Surgical Management of Hyperparathyroidism
Clinical Features and Indications for Surgery
Indications For Surgery
Patients with pHPT exist on a spectrum from being completely asymptomatic to morbidly symptomatic, based on duration and severity of hypercalcemia. For those who are symptomatic, there are several sited sequelae that remain clear indications for surgery: nephrolithiasis, osteoporosis which can result in pathologic bone fractures, progressive renal injury, neuromuscular and neurocognitive impairments such as fatigue, inability to concentrate, and depression, as well as the rare phenomenon of hypercalcemic crisis.1–3
The best treatment for totally asymptomatic patients is still a subject of controversy.1–3 The 2008 Workshop on Primary Hyperparathyroidism recommended criteria for surgical treatment in these asymptomatic patients4:
• 24-hour urinary calcium > 400 mg
• Creatinine clearance < 60 mL/min/1.73m2
This group certainly has no urgent need for surgery. Regular follow-up is indicated to avoid disease progression. Surgery is usually recommended for younger patients (<50 years), as symptoms will develop in approximately 20% of these patients with long-term follow-up.3,5 Neuromuscular symptoms such as weakness (20% to 60%) occur in patients with pHPT, and successful surgery reverses these symptoms in most patients.6
In general, surgery is indicated for all patients with clear biochemical evidence of pHPT and documented signs or symptoms of the disease. In apparently asymptomatic patients with pHPT, surgery is indicated for younger patients who have a low operative risk and long temporal exposure to the disease. In apparently asymptomatic older patients, surgery is reserved for patients in whom evidence of progression and/or symptoms develop. Progression is measured by a decrease in bone density (T score ≤ 2.5 at any site), elevated serum calcium (≥1 mg/dL above normal), or urinary levels of calcium greater than 400 mg/24 hours.4
Prognostic Indicators of Parathyroid Pathology
Certain symptoms and signs are useful predictors of different types of parathyroid pathology. Parathyroid adenoma is seldom if ever palpable, but parathyroid carcinoma is usually palpable.7 Exceptionally high concentrations of PTH or calcium in serum also may suggest parathyroid cancer.7 The diagnosis of familial multiple endocrine neoplasia type 1 (MEN1) or MEN2a predicts parathyroid hyperplasia7–9; however, there is wide variation in the size of the abnormal parathyroid glands in patients with MEN1, so there might be a failure of the surgeon to recognize hyperplasia.10 The presence of multiple lipomas can occur in MEN1. Studies have identified the genetic defect in patients with MEN1 to be a MENIN mutation on chromosome 11q1311 and in patients with MEN2, a RET proto-oncogene mutation on chromosome 10.12 MEN2 must be distinguished from familial MEN2b, because hyperparathyroidism is not part of the latter. Familial hypocalciuric hypercalcemia (FHH) is an autosomal dominant trait usually manifested as asymptomatic hypercalcemia and relative hypocalciuria.9,13 Mutations in the calcium-sensing receptor gene on chromosome 3 have been identified in a heterozygous form in benign FHH9 but not in sporadic adenomas. In patients with FHH, the hypercalcemia is PTH dependent and associated with mild parathyroid hyperplasia; however, subtotal parathyroidectomy is not effective and contraindicated. In such cases, measurement of urinary calcium excretion and detection of relative hypocalciuria (<100 mg/24 hours) should lead to cancellation of surgery and testing for hypercalcemia and hypocalciuria in relatives.13
Parathyroid Crisis
Parathyroid crisis is an unusual state of progressive, marked hyperparathyroidism producing anorexia, vomiting, dehydration, decrease in renal function, progressive hypercalcemia, deterioration of mental status, confusion, coma, and if untreated, death.14,15 Fatigue, muscle weakness, polyuria, and polydipsia are also frequent. Hypercalcemia may have been noted in the past but left untreated or inadequately treated. Often, no apparent reason can be found for the sudden worsening of hyperparathyroidism. Some cases are apparently precipitated by bacterial or viral infection, trauma, or recent surgery. Serum calcium should not be the only defining criterion for a hypercalcemic crisis, because asymptomatic patients with serum calcium of 20 mg/dL and patients in hypercalcemic crisis with serum calcium less than 14 mg/dL have been reported.16 Severe hyperparathyroidism may also be a manifestation of parathyroid carcinoma, which should be considered in the differential diagnosis.7
Parathyroid crisis is a potentially life-threatening disorder that requires vigorous medical management in preparation for definitive surgery. Attention must first be directed toward hydration, reduction of the hypercalcemia, and stabilization of the clinical state. Large amounts of intravenous saline are administered to ensure rehydration. This is followed by furosemide, which further reduces the hypercalcemia. At the same time, the diagnosis of hyperparathyroidism is established by measuring serum levels of calcium and intact PTH. If hydration and treatment with furosemide intravenously are not effective in reducing the hypercalcemia, treatment with bisphosphonates, calcitonin, or cinacalcet might be necessary, but surgery is indicated as quickly as the patient can be stabilized, because the crisis can worsen rapidly.14
Minimally Invasive Parathyroidectomy: Procedures for Preoperative and Intraoperative Localization of Abnormal Parathyroid Glands
Parathyroid Localization Before Initial Surgery
The overall trend in surgery over the past 10 years is towards minimally invasive techniques. The objective is to minimize length of incisions and the associated increased pain, higher rate of infection, and longer duration of hospital stay. With the ability to now reliably localize abnormal parathyroid glands and confirm their removal intraoperatively, minimally invasive parathyroidectomy (MIP) has become the procedure of choice for pHPT.17–23
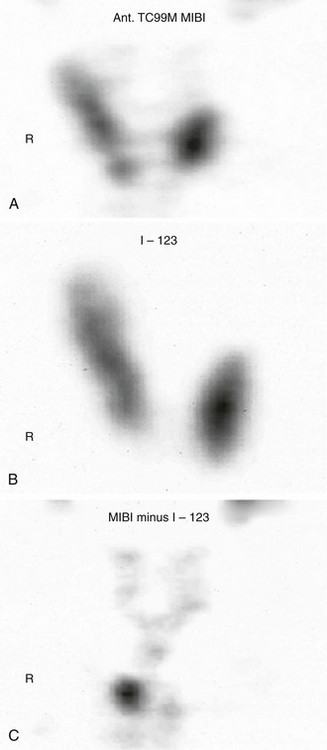
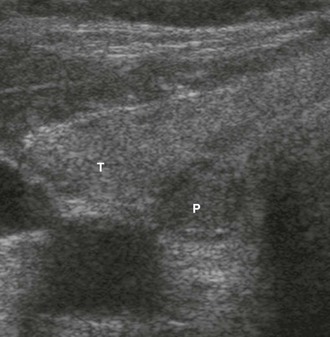
There is general consensus that the best localization procedures for initial parathyroid operations are the combination of ultrasound (US) and sestamibi scanning (Fig. 9-1). The single best preoperative localization study is sestamibi, which has a specificity of 98%, but there has been reported variation in localization sensitivity for pHPT from 43% to 91%.24,25 It has been recently reported that sestamibi optimization of acquisition and processing parameters may improve scan sensitivity in pHPT.26 Sestamibi is a monovalent lipophilic cation that diffuses passively across the cell membrane and concentrates in mitochondria. It is preferentially concentrated in abnormal parathyroid tissue because of increased blood supply, higher metabolic activity, and an absence of p-glycoprotein on the cell membrane. Sestamibi scans can be done preoperatively to plan a MIP (Fig. 9-2).20,27–34 High-resolution ultrasound is also a useful study for preoperative parathyroid localization.35–37 It images the abnormal parathyroid gland as a hypoechoic (sonolucent) mass compared to the more echo-dense thyroid tissue (Fig. 9-3). It is specific, but it is not as sensitive as sestamibi. Its specificity is 98% and sensitivity is 66%.38 Further, if both sestamibi and ultrasound are positive in the same location, the patient is virtually assured that an abnormal parathyroid gland will be found and removed.
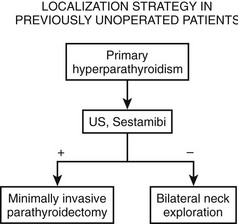
FIGURE 9-1 Flow diagram for localization strategy in previously unoperated patients. US, Ultrasound.
Parathyroid Localization Before Repeat Surgery
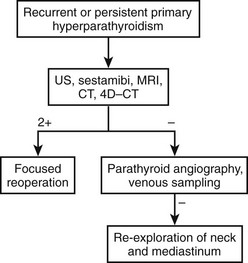
In patients with persistent or recurrent (normocalcemia for 6 months or longer, then recurrent hypercalcemia) hyperparathyroidism, the chance of successful surgery is reduced,39,40 and the incidence of complications is greater.41–43 Therefore, maximum effort at parathyroid gland localization is made, commencing with the noninvasive procedures (US, computed tomography [CT], magnetic resonance imaging [MRI], and sestamibi scanning) and proceeding, if necessary, to the more invasive studies (Fig. 9-4). Currently, noninvasive techniques localize an abnormal gland in about 75% to 80% of patients requiring repeat surgery,44 whereas invasive studies, such as venous sampling, selective angiography, or percutaneous ultrasound/CT-guided fine-needle aspiration, help with the remainder.45,46
Ultrasound
US, using a 10-MHz probe, is readily available, noninvasive, and the least expensive technique to preoperatively image abnormal parathyroid glands. It is particularly effective for localizing enlarged parathyroid glands in the neck and can be used to identify 60% of the abnormal glands in patients requiring reoperation.44 US identifies juxtathyroidal parathyroid glands (see Fig. 9-3).
US has some disadvantages. It is operator-dependent, making the accuracy variable.35,37,38,47 It may fail to image posterior glands in the tracheoesophageal groove and glands in the anterior mediastinum. In multiple-gland hyperplasia, it generally demonstrates only the dominant gland.
Sestamibi Scintigraphy
Technetium 99m–labeled sestamibi scanning has superior resolution and sensitivity (80% to 90%) in detecting hypercellular parathyroid glands prior to reoperations.48 Both the thyroid and parathyroid will take up sestamibi, but the uptake will be stronger and the signal will persist longer in parathyroid adenomas or hyperplasia (see Fig. 9-2). The combination of single-photon emission CT (SPECT) with sestamibi has improved the sensitivity to about 85%, especially for deep cervical and mediastinal parathyroid tumors.25 Sestamibi has been combined with the gamma probe for hand-held intraoperative localization of abnormal parathyroid glands.27,29–34 Advocates suggest that this approach is less invasive and can be done under local anesthesia as an outpatient procedure through a smaller incision. Sestamibi scans facilitate the dissection and make the surgery easier, but they have not been shown to affect the outcome in previously unoperated patients. One study demonstrated that there was no significant difference in cure rate between patients who had preoperative sestamibi scan and those who did not. The cure rate was 97.5% and 99%, respectively. However, there was a significant difference in cure rate between the negative sestamibi scan group (92.7%) and both the no-scan group (99.3%) and the positive-scan group (100%). Thus sestamibi scan can be used to identify those patients who are less likely to have successful surgery.49
Computed Tomography
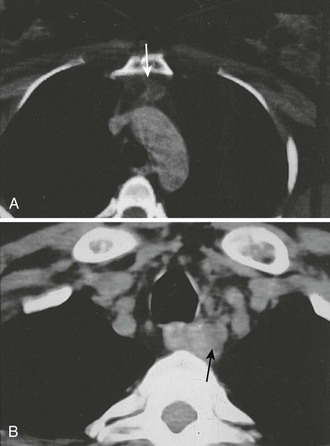
CT is particularly effective for identifying ectopic glands in the anterior mediastinum and enlarged glands in the tracheoesophageal groove. Ectopic glands in the anterior mediastinum often lie within the fat-replaced thymus, and even small adenomas are readily visualized (Fig. 9-5A). Ectopic glands in the tracheoesophageal groove are detected as a solid mass adjacent to the esophagus (Fig. 9-5B). Undescended glands near the carotid bifurcation are also identified by CT, provided that the examination is carried up to the level of the hyoid bone. On the other hand, CT is poor at detecting intrathyroid or juxtathyroid tumors and exposes the patient to risks associated with contrast media and radiation.
Magnetic Resonance Imaging
Initial experience with MRI of abnormal parathyroid glands has been successful for large parathyroid adenomas, which on T2-weighted or stir-pulse sequences produce a bright signal.50 In the mediastinum, this signal may be confused with fat, and a T1-weighted image is required to specifically identify the pathology. With gadolinium-enhanced MRI and T1- and T2-weighted images, MRI can now provide higher sensitivity than CT for identifying ectopic parathyroid tumors. It can be a useful study, and it may have more sensitivity than CT.
Selective Venous Sampling for Parathyroid Hormone
Selective venous sampling requires the greatest experience and is the most variably performed of all the localizing procedures in nonreferral centers. Contrast load, radiation exposure, and cost (15 to 20 PTH determinations), in addition to radiography costs, are all significant. Moreover, gradients determined by selective catheterization identify only the region of pathology (e.g., right side of the neck, mediastinum) but do not image the elusive gland. A new technique is to add the rapid PTH assay to selective venous sampling to provide a short turnaround time and allow the radiologist to obtain more selective samples in regions in which high concentrations of PTH are found. This combination of venous sampling and rapid PTH assay localized the abnormal parathyroid gland correctly in 6 of 7 patients who had negative noninvasive imaging and required reoperation for prior unsuccessful parathyroid surgery.51 It is indicated in only a small proportion of reoperative patients who have significant primary hyperparathyroidism and no apparent localizing information after completing all noninvasive studies and angiography.
Positron Emission Tomography
Regional body fluorodeoxyglucose positron emission tomography (FDG-PET) has been evaluated as a means of localizing pathology in recurrent hyperparathyroidism. Regional PET imaging of the neck and upper chest was able to identify 79% of parathyroid adenomas in 20 patients.52 PET appears to have potential; however, is relatively expensive compared to other more commonly utilized localization techniques. It is not used frequently for abnormal parathyroid gland localization.
Four-Dimensional Computed Tomography
4D-CT is the latest described technology for the preoperative evaluation of patients with parathyroid disease. 4D-CT adds to existing CT by detecting the perfusion characteristics of parathyroid and is able to differentiate between normal and hyperfunctioning glands (which have rapid uptake and quick washout). In a way, this is similar to CT angiography. This modality combines anatomic and functional localization in a single study, with preliminary data suggesting greater accuracy than sestamibi scan. Perhaps the greatest yield with 4D-CT imaging will be in localization of hyperfunctioning glands in the setting of persistent or recurrent parathyroid disease.53
Summary of Radiographic Localization
We suggest that sestamibi and US be used in patients undergoing initial exploration for pHPT. Accurate preoperative localization studies allow a minimally invasive parathyroidectomy that shortens hospital stay, minimizes scar, and provides a successful outcome. Furthermore, in patients undergoing reoperations, preoperative radiologic localization studies are necessary and helpful to plan the operative approach (see Fig. 9-4). We recommend liberal use of each of the noninvasive imaging studies (US, CT, 99mTc-sestamibi, and MRI) as an initial imaging cluster. If two studies identify the abnormal parathyroid gland in the same location, we proceed with surgery. Our institution is currently establishing 4D-CT technology, but it is not yet available. As such, if the noninvasive studies are equivocal, we then perform arteriography. If that study is positive, we perform surgery; if negative, we recommend selective venous sampling for rapid PTH measurements (as discussed above).51
Intraoperative Determination of Parathyroid Hormone
Intraoperative determination of PTH allows rapid monitoring of parathyroid status during parathyroid surgery.54 Generally, after successful removal of a single parathyroid adenoma or adequate resection of hyperplastic glands, serum PTH levels begin to fall immediately and reach a 50% drop from the baseline level or normal range within 10 to 15 minutes.55 Studies demonstrate that serum levels of intact PTH decline rapidly, only 5 minutes after resection of a parathyroid adenoma (Fig. 9-6).56,57 Furthermore, the rate of decline is less in patients with hyperplasia and may provide an additional intraoperative means of diagnosing hyperplasia (Fig. 9-7).58 A serum sample for PTH should be obtained just after the induction of anesthesia. Repeated serum samples are obtained intraoperatively immediately following resection of an enlarged gland, and then 10 minutes following removal. This protocol has been designed to take into account the half-life of PTH, which is 1 to 4 minutes, and avoid misleading results from a spike in concentration that may occur during handling and removal of the adenoma.57 A 50% reduction in the PTH level from the median baseline level indicates a successful outcome (see Fig. 9-6).56 Some also recommend a second criterion of a normal serum level of PTH. The operation can be terminated on this result without identification of other parathyroid glands. Furthermore, the assay can be used to diagnose an abnormal parathyroid gland by performing fine needle aspiration (FNA) of a mass lesion and then diluting the sample with heparinized saline and measuring PTH levels—which will be very high if the mass is parathyroid tissue.58
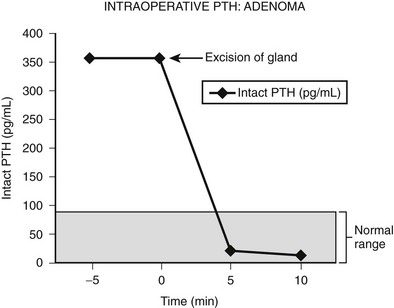
FIGURE 9-6 Intraoperative measurement of intact PTH in a patient undergoing surgery for an adenoma. After the parathyroid adenoma is removed, the serum PTH levels decrease more than 50% from the median baseline level, indicating a successful outcome, and the surgery is terminated.
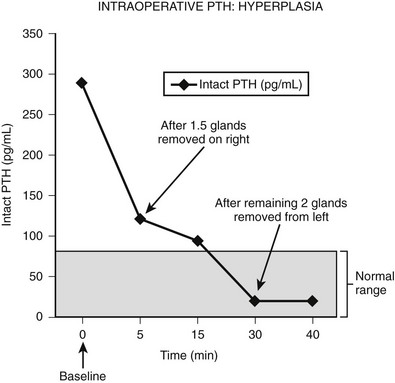
FIGURE 9-7 Intraoperative measurement of intact PTH in a patient who underwent neck exploration for pHPT. The right upper gland and half of the right lower gland were excised. Both appeared abnormally large, and frozen section analysis showed hypercellular tissue. The PTH decreased to less than 50% baseline, but it did not return to the normal range (<80 pg/mL). The left neck was explored, and both parathyroid glands were excised. The PTH level subsequently dropped within normal range.
Intraoperative determination of PTH levels appears to complement surgical skill and histopathologic information and has the potential to provide additional guidance regarding the extent and degree of neck exploration. However, false-negative results57 or technical difficulties may be encountered, and this information may be difficult to interpret in the case of double adenoma or hyperplasia. Nevertheless, its use has greatly facilitated minimally invasive parathyroidectomy, and operative failure rates with the use of intraoperative PTH assay have decreased from 6% to 1.5% for initial operations59 and from 24% to 6% for reoperations.60
Surgical Management of Primary Hyperparathyroidism
Surgery is the mainstay for treatment of pHPT. The methods of surgery are becoming less invasive. The possible causes of pHPT are adenoma (83%), hyperplasia (15%), double adenoma (1% to 2%), and carcinoma (1%).61 Some argue that double adenomas might represent undetected hyperplasia. However, studies have shown that recurrent disease does not develop in patients with removed double adenomas after long follow-up.62,63 This suggests that the diagnosis of double adenoma is real. A family history of parathyroid disease or associated endocrinopathies is associated with hyperplasia. A history of neck irradiation is associated with adenoma.64
Before performing surgery for pHPT, the surgeon must obtain informed consent, which requires careful discussion of the outcome and complications. A successful initial parathyroid surgery, using either minimally invasive techniques or bilateral cervical exploration, is expected in greater than 95% of patients undergoing initial operations21,22,64 and in 78% to 90% of reoperations.65,66 Udelsman et al.42 recently reported a success rate for reoperative surgery for pHPT as high as 96%. Recurrent laryngeal nerve injury occurs in less than 1% of initial operations64 and more than 5% of repeat operations.41 Fortunately, the symptoms in many of these nerve injuries are temporary, and full recovery may be seen at the 3- to 6-month follow-up. Hypoparathyroidism rarely occurs after initial explorations but may occur in 2.7% to 16% of reoperations.65,66
Anatomy
Facility in the surgical identification of normal and abnormal parathyroid glands is essential. Parathyroid glands vary in color from light yellow to reddish brown, and the consistency is usually soft and pliable.67 A reddish color and dense consistency reflect a high parenchymal cell content (abnormal gland); a yellowish white color is found with a high fat content (normal gland).67 Typically, four parathyroid glands are present.
Parathyroid glands differ in shape and size. Eighty-three percent are oval or bean-shaped, 11% are elongated, 5% are bilobate, and 1% are multilobated.67 Normal glands tend to be flat and ovoid; with enlargement, they become globular. Normal measurements are 3 × 5 × 7 mm. The combined weight of all parathyroid glands is 90 to 130 mg, and the superior glands are usually smaller than the inferior glands.64 Most parathyroid glands are suspended by a small vascular pedicle and enveloped by a pad of fatty tissue.68
Autopsy series demonstrate that four glands are found in 91% of subjects, five glands in 4%, and three glands in 5%.69 In studies done by serial sectioning of embryos, at least four parathyroid glands are found in every specimen.69 Approximately 5% of humans have supernumerary (more than four) parathyroid glands.70 Supernumerary glands and fragments of parathyroid glands are most commonly found within the thymus.
Although gland distribution may deviate widely, the location of parathyroid glands is predictable from knowledge of embryology.68 Originating from the fourth pharyngeal pouch,71 the superior parathyroid glands are commonly found along the posterior surface of the upper two-thirds of the thyroid gland (92%)67 (Fig. 9-8). Frequently (40%), superior parathyroid gland adenomas migrate posteriorly, behind the inferior thyroid artery to a position along the esophagus.61 Division of the superior thyroid artery and mobilization of the superior thyroid pole are usually unnecessary to expose the superior parathyroid glands, but the fascia connecting the lateral portion of the thyroid lobe to the carotid sheath must be incised. The location of the superior glands is relatively constant, and these glands can generally be identified quickly and easily. Superior parathyroid adenomas may have a unique relationship to the recurrent laryngeal nerve such that the nerve is embedded in the anterior medial capsule of the adenoma, or the gland can be rounded and tucked into the exact spot where the recurrent nerve enters the larynx.
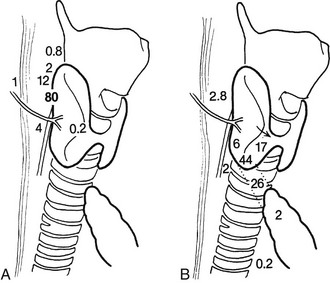
FIGURE 9-8 Diagram of potential locations of superior (A) and inferior (B) parathyroid glands. Numbers refer to the percentage of glands found at each location. (From Akerstrom G, Malmaeus J, Bergstrom R: Surgical anatomy of human parathyroid glands. Surgery 95:14, 1984.)
The inferior parathyroid glands have a more variable distribution than the superior ones (see Fig. 9-8). With the thymus, they originate from the third pharyngeal pouch.71 As the thymus migrates caudally, the lower glands migrate until they reach the lower pole of the thyroid gland. Seventeen percent of inferior parathyroid glands touch the inferior border of the thyroid, 44% are within 1 cm of the inferior border of the thyroid (also known as the thyrothymic ligament), 26% are within the superior horn of the thymus, and 2% are in the mediastinal thymus.67,68 The remainder are either within the thyroid or are undescended in the upper portion of the neck near the carotid bifurcation72 (see Fig. 9-8). This variable anatomic distribution makes the inferior glands more difficult to locate than the superior ones. An inferior parathyroid adenoma is generally bordered posteriorly and laterally by the recurrent laryngeal nerve and is inferior to the inferior thyroid artery.
General Technique of Exploration
It appears that some form of minimally invasive parathyroid surgery has replaced standard bilateral neck exploration for pHPT. This is still controversial, and some recent studies suggest that standard bilateral neck exploration is just as good and less expensive.73 However, most surgeons, endocrinologists, and patients now think that MIP is preferable because it is associated with similar excellent results, less pain, better cosmesis, and more rapid recovery.74 We recommend the use of intraoperative rapid PTH assay to assess outcome intraoperatively without extensive exploration, but this has been controversial; one group has demonstrated that this is unnecessary if sestamibi demonstrates a single abnormal gland.75 General endotracheal anesthesia is used, although regional block anesthesia has been advocated by some and is equally effective (Fig. 9-9).76,77 Local anesthesia or regional block has been advocated for the elderly undergoing targeted parathyroidectomy.76
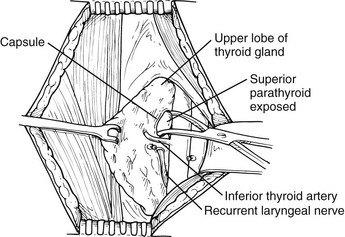
FIGURE 9-9 Identification of a left superior parathyroid adenoma. The thyroid gland is elevated with a Babcock clamp. The investing thyroid fascia is opened posterior to the upper pole of the left lobe. A left upper parathyroid adenoma is identified superior to the inferior thyroid artery and posterolateral to the recurrent laryngeal nerve. The left recurrent laryngeal nerve is shown in its usual location within the tracheoesophageal groove and posterior to the inferior thyroid artery.
Intraoperative PTH assay is performed as described previously. The patient is positioned in a manner as for thyroid surgery. A 3-cm transverse cervical incision is made approximately 2 cm above the sternal notch in a skin crease. It is centered on the side of the localization studies and the anterior border of the sternocleidomastoid muscle. A focused approach directly to the abnormal parathyroid gland is made based on the preoperative imaging results. Alternatively, some recommend an approach based on a hand-held gamma probe to detect labeled sestamibi within the adenoma.78 Because a high proportion (90%) of parathyroid adenomas are imaged by sestamibi scanning, the technique is used to guide intraoperative localization of the abnormal gland with a hand-held gamma detector.27,30–33 Then under local anesthesia, an incision is made in the area of highest radioactivity, and dissection is focused on the abnormal “hot” adenoma. This technique can be combined with immediate PTH assay to quickly determine whether all the abnormal parathyroid tissue has been removed. The method relies on radioactive detection and PTH measurement to guide surgery rather than precise operative identification. It may be limited in identification of multiple abnormal glands and glands adjacent to the thyroid, which may also be hot. Limited sub-platysmal flaps are raised, since the goal of surgery is to remove a solitary adenoma and not to perform a standard neck exploration. Because of this, limited mobilization of the thyroid gland is performed, and retraction of the strap muscles on the side of the dissection is done. Dissection is focused on the preoperative localization studies, and the abnormal parathyroid gland is removed.
The surgeon should be aware of the relationship of the parathyroid glands to the recurrent laryngeal nerves and avoid injury to the nerve. The upper glands are posterior and lateral to the recurrent laryngeal nerve (see Fig. 9-9), while the lower glands are anterior and medial to it (Fig. 9-10). The vascular pedicle is either ligated, clipped, or controlled with the harmonic scalpel, and the specimen is sent to pathology. The intraoperative PTH blood levels are measured while the wound is closed. The procedure is not terminated until the levels drop 50% from the median baseline level and/or into the normal range (see Fig. 9-6). The success of MIP has been confirmed to be the same as bilateral standard neck exploration. For example, in 255 consecutive MIPs, the cure rate was 99%, and the complication rate was 1.2%. MIP has been associated with a 50% reduction in operating room time and shorter hospitalization (Table 9-1).21 However, MIP may artificially increase the probability of adenoma and decrease the chance of detection of hyperplasia or multiple gland disease. Two recent independent studies documented that the rate of hyperplasia with MIP was lower than the expected rate of 15% for standard bilateral explorations.79,80 If the serum levels of PTH do not drop or there is evidence for hyperplasia on the preoperative imaging studies or by family history, the conventional bilateral neck exploration is performed.
Table 9-1
Success of Minimally Invasive Parathyroidectomy Compared to Bilateral Neck Exploration
| 656 Consecutive Parathyroidectomies 1990–2001 | 401 BNE | 255 MIP |
| Complications | 3% | 1.2% |
| Cure rate | 97% | 99% |
| Operating time | 2.4 hr | 1.3 hr |
| Length of stay (days) | 1.64 | 0.24 |
| Mean cost savings | — | $2,693 per procedure |
BNE, Bilateral Neck Exploration; MIP, minimally invasive parathyroidectomy.
Abstracted from Udelsman R: Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg 235:665–670; discussion 670–662, 2002.
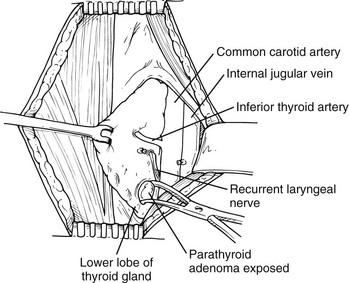
FIGURE 9-10 Identification of a left inferior parathyroid adenoma. The thyroid gland is elevated with a Babcock clamp. A left lower parathyroid adenoma is identified inferior to the inferior thyroid artery and anteromedial to the recurrent laryngeal nerve, which is the most common position for a left inferior parathyroid adenoma, although the inferior parathyroid gland can be located in other positions.
Special Issues In Surgery
If focused MIP is unsuccessful, bilateral neck exploration is recommended. Essentially, this is the standard parathyroid exploration that was done for years with consistent high probability of success and low complication rate. Any enlarged or abnormal glands are removed.64 The most important indicator of normal or abnormal parathyroid tissue is the appearance of the gland. Pathologists may find it difficult to differentiate normal from hypercellular parathyroid tissue or hyperplasia from adenoma, but they can reliably confirm that whatever tissue was biopsied is parathyroid.77 If two glands are enlarged (double adenoma), both are removed. Long-term results with this method of management have been highly satisfactory.63,64
Hyperplasia
In generalized four-gland enlargement or hyperplasia, surgical management is more difficult and the results less satisfactory. Intraoperative PTH levels may help distinguish between adenoma and hyperplasia and help guide the amount of parathyroid resection. Abnormal parathyroid glands should be removed until the levels drop 50% from the median baseline preoperative level and into the normal range (see Figs. 9-6 and 9-7). Studies suggest that subtotal (3.5-gland) parathyroidectomy is the procedure of choice for hyperplasia. This is primarily because of an unacceptably high incidence of hypoparathyroidism with 4-gland parathyroidectomy and transplant. The cervical thymus should also be removed because supernumerary glands or fragments of parathyroid glands are commonly found within it. The results of subtotal parathyroidectomy have been variable, with a 13% incidence of persistent disease, a 15% incidence of recurrent disease, and a similar incidence of hypoparathyroidism. The surgical approach is removal of 3.5 parathyroid glands, with approximately 30 to 50 mg of the most normal-appearing parathyroid tissue left and marked with a surgical clip in the neck. If recurrent hypercalcemia develops, a portion of the remaining marked hyperplastic parathyroid gland can be removed at a reoperation.81–84
Mediastinal Exploration
If a patient undergoes an unsuccessful operation for hyperparathyroidism, the patient should be reevaluated, and localization procedures should be performed (see Fig. 9-4). Median sternotomy is indicated in only 1% to 2% of patients undergoing initial exploration and approximately 20% to 30% of those requiring reoperation. In our series of 33 patients who underwent median sternotomy as part of a reoperation for pHPT,85 30% did not have abnormal parathyroid tissue in the mediastinum.86 Of the abnormal mediastinal glands found, most were discovered in the thymus (64%), and some were not visible during surgery. This mandates that a total thymectomy should be performed during any mediastinal exploration for pHPT. Wells and Cooper86 have reported the ability to remove the entire thymus (including the mediastinal component) without dividing the sternum by using a special retractor to elevate the sternum. This procedure may be used to remove mediastinal parathyroid adenomas less invasively. Mediastinal parathyroid adenomas have also been reported in the aortopulmonary window.
Secondary and Tertiary Hyperparathyroidism
Almost all patients with advanced renal failure who are maintained by chronic dialysis have evidence of bone disease secondary to hyperparathyroidism and elevated serum levels of PTH.87–89 Medical therapy has improved the course of these patients (see Chapter 14), but genetic evidence has shown that monoclonal transformations with specific genetic mutations sometimes arise in these patients, so that parathyroid gland growth independence has occurred that may explain resistant hypercalcemia. Clinically, additional potential indications for parathyroidectomy include (1) hypercalcemia in prospective renal transplant patients; (2) pathologic fractures secondary to renal osteodystrophy; (3) symptoms such as pruritus, bone pain, and extensive soft-tissue calcification and calciphylaxis; (4) hypercalcemia in patients with well-functioning renal transplants; and (5) a calcium-times-phosphate product greater than 70.89 Improvements in medical management have reduced the need for surgery. Successful parathyroid surgery plus appropriate supplementation with vitamin D and calcium provide a marked increase in lumbar bone marrow density and a modest increase in radial bone marrow density in renal failure patients with secondary HPT.90
Reoperations for Primary Hyperparathyroidism
Reoperations for pHPT should be classified as operations for either persistent disease or recurrent disease. Persistent disease means that hypercalcemia never resolved after the initial neck exploration. Recurrent disease means that hypercalcemia recurs after 6 months of normalization of serum calcium. The complexity of repeat neck surgery for primary hyperparathyroidism makes it imperative to confirm the diagnosis and presence of symptoms and to order preoperative localization studies (see Fig. 9-4).42,43,65 The prior operative record and pathology reports are reviewed.
The first operative report, pathology results, and localization studies are used to plan the re-exploration. For example, if two abnormal parathyroid glands were removed and the family history is positive for parathyroid disease, the working diagnosis is hyperplasia. A biopsy-proven normal gland found at the initial procedure and radiologic localization studies suggesting a mediastinal adenoma prompt a direct mediastinal approach. Designing the operation—right side, left side, median sternotomy, all or any—can be done only by putting all the data together. We use an alternative route in the neck along the medial border of the sternocleidomastoid muscle instead of between the strap muscles.42,43,53,65 This technique requires a separate approach on each side of the neck. It is especially important to look for intrathyroid, intrathymic, and paraesophageal parathyroid adenomas because ectopic locations are more common in reoperations (Fig. 9-11).
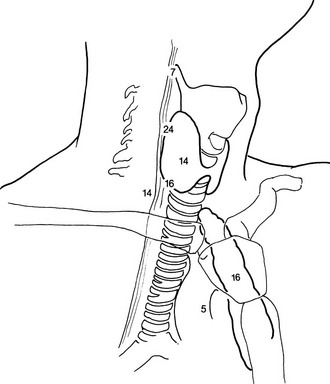
FIGURE 9-11 Location of abnormal parathyroid glands found during reoperations for pHPT. Numbers refer to the percentage of glands found at each location.
It should be remembered that even during reoperations for primary hyperparathyroidism, most abnormal glands can be removed through a cervical incision65 (see Fig. 9-11). Abnormal parathyroid glands may be retroesophageal or posterior along the tracheoesophageal groove, which is the most common missed position.50,53 They may be intrathyroidal,91 or they may be located in an undescended parathymic remnant high in the carotid sheath.92 A missed adenoma may be located in pharyngeal or adjacent structures like the vagus nerve.93 If these abnormal glands are not in the neck, they may be in the thymus. Slow, meticulous exploration in a bloodless field is generally necessary to find these “ectopic” glands (see Fig. 9-11).
Reoperative parathyroid disease remains a major challenge. It is clear that operative risk increases with each succeeding re-exploration. However, with careful attention to confirmation of the diagnosis, prior operative records, and judicious use of preoperative localization, a successful outcome may be achieved in approximately 90% of reoperations.50,92
Parathyroid Carcinoma
Parathyroid carcinoma should be considered in the working diagnosis of patients with pHPT when the serum level of calcium is very high (>14 mg/dL), in patients with vocal cord paralysis, in patients with evidence of local recurrence of the abnormal gland, or when a palpable neck mass is present.7,94 Parathyroid carcinoma may be familial.95
It is difficult to accurately assess the spectrum of clinical manifestations, degree of malignancy, and prognosis of parathyroid carcinoma. The incidence is very low, and the malignancy appears to be diagnosed at an earlier stage as a result of earlier detection of hypercalcemia. A major problem is failure to properly identify the correct pathologic diagnosis during the operation and, therefore, failure to perform adequate resection of the carcinomatous parathyroid tissue along with the ipsilateral lobe of the thyroid.7,96 Unequivocal pathologic features of parathyroid carcinoma include the identification of mitoses in several high-power microscopic fields, fibrous bands or desmoplasia, and evidence of distant metastases or direct local invasion of the capsule, adjacent structures, and blood vessels.97 However, not all patients with parathyroid carcinoma have all these features, so the diagnosis must be ascertained from clinical as well as pathologic evidence.7 Furthermore, the natural history of patients with parathyroid cancer appears to be variable. Some tumors disseminate rapidly and have a poor prognosis,98 whereas others tend to recur locally and have a long disease-free interval.7
Literature reports involving single cases tend to emphasize more serious tumors, either intrinsically malignant or longstanding, with clear evidence of extraglandular spread at the initial operation. The cancer is usually invading along the tracheoesophageal groove, and the patient may have hoarseness secondary to a recurrent laryngeal nerve injury. At neck exploration, the carcinomatous tissue appears gray with a thick, hard capsule. We recommend, based on suspicion (e.g., mass, local recurrence, high serum level of calcium), wide excision, including thyroid lobectomy in continuity with the tumor.7,96,97 If one has doubt about the diagnosis, biopsy of tumor extrinsic from the main tumor mass, either within lymph nodes or invading local strap muscle, provides clear evidence of cancer. Recurrent laryngeal nerve injury, either from the tumor itself or from the surgeon attempting to completely resect the tumor mass with the ipsilateral thyroid lobe, is probable and occurs in 75% of patients.7 Locally recurrent benign parathyroid adenomas may occur and be confused with parathyroid carcinomas. Recurrent adenomas generally have a longer disease-free interval, a lower serum level of calcium, and a history of either incomplete resection or spillage of tumor at the time of initial surgery.7 Nevertheless, both locally recurrent parathyroid adenoma and cancer appear to respond favorably to aggressive local re-resection, and most patients can be rendered either hypocalcemic or normocalcemic for a reasonable period.7 Once disease has spread to distant sites, surgery appears to have less of a role in treatment. Resection of pulmonary metastatic disease has been done without clear benefit.98 In patients with distant metastases, dacarbazine (DTIC) chemotherapy has been effective in some instances.97 Therapy for these patients has been primarily directed at controlling the severe hypercalcemia.
Postoperative Hypocalcemia
If the symptoms of hypocalcemia are severe and the patient appears to be on the verge of tetany (occurring most frequently in patients with “hungry bone syndrome”), the clinician may need to treat with intravenous calcium. These symptoms can usually be rapidly corrected by the infusion of 2 mg/kg of elemental calcium over a 15-minute period. Symptoms return unless a longer infusion is used. Approximately 15 mg/kg of elemental calcium is then infused over a 24-hour period, with half the total amount administered in the initial 6 hours. Serum levels of calcium should be monitored closely during the infusion, and infusion rates and amounts may be adjusted accordingly. Only approximately 13% of patients have severe symptoms of hypoparathyroidism after surgery. These patients appear to be older; have higher preoperative serum levels of calcium, PTH, alkaline phosphatase, and urea nitrogen; and have large adenomas removed at surgery16 and typically require intravenous calcium. Most patients do not need this type of calcium replacement.
When hypocalcemia persists despite maximal oral replacement doses, and hyperphosphatemia develops, 1,25(OH)2D3 (calcitriol) is initiated.99 This drug is recommended because of rapid onset of action and short duration of use. The usual initial dose of calcitriol is 0.25 to 1.0 µg/day given on a twice-daily schedule. The dose can be increased to a maximum of 2.0 µg/day, depending on the response in terms of serum levels of calcium and phosphorus. In general, the lowest possible dose that produces low normal serum levels and no hypocalcemic symptoms should be used. Serum levels of calcium should be monitored weekly after discharge to further adjust oral calcium and calcitriol doses.
References
1. NIH conference. Diagnosis and management of asymptomatic primary hyperparathyroidism: consensus development conference statement. Ann Intern Med. 1991;114:593–597.
2. Norton, JA. Controversies and advances in primary hyperparathyroidism. Ann Surg. 1992;215:297–299.
3. Scholz, DA, Purnell, DC. Asymptomatic primary hyperparathyroidism. 10-year prospective study. Mayo Clin Proc. 1981;56:473–478.
4. Bilezikian, JP, Khan, AA, Potts, JT, Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94(2):335–339.
5. Silverberg, SJ, Brown, I, Bilezikian, JP. Age as a criterion for surgery in primary hyperparathyroidism. Am J Med. 2002;113:681–684.
6. Delbridge, LW, Marshman, D, Reeve, TS, et al. Neuromuscular symptoms in elderly patients with hyperparathyroidism: improvement with parathyroid surgery. Med J Aust. 1988;149:74–76.
7. Fraker, DL, et al. Locally recurrent parathyroid neoplasms as a cause for recurrent and persistent primary hyperparathyroidism. Ann Surg. 1991;213:58–65.
8. Keiser, HR, Beaven, MA, Doppman, J, et al. Sipple’s syndrome: medullary thyroid carcinoma, pheochromocytoma, and parathyroid disease. Studies in a large family. NIH conference. Ann Intern Med. 1973;78:561–579.
9. Rizzoli, R, Green, J, 3rd., Marx, SJ. Primary hyperparathyroidism in familial multiple endocrine neoplasia type I. Long-term follow-up of serum calcium levels after parathyroidectomy. Am J Med. 1985;78:467–474.
10. Marx, SJ, et al. Heterogeneous size of the parathyroid glands in familial multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf). 1991;35:521–526.
11. The search for the MEN1 gene. The European Consortium on MEN-1. J Intern Med. 1998;243:441–446.
12. Lairmore, TC, et al. Familial medullary thyroid carcinoma and multiple endocrine neoplasia type 2B map to the same region of chromosome 10 as multiple endocrine neoplasia type 2A. Genomics. 1991;9:181–192.
13. Hosokawa, Y, Pollak, MR, Brown, EM, et al. Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors. J Clin Endocrinol Metab. 1995;80:3107–3110.
14. Bilezikian, JP. Management of acute hypercalcemia. N Engl J Med. 1992;326:1196–1203.
15. Fitzpatrick, LA, Bilezikian, JP. Acute primary hyperparathyroidism. Am J Med. 1987;82:275–282.
16. Brasier, AR, Nussbaum, SR. Hungry bone syndrome: clinical and biochemical predictors of its occurrence after parathyroid surgery. Am J Med. 1988;84:654–660.
17. Chen, H. Surgery for primary hyperparathyroidism: what is the best approach? Ann Surg. 2002;236:552–553.
18. Irvin, GL, 3rd., Carneiro, DM, Solorzano, CC. Progress in the operative management of sporadic primary hyperparathyroidism over 34 years. Ann Surg. 2004;239:704–708.
19. Palazzo, FF, Delbridge, LW. Minimal-access/minimally invasive parathyroidectomy for primary hyperparathyroidism. Surg Clin North Am. 2004;84:717–734.
20. Sosa, JA, Udelsman, R. Minimally invasive parathyroidectomy. Surg Oncol. 2003;12:125–134.
21. Udelsman, R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235:665–670.
22. Udelsman, R, Donovan, PI, Sokoll, LJ. One hundred consecutive minimally invasive parathyroid explorations. Ann Surg. 2000;232:331–339.
23. Slepavicius, A, Beisa, V, Janusonis, V, et al. Focused versus conventional parathyroidectomy for primary hyperparathyroidism: a prospective, randomized, blinded trial. Langenbecks Arch Surg. 2008.
24. Denham, DW, Norman, J. Cost-effectiveness of preoperative sestamibi scan for primary hyperparathyroidism is dependent solely upon the surgeon’s choice of operative procedure. J Am Coll Surg. 1998;186:293–305.
25. McBiles, M, Lambert, AT, Cote, MG, et al. Sestamibi parathyroid imaging. Semin Nucl Med. 1995;25:221–234.
26. Pappu, S, Donovan, P, Cheng, D, et al. Sestamibi scans are not all created equally. Arch Surg. 2005;140:383–386.
27. Rubello, D, Mariani, G. Hand-held gamma probe or hand-held miniature gamma camera for minimally invasive parathyroidectomy: competition, evolution or synergy? Eur J Nucl Med Mol Imaging. 2007;34:162–164.
28. Fuchs, SP, et al. Minimally-invasive parathyroidectomy: a good operative procedure for primary hyperparathyroidism even without the use of intraoperative parathyroid-hormone assessment or a gamma probe. Ned Tijdschr Geneeskd. 2005;149:1463–1467.
29. Bekis, R, et al. The role of gamma probe activity counts in minimally invasive parathyroidectomy. Preliminary results. Nuklearmedizin. 2004;43:190–194.
30. Ugur, O, et al. Clinicopathologic and radiopharmacokinetic factors affecting gamma probe-guided parathyroidectomy. Arch Surg. 2004;139:1175–1179.
31. Bozkurt, MF, Ugur, O, Hamaloglu, E, et al. Optimization of the gamma probe-guided parathyroidectomy. Am Surg. 2003;69:720–725.
32. Rubello, D, et al. Importance of radio-guided minimally invasive parathyroidectomy using hand-held gamma probe and low (99m)Tc-MIBI dose. Technical considerations and long-term clinical results. Q J Nucl Med. 2003;47:129–138.
33. Burkey, SH, et al. Will directed parathyroidectomy utilizing the gamma probe or intraoperative parathyroid hormone assay replace bilateral cervical exploration as the preferred operation for primary hyperparathyroidism? World J Surg. 2002;26:914–920.
34. Singer, JA, Sardi, A, Conaway, G, et al. Minimally invasive parathyroidectomy utilizing a gamma detecting probe intraoperatively. MD Med J. 1999;48:55–58.
35. Davis, ML, et al. Ultrasound facilitates minimally invasive parathyroidectomy in patients lacking definitive localization from preoperative sestamibi scan. Am J Surg. 2007;194:785–790.
36. Abraham, D, et al. Utility of ultrasound-guided fine-needle aspiration of parathyroid adenomas for localization before minimally invasive parathyroidectomy. Endocr Pract. 2007;13:333–337.
37. Kell, MR, et al. Minimally invasive parathyroidectomy with operative ultrasound localization of the adenoma. Surg Endosc. 2004;18:1097–1098.
38. Purcell, GP, et al. Parathyroid localization with high-resolution ultrasound and technetium Tc 99m sestamibi. Arch Surg. 1999;134:824–828.
39. Carty, SE, Norton, JA. Management of patients with persistent or recurrent primary hyperparathyroidism. World J Surg. 1991;15:716–723.
40. Shen, W, et al. Reoperation for persistent or recurrent primary hyperparathyroidism. Arch Surg. 1996;131:861–867.
41. Patow, CA, Norton, JA, Brennan, MF. Vocal cord paralysis and reoperative parathyroidectomy. A prospective study. Ann Surg. 1986;203:282–285.
42. Udelsman, R, Donovan, PI. Remedial parathyroid surgery: changing trends in 130 consecutive cases. Ann Surg. 2006;244:471–479.
43. Wang, TS, Udelsman, R. Remedial surgery for primary hyperparathyroidism. Adv Surg. 2007;41:1–15.
44. Miller, DL, et al. Localization of parathyroid adenomas in patients who have undergone surgery. Part I. Noninvasive imaging methods. Radiology. 1987;162:133–137.
45. Miller, DL. Pre-operative localization and interventional treatment of parathyroid tumors: when and how? World J Surg. 1991;15:706–715.
46. MacFarlane, MP, et al. Use of preoperative fine-needle aspiration in patients undergoing reoperation for primary hyperparathyroidism. Surgery. 1994;116:959–964.
47. Jaskowiak, N, et al. A prospective trial evaluating a standard approach to reoperation for missed parathyroid adenoma. Ann Surg. 1996;224:308–320.
48. Wei, JP, Burke, GJ, Mansberger, AR, Jr. Preoperative imaging of abnormal parathyroid glands in patients with hyperparathyroid disease using combination Tc-99m-pertechnetate and Tc-99m-sestamibi radionuclide scans. Ann Surg. 1994;219:568–572.
49. Allendorf, J, et al. The impact of sestamibi scanning on the outcome of parathyroid surgery. J Clin Endocrinol Metab. 2003;88:3015–3018.
50. Lange, JR, Norton, JA. Surgery for persistent or recurrent primary hyperparathyroidism. Curr Pract Surg. 1992;4:26.
51. Udelsman, R, et al. Rapid parathyroid hormone analysis during venous localization. Ann Surg. 2003;237:714–719.
52. Neumann, DR, et al. Regional body FDG-PET in postoperative recurrent hyperparathyroidism. J Comput Assist Tomogr. 1997;21:25–28.
53. Mittendorf, EA, Perrier, NP. Persistent or recurrent hyperparathyroidism. In: Cameron JL, ed. Current Surgical Therapy. ed 9. St Louis: Mosby; 2008:630.
54. Patel, PC, Pellitteri, PK, Patel, NM, et al. Use of a rapid intraoperative parathyroid hormone assay in the surgical management of parathyroid disease. Arch Otolaryngol Head Neck Surg. 1998;124:559–562.
55. Bergenfelz, A, Isaksson, A, Lindblom, P, et al. Measurement of parathyroid hormone in patients with primary hyperparathyroidism undergoing first and reoperative surgery. Br J Surg. 1998;85:1129–1132.
56. Garner, SC, Leight, GS, Jr. Initial experience with intraoperative PTH determinations in the surgical management of 130 consecutive cases of primary hyperparathyroidism. Surgery. 1999;126:1132–1137.
57. Yang, GP, Levine, S, Weigel, RJ. A spike in parathyroid hormone during neck exploration may cause a false-negative intraoperative assay result. Arch Surg. 2001;136:945–949.
58. Westra, WH, Pritchett, DD, Udelsman, R. Intraoperative confirmation of parathyroid tissue during parathyroid exploration: a retrospective evaluation of the frozen section. Am J Surg Pathol. 1998;22:538–544.
59. Boggs, JE, Irvin, GL, 3rd., Carneiro, DM, et al. The evolution of parathyroidectomy failures. Surgery. 1999;126:998–1002.
60. Irvin, GL, 3rd., Molinari, AS, Figueroa, C, et al. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. 1999;229:874–878.
61. Thompson, NW, Eckhauser, FE, Harness, JK. The anatomy of primary hyperparathyroidism. Surgery. 1982;92:814–821.
62. Attie, JN, Bock, G, Auguste, LJ. Multiple parathyroid adenomas: report of thirty-three cases. Surgery. 1990;108:1014–1019.
63. Roses, DF, et al. Primary hyperparathyroidism associated with two enlarged parathyroid glands. Arch Surg. 1989;124:1261–1265.
64. Wells, SA, Jr., Leight, GS, Ross, AJ, 3rd. Primary hyperparathyroidism. Curr Probl Surg. 1980;17:398–463.
65. Brennan, MF, Norton, JA. Reoperation for persistent and recurrent hyperparathyroidism. Ann Surg. 1985;201:40–44.
66. Grant, CS, van Heerden, JA, Charboneau, JW, et al. Clinical management of persistent and/or recurrent primary hyperparathyroidism. World J Surg. 1986;10:555–565.
67. Akerstrom, G, Malmaeus, J, Bergstrom, R. Surgical anatomy of human parathyroid glands. Surgery. 1984;95:14–21.
68. Wang, C. The anatomic basis of parathyroid surgery. Ann Surg. 1976;183:271–275.
69. Alveryd, A. Parathyroid glands in thyroid surgery. I. Anatomy of parathyroid glands. II. Postoperative hypoparathyroidism—identification and autotransplantation of parathyroid glands. Acta Chir Scand. 1968;389:1–120.
70. Wang, C, Mahaffey, JE, Axelrod, L, et al. Hyperfunctioning supernumerary parathyroid glands. Surg Gynecol Obstet. 1979;148:711–714.
71. Gilmour, J. The gross anatomy of the parathyroid glands. J Pathology. 1938;46:133.
72. Edis, AJ, Purnell, DC, van Heerden, JA. The undescended “parathymus.” An occasional cause of failed neck exploration for hyperparathyroidism. Ann Surg. 1979;190:64–68.
73. Schell, SR, Dudley, NE. Clinical outcomes and fiscal consequences of bilateral neck exploration for primary idiopathic hyperparathyroidism without preoperative radionuclide imaging or minimally invasive techniques. Surgery. 2003;133:32–39.
74. Burkey, SH, Snyder, WH, 3rd., Nwariaku, F, et al. Directed parathyroidectomy: feasibility and performance in 100 consecutive patients with primary hyperparathyroidism. Arch Surg. 2003;138:604–608.
75. Goldstein, RE, Billheimer, D, Martin, WH, et al. Sestamibi scanning and minimally invasive radioguided parathyroidectomy without intraoperative parathyroid hormone measurement. Ann Surg. 2003;237:722–730.
76. Biertho, L, Chu, C, Inabnet, WB. Image-directed parathyroidectomy under local anaesthesia in the elderly. Br J Surg. 2003;90:738–742.
77. Saxe, AW, Brown, E, Hamburger, SW. Thyroid and parathyroid surgery performed with patient under regional anesthesia. Surgery. 1988;103:415–420.
78. Norman, J, Denham, D. Minimally invasive radioguided parathyroidectomy in the reoperative neck. Surgery. 1998;124:1088–1092.
79. Genc, H, et al. Differing histologic findings after bilateral and focused parathyroidectomy. J Am Coll Surg. 2003;196:535–540.
80. Lee, NC, Norton, JA. Multiple-gland disease in primary hyperparathyroidism: a function of operative approach? Arch Surg. 2002;137:896–899.
81. Wells, SA, Jr., Ellis, GJ, Gunnells, JC, et al. Parathyroid autotransplantation in primary parathyroid hyperplasia. N Engl J Med. 1976;295:57–62.
82. Wells, SA, Jr., Farndon, JR, Dale, JK, et al. Long-term evaluation of patients with primary parathyroid hyperplasia managed by total parathyroidectomy and heterotopic autotransplantation. Ann Surg. 1980;192:451–458.
83. Saxe, AW, Brennan, MF. Reoperative parathyroid surgery for primary hyperparathyroidism caused by multiple-gland disease: total parathyroidectomy and autotransplantation with cryopreserved tissue. Surgery. 1982;91:616–621.
84. Senapati, A, Young, AE. Parathyroid autotransplantation. Br J Surg. 1990;77:1171–1174.
85. Norton, JA, Venzon, DJ, Berna, MJ, et al. Prospective study of surgery for primary hyperparathyroidism (HPT) in multiple endocrine neoplasia-type 1 and Zollinger-Ellison syndrome: long-term outcome of a more virulent form of HPT. Ann Surg. 2008;247(3):501–510.
86. Wells, SA, Jr., Cooper, JD. Closed mediastinal exploration in patients with persistent hyperparathyroidism. Ann Surg. 1991;214:555–561.
87. Johnson, WJ, et al. Results of subtotal parathyroidectomy in hemodialysis patients. Am J Med. 1988;84:23–32.
88. Arnold, A, et al. Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest. 1995;95:2047–2053.
89. Andress, DL, Ott, SM, Maloney, NA, et al. Effect of parathyroidectomy on bone aluminum accumulation in chronic renal failure. N Engl J Med. 1985;312:468–473.
90. Yano, S, et al. Effect of parathyroidectomy on bone mineral density in hemodialysis patients with secondary hyperparathyroidism: possible usefulness of preoperative determination of parathyroid hormone level for prediction of bone regain. Horm Metab Res. 2003;35:259–264.
91. Wang, C, Gaz, RD, Moncure, AC. Mediastinal parathyroid exploration: a clinical and pathologic study of 47 cases. World J Surg. 1986;10:687–695.
92. Fraker, DL, et al. Undescended parathyroid adenoma: an important etiology for failed operations for primary hyperparathyroidism. World J Surg. 1990;14:342–348.
93. Chan, TJ, et al. Persistent primary hyperparathyroidism caused by adenomas identified in pharyngeal or adjacent structures. World J Surg. 2003;27:675–679.
94. Wang, CA, Gaz, RD. Natural history of parathyroid carcinoma. Diagnosis, treatment, and results. Am J Surg. 1985;149:522–527.
95. Streeten, EA, Weinstein, LS, Norton, JA, et al. Studies in a kindred with parathyroid carcinoma. J Clin Endocrinol Metab. 1992;75:362–366.
96. Cohn, K, Silverman, M, Corrado, J, et al. Parathyroid carcinoma: the Lahey Clinic experience. Surgery. 1985;98:1095–1100.
97. Calandra, DB, Chejfec, G, Foy, BK, et al. Parathyroid carcinoma: biochemical and pathologic response to DTIC. Surgery. 1984;96:1132–1137.
98. Flye, MW, Brennan, MF. Surgical resection of metastatic parathyroid carcinoma. Ann Surg. 1981;193:425–435.
99. Reichel, H, Koeffler, HP, Norman, AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980–991.