Chapter 110 Surgical Management of Extratemporal Lobe Epilepsy
Extratemporal lobe epilepsy comprises debilitating conditions of heterogeneous symptomatology and pathology, both challenging to treat and yet amenable to several surgical interventions. It can coexist with mesial temporal sclerosis as dual pathology. A total of 4% to 15% of adult temporal lobe resections involve dual temporal and extratemporal pathology. The extratemporal pathology is most commonly cortical dysplasia followed by vascular malformations, infarction, and dysembryoplastic neuroepithelial tumors in descending order of frequency.1–3 The higher childhood prevalence of dual pathology suggests earlier progression to intractable epilepsy and hence surgery.4
History
Little was understood about anatomic localization of brain function and thus epilepsy until the late 19th century when David Ferrier and John Hughlings Jackson characterized cerebral functions in monkeys and humans, respectively.6,7 Hughlings Jackson rightly commented that “a convulsion is but a symptom, and implies only that there is an occasional, an excessive, and a disorderly discharge of nerve tissue on the muscles. This discharge occurs in all degrees; it occurs with all sorts of conditions of ill health, at all ages and under innumerable circumstances.” A prevailing British culture of cerebral localization emboldened first the Glaswegian William Macewen in 1879 and then the Englishman Sir Rickman Godlee in 1884 to perform exploratory craniotomies upon young patients with contralateral focal seizures.8,9 Both were vindicated, Macewen discovering of an acute subdural haematoma and Godlee finding a brain tumor. Sir Victor Horsley also performed extratemporal surgery for focal seizures in the late 19th century, describing in 10 cases a combination of subpial and lobar resections.10 Horsley’s cortical escharotomies of his patients led Hughlings Jackson to conclude of three cases that “there was in every case of epileptiform seizures a very local change of some kind,” and that because “the starting point of the fit was the sign to us of a discharging lesion, he would advise cutting out that lesion, whether it was produced by tumour or not.”11 Thus, resective extratemporal epilepsy surgery was commenced.
Corpus callosotomy arose in the 1930s from Van Wagenen’s serendipitous observation that patients with stroke involving the corpus callosum often had improvements in seizures. “It was decided to divide the corpus callosum surgically in an effort to limit the spread of a convulsive wave to one half of the cerebrum.”12 In both children and adults, corpus callosotomy appears, on average, to improve drop attacks and generalized tonic and tonic-clonic seizure frequency by 80% in 70% of patients and complex partial, myoclonic and absence seizure frequency by 50% in 50% of patients.13 Hemispherectomy was first described for infantile hemiplegia by Krynauw in 1950.14 Having late complications of hemosiderosis, hydrocephalus, brain shift and hemorrhagic membrane formation, it was abandoned in the 1970s in favor of functional hemispherectomy advocated by Rasmussen.15 Most studies relate to children, three published adult studies reporting, respectively, 4 of 4, 5 of 9, and 5 of 12 patients becoming seizure free.16–18 Multiple subpial transection aims to limit the horizontal spread of epileptiform activity across functional columns of eloquent cortex. A meta-analysis of adults and children has shown greater than 95% seizure frequency reduction in 87% of patients with generalized seizures and 68% with partial seizures, compared to cortical transections alone.19 It has been recommended for acquired epileptic aphasia (Landau-Kleffner syndrome).20 Late seizure recurrence in adults has, however, been reported in other adult groups.21
Electrical neuromodulatory approaches to extratemporal epilepsy are indicated where epilepsy persists despite resection of epileptogenic foci or in the palliative circumstance where no seizure focus is demonstrated using scalp recording. Nonnoninvasive neuroimaging and invasive recording are described elsewhere. Vagus nerve stimulation has been approved in many countries for partial seizures with or without secondary generalization based on trials showing differences in seizure frequency reduction between high-frequency (25%–30% reduction) and low-frequency (6%–15% reduction) stimulation groups.22 Median seizure reductions in 454 patients were 44% from baseline after 3 years, with 43% of patients having at least 50% seizure frequency reduction, although 20% had persisting hoarseness at 2 years of follow-up.23 Trigeminal nerve stimulation has also recently been reported, 12 patients having a median 66% seizure frequency reduction at 3 months with occasional side effects of orbicularis oculi twitching and dental discomfort and paraesthesia. The results augur for larger trials given its potential advantages over vagus nerve stimulation or transcutaneous test stimulation.24
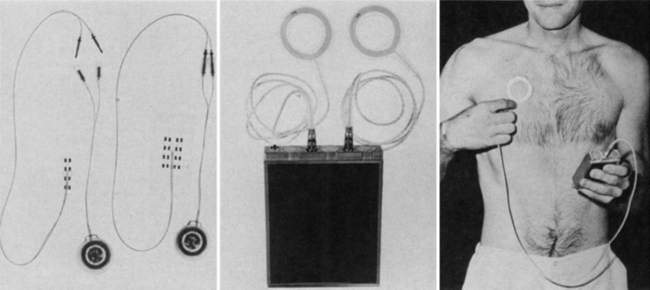
DBS for epilepsy is almost as old as human stereotactic surgery, having first been attempted acutely in 1952 by Heath. He recorded interictal spikes from the septum in a patient with complex partial seizures and then stimulated him. “Almost instantly, his behavioural state changed from one of disorganisation, rage and persecution to one of happiness and mild euphoria.”25 Cooper first treated epilepsy with implantable DBS throughout the 1970s, targeting the superomedial cerebellar cortex and reporting seizure reduction first in 6 of 7 and then later 18 of 32 patients (Fig. 110-1).26 Eleven unblinded case series have reported benefits in 88 of 116 patients (76%),27 but two small double-blinded studies comprising 14 patients have shown no benefit.28 Cerebellar stimulation therefore fell out of favor, with the exception of one recent report of five patients with generalized tonic-clonic seizures showing mean seizure frequency reduction of 59% at 6 months after surgery.29 Current deep brain targets under evaluation for epilepsy are the anterior thalamic nucleus, centromedian thalamus, subthalamic nucleus, posterior hypothalamus, hippocampus caudate, corpus callosum, and brain stem.30,31 We review current clinical outcomes information for these brain regions below.
Anterior Thalamic Nucleus Stimulation
Bilateral DBS of the anterior thalamic nuclei has been undertaken by several groups and is currently being investigated in a multicenter, double-blind, randomized clinical trial–stimulation of the anterior nucleus of the thalamus for epilepsy (SANTE), the first results of which were published in 2010.32 Several small recent studies showed clinical benefits leading to the trial. Lim et al. found seizure frequency reduced 49% from baseline on average in four patients with a mean 44 months of follow-up.33 Osorio et al. showed mean reductions of 75% in four patients delivering stimulation only with detection of electroencephalogram (EEG) changes.34 Lee et al. studied three patients, finding a 75% reduction in seizure frequency at a mean follow-up of 13 months.35
Ten patients were studied in two centers as pilot data for SANTE. Kerrigan et al. reported four of five patients showing significant reductions in frequency and severity of seizures after 6 to 36 months without complications.36 Hodaie et al. reported a mean reduction from baseline of 54% in seizure frequency at mean follow-up of 15 months, also without adverse effects and with a stun effect reducing seizure frequency before turning on stimulation.37 Andrade et al. later described five of six patients from the latter center with improvements of at least 50% in their seizure frequency over a mean follow-up period of 5 years.38
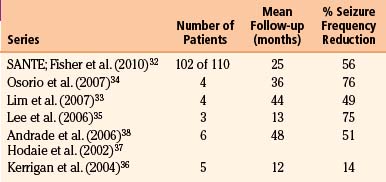
SANTE recruited 110 patients with medically refractory partial or secondarily generalized seizures.32 Bilateral DBS was standardized to monopolar stimulation at a frequency of 145 Hz, pulse width of 90 μs and cycle time on for 1 minute, and then off for 5 minutes using quadripolar electrodes (Medtronic Inc., Minneapolis, MN, USA). Significant seizure reduction from a median baseline of 19.5 seizures per month was seen in the group stimulated at an amplitude of 5 volts compared to the placebo-implanted group stimulated at 0 volts, with a 29% seizure reduction in the last month of a 3-month blinded phase. After 3 months, all patients were transferred to 5-volt DBS. Median seizure frequency reduction continued to improve with DBS throughout 3 years of the trial, with a 41% median seizure frequency reduction at 1 year, 56% reduction in 102 patients at 2 years, and 68% reduction in 57 patients at 3 years, 54% of patients having a seizure reduction of at least 50%, and 14 patients being seizure free at 6 months. No surgery-related symptomatic hemorrhages or deaths were reported, although two participants had transient, stimulation-induced seizures. Results of SANTE and other clinical studies are summarized in Table 110-1.
Table 110-1 Case Series of Bilateral, Anterior, Thalamic Nucleus Deep Brain Stimulation for Partial or Secondarily Generalized Epilepsy
The mechanisms of anterior thalamic nucleus stimulation remain unclear. Animal models have shown seizure reduction in drug-induced seizure models, postulating current dependent and serotonin mediated effects.39,40 Anatomic evidence suggests widespread limbic system sclerosis in epilepsy, including projections to and from anterior thalamic nucleus to cingulate cortex and hippocampus.41 Concurrent thalamic and scalp EEG recording studies after surgery suggest a recruiting rhythm, elicited with low-frequency stimulation, correlating with clinical improvement.42 The nucleus is small and projects to and from many limbic and cortical structures, yet is less deep and proximal to subarachnoid vessels than the mammillary bodies, enabling safer targeting. Stereotactic ablation of the target demonstrated seizure reduction four decades ago,43 motivating its intensive experimental and clinical study.
Centromedian Thalamic Stimulation
Wilder Penfield first postulated that the centromedian thalamic nucleus could be modulated to ameliorate seizures, noting its diffuse projections from the brain stem to the cerebral cortex.44 Targeting the parvocellular division of the centromedian nucleus bilaterally, Velasco and colleagues have found seizure frequency reduction in 13 patients with Lennox-Gastaut syndrome.45 Low-frequency (6–8 Hz) bipolar stimulation was delivered through pairs of adjacent quadripolar electrode contacts while assessing responses in concomitant scalp EEG. Implanted DBS settings were low frequency or high (130 Hz), pulse width 450 μs and amplitude of 6 to 10 volts. Their results from unblinded case series showed significant improvements in generalized seizures, less so in complex partial seizures, and seizure frequency increases with depleted battery life of implantable pulse generators.46 The results have, however, not yet been replicated by others for this brain target. A double-blinded, crossover design, trial in seven patients by Fisher et al. revealed no clinical improvement in seizure frequency with centromedian thalamic stimulation,47 nor did a recent report of two patients with longer-term follow-up.38
Subthalamic Nucleus Stimulation
The subthalamic nucleus has been established of late as the surgical target of choice in DBS for Parkinson’s disease, and is therefore appealing, as functional neurosurgeons should have most experience with it. A mechanistic rationale for its stimulation has arisen from observations that the substantia nigra pars reticulara may gate seizures through inhibitory nigrotectal projections to the superior colliculi, its inhibition suppressing seizures in animal models.48 Subthalamic nucleus inhibition has also been shown to suppress seizures in animals.49 Clinically, Chabardès et al. demonstrated mean 64% seizure frequency reduction in four of five patients.50 Other small studies have also shown improvements, two of five patients reported by Loddenkemper et al. as having 80% improvement in their partial seizures and two patients having their seizures mildly reduced according to Handforth et al.51,52 Despite this evidence and Benabid’s promising case report of a child showing 81% seizure improvement at 30 months,53 larger, blinded trials are required to demonstrate efficacy.
Other Deep Brain Targets
The posterior hypothalamus has been targeted by DBS based on observations that the mammillary bodies show epileptiform activity with depth electrode recording.54 A report of two patients by Franzini et al. showed seizure frequency reduced by up to 80% from baseline after 9 months follow-up.55 The mammillothalamic tract has also been stimulated to relieve gelastic seizures secondary to hypothalamic hamartomas with improvements in seizure frequency.56 Growing case series of DBS for cluster headache targeting the posterior hypothalamus increase our experience and confidence of this challenging deep brain target adjacent to the Circle of Willis,57 with initial evidence pointing towards specific indications of epilepsy with concomitant aggression or gelastic seizures.
DBS of the head of the caudate has been studied as an epilepsy treatment after nonhuman primate studies showing reductions in induced seizures with stimulation.58 Sramka et al. have published a study of 74 patients showing reduced interictal EEG activity with both caudate and dentate nucleus stimulation.59 Chkhenkeli et al. have stimulated the ventral caudate head at low frequency (4–8 Hz) in 38 patients, impressively eliminating seizures in 21 patients and improving 35 patients overall (92%).60 These results both encourage further, more controlled, clinical trials of caudate DBS.
We will not discuss hippocampal DBS as it relates largely to mesial temporal rather than extratemporal lobe epilepsy. However, two other deep brain targets deserve brief mention. The corpus callosum has been stimulated in 10 patients, perhaps unsurprisingly without benefit,61 and the locus coeruleus has been stimulated unilaterally in 2 patients, seemingly improving seizure frequency.62
Operative Technique
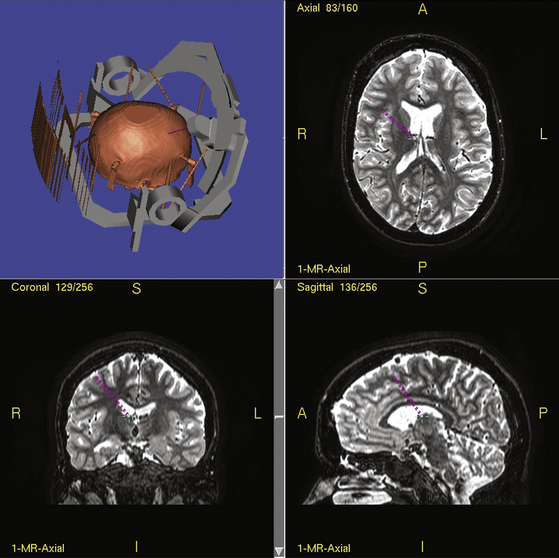
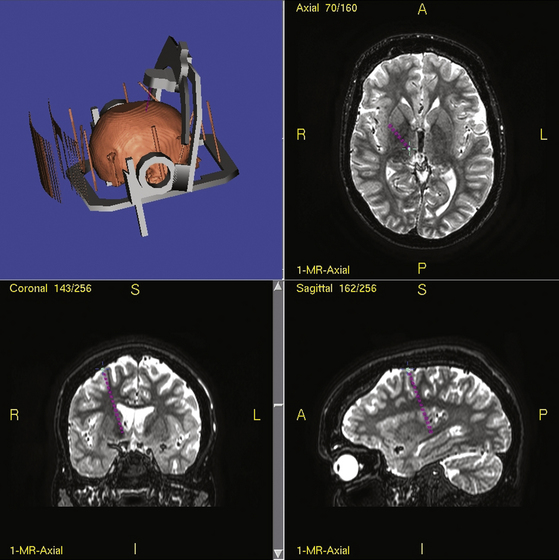
Stereotactic techniques differ considerably between surgeons regarding frame used (if indeed used at all), targeting software used, whether the patient is awake or asleep, whether microelectrode recording is performed, and now whether DBS equipment is used. Our technique is detailed elsewhere.63,64 Details of how to perform stereotactic DBS procedures can be found elsewhere in this book. To summarize our technique, all patients now have several MRI scans prior to epilepsy surgery including T1, T2, FLAIR, inversion-recovery, and DTI sequences in 1-mm thick axial slices. For surgery, a Cosman-Roberts-Wells (CRW) base ring is applied to the patient’s head under general anesthesia. A stereotactic CT scan is then performed and the MRI scan volumetrically fused to it using Radionics Image Fusion and Stereoplan programs to eliminate spatial distortions using MRI stereotaxy alone, that arise from magnetic field effects.65,66 The coordinates of the thalamic target are then calculated. Each electrode is passed to target via a twist-drill craniostomy following a linear parasagittal scalp incision after impedance checks confirming an extraventricular approach through grey matter. Implanted electrodes are then secured to the skull using the BIOPLATE system (Codman, Raynham, MA).
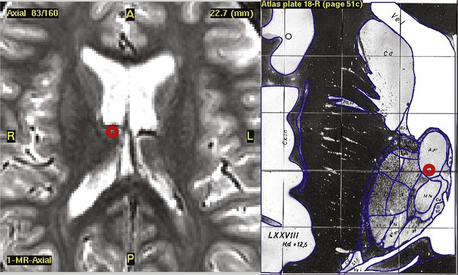
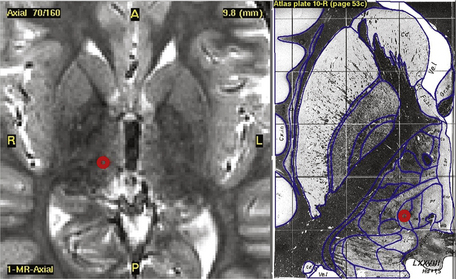
Typical coordinates for the anterior thalamic nucleus are 5 to 6 mm lateral to the midcommissural point, 8 mm anterior to the posterior commissure and 12 mm above the anterior commissure/posterior commissure (AC/PC) line, the angle trajectory being approximately 60 degrees posteroinferiorly from the coronal plane (Figs. 110-2 and 110-3). As the anterior thalamic nuclei are visible in the floor of the lateral ventricles on MRI, direct localization is also possible. Common coordinates for the centromedian thalamic nucleus are 11 mm lateral to the midcommissural point, 2 mm above the AC/PC line at the midpoint of the third ventricle (Figs. 110-4 and 110-5). We aim to place at least two electrode contacts in the parvocellular subdivision of the centromedian nucleus, its most lateral, anterior, and ventral part to optimize efficacy (Fig. 110-4). Indirect localization of either nucleus is verified using the Schaltenbrand atlas (Figs. 110-2 and 110-4).67
Complications
While stereotactic ablation for epilepsy has been successfully performed in the past,43–68 DBS confers adjustability and reversibility such that unwanted adverse effects can be titrated out and avoided. DBS supersedes lesioning surgery due to lower mortality rates and reductions in both serious complications such as hemorrhage and adverse effects upon cognition, speech, and swallowing.69 However, the DBS technique confers higher risks of infection due to the neuroprosthesis and of complications related to the frequently longer operative duration. Thalamic nucleus DBS for epilepsy appears safe and well-tolerated with reported adverse effects being transient and mild. These include episodic nystagmus with cycling stimulation, auditory hallucinations, paranoid ideation, anorexia, and lethargy. Complications in the epilepsy case series cited above of an asymptomatic small right frontal hematoma, scalp erosion requiring DBS removal, implantable pulse generator infection and accidental switching off of the stimulator are all well described in DBS for other conditions and do not seem to occur more frequently than the norm.
Whether described as “neuromodulation,” “neurostimulation,” or “functional,” it should be emphasized that DBS is an intracranial surgical procedure that brings small but significant risks. Aside from multidisciplinary assessment to determine suitability for the procedure, the patient must be refractory to medical treatment and able to give informed consent (where appropriate) to risks of stroke (1%–3%), worsening of seizures (<1%), death (0.1%), skin erosion, lead breakage, and the need for implantable pulse generator revision surgery every 1 to 10 years regardless of whether rechargeable equipment is used, and infection (3%)—a small proportion of cases requiring complete removal of the DBS system.64,69,70 Patients should also be counseled for the possibility that they may derive no benefit from DBS or not tolerate it well, again necessitating its removal. The likelihood of this varies from indication to indication, but it should be emphasized when treating less established nonmovement disorder indications such as epilepsy.
Closed-Loop Stimulation
Closed-loop also known as demand-driven or “smart” stimulation describes devices that respond to and terminate epileptiform activity. They have potential advantages not just in terms of efficacy but also in increased efficiency prolonging device and battery longevity and reduced adverse effects.71 A recent trial of automated seizure detection equipment delivering stimulation to the anterior thalamic nucleus and cortex reduced seizure frequency in three of four cortically stimulated and two of four thalamically stimulated patients.72 A multicenter trial of a closed-loop stimulator is currently underway for patients with partial onset seizures,73 having shown a 45% seizure frequency reduction in seven of eight patients at 9 months follow-up.74 Such technology holds much promise and seems naturally suited to ameliorating epileptic attacks.
Conclusions
The three established resective surgical approaches to extratemporal epilepsy–hemispherectomy, corpus callosotomy, and multiple subpial transection offer good clinical outcomes for seizure reduction and freedom with low morbidity in adults with medically refractory epilepsy. Their indications vary and are detailed elsewhere. DBS is emerging as a viable neurosurgical therapy for epilepsy of partial onset with or without secondary generalization for the cohort of patients not suitable for or having failed resective surgery. While bilateral anterior thalamic DBS presently holds the most promise presently, other putative targets of stimulation may depend upon seizure localization and also include the subthalamic nucleus, caudate, hippocampus, cerebellum, hypothalamus, and centromedian thalamus. Continued research is necessary to clarify the potential patient populations for whom the techniques are best indicated, which targets are most efficacious, optimal stimulation parameters, and the best mode of stimulation, whether cyclical, continuous, or smarter.
Andrade D.M., Zumsteg D., Hamani C., et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology. 2006;66(10):1571-1573.
Benabid A.L., Minotti L., Koudsie A., et al. Antiepileptic effect of high-frequency stimulation of the subthalamic nucleus (corpus luysi) in a case of medically intractable epilepsy caused by focal dysplasia: a 30-month follow-up: technical case report. Neurosurgery. 2002;50(6):1385-1391. discussion 1391–1392
Chabardes S., Kahane P., Minotti L., et al. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4(suppl 3):S83-S93.
Chkhenkeli S.A., Sramka M., Lortkipanidze G.S., et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106(4):318-329.
Cooper I.S., Amin I., Gilman S. The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc. 1973;98:192-196.
Cukiert A., Baumel S.W., Andreolli M., Marino R.Jr. Effects of corpus callosum stimulation on the morphology and frequency of epileptic bursts in the feline topical penicillin generalized model. Stereotact Funct Neurosurg. 1989;52(1):18-25.
Feinstein B., Gleason C.A., Libet B. Stimulation of locus coeruleus in man. Preliminary trials for spasticity and epilepsy. Stereotact Funct Neurosurg. 1989;52(1):26-41.
Fisher R., Salanova V., Witt T., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
Fisher R.S., Uematsu S., Krauss G.L., et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33(5):841-851.
Fountas K.N., Smith J.R., Murro A.M., et al. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 2005;83(4):153-158.
Franzini A., Messina G., Marras C., et al. Deep brain stimulation of two unconventional targets in refractory nonresectable epilepsy. Stereotact Funct Neurosurg. 2008;86(6):373-381.
Handforth A., DeSalles A.A., Krahl S.E. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. 2006;47(7):1239-1241.
Hodaie M., Wennberg R.A., Dostrovsky J.O., Lozano A.M. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43(6):603-608.
Khan S., Wright I., Javed S., et al. High frequency stimulation of the mamillothalamic tract for the treatment of resistant seizures associated with hypothalamic hamartoma. Epilepsia. 2009;50(6):1608-1611.
Kerrigan J.F., Litt B., Fisher R.S., et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45(4):346-354.
Lee K.J., Jang K.S., Shon Y.M. Chronic deep brain stimulation of subthalamic and anterior thalamic nuclei for controlling refractory partial epilepsy. Acta Neurochir Suppl. 2006;99:87-91.
Lim S.N., Lee S.T., Tsai Y.T., et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia. 2007;48(2):342-347.
Loddenkemper T., Pan A., Neme S., et al. Deep brain stimulation in epilepsy. J Clin Neurophysiol. 2001;18(6):514-532.
Oakley J.C., Ojemann G.A. Effects of chronic stimulation of the caudate nucleus on a preexisting alumina seizure focus. Exp Neurol. 1982;75(2):360-367.
Osorio I., Overman J., Giftakis J., Wilkinson S.B. High frequency thalamic stimulation for inoperable mesial temporal epilepsy. Epilepsia. 2007;48(8):1561-1571.
Pereira E.A., Green A.L., Nandi D., Aziz T.Z. Deep brain stimulation: indications and evidence. Expert Rev Med Devices. 2007;4(5):591-603.
Sramka M., Chkhenkeli S.A. Clinical experience in intraoperational determination of brain inhibitory structures and application of implanted neurostimulators in epilepsy. Stereotact Funct Neurosurg. 1990;54-55:56-59.
Velasco A.L., Velasco F., Jimenez F., et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47(7):1203-1212.
Velasco F., Velasco A.L., Velasco M., et al. Deep brain stimulation for treatment of the epilepsies: the centromedian thalamic target. Acta Neurochir Suppl. 2007;97(Pt 2):337-342.
Vercueil L., Benazzouz A., Deransart C., et al. High-frequency stimulation of the subthalamic nucleus suppresses absence seizures in the rat: comparison with neurotoxic lesions. Epilepsy Res. 1998;31(1):39-46.
1. Salanova V., Markand O., Worth R. Temporal lobe epilepsy: analysis of patients with dual pathology. Acta Neurol Scand. 2004;109(2):126-131.
2. Li L.M., Cendes F., Andermann F., et al. Surgical outcome in patients with epilepsy and dual pathology. Brain. 1999;122(Pt 5):799-805.
3. Cascino G.D., Jack C.R.Jr., Parisi J.E., et al. Operative strategy in patients with MRI-identified dual pathology and temporal lobe epilepsy. Epilepsy Res. 1993;14(2):175-182.
4. Harkness W. Temporal lobe resections. Childs Nerv Syst. 2006;22(8):936-944.
5. Chadwick J., Mann W.N. The Medical Works of Hippocrates: A New Translation from the Original Greek Made Especially for English Readers. Oxford: Blackwell Scientific; 1950.
6. Ferrier D. The Functions of the Brain. London: Smith Elder & Co; 1876.
7. Jackson J.H. A Study of Convulsions. London: Printed by Odell & Ives; 1870.
8. Macewen W. An address on the surgery of the brain and spinal cord delivered at the Annual Meeting of the British Medical Association, held in Glasgow, August 9th, 1888. BMJ. 1888;2:302-309.
9. Bennett A.H., Godlee R.J. Case of cerebral tumour. Med Chir Trans (2nd ser). 1885;68:243-275.
10. Horsley V. Remarks on ten consecutive cases of operations upon the brain and cranial cavity to illustrate the details and safety of the method employed. BMJ. 1887;1(1373):863-865.
11. Horsley V. Brain surgery. BMJ. 2, 1886. 670–675
12. Van Wagenen W.P., Harren R.Y. Surgical division of the commissural pathways in the corpus callosum: relation to spread of an epileptic attack. Arch Neurol Psychiatry. 1940;44:740-759.
13. Spencer S., Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525-537.
14. Krynauw R.A. Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13:243-267.
15. Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10(2):71-78.
16. Cukiert A., Cukiert C.M., Argentoni M., et al. Outcome after hemispherectomy in hemiplegic adult patients with refractory epilepsy associated with early middle cerebral artery infarcts. Epilepsia. 2009;50(6):1381-1384.
17. McClelland S.3rd, Maxwell R.E. Hemispherectomy for intractable epilepsy in adults: the first reported series. Ann Neurol. 2007;61(4):372-376.
18. Steinhoff B.J., Staack A.M., Bilic S., et al. Functional hemispherectomy in adults with intractable epilepsy syndromes: a report of 4 cases. Epileptic Disord. 2009;11(3):251-257.
19. Spencer S.S., Schramm J., Wyler A., et al. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43(2):141-145.
20. Irwin K., Birch V., Lees J., et al. Multiple subpial transection in Landau-Kleffner syndrome. Dev Med Child Neurol. 2001;43(4):248-252.
21. Orbach D., Romanelli P., Devinsky O., Doyle W. Late seizure recurrence after multiple subpial transections. Epilepsia. 2001;42(10):1316-1319.
22. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995;45(2):224-230.
23. Morris G.L.3rd, Mueller W.M. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53(8):1731-1735.
24. DeGiorgio C.M., Murray D., Markovic D., Whitehurst T. Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology. 2009;72(10):936-938.
25. Heath R.G. Electrical self-stimulation of the brain in man. Am J Psychiatry. 1963;120:571-577.
26. Cooper I.S., Amin I., Gilman S. The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc. 1973;98:192-196.
27. Davis R. Cerebellar stimulation for seizure control. In: Lozano A.M., Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. Heidelberg: Springer-Verlag; 2009:2823-2837.
28. Wright G.D., McLellan D.L., Brice J.G. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47(8):769-774.
29. Velasco F., Carrillo-Ruiz J.D., Brito F., et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. 2005;46(7):1071-1081.
30. Theodore W.H., Fisher R.S. Brain stimulation for epilepsy. Lancet Neurol. 2004;3(2):111-118.
31. Lockman J., Fisher R.S. Therapeutic brain stimulation for epilepsy. Neurol Clin. 2009;27(4):1031-1040.
32. Fisher R., Salanova V., Witt T., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
33. Lim S.N., Lee S.T., Tsai Y.T., et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia. 2007;48(2):342-347.
34. Osorio I., Overman J., Giftakis J., Wilkinson S.B. High frequency thalamic stimulation for inoperable mesial temporal epilepsy. Epilepsia. 2007;48(8):1561-1571.
35. Lee K.J., Jang K.S., Shon Y.M. Chronic deep brain stimulation of subthalamic and anterior thalamic nuclei for controlling refractory partial epilepsy. Acta Neurochir Suppl. 2006;99:87-91.
36. Kerrigan J.F., Litt B., Fisher R.S., et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45(4):346-354.
37. Hodaie M., Wennberg R.A., Dostrovsky J.O., Lozano A.M. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43(6):603-608.
38. Andrade D.M., Zumsteg D., Hamani C., et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology. 2006;66(10):1571-1573.
39. Mirski M.A., Ziai W.C., Chiang J., et al. Anticonvulsant serotonergic and deep brain stimulation in anterior thalamus. Seizure. 2009;18(1):64-70.
40. Hamani C., Hodaie M., Chiang J., et al. Deep brain stimulation of the anterior nucleus of the thalamus: effects of electrical stimulation on pilocarpine-induced seizures and status epilepticus. Epilepsy Res. 2008;78(2-3):117-123.
41. Oikawa H., Sasaki M., Tamakawa Y., Kamei A. The circuit of Papez in mesial temporal sclerosis: MRI. Neuroradiology. 2001;43(3):205-210.
42. Zumsteg D., Lozano A.M., Wennberg R.A. Rhythmic cortical EEG synchronization with low frequency stimulation of the anterior and medial thalamus for epilepsy. Clin Neurophysiol. 2006;117(10):2272-2278.
43. Mullan S., Vailati G., Karasick J., Mailis M. Thalamic lesions for the control of epilepsy. A study of nine cases. Arch Neurol. 1967;16(3):277-285.
44. Penfield W. The cerebral cortex in man. I. The cerebral cortex and consciousness (Harvey Lecture, 1936). Arch Neurol Psychiatry. 1938;38:725-743.
45. Velasco A.L., Velasco F., Jimenez F., et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47(7):1203-1212.
46. Velasco F., Velasco A.L., Velasco M., et al. Deep brain stimulation for treatment of the epilepsies: the centromedian thalamic target. Acta Neurochir Suppl. 2007;97(Pt 2):337-342.
47. Fisher R.S., Uematsu S., Krauss G.L., et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33(5):841-851.
48. Gale K. Role of the substantia nigra in GABA-mediated anticonvulsant actions. Adv Neurol. 1986;44:343-364.
49. Vercueil L., Benazzouz A., Deransart C., et al. High-frequency stimulation of the subthalamic nucleus suppresses absence seizures in the rat: comparison with neurotoxic lesions. Epilepsy Res. 1998;31(1):39-46.
50. Chabardes S., Kahane P., Minotti L., et al. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4(suppl 3):S83-S93.
51. Handforth A., DeSalles A.A., Krahl S.E. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. 2006;47(7):1239-1241.
52. Loddenkemper T., Pan A., Neme S., et al. Deep brain stimulation in epilepsy. J Clin Neurophysiol. 2001;18(6):514-532.
53. Benabid A.L., Minotti L., Koudsie A., et al. Antiepileptic effect of high-frequency stimulation of the subthalamic nucleus (corpus luysi) in a case of medically intractable epilepsy caused by focal dysplasia: a 30-month follow-up: technical case report. Neurosurgery. 2002;50(6):1385-1391. discussion 1391–1392
54. van Rijckevorsel K., Abu Serieh B., de Tourtchaninoff M., Raftopoulos C. Deep EEG recordings of the mammillary body in epilepsy patients. Epilepsia. 2005;46(5):781-785.
55. Franzini A., Messina G., Marras C., et al. Deep brain stimulation of two unconventional targets in refractory nonresectable epilepsy. Stereotact Funct Neurosurg. 2008;86(6):373-381.
56. Khan S., Wright I., Javed S., et al. High frequency stimulation of the mamillothalamic tract for the treatment of resistant seizures associated with hypothalamic hamartoma. Epilepsia. 2009;50(6):1608-1611.
57. Grover P.J., Pereira E.A., Green A.L., et al. Deep brain stimulation for cluster headache. J Clin Neurosci. 2009;16(7):861-866.
58. Oakley J.C., Ojemann G.A. Effects of chronic stimulation of the caudate nucleus on a preexisting alumina seizure focus. Exp Neurol. 1982;75(2):360-367.
59. Sramka M., Chkhenkeli S.A. Clinical experience in intraoperational determination of brain inhibitory structures and application of implanted neurostimulators in epilepsy. Stereotact Funct Neurosurg. 1990;54-55:56-59.
60. Chkhenkeli S.A., Sramka M., Lortkipanidze G.S., et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106(4):318-329.
61. Cukiert A., Baumel S.W., Andreolli M. Marino Jr R. Effects of corpus callosum stimulation on the morphology and frequency of epileptic bursts in the feline topical penicillin generalized model. Stereotact Funct Neurosurg. 1989;52(1):18-25.
62. Feinstein B., Gleason C.A., Libet B. Stimulation of locus coeruleus in man. Preliminary trials for spasticity and epilepsy. Stereotact Funct Neurosurg. 1989;52(1):26-41.
63. Bittar R.G., Burn S.C., Bain P.G., et al. Deep brain stimulation for movement disorders and pain. J Clin Neurosci. 2005;12(4):457-463.
64. Joint C., Nandi D., Parkin S., et al. Hardware-related problems of deep brain stimulation. Movement Disord. 2002;17(suppl 3):S175-S180.
65. Papanastassiou V., Rowe J., Scott R., et al. Use of the Radionics Image Fusion and Stereoplan programs for target localization in functional neurosurgery. J Clin Neurosci. 1998;5(1):28-32.
66. Orth R.C., Sinha P., Madsen E.L., et al. Development of a unique phantom to assess the geometric accuracy of magnetic resonance imaging for stereotactic localization. Neurosurgery. 1999;45(6):1423-1431.
67. Schaltenbrand G., Wahren W. Atlas for Stereotaxy of the Human Brain. Stuttgart: Thieme; 1977.
68. Gillingham F.J., Watson W.S., Donaldson A.A., Cairns V.M.. Stereotactic lesions for the control of intractable epilepsy. Acta Neurochir (Wien), 1976;suppl 23:263-269
69. Pereira E.A., Green A.L., Nandi D., Aziz T.Z. Deep brain stimulation: indications and evidence. Expert Rev Med Devices. 2007;4(5):591-603.
70. Lyons K.E., Wilkinson S.B., Overman J., Pahwa R. Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology. 2004;63(4):612-616.
71. Litt B., Echauz J. Prediction of epileptic seizures. Lancet Neurol. 2002;1(1):22-30.
72. Osorio I., Frei M.G., Sunderam S., et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57(2):258-268.
73. Fountas K.N., Smith J.R. A novel closed-loop stimulation system in the control of focal, medically refractory epilepsy. Acta Neurochir Suppl. 97(Pt 2), 2007. 357–262
74. Fountas K.N., Smith J.R., Murro A.M., et al. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 2005;83(4):153-158.