection of selected superficial (mostly cerebellar) lesions is feasible and follows a standard technique of circumferential dissection with careful identification of feeding arteries and draining veins. Intraoperative angiography is critical to avoid incomplete resection. Finally, successful treatment of infratentorial AVMs requires a multimodality approach, combining diagnostic and interventional neuroradiology, microsurgical resection, and SRS in carefully selected patients.
Akdemir H., Oktem S., Menku A., et al. Image-guided microneurosurgical management of small arteriovenous malformation: role of neuronavigation and intraoperative Doppler sonography. Minim Invasive Neurosurg. 2007;50:163-169.
ApSimon H.T., Reef H., Phadke R.V., Popovic E.A. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33:2794-2800.
Arnaout O.M., Gross B.A., Eddleman C.S., et al. Posterior fossa arteriovenous malformations. Neurosurg Focus. 2009;26:E12.
Batjer H.H., Devous M.D.Sr., Meyer Y.J., et al. Cerebrovascular hemodynamics in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Neurosurgery. 1988;22:503-509.
Batjer H., Samson D. Arteriovenous malformations of the posterior fossa. Clinical presentation, diagnostic evaluation, and surgical treatment. J Neurosurg. 1986;64:849-856.
Chang S.D., Lopez J.R., Steinberg G.K. The usefulness of electrophysiological monitoring during resection of central nervous system vascular malformations. J Stroke Cerebrovasc Dis. 1999;8:412-422.
da Costa L., Thines L., Dehdashti A.R., et al. Management and clinical outcome of posterior fossa arteriovenous malformations: report on a single-centre 15-year experience. J Neurol Neurosurg Psychiatry. 2009;80:376-379.
da Costa L., Wallace M.C., Ter Brugge K.G., et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40:100-105.
Guidetti B., Delitala A. Intracranial arteriovenous malformations. Conservative and surgical treatment. J Neurosurg. 1980;53:149-152.
Hernesniemi J.A., Dashti R., Juvela S., et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63:823-829.
Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001;95:633-637.
Jessurun G.A., Kamphuis D.J., van der Zande F.H., Nossent J.C. Cerebral arteriovenous malformations in The Netherlands Antilles. High prevalence of hereditary hemorrhagic telangiectasia-related single and multiple cerebral arteriovenous malformations. Clin Neurol Neurosurg. 1993;95:193-198.
Kashiwagi S., van Loveren H.R., Tew J.M.Jr., et al. Diagnosis and treatment of vascular brain-stem malformations. J Neurosurg. 1990;72:27-34.
Khaw A.V., Mohr J.P., Sciacca R.R., et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004;35:660-663.
Osborn A., Blaser S., Provenzale J., et al. Arteriovenous malformations. In: Osborn A., editor. Diagnostic Imaging. Brain. Altona, Manitoba: Amirsys; 2004:I5.
O’Shaughnessy B.A., Getch C.C., Bendok B.R., Batjer H.H. Microsurgical resection of infratentorial arteriovenous malformations. Neurosurg Focus. 2005;19:E5.
Pollock B.E., Gorman D.A., Brown P.D. Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg. 2004;100:210-214.
Samson D., Kopitnik T.A.Jr., Batjer H., Purdy P.D. Technical Features of the Management of Arteriovenous Malformations of the Brainstem and Cerebellum. In: Batjer H., editor. Cerebrovascular Disease. Philadelphia: Lippincott-Raven Publishers; 1997:811-821.
Sanchez-Mejia R.O., McDermott M.W., Tan J., et al. Radiosurgery facilitates resection of brain arteriovenous malformations and reduces surgical morbidity. Neurosurgery. 2009;64:231-238.
Sinclair J., Kelly M.E., Steinberg G.K. Surgical management of posterior fossa arteriovenous malformations. Neurosurgery. 2006;58::189-201.
Spetzler R.F., Martin N.A. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
Stapf C., Mast H., Sciacca R.R., et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
Taylor C.L., Dutton K., Rappard G., et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810-812.
Unsgaard G., Ommedal S., Rygh O.M., Lindseth F. Operation of arteriovenous malformations assisted by stereoscopic navigation-controlled display of preoperative magnetic resonance angiography and intraoperative ultrasound angiography. Neurosurgery. 2007;61:407-415.
Zabel-du Bois A., Milker-Zabel S., Huber P., et al. Stereotactic LINAC-based radiosurgery in the treatment of cerebral arteriovenous malformations located deep, involving corpus callosum, motor cortex, or brainstem. Int J Radiat Oncol Biol Phys. 2006;64:1044-1048.
1. da Costa L., Thines L., Dehdashti A.R., et al. Management and clinical outcome of posterior fossa arteriovenous malformations: report on a single-centre 15-year experience. J Neurol Neurosurg Psychiatry. 2009;80:376-379.
2. Pollock B.E., Gorman D.A., Brown P.D. Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg. 2004;100:210-214.
3. O’Shaughnessy B.A., Getch C.C., Bendok B.R., Batjer H.H. Microsurgical resection of infratentorial arteriovenous malformations. Neurosurg Focus. 2005;19:E5.
4. Nozaki K., Hashimoto N., Kikuta K., et al. Surgical applications to arteriovenous malformations involving the brainstem. Neurosurgery. 2006;58:ONS270-278.
5. Massager N., Regis J., Kondziolka D., et al. Gamma knife radiosurgery for brainstem arteriovenous malformations: preliminary results. J Neurosurg. 2000;93(suppl 3):102-103.
6. Batjer H., Samson D. Arteriovenous malformations of the posterior fossa. Clinical presentation, diagnostic evaluation, and surgical treatment. J Neurosurg. 1986;64:849-856.
7. Olivecrona H., Riives J. Arteriovenous aneurysms of the brain, their diagnosis and treatment. Arch Neurol Psychiatry. 1948;59:567-602.
8. Kelly M.E., Guzman R., Sinclair J., et al. Multimodality treatment of posterior fossa arteriovenous malformations. J Neurosurg. 2008;108:1152-1161.
9. Solomon R.A., Stein B.M. Management of arteriovenous malformations of the brain stem. J Neurosurg. 1986;64:857-864.
10. Zabel-du Bois A., Milker-Zabel S., Huber P., et al. Stereotactic LINAC-based radiosurgery in the treatment of cerebral arteriovenous malformations located deep, involving corpus callosum, motor cortex, or brainstem. Int J Radiat Oncol Biol Phys. 2006;64:1044-1048.
11. Maruyama K., Kondziolka D., Niranjan A., et al. Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg. 2004;100:407-413.
12. Drake C.G., Friedman A.H., Peerless S.J. Posterior fossa arteriovenous malformations. J Neurosurg. 1986;64:1-10.
13. Fults D., Kelly D.L.Jr. Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658-662.
14. Samson D., Kopitnik T.A.Jr, Batjer H., Purdy P.D. Technical Features of the Management of Arteriovenous Malformations of the Brainstem and Cerebellum. In: Batjer H., editor. Cerebrovascular Disease. Philadelphia: Lippincott-Raven Publishers; 1997:811-821.
15. Chang S., Steinberg G.K. Brainstem Arteriovenous Malformations. In: Stieg P., Batjer H., Samson D. Intracranial Arteriovenous Malformations. New York, NY: Informa Healthcare USA, Inc.; 2007:315-327.
16. Spetzler R.F., Martin N.A. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
17. Brown R.D.Jr, Wiebers D.O., Torner J.C., O’Fallon W.M. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted County, Minnesota. J Neurosurg. 1996;85:29-32.
18. Jessurun G.A., Kamphuis D.J., van der Zande F.H., Nossent J.C. Cerebral arteriovenous malformations in The Netherlands Antilles. High prevalence of hereditary hemorrhagic telangiectasia-related single and multiple cerebral arteriovenous malformations. Clin Neurol Neurosurg. 1993;95:193-198.
19. Brown R.D.Jr, Wiebers D.O., Torner J.C., O’Fallon W.M. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology. 1996;46:949-952.
20. Al-Shahi R., Bhattacharya J.J., Currie D.G., et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163-1169.
21. ApSimon H.T., Reef H., Phadke R.V., Popovic E.A. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33:2794-2800.
22. Berman M.F., Sciacca R.R., Pile-Spellman J., et al. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47:389-396.
23. Courville C.B. Pathology of the Central Nervous System: A Study Based Upon a Survey of Lesions Found in a Series of Forty Thousand Autopsies, 3rd ed. Mountain View, Calif: Pacific Press Publishing Association; 1950. 142-152
24. Gross C.R., Kase C.S., Mohr J.P., et al. Stroke in south Alabama: incidence and diagnostic features—a population based study. Stroke. 1984;15:249-255.
25. Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001;95:633-637.
26. Jellinger K. The morphology of centrally-situated angiomas. In: Pia H., Gleave J., Grote E., Zierski J. Cerebral Angiomas. Berlin: Springer-Verlag; 1975:9-20.
27. Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev. 1986;9:177-216.
28. Karhunen P.J., Penttila A., Erkinjuntti T. Arteriovenous malformation of the brain: imaging by postmortem angiography. Forensic Sci Int. 1990;48:9-19.
29. Luessenhop A. Natural history of cerebral arteriovenous malformations. In: Wilson C., Stein B.M. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:12-23.
30. McCormick W. Pathology of vascular malformations of the brain. In: Wilson C.B., Stein B.M. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:44-63.
31. Michelsen W.J. Natural history and pathophysiology of arteriovenous malformations. Clin Neurosurg. 1979;26:307-313.
32. Mohr J.P., Pile-Spellman J., Stein B.M. Arteriovenous malformations and other vascular anomalies. In: Barnett H.J.M., editor. Stroke: pathophysiology, Diagnosis, and Management. 3rd ed. New York: Churchill Livingstone; 1998:725-750.
33. Stapf C., Mast H., Sciacca R.R., et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:e29-e33.
34. Stapf C., Mohr J.P., Pile-Spellman J., et al. Epidemiology and natural history of arteriovenous malformations. Neurosurg Focus. 2001;11:e1.
35. Arnaout O.M., Gross B.A., Eddleman C.S., et al. Posterior fossa arteriovenous malformations. Neurosurg Focus. 2009;26:E12.
36. Perret G., Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966;25:467-490.
37. Al-Shahi R., Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001;124:1900-1926.
38. Symon L., Tacconi L., Mendoza N., Nakaji P. Arteriovenous malformations of the posterior fossa: a report on 28 cases and review of the literature. Br J Neurosurg. 1995;9:721-732.
39. Khaw A.V., Mohr J.P., Sciacca R.R., et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004;35:660-663.
40. Hernesniemi J.A., Dashti R., Juvela S., et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63:823-829.
41. da Costa L., Wallace M.C., Ter Brugge K.G., et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40:100-105.
42. Mine S., Hirai S., Ono J., Yamaura A. Risk factors for poor outcome of untreated arteriovenous malformation. J Clin Neurosci. 2000;7:503-506.
43. Stefani M.A., Porter P.J., terBrugge K.G., et al. Large and deep brain arteriovenous malformations are associated with risk of future hemorrhage. Stroke. 2002;33:1220-1224.
44. Stapf C., Mast H., Sciacca R.R., et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
45. Willinsky R., Lasjaunias P., Terbrugge K., Pruvost P. Brain arteriovenous malformations: analysis of the angio-architecture in relationship to hemorrhage (based on 152 patients explored and/or treated at the Hopital de Bicetre between 1981 and 1986). J Neuroradiol. 1988;15:225-237.
46. Vinuela F., Nombela L., Roach M.R., et al. Stenotic and occlusive disease of the venous drainage system of deep brain AVM’s. J Neurosurg. 1985;63:180-184.
47. Guidetti B., Delitala A. Intracranial arteriovenous malformations. Conservative and surgical treatment. J Neurosurg. 1980;53:149-152.
48. Silber M.H., Sandok B.A., Earnest F.T. Vascular malformations of the posterior fossa. Clinical and radiologic features. Arch Neurol. 1987;44:965-969.
49. Osborn A., Blaser S., Provenzale J., et al. Arteriovenous Malformations. In: Osborn A., editor. Diagnostic imaging. Brain. Altona, Manitoba: Amirsys, 2004. I5-5 to I5-7
50. Kashiwagi S., van Loveren H.R., Tew J.M.Jr, et al. Diagnosis and treatment of vascular brain-stem malformations. J Neurosurg. 1990;72:27-34.
51. Kaptain G.J., Lanzino G., Do H.M., Kassell N.F. Posterior inferior cerebellar artery aneurysms associated with posterior fossa arteriovenous malformation: report of five cases and literature review. Surg Neurol. 1999;51:146-152.
52. Wikholm G., Lundqvist C., Svendsen P. Embolization of cerebral arteriovenous malformations: part I—Technique, morphology, and complications. Neurosurgery. 1996;39:448-459.
53. Sanchez-Mejia R.O., McDermott M.W., Tan J., et al. Radiosurgery facilitates resection of brain arteriovenous malformations and reduces surgical morbidity. Neurosurgery. 2009;64:231-238.
54. Chang S.D., Lopez J.R., Steinberg G.K. The usefulness of electrophysiological monitoring during resection of central nervous system vascular malformations. J Stroke Cerebrovasc Dis. 1999;8:412-422.
55. Sinclair J., Kelly M.E., Steinberg G.K. Surgical management of posterior fossa arteriovenous malformations. Neurosurgery. 2006;58:ONS189-201.
56. Akdemir H., Oktem S., Menku A., et al. Image-guided microneurosurgical management of small arteriovenous malformation: role of neuronavigation and intraoperative Doppler sonography. Minim Invasive Neurosurg. 2007;50:163-169.
57. Unsgaard G., Ommedal S., Rygh O.M., Lindseth F. Operation of arteriovenous malformations assisted by stereoscopic navigation-controlled display of preoperative magnetic resonance angiography and intraoperative ultrasound angiography. Neurosurgery. 2007;61:407-416.
58. Young W.L., Kader A., Ornstein E., et al. Cerebral hyperemia after arteriovenous malformation resection is related to “breakthrough” complications but not to feeding artery pressure. The Columbia University Arteriovenous Malformation Study Project. Neurosurgery. 1996;38:1085-1095.
59. Batjer H.H., Devous M.D.Sr, Meyer Y.J., et al. Cerebrovascular hemodynamics in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Neurosurgery. 1988;22:503-509.
60. Sekhon L.H., Morgan M.K., Spence I. Normal perfusion pressure breakthrough: the role of capillaries. J Neurosurg. 1997;86:519-524.
61. al-Rodhan N.R., Sundt T.M.Jr, Piepgras D.G., et al. Occlusive hyperemia: a theory for the hemodynamic complications following resection of intracerebral arteriovenous malformations. J Neurosurg. 1993;78:167-175.
62. Bosch D.A., Beute G.N. Successful stereotaxic evacuation of an acute pontomedullary hematoma. Case report. J Neurosurg. 1985;62:153-156.
63. Davis D.H., Kelly P.J. Stereotactic resection of occult vascular malformations. J Neurosurg. 1990;72:698-702.
64. Konovalov A.N., Spallone A., Makhmudov U.B., et al. Surgical management of hematomas of the brain stem. J Neurosurg. 1990;73:181-186.
65. Taylor C.L., Dutton K., Rappard G., et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810-812.